Understanding the Inhibition Mechanism of Lignin Adsorption to Cellulase in Terms of Changes in Composition and Conformation of Free Enzymes
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Enzymatic Lignin Samples
2.3. Lignin Adsorption
2.4. Characterization of Enzymatic Lignin
2.5. Characterization of Free Enzyme
3. Results and Discussion
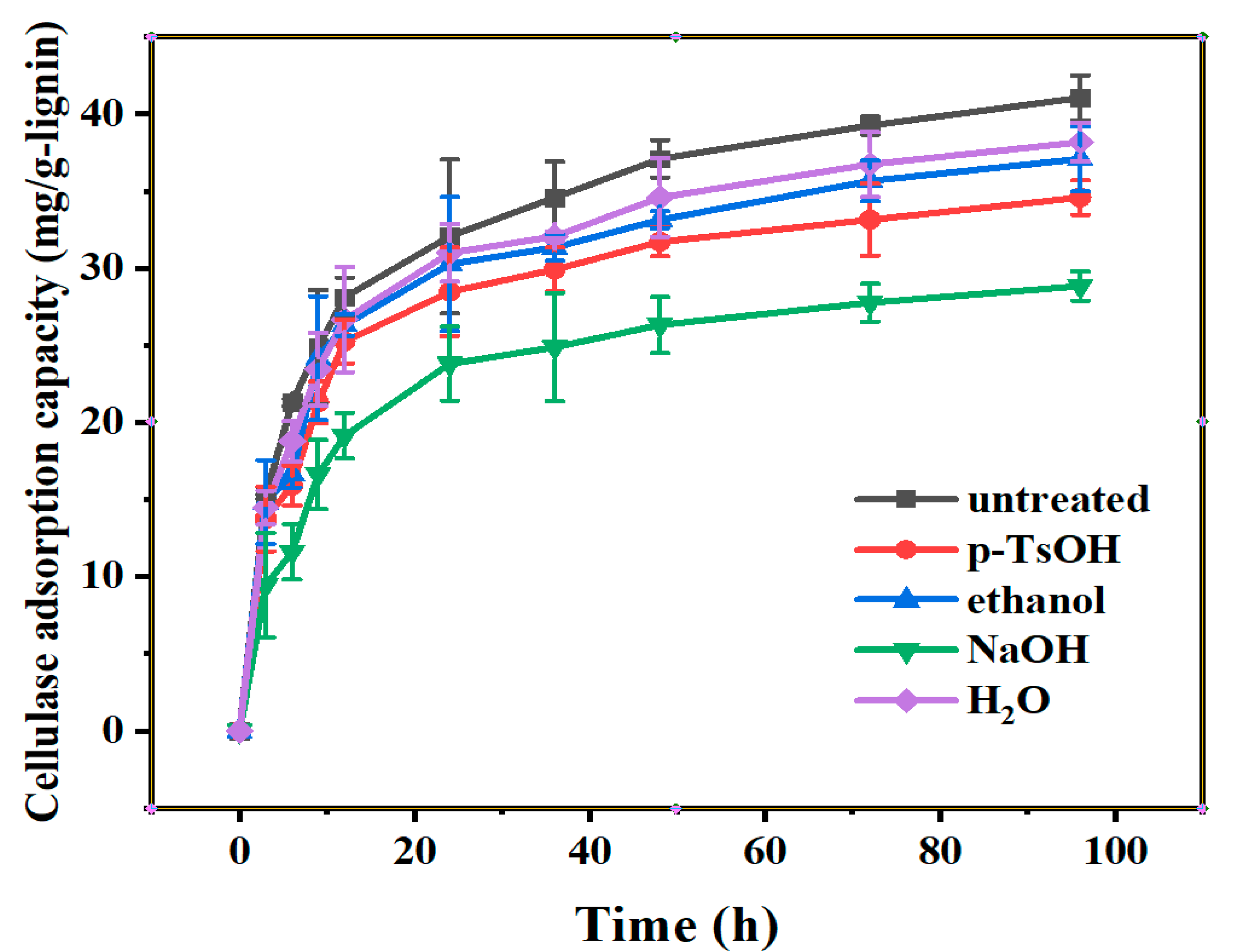
3.1. The Adsorption Curves of Lignin to Cellulase
3.1.1. Adsorption Curves of Cellulase on Lignin
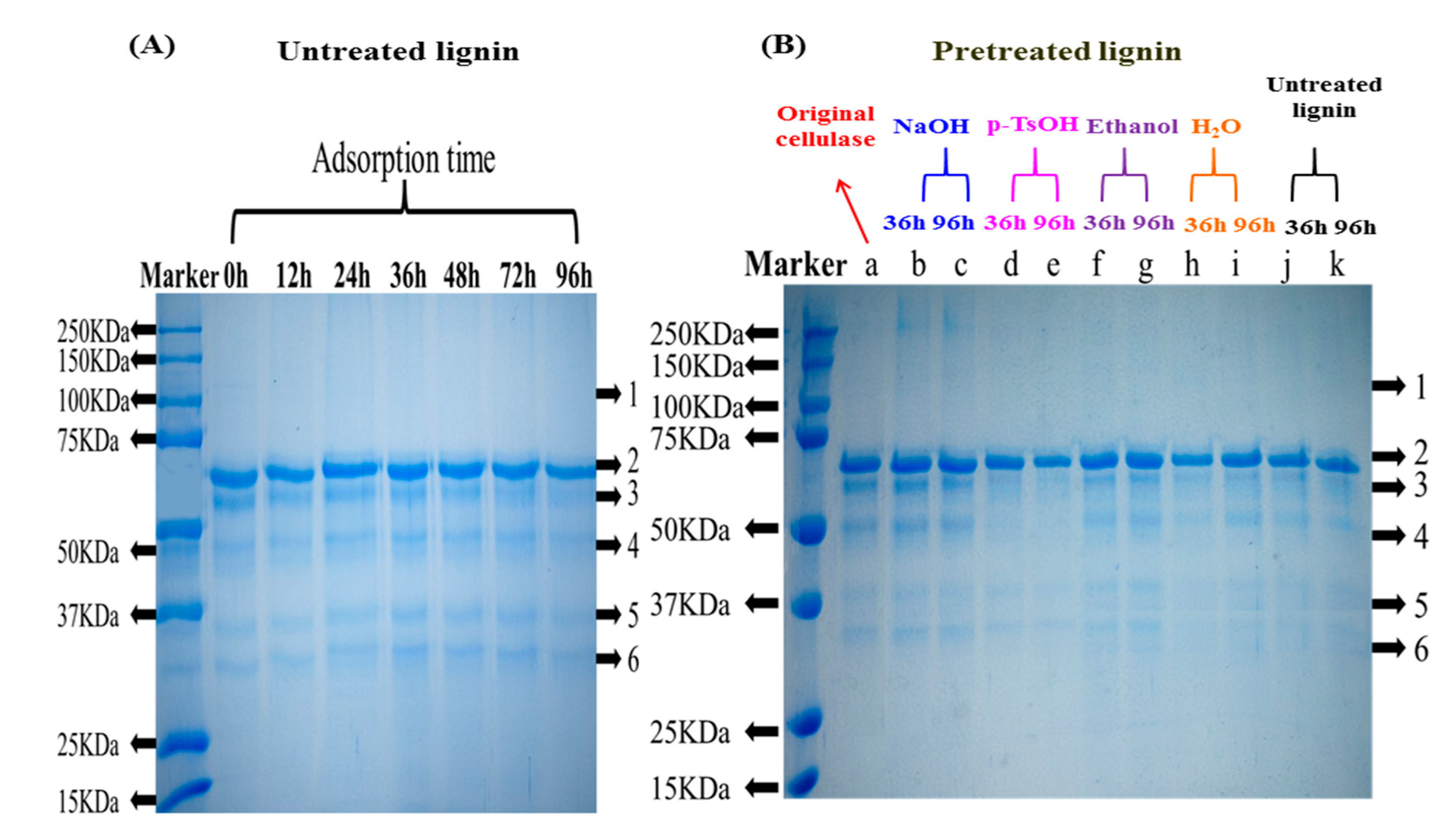
3.1.2. Composition Analysis of Free Enzymes
3.2. The Characterization of Lignin
3.2.1. Composition Analysis of Lignin
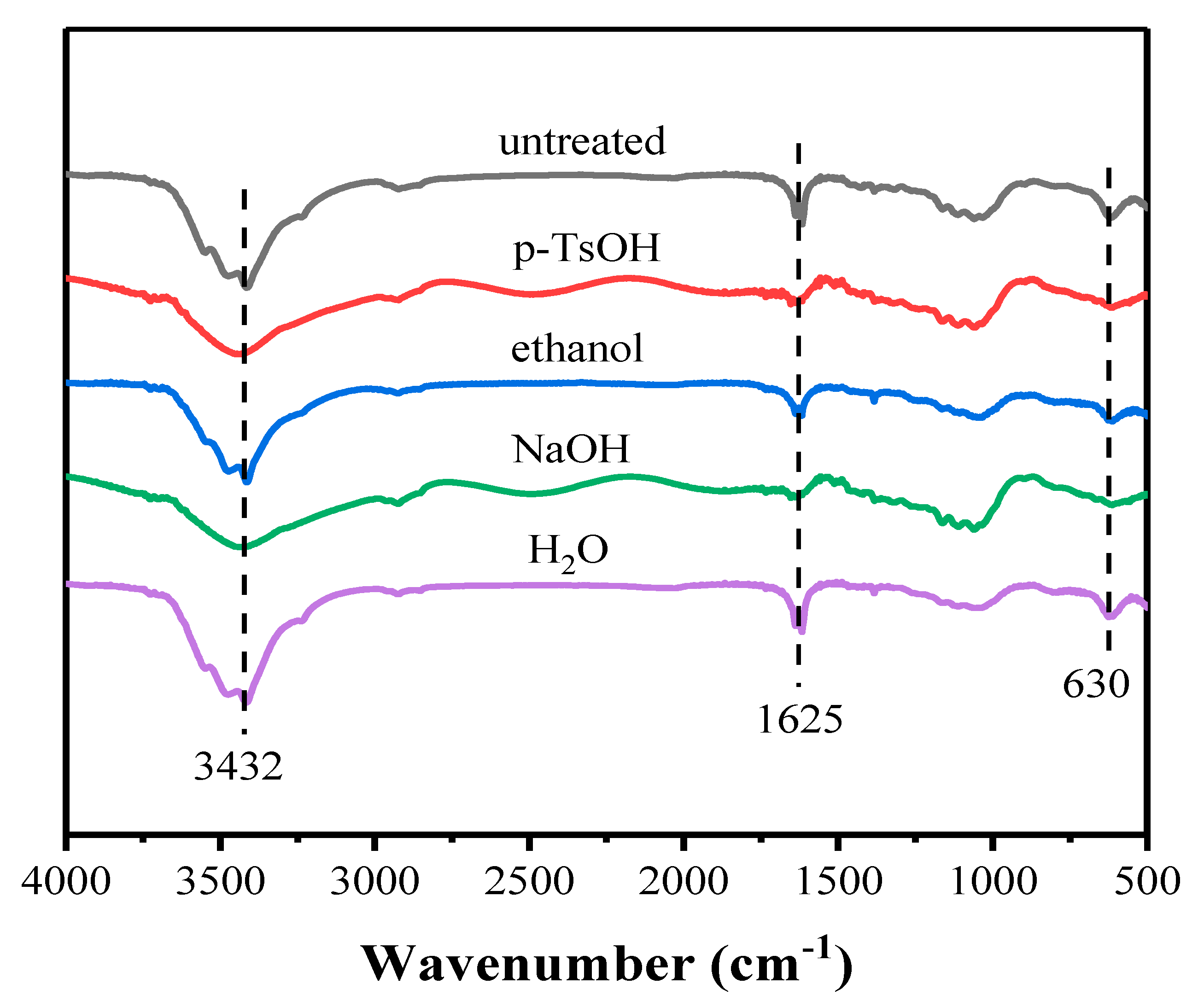
3.2.2. FT-IR Analysis of Lignin
3.2.3. XPS Analysis of Lignin
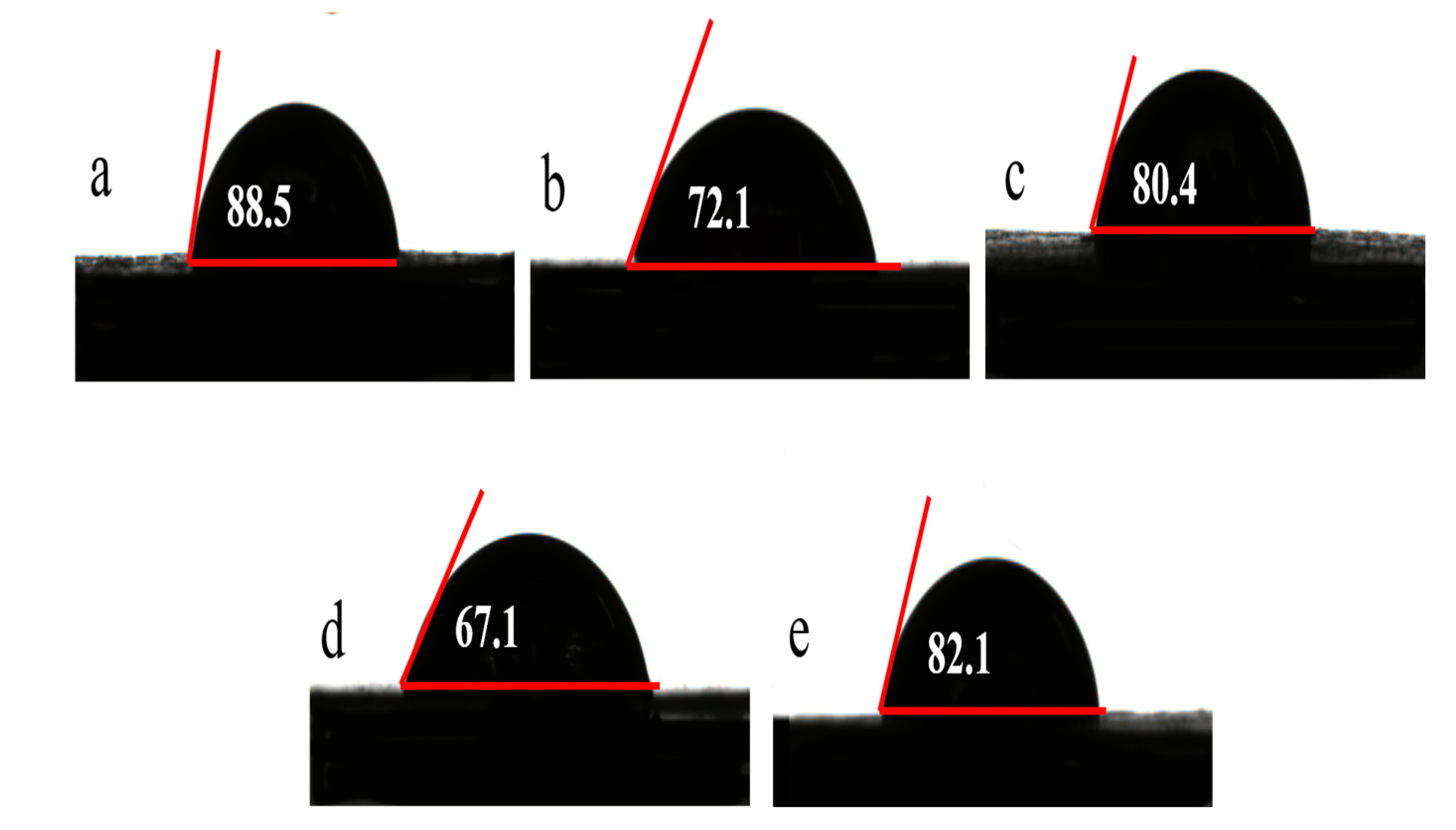
3.2.4. Contact Angle and Zeta Potential of Lignin
3.3. The Characterization of free Enzymes after Lignin Adsorption
3.3.1. Fluorescence Spectroscopy of Free Enzymes
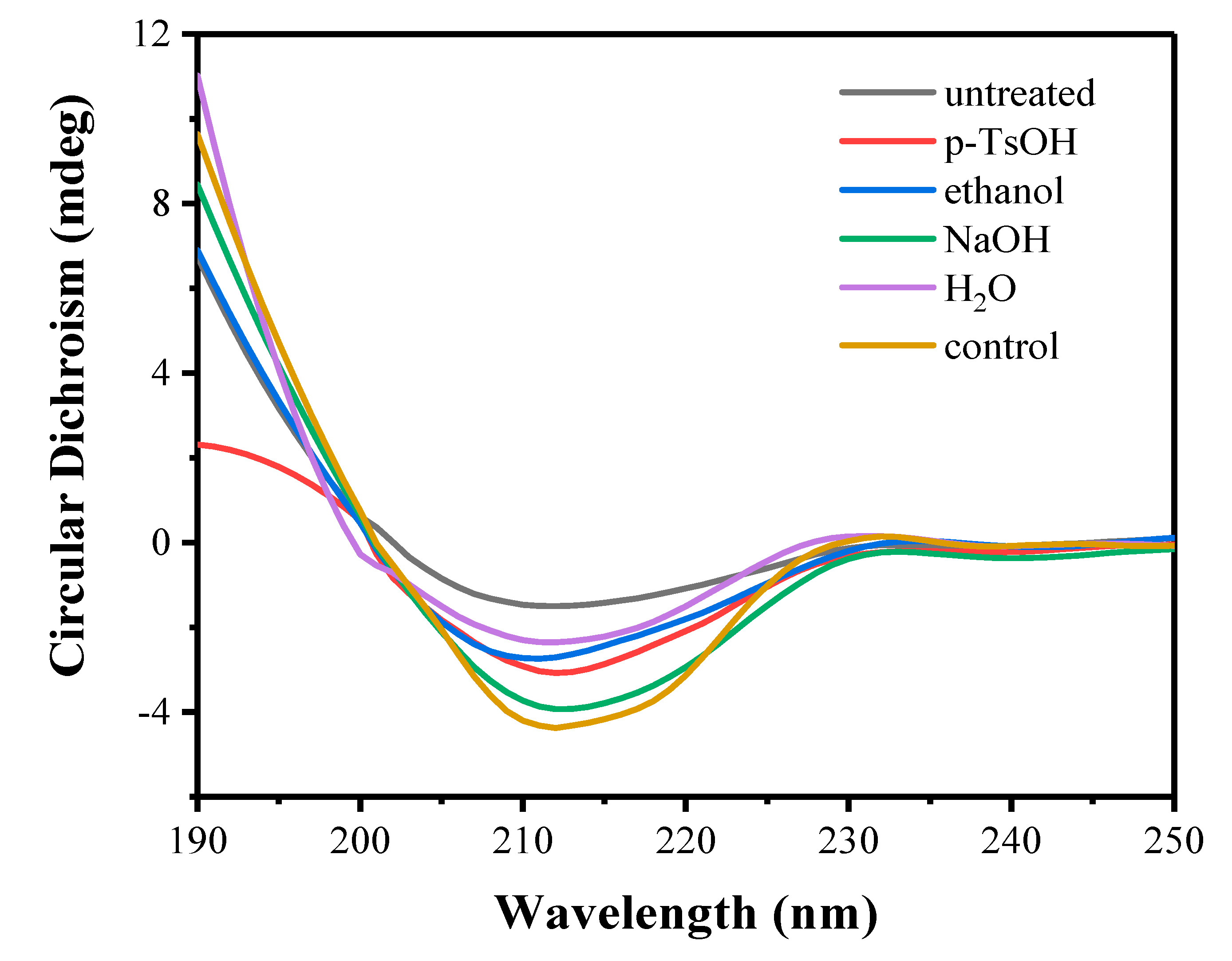
3.3.2. Circular Dichroism of Free Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Kim, S.-H. Lignocellulosic biomass as renewable feedstock for biodegradable and recyclable plastics production: A sustainable approach. Renew. Sustain. Energy Rev. 2022, 158, 112130. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Hou, J.; Ni, Y.; Liu, S.; Liu, Y.; Yu, S.; Nie, S.; Wu, Q.; Wu, C. Efficient Fractionation of Corn Stover for Biorefinery Using a Sustainable Pathway. ACS Sustain. Chem. Eng. 2020, 8, 3454–3464. [Google Scholar] [CrossRef]
- Su, Y.; Huang, C.; Lai, C.; Yong, Q. Green solvent pretreatment for enhanced production of sugars and antioxidative lignin from poplar. Bioresour. Technol. 2021, 321, 124471. [Google Scholar] [CrossRef] [PubMed]
- Rajak, R.C.; Saha, P.; Singhvi, M.; Kwak, D.; Kim, D.; Lee, H.; Deshmukh, A.R.; Bu, Y.; Kim, B.S. An eco-friendly biomass pretreatment strategy utilizing reusable enzyme mimicking nanoparticles for lignin depolymerization and biofuel production. Green Chem. 2021, 23, 5584–5599. [Google Scholar] [CrossRef]
- Saini, J.K.; Patel, A.K.; Adsul, M.; Singhania, R.R. Cellulase adsorption on lignin: A roadblock for economic hydrolysis of biomass. Renew. Energy 2016, 98, 29–42. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Martin-Sampedro, R.; Heikkinen, H.; Rovio, S.; Marjamaa, K.; Tamminen, T.; Rojas, O.J.; Kruus, K. Inhibitory effect of lignin during cellulose bioconversion: The effect of lignin chemistry on non-productive enzyme adsorption. Bioresour. Technol. 2013, 133, 270–278. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, R.; Tong, W.; Jin, Y.; Hu, J.; Song, K. Improving Enzymatic Saccharification and Ethanol Production from Hardwood by Deacetylation and Steam Pretreatment: Insight into Mitigating Lignin Inhibition. ACS Sustain. Chem. Eng. 2020, 8, 17967–17978. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Lu, Z.; Zhang, J. Adsorption and desorption of cellulase on/from enzymatic residual lignin after alkali pretreatment. Ind. Crop Prod. 2020, 155, 112811. [Google Scholar] [CrossRef]
- Yu, H.; Hou, J.; Yu, S.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Jiang, J.; Nie, S. Comprehensive understanding of the non-productive adsorption of cellulolytic enzymes onto lignins isolated from furfural residues. Cellulose 2019, 26, 3111–3125. [Google Scholar] [CrossRef]
- Nakagame, S.; Chandra, R.P.; Saddler, J.N. The effect of isolated lignins, obtained from a range of pretreated lignocellulosic substrates, on enzymatic hydrolysis. Biotechnol. Bioeng 2010, 105, 871–879. [Google Scholar] [CrossRef]
- Kellock, M.; Rahikainen, J.; Marjamaa, K.; Kruus, K. Lignin-derived inhibition of monocomponent cellulases and a xylanase in the hydrolysis of lignocellulosics. Bioresour. Technol. 2017, 232, 183–191. [Google Scholar] [CrossRef]
- Siqueira, G.; Arantes, V.; Saddler, J.N.; Ferraz, A.; Milagres, A.M.F. Limitation of cellulose accessibility and unproductive binding of cellulases by pretreated sugarcane bagasse lignin. Biotechnol. Biofuels 2017, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Qin, C.; Clarke, K.; Li, K. Interactive forces between lignin and cellulase as determined by atomic force microscopy. Biotechnol. Biofuels 2014, 7, 65. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Rahikainen, J.L.; Evans, J.D.; Mikander, S.; Kalliola, A.; Puranen, T.; Tamminen, T.; Marjamaa, K.; Kruus, K. Cellulase-lignin interactions-the role of carbohydrate-binding module and pH in non-productive binding. Enzym. Microb Technol. 2013, 53, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Fu, H.; Lee Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Rahikainen, J.; Mikander, S.; Marjamaa, K.; Tamminen, T.; Lappas, A.; Viikari, L.; Kruus, K. Inhibition of enzymatic hydrolysis by residual lignins from softwood--study of enzyme binding and inactivation on lignin-rich surface. Biotechnol. Bioeng 2011, 108, 2823–2834. [Google Scholar] [CrossRef]
- Wang, J.; Hao, X.; Wen, P.; Zhang, T.; Zhang, J. Adsorption and desorption of cellulase on/from lignin pretreated by dilute acid with different severities. Ind. Crop Prod. 2020, 148, 112309. [Google Scholar] [CrossRef]
- Yoo, C.G.; Li, M.; Meng, X.; Pu, Y.; Ragauskas, A.J. Effects of organosolv and ammonia pretreatments on lignin properties and its inhibition for enzymatic hydrolysis. Green Chem. 2017, 19, 2006–2016. [Google Scholar] [CrossRef]
- Pang, C.; Xie, T.; Lin, L.; Zhuang, J.; Liu, Y.; Shi, J.; Yang, Q. Changes of the surface structure of corn stalk in the cooking process with active oxygen and MgO-based solid alkali as a pretreatment of its biomass conversion. Bioresour. Technol. 2012, 103, 432–439. [Google Scholar] [CrossRef] [PubMed]
- van de Weert, M. Fluorescence quenching to study protein-ligand binding: Common errors. J. Fluoresc. 2010, 20, 625–629. [Google Scholar] [CrossRef]
- Anand, U.; Jash, C.; Boddepalli, R.K.; Shrivastava, A.; Mukherjee, S. Exploring the mechanism of fluorescence quenching in proteins induced by tetracycline. J. Phys. Chem. B 2011, 115, 6312–6320. [Google Scholar] [CrossRef]
- Payne, C.M.; Bomble, Y.J.; Taylor, C.B.; McCabe, C.; Himmel, M.E.; Crowley, M.F.; Beckham, G.T. Multiple functions of aromatic-carbohydrate interactions in a processive cellulase examined with molecular simulation. J. Biol. Chem. 2011, 286, 41028–41035. [Google Scholar] [CrossRef]
- Nakamura, A.; Tsukada, T.; Auer, S.; Furuta, T.; Wada, M.; Koivula, A.; Igarashi, K.; Samejima, M. The tryptophan residue at the active site tunnel entrance of Trichoderma reesei cellobiohydrolase Cel7A is important for initiation of degradation of crystalline cellulose. J. Biol. Chem. 2013, 288, 13503–13510. [Google Scholar] [CrossRef]


| Methods | Temperature (°C) | Concentration (%) | Glucan (%) | Xylan (%) | Klason Lignin (%) | Acid-Soluble Lignin (%) | Ash (%) |
|---|---|---|---|---|---|---|---|
| Untreated | - | - | 5.36 | 3.91 | 80.72 | 1.10 | 2.86 |
| p-TsOH | 100 | 30 | 1.51 | 3.24 | 86.67 | 0.41 | 4.02 |
| Ethanol | 180 | 50 | 3.99 | 3.66 | 84.64 | 1.03 | 3.00 |
| NaOH | 80 | 2 | 0.36 | 2.82 | 93.40 | 0.87 | 0.97 |
| H2O | 180 | - | 4.43 | 3.71 | 81.39 | 0.80 | 1.96 |
| Lignin Samples | C1 (%) | C2 (%) | C3 (%) | C4 (%) |
|---|---|---|---|---|
| Untreated | 39.56 | 30.92 | 17.33 | 12.19 |
| p-TsOH | 48.31 | 33.41 | 9.33 | 8.95 |
| Ethanol | 48.25 | 32.25 | 13.68 | 5.82 |
| NaOH | 57.33 | 28.59 | 8.72 | 5.35 |
| Hot water | 45.07 | 30.44 | 16.08 | 8.42 |
| Lignin Samples | Zeta (mV) |
|---|---|
| Untreated | −14.50 ± 1.42 |
| p-TsOH | −9.21 ± 3.13 |
| Ethanol | −10.20 ± 1.77 |
| NaOH | −6.71 ± 2.50 |
| H2O | −13.50 ± 1.87 |
| Lignin Samples | Enzyme | α-Helix (%) | β-Sheet (%) | β-Turns (%) | Unordered (%) |
|---|---|---|---|---|---|
| untreated | Cell | 15.80 | 35.40 | 16.80 | 33.10 |
| p-TsOH | Cell | 20.30 | 33.30 | 17.80 | 30.50 |
| ethanol | Cell | 19.50 | 32.40 | 16.30 | 33.70 |
| NaOH | Cell | 24.50 | 27.80 | 17.70 | 29.60 |
| H2O | Cell | 21.10 | 28.00 | 17.60 | 33.40 |
| control | Cell | 26.00 | 27.30 | 18.40 | 29.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, C.; Yan, C.; Wang, A.; Chen, C.; Chen, D.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.; et al. Understanding the Inhibition Mechanism of Lignin Adsorption to Cellulase in Terms of Changes in Composition and Conformation of Free Enzymes. Sustainability 2023, 15, 6057. https://doi.org/10.3390/su15076057
Cui C, Yan C, Wang A, Chen C, Chen D, Liu S, Li L, Wu Q, Liu Y, Liu Y, et al. Understanding the Inhibition Mechanism of Lignin Adsorption to Cellulase in Terms of Changes in Composition and Conformation of Free Enzymes. Sustainability. 2023; 15(7):6057. https://doi.org/10.3390/su15076057
Chicago/Turabian StyleCui, Can, Cancan Yan, Ailin Wang, Cui Chen, Dan Chen, Shiwei Liu, Lu Li, Qiong Wu, Yue Liu, Yuxiang Liu, and et al. 2023. "Understanding the Inhibition Mechanism of Lignin Adsorption to Cellulase in Terms of Changes in Composition and Conformation of Free Enzymes" Sustainability 15, no. 7: 6057. https://doi.org/10.3390/su15076057
APA StyleCui, C., Yan, C., Wang, A., Chen, C., Chen, D., Liu, S., Li, L., Wu, Q., Liu, Y., Liu, Y., Nie, G., Jiang, X., Nie, S., Yao, S., & Yu, H. (2023). Understanding the Inhibition Mechanism of Lignin Adsorption to Cellulase in Terms of Changes in Composition and Conformation of Free Enzymes. Sustainability, 15(7), 6057. https://doi.org/10.3390/su15076057







