Abstract
Industrial sites are typically located in close proximity to bodies of water, making industrial wastewater a prevalent source of pollution. Microplastics, which are plastic fragments generated from everyday activities or industrial operations and are smaller than 5 mm in size, can readily find their way into wastewater treatment plants (WWTPs). The objective of this research was to offer extensive insight into the fate of microplastics in industrial WWTPs worldwide, as well as to explore the effectiveness of diverse advanced treatment technologies in eliminating microplastics. The prevalence of microplastics and their negative impact on aquatic environments has been acknowledged in recent years. The progressive discharge of plastic waste, insufficient detection processes with specialized elimination methods and a sluggish disposal rate have led to the continuous presence of microplastics in various ecosystems worldwide, such as domestic wastewater and industrial wastewater. Research outcomes have revealed that they can adsorb a variety of pathogens, heavy metals and chemical substances that are commonly used in production processes. Microplastics can be consumed by aquatic life, which might lead them up the food chain to human bodies, resulting in potential digestion tract blockage, digestion disturbance and diminished reproductive growth. Microplastics have thus become a growing threat and cause for concern, demanding the containment of their dispersion. This work offers a critical evaluation of current and developing techniques for microplastic detection and separation from industrial wastewater, which are the most challenging endeavors when treating systems containing microplastics. A review of the effect of microplastics on aquatic environments and human health is also conducted. This analysis offers a comprehensive view of the full microplastic detection and removal strategies and their related concerns in order to establish a waste disposal standard that minimizes the potential hazardous effects of microplastics in aquatic systems.
1. Introduction
The pollution of aquatic ecosystems with plastic has been a pressing and enduring concern [1]. The lightweight nature of plastic enables it to be carried over vast distances by wind and ocean currents [2]. ISO technical report 21,960 provides a definition of microplastics, referring to particles ranging from 1 µm to 1 mm in size [3] which may be categorized into primary and secondary types [4,5]. Among microplastics, particles with dimensions from 1 mm to 5 mm are known as large microplastics. On the other hand, particles with dimensions in the range of 1 nm to 1 µm are defined as nanoplastics [3]. The former are deliberately constructed to be microscopic and can be found in various cosmetic products, while the latter result from the degradation of larger debris.
Industrial and residential activity are responsible for microplastic (MP) pollution [6,7]. The correlation of the level of industrialization to MP is widely accepted [8]. According to research [9,10] and model studies [11], industrial microplastic emissions are higher than from other areas. Industrial waste discharge is one of the leading causes of MP pollution in rivers [12] and oceans [13]. Microplastics are found in various environments [14], including the atmosphere [15], ground [16], ocean [17] and freshwater [18]. due to their small dimensions and high specific surface area [19], MPs are capable of adsorbing various pollutants, including heavy metals [20], polycyclic hydrocarbons [21], polybrominated diphenyl ethers [22], drugs and personal care products, from the environment [20,22,23]. Moreover, they offer a large surface area for the adsorption of various chemicals [24,25]. These particles, in addition to nanoplastics, which can potentially enter cells and disrupt cellular processes, are of great concern for environmental removal efforts [26]. There is a lack of literature regarding the impact of industrial sources on the microplastic contamination of urban wastewater, as indicated by prior studies [27]. As a result, researchers have shifted their attention toward identifying and regulating the sources of MPs, particularly in industrial zones [28,29]. In order to effectively manage MP pollution, it is crucial to obtain a deeper understanding of the significance of their sources and means of reaching sewer systems [11].
The effluents of urban WWTPs or facilities treating sewage are known to be significant recipients of microplastic (MP) discharges, which can consist of primary and secondary microplastics [30]. The appearance of microplastics has been observed in both the influent (ranging from approximately 645 to 1567 MPs/L) and effluent (ranging from approximately 16 to 131 MPs/L) of WWTPs, as reported in a study cited in Franco et al. [31]. Additionally, recent research has revealed the existence of MPs in sludge (ranging from 2742 to 15385 MPs/kg) [32]. Although numerous factors [33] can influence the quantity, composition and morphology of microplastics, the main factor is the source of the MPs [34]. Typically, MPs found in urban wastewater arise from daily activities, such as the use of products such as toothpaste, cleansers and shower gels [35]. Additionally, plastics in waste can be broken down by microorganisms in the leachate and ultimately end up in WWTPs [36].
Information about the variety of microplastics (MPs) from different sources is scarce. Specifically, there is limited knowledge about the traits of microplastics in industrial wastewater [37]. Although the concentrations of microplastics (MPs) in treated wastewater are lower than in non-treated wastewater, they still contribute significantly to environmental contamination [13]. Research by Conley et al. [38] estimated that three wastewater treatment plants (WWTPs) in the USA release 500–1000 million MPs daily. Similarly, Wolff et al. [39] reported the release of approximately 3000 to 5900 MPs/m3 of treated effluent from a German plant. Various techniques have been studied for removing MPs from water, including coagulation/flocculation, sedimentation, magnetic extraction and membrane filtration, which can reduce the quantity of MPs by approximately 80% [40]. Nanofiltration (NF) and reverse osmosis (RO) were previously also utilized in potable water treatment; however, they are prone to fouling caused by MPs [41]. Despite the availability of advanced treatment technologies, further optimization is needed before they are mandated by wastewater treatment legislation [42]. There are still gaps in understanding the effectiveness of wastewater treatment processes in eliminating MPs and the effect of MPs on these processes.
Moreover, there are inadequate data on the heterogeneity of microplastics (MPs) originating from diverse sources, particularly with respect to the attributes of microplastics found in industrial wastewater [43]. As a result, the exact share of contamination owed to industrial sources remains imprecise [44]. One possible explanation for this is that only a limited number of countries have established policies on these issues, resulting in MP pollution becoming a worldwide issue, as stated in [45]. The presence and distribution of microplastics (MPs) in various settings have been thoroughly studied, but their causes and fates in certain cases are still unclear [32]. Wastewater treatment plants (WWTPs) are some of the significant contributors of MPs [46]. However, while removal methods for other pollutants have been thoroughly investigated, there are still gaps in the knowledge regarding emerging pollutants such as MPs [32]. The detection and characterization of microplastics in the environment poses a multifaceted problem that has yet to be fully resolved. Early efforts in this area delved into characterizing the extent of microplastic pollution, but the research since then has broadened to include investigations into the processes and fate of microplastics [47]. The high quantities detected in the environment underscore the importance of conducting studies on their global occurrence and distribution [27]. Furthermore, the development of effective removal technologies remains a significant challenge in preventing the release of large quantities of MPs into the environment [48].
Acquiring a comprehensive understanding of the behavior of microplastics in wastewater treatment plants (WWTPs) is of the utmost urgency, as WWTPs are widely recognized as the primary contributors of microplastic contamination in aquatic ecosystems. [29]. The main objective of this work was to comprehend the fate of microplastics in industrial treatment plants globally, in addition to ascertaining the microplastics removal efficiency of different advanced treatments. This review aims to address this gap by examining literature from various WWTPs in seven countries to provide reliable and comprehensive information on the characteristics of microplastics and their removal technologies. The selection of different types of WWTPs serves a threefold purpose: (i) to gain an in-depth understanding of MPs in different WWTPs, (ii) to analyze the elimination patterns of different microplastic shapes, polymer categories and particle sizes and (iii) to evaluate treatment technologies worldwide. This work is unique in its objective to review current works and present up-to-date information on the efficiency of microplastics removal, highlighting the factors that influence the process and providing informative results to better comprehend the fate of microplastics in WWTPs.
2. Fate of Microplastics
The fate of microplastics (MPs) is determined by a variety of factors, such as biodegradation, physical abrasion and chemical oxidation [49]. The interplay between these processes and the transport of MPs determines their fate [50]. To evaluate the environmental hazards of MPs, regulators and researchers rely on environmental fate modeling [51]. There are three primary fates for MPs: aquatic environments [52,53], air environments (e.g., humans and sea birds) [54] and terrestrial environments (such as soil and landfills) [55]. The fate of MPs in different environments is affected by a range of natural factors, such as temperature and sunlight, and their physicochemical properties, including size and density [56]. The characteristics of microplastic polymers play a crucial role in their persistence, fate, degradation and ability to adsorb or release organic contaminants [9]. Understanding the fate of MPs is complex and challenging due to the tedious and cost-intensive analysis involved [57]. Several studies have explored the partitioning of microplastics during wastewater treatment [35,38,58,59]. Specifically, the fate of microplastics is largely dependent on their physicochemical properties and the treatment they undergo. Adsorption, for instance, refers to the transformation of pollutants from a liquid to a solid phase; thus, the fate of adsorbed substances is linked to the fate of solids (e.g., agriculture) [60].
There are numerous aspects that have an effect on the transportation of microplastics in water systems, including their density, shape, size and interactions with biota. Additionally, various characteristics [61] of the host environment, such as how fast the flow is, how deep it is, the topography, weather conditions and human activities in the vicinity [41], can also play a role. Different studies have reported varying levels of efficiency in removing MPs from water, with removal rates ranging from about 60% to almost 100%. The efficiency of removing MPs is affected by a range of factors, such as the plant layout and technology, influent attributes, pre- and primary treatments and secondary treatments.
Ngo et al. [62] noticed that fibers are usually not easy to remove. Additionally, polyethylene (PET) and polypropylene (PP) are commonly found in the influent but are often encountered in the effluent because of their low density, making them easier to remove by skimming. Fahrenfeld et al. [63] argued that films and fibers are the most successfully eliminated items. This discrepancy is likely due to differences in plant topography and research protocols followed during the studies.
In pretreatments and primary treatments, the elimination efficiency can vary from 40.7% to 91.7% [62], depending on whether an aerated grit chamber is present or not and on the polymer categories detected in the influent. Fibers are often the least-removed items at these steps. Pre-treatments have the highest impact on the relative size distribution of MPs, as they are able to remove particles of a larger size. Secondary treatments, such as activated sludge and sedimentation, can lead to removal efficiencies in the range of 28.1% to 66.7% [62], depending on their diverse configurations and retention time. Longer retention times can boost the development of biofilms on microplastics, leading to a higher weight and thus better results. Some studies have reported a total removal rate of additional treatments reaching higher than 90% [64,65]. Nevertheless, further research is required on this subject.
Fragment removal appears to be higher than fiber removal in secondary treatments, with some studies reporting higher amounts of fibers after secondary treatments. This discrepancy may be attributed to undiscovered interactions among microplastics, microbes and flocculants. Additionally, recent studies have confirmed that secondary treatments have lower elimination rates for fibers. In some studies [66,67], the relative abundance of fibers in the effluent was found to be at higher levels than in the influent [68], which may due to the diverse sampling mechanisms applied to each stream.
In conclusion, the efficiency of MPs removal from water is influenced by multiple factors, and different types of MPs may be more or less effectively removed depending on the treatment process and operational conditions. Additional research is required to comprehend the movements of MPs in water systems and to develop more effective treatment technologies for their elimination.
Ngo et al. [62] have stated that using a membrane biological reactor (MBR) is the most efficient option for the extraction of MPs, steadily achieving an elimination rate higher than 99.5%. However, the researchers have emphasized the importance of studying fouling after a certain time of operation [62]. Bayo et al. [69] observed that during an 18-month period in a WWTP in Spain, the MBR method demonstrated better removal rates for MPs than the typical activated sludge (CAS) and rapid sand filtration (RSF) process (79% vs. 75%), although there was no statistical significance in the difference. This work found that the elimination of MPs was not as high as expected, but it was comparable to findings by Raju et al. [66]. Bayo et al. [69] observed considerable differences in elimination rates when they used activated sludge and rapid sand filtration in combination with an MBR. Sun et al. [13] have confirmed that membrane filtration is considered the most effective for MPs removal. Membrane fouling is a major challenge associated with membrane processes. Microplastics (MPs) can contribute to this issue because of their small dimensions and surface attributes. Inadequate control of fouling can lead to significant operational and financial concerns. As a result, many studies have been conducted to address membrane fouling [70]. Ngo et al. [62] found major inconsistencies in MP removal using RSF, and Sun et al. [13] discovered that biological aerated filters did not cause a significant change in MPs in the effluent. Park et al. found that wastewater treatment plants (WWTPs) utilizing advanced phosphorus removal techniques demonstrated a greater removal efficiency compared to those without such processes [68].
The research outcomes from Freeman et al. [45] and Sun et al. [13] suggest that polyester polyethylene MPs are the most common pollutants detected in effluents from WWTPs. Moreover, some authors have found that the smallest size classes of these microparticles have the highest frequency [64], while others have not confirmed this finding [67]. Bayo et al. [67] identified no significant change in effluent concentrations among seasons, but Mintenig et al. [71] noted a heightened concentration during a strong rain event. However, the impact on the size variations and polymeric variety have yet to be fully understood. The removal of MPs can be improved through pretreatment and primary treatments and secondary and tertiary treatments can also be effective, depending on the technology employed. Temporal patterns exist, but further research is needed to confirm them.
3. Water Treatment Technologies for the Removal of Microplastics
According to the literature [72], WWTPs are not created for the purpose of degrading plastic materials. However, there are various technologies available for removing microplastics (MPs) from wastewater, including pretreatments such as screening and grit chambers, initial sedimentation, activated sludge processes, coagulation, constructed wetlands and filtration. Based on their installation and maintenance costs, these technologies can be categorized from low-cost to high cost categories [73].
Figure 1 depicts a typical wastewater treatment plant (WWTP) system and the MPs/NPs elimination rates [7].
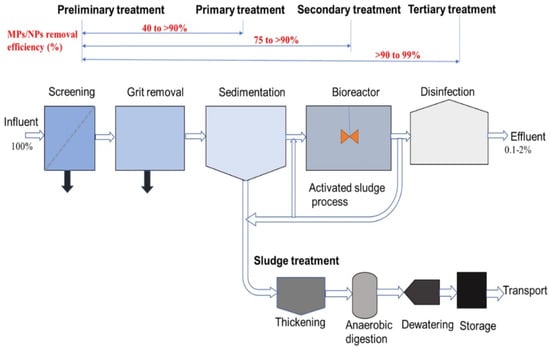
Figure 1.
Microplastics elimination rates in typical WWTPs. Reprinted with permission [7], Elsevier Ltd©.
3.1. Primary Treatment
Numerous studies have shown that primary treatment processes such as skimming and settling can significantly reduce the amount of microplastics found in WWTPs [65,74]. The elimination efficiency during this stage relies on the physical properties of the MPs, such as their density, dimensions and shape, and the characteristics of the water sample, such as the suspended solid concentration. Temperature plays a critical role in the implementation of a microplastics (MPs) procedure, as higher temperatures can enhance the efficiency of the oxidation process. However, there is a risk of modifying the characteristics of the plastic particles, potentially resulting in morphological alterations and causing them to break down [75]. Floatation and gravity settling affect the elimination of the MPs at this phase. Gravity can cause MPs with higher densities to settle quickly, while those with lower densities may float and be separated [66]. In the presence of elevated levels of suspended solids, microplastics (MPs) have the potential to aggregate, leading to increased sedimentation rates and the improved extraction of MPs from wastewater. As a result, this may help alleviate the burden of MPs on other treatment processes by reducing their overall load [67]. However, MPs connected to unstable flocs could potentially not settle as a solid bulk and may consequently escape, resulting in a lower removal of MPs [76]. In addition, the retention of MPs is influenced by the hydraulic retention time and turbulence. As primary treatment is highly effective at removing large and fragmented MPs [77], it should be the primary area of focus for improving the removal of MPs [78]. Retrofitting secondary plants with primary clarifiers can likewise enhance the efficiency of microplastics elimination [38], but this strategy might be affected by the locale, availability of land, current facility architecture, influent load on WWTPs and cost [38].
3.2. Secondary Treatment
Studies have shown that the activated sludge method is successful at eliminating MPs, suspended solids, nutrients and dissolved organic matter [59,79]. It has been observed that sludge contains more microfibers and foams (which are round or irregular in shape) than the secondary effluent, indicating a greater tendency for fibers to bio-foul in sludge. In contrast, small openings in foam surfaces attract particulates, thus augmenting their weight and allowing them to settle in the sludge quickly [66,80]. For granular MPs, settling is determined by Stoke’s law [81].
3.3. Tertiary Treatment
The existing treatment systems primarily aim to eliminate solids, organic substances, nutrients, pathogens and emerging pollutants, and they are not designed to specifically metabolize plastic materials [72]. Although the conventional treatment processes exhibit a reasonable level of MPs elimination, there are still some small-sized MPs in the effluent which can be extracted before discharge [72]. The available options were devised with the intention to eliminate the remaining solids and organics in the wastewater, including—among others—coagulation, membrane filtration and bioreactors, constructed wetlands and advanced oxidation processes [72]. Incorporating advanced treatment methods such as tertiary treatment is necessary to improve the elimination efficiency of MPs/NPs, resulting in low concentrations of MPs in the effluent from tertiary treatment systems [82].
Coagulation involves the destabilization of colloidal particles and their subsequent agglomeration in floccules through the addition of chemical reagents, succeeded by a physical separation. Coagulation neutralizes the charge of colloidal particles and facilitates sweep flocculation, resulting in stable flocs that settle quickly. The efficiency rate of coagulation is impacted by the physicochemical attributes of the material, such as its dimensions, shape, pH and organic content. The category and quantity of coagulant employed and the concentration of MPs in the water also impact the results of the method [7]. The existing literature provides information on the effectiveness of coagulation/flocculation for the removal of various microplastic particle types (including polyethylene, polyester etc.), each with diverse dimensions and shapes, either untreated or aged. The experiments employed frequently utilized coagulants such as iron and aluminum salts. Synthetic matrices with well-defined properties are frequently used to study the coagulation/flocculation mechanism under laboratory conditions [83].
The results obtained, however, differ based on the conditions of the coagulation process, the polymers and their properties, as well as the characteristics of the matrix in which microplastics are found [84]. In their research, Vasiljević et al. examined the use of FeCl3 for coagulation and flocculation in a synthetic matrix and reached an elimination efficiency of 67–99% for textile fibers as opposed to the starting concentration. The maximum rate was observed at a FeCl3 concentration of approximately 3.9 mM, whereas the minimum was at a concentration of 0.7 mM. With the exception of the highest dose, all applied doses exhibited similar removal efficiency values, indicating complete elimination. Using PACl as a coagulant resulted in marginally lower values ranging from approximately 57% to 91%. For the combination of coagulants, elimination efficiencies of 35% were reached for Fe/Al, whereas an elimination efficiency of 66% was achieved for Al/Fe and 78% for Fe + Al. These results suggest that combining coagulants does not improve the elimination rate of textile fibers as opposed to single materials [85].
Jachimowicz et al. studied the removal of microplastics (MPs) from wastewater in a wastewater treatment plant (WWTP), using different coagulants and flocculants at varying dosages prior to the initial primary. The study found that the best elimination rate (90%) was reached when using a dose of 2.5 mL PAX/m3, and the initial sludge produced had a low MP concentration that was easy to manage in ensuing treatment phases. At the higher dosage, PIX was able to substantially improve the elimination of P-PO4 (reaching 94%) and COD (approximately 73%) [86]. Mathieu Lapointe et al. have made a valiant effort to explain the coagulation and flocculation processes involved in microplastics (MPs) removal by studying both pristine and worn-down MPs. They found that worn-down MPs exhibited an altered surface chemistry and roughness, which in turn affected their affinity for coagulants and flocculants. The results showed that coagulant efficiency increased in every case of worn-down MPs. For instance, elimination rates of approximately 97% and 99% were noted for PEST and worn-down PE. However, larger, pristine PE microplastics posed the highest resistance to coagulation and flocculation, reaching an elimination of only 82%, even under optimal conditions [87]. In conclusion, the coagulation process appears to hold relatively new, promising and still-evolving ground, given its numerous benefits. Scientific research teams have been making strides toward advancing this method.
According to one study [88], the data reported on the removal of microplastics (MPs) through disc filters yielded inconsistent outcomes because of variations in filter sizes, types of MPs studied and the methods of characterization employed. Disc filters typically consist of fiber materials with varied mesh pore dimensions and are assumed to remove particles larger than the mesh size. These filters have been found to effectively eliminate MPs at rates ranging from 40 to 98.5%, in addition to fine particulate matter [88]. Larger MPs are retained on the filter, while small-sized ones may go through [7].
The use of sand filtration in tertiary wastewater treatment may require physicochemical and biological interactions to eliminate particulates, nutrients and microorganisms, as well as microplastics (MPs) and nanoplastics (NPs) [59]. Sand filters, including rapid and gravity sand filters, act as a fence, preventing the percolation of MPs and NPs. While very small pores between the sand grains trap larger MPs on the surface, smaller MPs may pass through [59]. The confinement of MPs on the filter surface depends on their size and the employment of coagulation as a first treatment stage to form flocs that can be retained by the sand layer. Studies have reported an MPs removal efficiency of up to 73.8% using coagulation and sand filtration [59,72].
Figure 2 depicts the effect of MPs on various WWTP treatments, ultimately leading to reduced treatment efficiency [7].
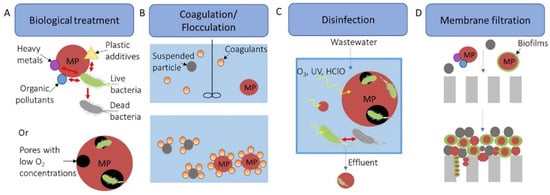
Figure 2.
Impact of microplastics on various treatments in WWTPs. Reprinted with permission. (A) Plastic additives and adsorbates in biological treatment have the potential to be detrimental to microorganisms. Additionally, microplastics with pores can provide a habitat for microorganisms, creating an environment with low oxygen levels that could impede the nitrification treatment process while promoting denitrification. (B) The coagulation/flocculation stage may require higher amounts of coagulants/flocculants due to the presence of microplastics. (C) Microplastics may interfere with disinfection by using up disinfectants or acting as a barrier to protect bacteria from disinfectants. (D) The effectiveness of membrane filtrations could be compromised by the presence of microplastics. Reprinted from Ref. [7], Elsevier Ltd©.
Ozone disinfection, another wastewater treatment mechanism, effectively destroys microorganisms and oxidizing compounds. Additionally, ozone has proven to be highly effective in removing micropollutants, including MPs, from wastewater, achieving a removal efficiency of almost 90% in some studies [59]. Constructed wetlands are considered a sustainable option for secondary or tertiary treatment in WWTPs, according to surface, horizontal subsurface or vertical flow. They have been found to enhance the elimination of MPs in WWTPs. Long et al. [89] verified removal efficiencies of approximately 26% and 10% for MPs in rural WWTPs using a horizontal subsurface flow, while Zhou et al. [90] verified a removal of MPs of more than 89%. The efficiency of constructed wetlands in removing MPs is affected by the plant’s architecture and environmental conditions. Membrane bioreactors (MBRs) combine bioreactors with filtration to eliminate suspended or dissolved inorganic and organic materials as well as MPs and NPs [91]. The mechanisms of MPs removal in MBRs include trapping the MPs and stopping them from infiltrating the effluent. Sedimentation is the main mechanism for removing larger particles in anaerobic conditions, while the removal mechanism for small-sized particles [92] in aerobic conditions is sludge adsorption interception.
Dissolved air flotation (DAF) can take place before or after the initial/secondary/final steps to remove lightweight particles, oils and greases. Although its main purpose is not to eliminate MPs and NPs, it has been found to have an efficiency of 95% in removing MPs [72]. Advanced oxidation processes are promising options for the tertiary treatment of WWTPs to mitigate the newly found contaminants, including MPs and NPs. Kang et al. [93] studied the decomposition of microplastics using magnetic carbon nanotubes (CNTs) under hydrothermal conditions; these release products that microorganisms can use as a source of carbon in the water. Membrane technology is effective in removing MPs from wastewater and has several advantages in treating various pollutants [32]. Microfiltration, ultrafiltration, nanofiltration and reverse osmosis can be utilized either as a secondary step or in combination with biological processes for the treatment of the primary effluent [94,95]. Considering the aforementioned details, it is evident that each treatment method has both advantages and limitations (Table 1) that are unique to the individual technique.

Table 1.
Advantages and drawbacks of treatment options used for the elimination of MPs from aquatic environments.
Overall, the removal process is affected by various factors, including the treatment technology, environmental conditions and the type of MP. Table 2 summarizes the location, MPs concentration in influent and effluent, MPs removal and primary treatment options of the WWTPs in the studies discussed above.

Table 2.
Information on the removal efficiencies of microplastics.
Bayo et al. [67] investigated the abundance, concentration and removal efficiency of MPs in a classical WWTP with an activated sludge process. The WWTP exhibited an efficiency of practically 90% in the final effluent, identifying more than 540 MPs, including fragments (~47%), films (~35%), beads, foam and fibers. The highest concentration was achieved by low- and high-density polyethylene (LDPE and HDPE).
Mintenig et al. [71] conducted a study to examine the appearance of microplastics (MPs) in the effluent of 12 plants in Germany. The WWTPs treated both municipal and industrial wastewater. The findings revealed that all the effluents contained MPs with sizes ranging from >500 μm to <500 μm. The concentration of microplastics larger than 500 μm varied between 0 and 5 × 101 m−3, while the concentration of microplastics that were smaller than that ranged from 1 × 101 to 9 × 103 m−3. There was no significant correlation between the number, size, or polymer type of MPs found in the different WWTPs. However, MPs larger than 500 μm were absent in the effluents of only two WWTPs, which were located in Schillig and Oldenburg (ap). Polyethylene (PE) was the most common plastic particle found in MPs > 500 μm, accounting for 59% of the total, followed by polypropylene (PP), with an average of 16%. In MPs < 500 μm, PE was also the prevailing polymer, accounting for 40% of the total [71].
Wang et al. [107] determined that the majority of the microplastics in the influent and effluent of various wastewater treatment plants in Changzhou, China, were polyethylene (PE), polypropylene (PP) and polystyrene (PS), comprising nearly 83% of all detected MPs. Most of these MPs were fragments and films less than 500 μm in size. Additionally, the study identified the presence of plastic particles in wastewater from four livestock farms; these particles were composed mainly of PE (38%) and polyamide (PAM) (32%). All the samples from the fish ponds contained MPs as well. There were no marked differences in the concentration of MPs from the various sources, implying that they could all potentially contribute to MPs pollution. However, there were variations in the size and color of the MPs detected in them, although there were no significant differences in the type and morphology of the polymer present [107].
In their study, Van Do et al. [103] examined the occurrence and dissemination of microplastics (MPs) in the influent and effluent of three typical industrial treatment facilities located in Danang city, Vietnam. The study showed that the quantity of MPs in the influent and effluent varied between approximately 180–440 and 140–340 particles/L, respectively. The average elimination rates of the three plants were found to be approximately 25%, 22% and 25% respectively [103]. The main types of polymers detected in the MPs were polyethylene terephthalate (PET), polyethylene (PE), nylon and polyvinyl chloride (PVC).
Dris et al. [104] observed the presence of MPs, mainly fibers, in total atmospheric fallout. High levels of fibers were also found in wastewater (260–320 × 103 particles/m3); however, after treatment, the contamination was reduced to approximately 15–50 × 103 particles m3. The study also investigated the river Seine, using two sampling devices. The findings indicated that the plankton net (0.8 cm mesh) mainly caught fibers.
Similarly, Franco et al. [27] examined influents and effluents from five municipal wastewater treatment plants (MWWTPs) and two industrial plants in Cadiz. The study showed that quantities of MPs were higher in industrial than in municipal plants, with elimination rates varying between 78% and 97%. The dimensions and shapes of the microplastics in the effluents were similar to those in the influents, implying that no shapes and sizes are preferred during the elimination process. The most frequently identified MPs in the influents were Polyvinyl chloride (PVC), high-density polyethylene (HDPE), Poly (ethyl methacrylate) (PEMA), Polypropylene (PP), Polystyrene (PS) and Polyethylene (PE). The study claimed that approximately 8.5 × 1011 microplastics may be released annually by the treated wastewaters [27].
Hidayaturrahman et al. [59] conducted a study to investigate the elimination efficiency of microplastics at various phases of treatment in three facilities and to evaluate the effectiveness of tertiary treatment using coagulation, ozone, membrane and rapid sand filtration. The study found that the first two treatments were successful in extracting MPs, with elimination rates varying between 75% and 92%. The percentage reached over 98% after the third treatment. The most frequently encountered MPs in all the samples were microbeads from personal care products and larger fragments of plastics. Microplastics were, however, still detected in significant quantities in the final effluent [59].
The results obtained by Long et al. [106] are consistent with those of this study. Long et al. used a better sampling process, which involved an electromagnetic flowmeter and a fast digital camera, to analyze sixty samples. The study found that the concentration of MPs in the influent ranged from 1.57 to 13.69 items/L, while in the effluent, approximately 79% to 98% of MPs were removed. The study estimated that approximately 6.5 × 108 MPs were released from the seven plants daily. The micro-Raman spectroscopic and light microscopic analyses revealed that plastic polymers accounted for approximately 62% of the particles, with polypropylene (~31%) and polyethylene (~22%) being the most commonly detected polymers. Additionally, the majority of the MPs were white (27%) or clear (about 26%) in color [106].
3.4. Color and Range of Size and Shape of MPs
The various dimensions, shapes and colors that were found in the collected samples from the WWTPs are described in Table 3.

Table 3.
Characteristics of MPs in industrial wastewater influent.
The final effluent, which is discharged from the WWTPs after undergoing various treatment processes, can exhibit different characteristics and concentrations of microplastics (MPs), which are determined by the treatment used. Therefore, it is essential to assess the behavior of MPs in treatment plants and the associated exposure risks in the environment through the disposed effluent or sludge. In WWTPs, the secondary treatment process, namely, biological treatment, is considered the most critical technology, with advanced oxidation being the most widely used method. Most WWTPs also employ additional treatments, such as advanced oxidation and membrane filtration, to further remove contaminants.
Microplastics are a diverse group of polymer mixtures with diverse dimensions and structure, and each shape has its unique physicochemical and toxicity properties [108]. The shape of microplastics is a significant classification factor and impacts their elimination efficiency in WWTPs [109]. Fibers, fragments and films are the most widely detected microplastics in wastewater [59,67]. Among the various shapes, fibers, which are filamentary microstructures, are the most frequently encountered types. Additionally, different types of polymers, including polyethene, polypropylene and polyester, have been identified as some of the most frequently identified microplastics in the wastewater [59,67].
The abundance of microplastics gradually decreases with additional treatments. Primary treatments, which rely on physical mechanisms, are the initial barrier to remove MPs in WWTPs and are more effective in extracting fiber microplastics because fibers are easily trapped through flocculation and settling [109]. The microplastic composition in the effluent of WWTPs is diverse [67,69], and even post-treatment, MPs are still detected in the resulting effluent and are continuously disposed of in bodies of water, thereby causing environmental pollution [59,72]. Therefore, these findings emphasize the importance of considering the environmental pollution caused by MPs/NPs released from WWTPs [59,71].
4. Challenges and Future Perspectives
The application of membrane technology has demonstrated impressive results in the elimination of MPs. However, the issue of membrane blocking and fouling must be addressed for large-scale implementation. The presence of other pollutants in wastewater may adsorb and interact with MPs through various mechanisms, such as electrostatic or hydrophobic interactions and ion exchanges. Further research is needed to explore surface modifications or coatings to enhance the sorption of MPs. Pilot studies and cost analyses are necessary to assess the feasibility of diverse treatment methods for mitigating MPs. The scientific community has reported concentrations of MPs from various WWTPs worldwide, but the standardization of sampling, purification and characterization processes is essential for producing reliable research results. Further analysis is needed to comprehend the concentration and characteristic profiles of MPs across different WWTPs. Geographical variations and human behaviors and developmental activities can be significant factors contributing to MPs pollution; thus, data from various regions can inform effective strategies for controlling MPs at their source.
5. Conclusions
Plastic pollution is a global problem that poses a significant threat to aquatic health. The presence of microplastics (MPs) is indicative of significant plastic pollution. As the global production and consumption of plastics continue to increase, the contamination of the environment with microplastics is expected to rise, leading to severe damage to ecosystems. Therefore, it is essential to control the emission of plastic at the source to prevent plastic pollution. Once plastics enter marine and freshwater environments, they break down into smaller pieces that can travel thousands of kilometers, causing harm to ecosystems far from the emission source. To address plastic pollution, an integrated and interdisciplinary approach that considers various disciplines is required. Recent research has shed light on the fate and effect of MPs on the environment.
There are two types of MPs: primary and secondary. Primary MPs are intentionally manufactured as micro-sized plastics, while secondary MPs are the byproduct of the fragmentation and degradation of larger plastics in the aquatic environment. Secondary MPs make up the majority of MPs found in aquatic environments. The issue of MPs generated in WWTPs of various sizes requires further investigation. Some microplastics from WWTPs can be transferred and disseminated in the aquatic environment, necessitating collaborations at local and global levels to create management plans that define the highest allowed levels of MPs in effluent. The efficiency of microplastic (MP) removal is influenced by their physical characteristics such as size, shape and density. Previous studies have shown that the most common types of MPs found in water samples are secondary microplastics, consisting of fibers and fragments, with the primary polymers being polyethylene terephthalate, polystyrene, polypropylene and polyethylene. The range of removal efficiency reported in the literature is 21.8–97.8%. In order to better understand the fate of microplastics in WWTPs and other environmental matrices, it is important to develop standardized methods for their sampling and analysis. Furthermore, research should focus on specific types of microplastics, especially in industrial areas, and their effects on plant roots. This study contributes to the critical understanding of treatment technologies for the removal of microplastics. Although treatment plants have demonstrated substantial removal efficiency, the complete removal of MPs has not been achieved. MP abundance has been observed to accumulate or be stored in WWTP sludge without proper treatment. Among the various shapes of MPs, fibers are the most commonly found MPs, while black, blue and red are the most frequently observed colors. To mitigate the risks posed, it is crucial to determine the sources of the polymers detected and their transportation pathways. However, additional research and established techniques are necessary to accurately identify these sources.
Author Contributions
Methodology, D.A.G., A.K.T., A.C.M., E.E., D.N.B., D.A.L., I.K.K. and G.Z.K..; writing—original draft preparation, D.A.G., A.K.T., A.C.M., E.E., D.N.B., D.A.L., I.K.K. and G.Z.K..; writing—review and editing, D.A.G., A.K.T., A.C.M., E.E., D.N.B., D.A.L., I.K.K. and G.Z.K.; supervision, D.N.B., I.K.K. and G.Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data analyzed during this study are included in this published article.
Acknowledgments
The financial support received for this study from the Greek Ministry of Development and Investments (General Secretariat for Research and Technology) through the research project “Intergovernmental International Scientific and Technological Innovation-Cooperation. Joint declaration of Science and Technology Cooperation between China and Greece” with the topic “Development of monitoring and removal strategies of emerging micro-pollutants in wastewaters” (grant No. T7DKI-00220) and the support is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bäuerlein, P.S.; Hofman-Caris, R.C.H.M.; Pieke, E.N.; ter Laak, T.L. Fate of Microplastics in the Drinking Water Production. Water Res. 2022, 221, 118790. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- ISO/TR 21960:2020(en)Plastics—Environmental Aspects—State of Knowledge and Methodologies. Available online: https://www.iso.org/obp/ui/#iso:std:iso:tr:21960:ed-1:v1:en (accessed on 25 February 2023).
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics Removal in Wastewater Treatment Plants: A Critical Review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Sherrell, P.C.; Chen, J.; Wang, H.; Zhang, W.; Yang, J. How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling. Adv. Sci. 2022, 9, 2103764. [Google Scholar] [CrossRef]
- Deng, H.; Wei, R.; Luo, W.; Hu, L.; Li, B.; Di, Y.; Shi, H. Microplastic Pollution in Water and Sediment in a Textile Industrial Area. Environ. Pollut. 2020, 258, 113658. [Google Scholar] [CrossRef]
- Reddy, A.S.; Nair, A.T. The Fate of Microplastics in Wastewater Treatment Plants: An Overview of Source and Remediation Technologies. Environ. Technol. Innov. 2022, 28, 102815. [Google Scholar] [CrossRef]
- Townsend, K.R.; Lu, H.-C.; Sharley, D.J.; Pettigrove, V. Associations between Microplastic Pollution and Land Use in Urban Wetland Sediments. Environ. Sci. Pollut. Res. 2019, 26, 22551–22561. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Cai, C.; He, Y.; Chen, L.; Xiong, X.; Huang, H.; Tao, S.; Liu, W. Occurrence and Characteristics of Microplastics in the Haihe River: An Investigation of a Seagoing River Flowing through a Megacity in Northern China. Environ. Pollut. 2020, 262, 114261. [Google Scholar] [CrossRef]
- Wu, P.; Tang, Y.; Dang, M.; Wang, S.; Jin, H.; Liu, Y.; Jing, H.; Zheng, C.; Yi, S.; Cai, Z. Spatial-Temporal Distribution of Microplastics in Surface Water and Sediments of Maozhou River within Guangdong-Hong Kong-Macao Greater Bay Area. Sci. Total Environ. 2020, 717, 135187. [Google Scholar] [CrossRef]
- Piehl, S.; Hauk, R.; Robbe, E.; Richter, B.; Kachholz, F.; Schilling, J.; Lenz, R.; Fischer, D.; Fischer, F.; Labrenz, M.; et al. Combined Approaches to Predict Microplastic Emissions Within an Urbanized Estuary (Warnow, Southwestern Baltic Sea). Front. Environ. Sci. 2021, 9, 616765. [Google Scholar] [CrossRef]
- Woodward, J.; Li, J.; Rothwell, J.; Hurley, R. Acute Riverine Microplastic Contamination Due to Avoidable Releases of Untreated Wastewater. Nat. Sustain. 2021, 4, 793–802. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Manjón, A.; Martínez-Díez, R.; Sol, D.; Laca, A.; Laca, A.; Rancaño, A.; Díaz, M. Long-Term Occurrence and Fate of Microplastics in WWTPs: A Case Study in Southwest Europe. Appl. Sci. 2022, 12, 2133. [Google Scholar] [CrossRef]
- Abbasi, S.; Keshavarzi, B.; Moore, F.; Turner, A.; Kelly, F.J.; Dominguez, A.O.; Jaafarzadeh, N. Distribution and Potential Health Impacts of Microplastics and Microrubbers in Air and Street Dusts from Asaluyeh County, Iran. Environ. Pollut. 2019, 244, 153–164. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Zhou, X.; Tian, Y.; Lin, C.; Wang, W.; Zhou, K.; Zhang, Y.; Lin, H. Microplastic Abundance, Distribution and Composition in the Mid-West Pacific Ocean. Environ. Pollut. 2020, 264, 114125. [Google Scholar] [CrossRef]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.-T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of Microplastics in Surface Water of the Lower Yellow River near Estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, S.-H.; Ge, C.; Gao, Q.; Huang, S.; Kang, Y.; Luo, G.; Zhang, Z.; Fan, L.; Zhu, Y.; et al. Removing Microplastics from Aquatic Environments: A Critical Review. Environ. Sci. Ecotechnology 2023, 13, 100222. [Google Scholar] [CrossRef]
- Yazdani Foshtomi, M.; Oryan, S.; Taheri, M.; Darvish Bastami, K.; Zahed, M.A. Composition and Abundance of Microplastics in Surface Sediments and Their Interaction with Sedimentary Heavy Metals, PAHs and TPH (Total Petroleum Hydrocarbons). Mar. Pollut. Bull. 2019, 149, 110655. [Google Scholar] [CrossRef]
- Sørensen, L.; Rogers, E.; Altin, D.; Salaberria, I.; Booth, A.M. Sorption of PAHs to Microplastic and Their Bioavailability and Toxicity to Marine Copepods under Co-Exposure Conditions. Environ. Pollut. 2020, 258, 113844. [Google Scholar] [CrossRef]
- Singla, M.; Díaz, J.; Broto-Puig, F.; Borrós, S. Sorption and Release Process of Polybrominated Diphenyl Ethers (PBDEs) from Different Composition Microplastics in Aqueous Medium: Solubility Parameter Approach. Environ. Pollut. 2020, 262, 114377. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of Microplastic Size on the Adsorption Behavior and Mechanism of Triclosan on Polyvinyl Chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef]
- Tang, K.H.D. Interactions of Microplastics with Persistent Organic Pollutants and the Ecotoxicological Effects: A Review. Trop. Aquat. Soil Pollut. 2021, 1, 24–34. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of Nanomaterials in the Treatment of Wastewater: A Review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Ho Daniel Tang, K. Ecotoxicological Impacts of Micro and Nanoplastics on Marine Fauna. Examines Mar. Biol. Oceanogr. 2020, 3. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Zahedi, S.; Quiroga, J.M.; Coello, M.D. Mapping Microplastics in Cadiz (Spain): Occurrence of Microplastics in Municipal and Industrial Wastewaters. J. Water Process Eng. 2020, 38, 101596. [Google Scholar] [CrossRef]
- Lechner, A.; Ramler, D. The Discharge of Certain Amounts of Industrial Microplastic from a Production Plant into the River Danube Is Permitted by the Austrian Legislation. Environ. Pollut. 2015, 200, 159–160. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A Review of the Removal of Microplastics in Global Wastewater Treatment Plants: Characteristics and Mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.-W. Conversion and Removal Strategies for Microplastics in Wastewater Treatment Plants and Landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Microplastic Pollution in Wastewater Treatment Plants in the City of Cádiz: Abundance, Removal Efficiency and Presence in Receiving Water Body. Sci. Total Environ. 2021, 776, 145795. [Google Scholar] [CrossRef]
- Hamidian, A.H.; Ozumchelouei, E.J.; Feizi, F.; Wu, C.; Zhang, Y.; Yang, M. A Review on the Characteristics of Microplastics in Wastewater Treatment Plants: A Source for Toxic Chemicals. J. Clean. Prod. 2021, 295, 126480. [Google Scholar] [CrossRef]
- Mason, S.A.; Garneau, D.; Sutton, R.; Chu, Y.; Ehmann, K.; Barnes, J.; Fink, P.; Papazissimos, D.; Rogers, D.L. Microplastic Pollution Is Widely Detected in US Municipal Wastewater Treatment Plant Effluent. Environ. Pollut. 2016, 218, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Narvaez, O.M.; Goonetilleke, A.; Perez, L.; Bandala, E.R. Engineered Technologies for the Separation and Degradation of Microplastics in Water: A Review. Chem. Eng. J. 2021, 414, 128692. [Google Scholar] [CrossRef]
- Magni, S.; Binelli, A.; Pittura, L.; Avio, C.G.; Della Torre, C.; Parenti, C.C.; Gorbi, S.; Regoli, F. The Fate of Microplastics in an Italian Wastewater Treatment Plant. Sci. Total Environ. 2019, 652, 602–610. [Google Scholar] [CrossRef]
- Durenkamp, M.; Pawlett, M.; Ritz, K.; Harris, J.A.; Neal, A.L.; McGrath, S.P. Nanoparticles within WWTP Sludges Have Minimal Impact on Leachate Quality and Soil Microbial Community Structure and Function. Environ. Pollut. 2016, 211, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric Microplastic Deposition in an Urban Environment and an Evaluation of Transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.; Clum, A.; Deepe, J.; Lane, H.; Beckingham, B. Wastewater Treatment Plants as a Source of Microplastics to an Urban Estuary: Removal Efficiencies and Loading per Capita over One Year. Water Res. X 2019, 3, 100030. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.; Kerpen, J.; Prediger, J.; Barkmann, L.; Müller, L. Determination of the Microplastics Emission in the Effluent of a Municipal Waste Water Treatment Plant Using Raman Microspectroscopy. Water Res. X 2019, 2, 100014. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Barchiesi, M.; Chiavola, A.; Di Marcantonio, C.; Boni, M.R. Presence and Fate of Microplastics in the Water Sources: Focus on the Role of Wastewater and Drinking Water Treatment Plants. J. Water Process Eng. 2021, 40, 101787. [Google Scholar] [CrossRef]
- Tursi, A.; Baratta, M.; Easton, T.; Chatzisymeon, E.; Chidichimo, F.; De Biase, M.; De Filpo, G. Microplastics in Aquatic Systems, a Comprehensive Review: Origination, Accumulation, Impact, and Removal Technologies. RSC Adv. 2022, 12, 28318–28340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Xie, Y.; Zhong, S.; Gao, P. Occurrence and Removal of Microplastics from Wastewater Treatment Plants in a Typical Tourist City in China. J. Clean. Prod. 2021, 291, 125968. [Google Scholar] [CrossRef]
- Mallow, O.; Spacek, S.; Schwarzböck, T.; Fellner, J.; Rechberger, H. A New Thermoanalytical Method for the Quantification of Microplastics in Industrial Wastewater. Environ. Pollut. 2020, 259, 113862. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.; Booth, A.M.; Sabbah, I.; Tiller, R.; Dierking, J.; Klun, K.; Rotter, A.; Ben-David, E.; Javidpour, J.; Angel, D.L. Between Source and Sea: The Role of Wastewater Treatment in Reducing Marine Microplastics. J. Environ. Manag. 2020, 266, 110642. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater Treatment Plants as a Pathway for Microplastics: Development of a New Approach to Sample Wastewater-Based Microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. J. Geophys. Res. Oceans 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Sol, D.; Laca, A.; Laca, A.; Díaz, M. Microplastics in Wastewater and Drinking Water Treatment Plants: Occurrence and Removal of Microfibres. Appl. Sci. 2021, 11, 10109. [Google Scholar] [CrossRef]
- Zha, F.; Shang, M.; Ouyang, Z.; Guo, X. The Aging Behaviors and Release of Microplastics: A Review. Gondwana Res. 2022, 108, 60–71. [Google Scholar] [CrossRef]
- Wang, C.; O’Connor, D.; Wang, L.; Wu, W.-M.; Luo, J.; Hou, D. Microplastics in Urban Runoff: Global Occurrence and Fate. Water Res. 2022, 225, 119129. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, Sources, Human Health Impacts and Mitigation of Microplastic Pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Du, S.; Zhu, R.; Cai, Y.; Xu, N.; Yap, P.-S.; Zhang, Y.; He, Y.; Zhang, Y. Environmental Fate and Impacts of Microplastics in Aquatic Ecosystems: A Review. RSC Adv. 2021, 11, 15762–15784. [Google Scholar] [CrossRef] [PubMed]
- Kane, I.A.; Clare, M.A. Dispersion, Accumulation, and the Ultimate Fate of Microplastics in Deep-Marine Environments: A Review and Future Directions. Front. Earth Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An Emerging Class of Air Pollutants: Potential Effects of Microplastics to Respiratory Human Health? Sci. Total Environ. 2020, 749, 141676. [Google Scholar] [CrossRef]
- Wang, W.; Ge, J.; Yu, X.; Li, H. Environmental Fate and Impacts of Microplastics in Soil Ecosystems: Progress and Perspective. Sci. Total Environ. 2020, 708, 134841. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Xing, B. Environmental Source, Fate, and Toxicity of Microplastics. J. Hazard. Mater. 2021, 407, 124357. [Google Scholar] [CrossRef]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. In Physico-Chemical Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3129-8. [Google Scholar]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.-G. A Study on Characteristics of Microplastic in Wastewater of South Korea: Identification, Quantification, and Fate of Microplastics during Treatment Process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A Review of the Fate of Micropollutants in Wastewater Treatment Plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ahmad, A.; Ibrahim, M.N.M.; Alshammari, M.B. Advanced Technologies for Wastewater Treatment. In Green Chemistry for Sustainable Water Purification; Shahid-ul-Islam, Shalla, A.H., Shahadat, M., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 179–202. ISBN 978-1-119-85229-2. [Google Scholar]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, Classification and Removal Efficiency of Microplastics in Wastewater Treatment Plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef]
- Fahrenfeld, N.L.; Arbuckle-Keil, G.; Naderi Beni, N.; Bartelt-Hunt, S.L. Source Tracking Microplastics in the Freshwater Environment. TrAC Trends Anal. Chem. 2019, 112, 248–254. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of Microplastics in Wastewater Treatment Plants and Their Environmental Dispersion with Effluent and Sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Xu, X.; Jian, Y.; Xue, Y.; Hou, Q.; Wang, L. Microplastics in the Wastewater Treatment Plants (WWTPs): Occurrence and Removal. Chemosphere 2019, 235, 1089–1096. [Google Scholar] [CrossRef]
- Raju, S.; Carbery, M.; Kuttykattil, A.; Senthirajah, K.; Lundmark, A.; Rogers, Z.; Scb, S.; Evans, G.; Palanisami, T. Improved Methodology to Determine the Fate and Transport of Microplastics in a Secondary Wastewater Treatment Plant. Water Res. 2020, 173, 115549. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; Olmos, S.; López-Castellanos, J. Microplastics in an Urban Wastewater Treatment Plant: The Influence of Physicochemical Parameters and Environmental Factors. Chemosphere 2020, 238, 124593. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Oh, M.-J.; Kim, P.-G.; Kim, G.; Jeong, D.-H.; Ju, B.-K.; Lee, W.-S.; Chung, H.-M.; Kang, H.-J.; Kwon, J.-H. National Reconnaissance Survey of Microplastics in Municipal Wastewater Treatment Plants in Korea. Environ. Sci. Technol. 2020, 54, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane Bioreactor and Rapid Sand Filtration for the Removal of Microplastics in an Urban Wastewater Treatment Plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Golgoli, M.; Khiadani, M.; Shafieian, A.; Sen, T.K.; Hartanto, Y.; Johns, M.L.; Zargar, M. Microplastics Fouling and Interaction with Polymeric Membranes: A Review. Chemosphere 2021, 283, 131185. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low Numbers of Microplastics Detected in Drinking Water from Ground Water Sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Masiá, P.; Sol, D.; Ardura, A.; Laca, A.; Borrell, Y.J.; Dopico, E.; Laca, A.; Machado-Schiaffino, G.; Díaz, M.; Garcia-Vazquez, E. Bioremediation as a Promising Strategy for Microplastics Removal in Wastewater Treatment Plants. Mar. Pollut. Bull. 2020, 156, 111252. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.M.; Waldron, S.; Gauchotte-Lindsay, C. Average Daily Flow of Microplastics through a Tertiary Wastewater Treatment Plant over a Ten-Month Period. Water Res. 2019, 163, 114909. [Google Scholar] [CrossRef] [PubMed]
- Ainali, N.M.; Kalaronis, D.; Kontogiannis, A.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microplastics in the Environment: Sampling, Pretreatment, Analysis and Occurrence Based on Current and Newly-Exploited Chromatographic Approaches. Sci. Total Environ. 2021, 794, 148725. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and Fate of Microplastics in Wastewater and Sludge Filter Cake from a Wastewater Treatment Plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Yurtsever, M.; Karadagli, F. Microplastic Removal by Aerated Grit Chambers versus Settling Tanks of a Municipal Wastewater Treatment Plant. J. Water Process Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Lv, X.; Dong, Q.; Zuo, Z.; Liu, Y.; Huang, X.; Wu, W.-M. Microplastics in a Municipal Wastewater Treatment Plant: Fate, Dynamic Distribution, Removal Efficiencies, and Control Strategies. J. Clean. Prod. 2019, 225, 579–586. [Google Scholar] [CrossRef]
- Castelluccio, S.; Alvim, C.B.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Fiore, S. Assessment of Microplastics Distribution in a Biological Wastewater Treatment. Microplastics 2022, 1, 141–155. [Google Scholar] [CrossRef]
- Khatmullina, L.; Isachenko, I. Settling Velocity of Microplastic Particles of Regular Shapes. Mar. Pollut. Bull. 2017, 114, 871–880. [Google Scholar] [CrossRef]
- Kittipongvises, S.; Phetrak, A.; Hongprasith, N.; Lohwacharin, J. Unravelling Capability of Municipal Wastewater Treatment Plant in Thailand for Microplastics: Effects of Seasonality on Detection, Fate and Transport. J. Environ. Manag. 2022, 302, 113990. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Q.; Li, J.; Li, Q.; Xu, H.; Ye, Q.; Wang, Y.; Shu, S.; Zhang, J. Removal of Polystyrene and Polyethylene Microplastics Using PAC and FeCl3 Coagulation: Performance and Mechanism. Sci. Total Environ. 2021, 752, 141837. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Q.; Xing, Y.; Liu, K.; Ling, W.; Yang, J.; Yang, Q.; Wu, T.; Zhang, J.; Pei, Z.; et al. Review of Research on Migration, Distribution, Biological Effects, and Analytical Methods of Microfibers in the Environment. Sci. Total Environ. 2023, 855, 158922. [Google Scholar] [CrossRef] [PubMed]
- Vasiljević, S.; Vujić, M.; Agbaba, J.; Federici, S.; Ducoli, S.; Tomić, R.; Tubić, A. Efficiency of Coagulation/Flocculation for the Removal of Complex Mixture of Textile Fibers from Water. Process. 2023, 11, 820. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Cydzik-Kwiatkowska, A. Coagulation and Flocculation before Primary Clarification as Efficient Solutions for Low-Density Microplastic Removal from Wastewater. Int. J. Environ. Res. Public. Health 2022, 19, 13013. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 Μm Microplastic Particles from Treated Wastewater by a Disc Filter. Water 2019, 11, 1935. [Google Scholar] [CrossRef]
- Long, Y.; Zhou, Z.; Yin, L.; Wen, X.; Xiao, R.; Du, L.; Zhu, L.; Liu, R.; Xu, Q.; Li, H.; et al. Microplastics Removal and Characteristics of Constructed Wetlands WWTPs in Rural Area of Changsha, China: A Different Situation from Urban WWTPs. Sci. Total Environ. 2022, 811, 152352. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, R.; Liu, Y.; Zhang, Y.; Zhou, J.; Qu, G.; Tang, S.; Wang, T. Plasma-Induced Conversion of Polystyrene Nanoplastics in Water: Intermediates Release, Toxicity, and Disinfection Byproducts Formation. Chem. Eng. J. 2022, 433, 134543. [Google Scholar] [CrossRef]
- Leslie, H.A.; Brandsma, S.H.; van Velzen, M.J.M.; Vethaak, A.D. Microplastics En Route: Field Measurements in the Dutch River Delta and Amsterdam Canals, Wastewater Treatment Plants, North Sea Sediments and Biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef]
- Wei, F.; Xu, C.; Chen, C.; Wang, Y.; Lan, Y.; Long, L.; Xu, M.; Wu, J.; Shen, F.; Zhang, Y.; et al. Distribution of Microplastics in the Sludge of Wastewater Treatment Plants in Chengdu, China. Chemosphere 2022, 287, 132357. [Google Scholar] [CrossRef]
- Kang, J.; Zhou, L.; Duan, X.; Sun, H.; Ao, Z.; Wang, S. Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings. Matter 2019, 1, 745–758. [Google Scholar] [CrossRef]
- Siddique, A.; Yaqoob, A.A.; Mirza, M.A.; Kanwal, A.; Ibrahim, M.N.M.; Ahmad, A. Potential Use of Ultrafiltration (UF) Membrane for Remediation of Metal Contaminants. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 341–364. ISBN 978-0-12-822880-7. [Google Scholar]
- Yaqoob, A.A.; Kanwal, A.; Ibrahim, M.N.M.; Mohammad, S.A.G.; Ahmad, A. Application and Fabrication of Nanofiltration Membrane for Separation of Metal Ions from Wastewater. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 365–398. ISBN 978-0-12-822880-7. [Google Scholar]
- Rybachuk, Y.; Jodłowski, A. Mathematical Model of Dissolved Air Flotation (DAF) Based on Impulse Conservation Law. SN Appl. Sci. 2019, 1, 541. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.L.; Smith, R.W. Overview of Flotation as a Wastewater Treatment Technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Wang, Q.; Hernández-Crespo, C.; Du, B.; Van Hulle, S.W.H.; Rousseau, D.P.L. Fate and Removal of Microplastics in Unplanted Lab-Scale Vertical Flow Constructed Wetlands. Sci. Total Environ. 2021, 778, 146152. [Google Scholar] [CrossRef] [PubMed]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Cvetnić, M.; Kušić, H.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Potential of Advanced Oxidation as Pretreatment for Microplastics Biodegradation. Separations 2023, 10, 132. [Google Scholar] [CrossRef]
- Dhodapkar, R.S.; Gandhi, K.N. Pharmaceuticals and Personal Care Products in Aquatic Environment: Chemicals of Emerging Concern? In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–85. ISBN 978-0-12-816189-0. [Google Scholar]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Cheng, Y.-X.; Chen, J.; Wu, D.; Liu, Y.-S.; Yang, Y.-Q.; He, L.-X.; Ye, P.; Zhao, J.-L.; Liu, S.-S.; Yang, B.; et al. Highly Enhanced Biodegradation of Pharmaceutical and Personal Care Products in a Novel Tidal Flow Constructed Wetland with Baffle and Plants. Water Res. 2021, 193, 116870. [Google Scholar] [CrossRef]
- Van Do, M.; Le, T.X.T.; Vu, N.D.; Dang, T.T. Distribution and Occurrence of Microplastics in Wastewater Treatment Plants. Environ. Technol. Innov. 2022, 26, 102286. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic Contamination in an Urban Area: A Case Study in Greater Paris. Environ. Chem. 2015, 12, 592. [Google Scholar] [CrossRef]
- Fryczkowska, B.; Przywara, L. Removal of Microplastics from Industrial Wastewater Utilizing an Ultrafiltration Composite Membrane RGO/PAN Application. Desalination Water Treat. 2021, 214, 252–262. [Google Scholar] [CrossRef]
- Long, Z.; Pan, Z.; Wang, W.; Ren, J.; Yu, X.; Lin, L.; Lin, H.; Chen, H.; Jin, X. Microplastic Abundance, Characteristics, and Removal in Wastewater Treatment Plants in a Coastal City of China. Water Res. 2019, 155, 255–265. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Duan, L.; Zhang, Y.; Zhou, Y.; Sui, Q.; Xu, D.; Qu, H.; Yu, G. Occurrence and Distribution of Microplastics in Domestic, Industrial, Agricultural and Aquacultural Wastewater Sources: A Case Study in Changzhou, China. Water Res. 2020, 182, 115956. [Google Scholar] [CrossRef] [PubMed]
- Lehtiniemi, M.; Hartikainen, S.; Näkki, P.; Engström-Öst, J.; Koistinen, A.; Setälä, O. Size Matters More than Shape: Ingestion of Primary and Secondary Microplastics by Small Predators. Food Webs 2018, 17, e00097. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic Is an Abundant and Distinct Microbial Habitat in an Urban River. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).