Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of NPAM Solution
2.3. Viscosity Measurement and Morphology Observation of NPAM Treated Wastewater
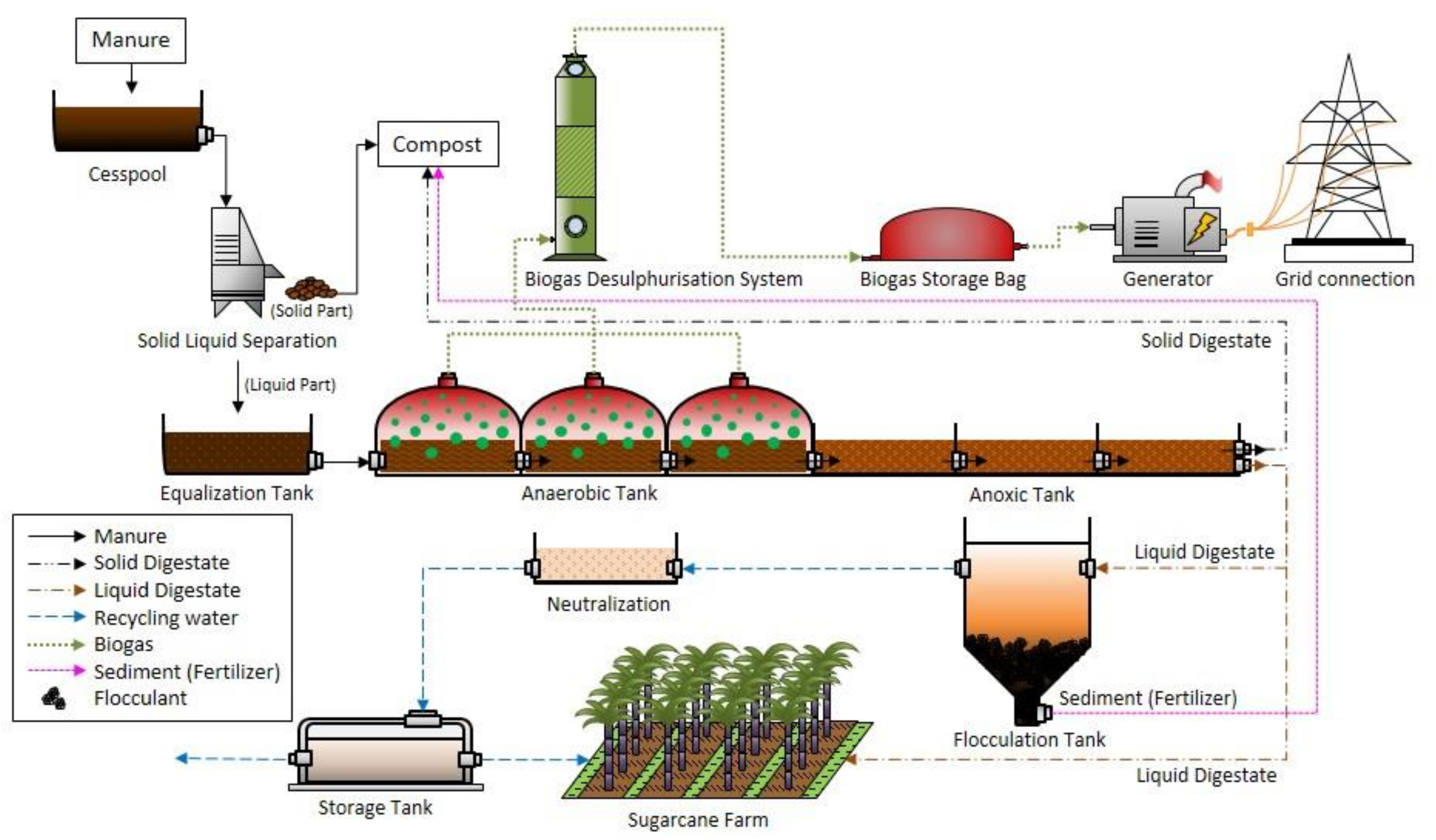
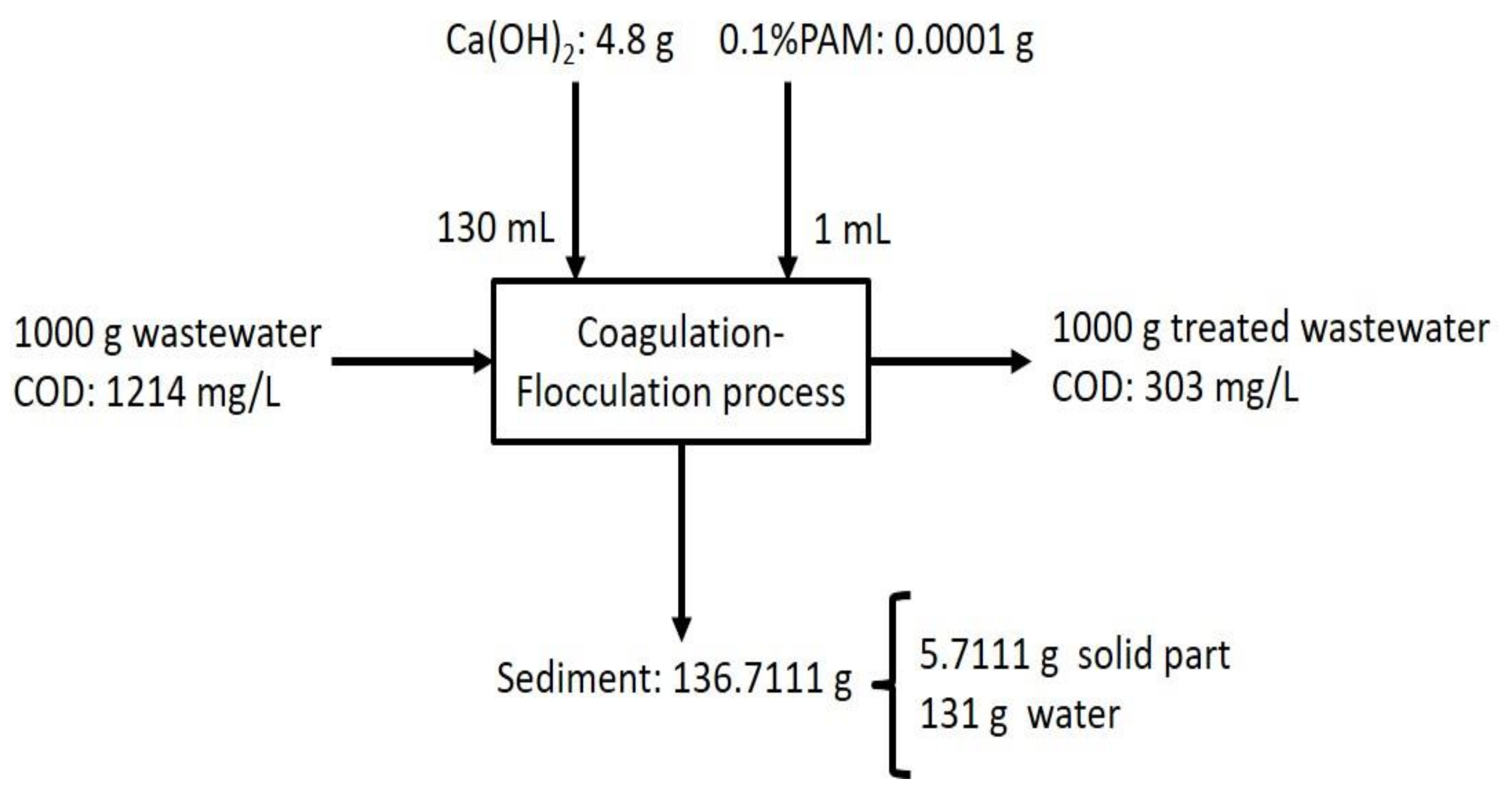
2.4. Wastewater Treatment and Analysis
3. Results and Discussion
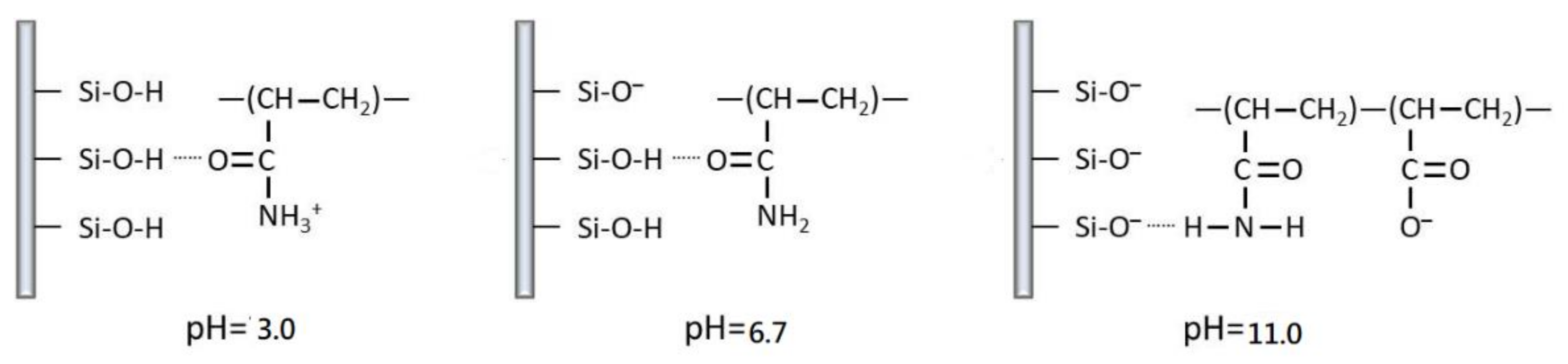
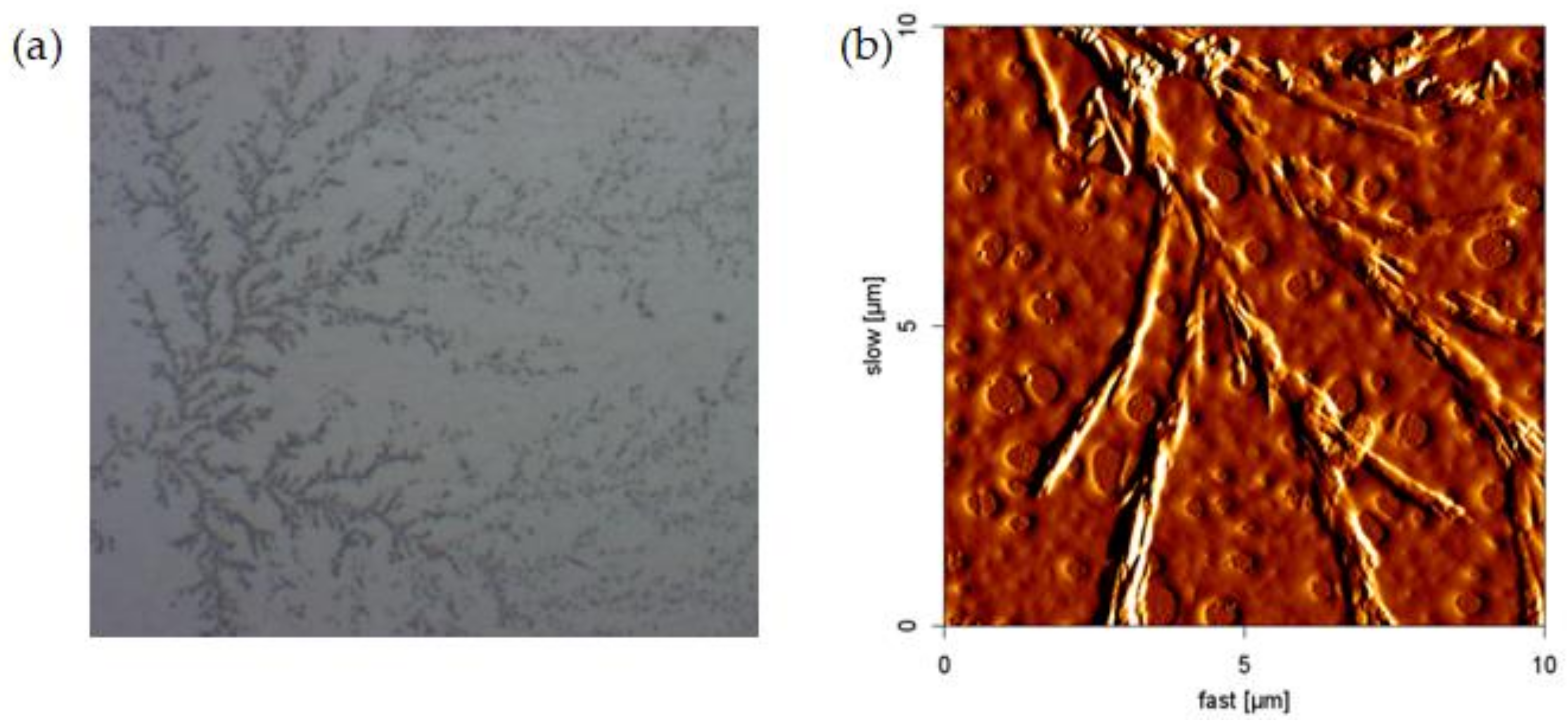
3.1. Effect of pH on Viscosity of NPAM
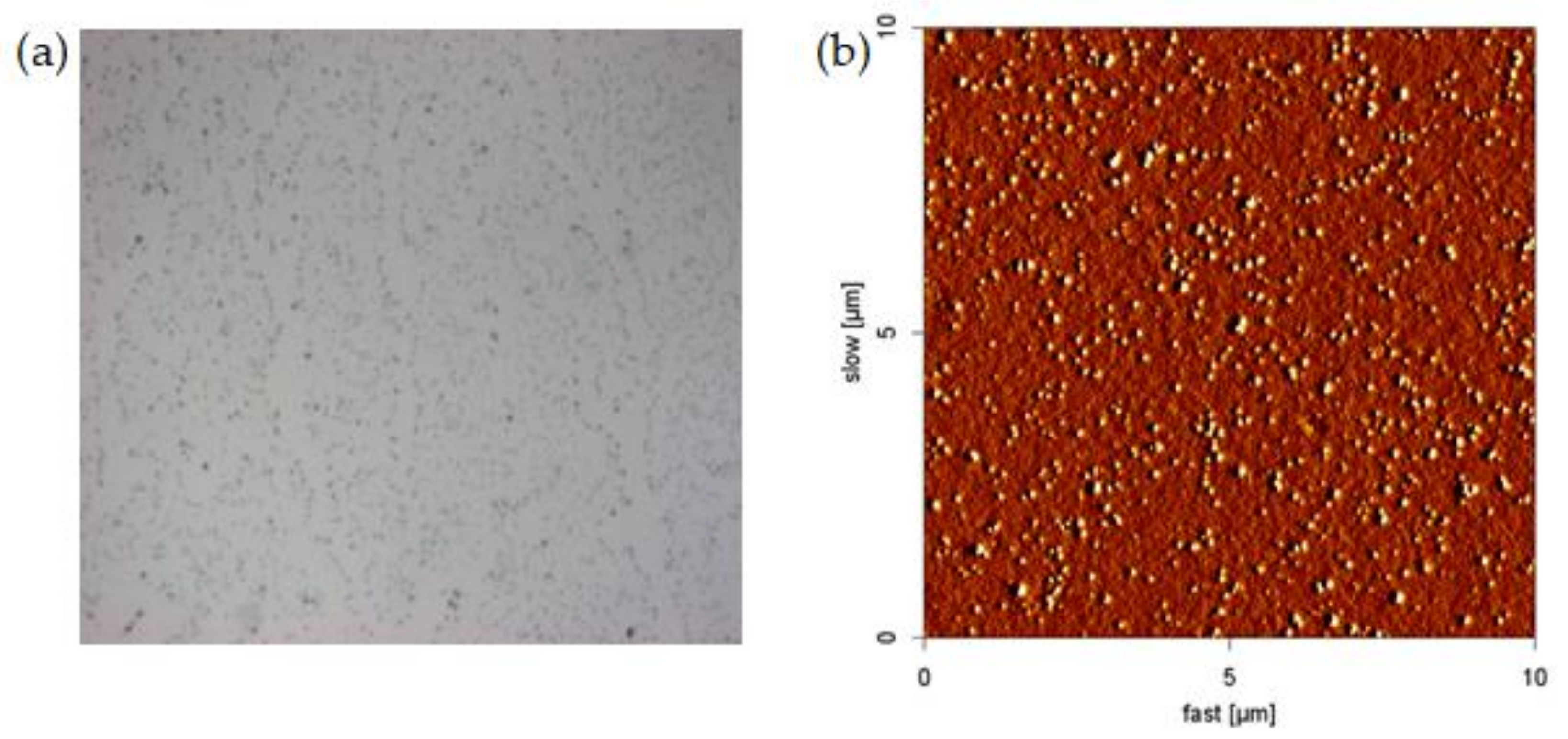
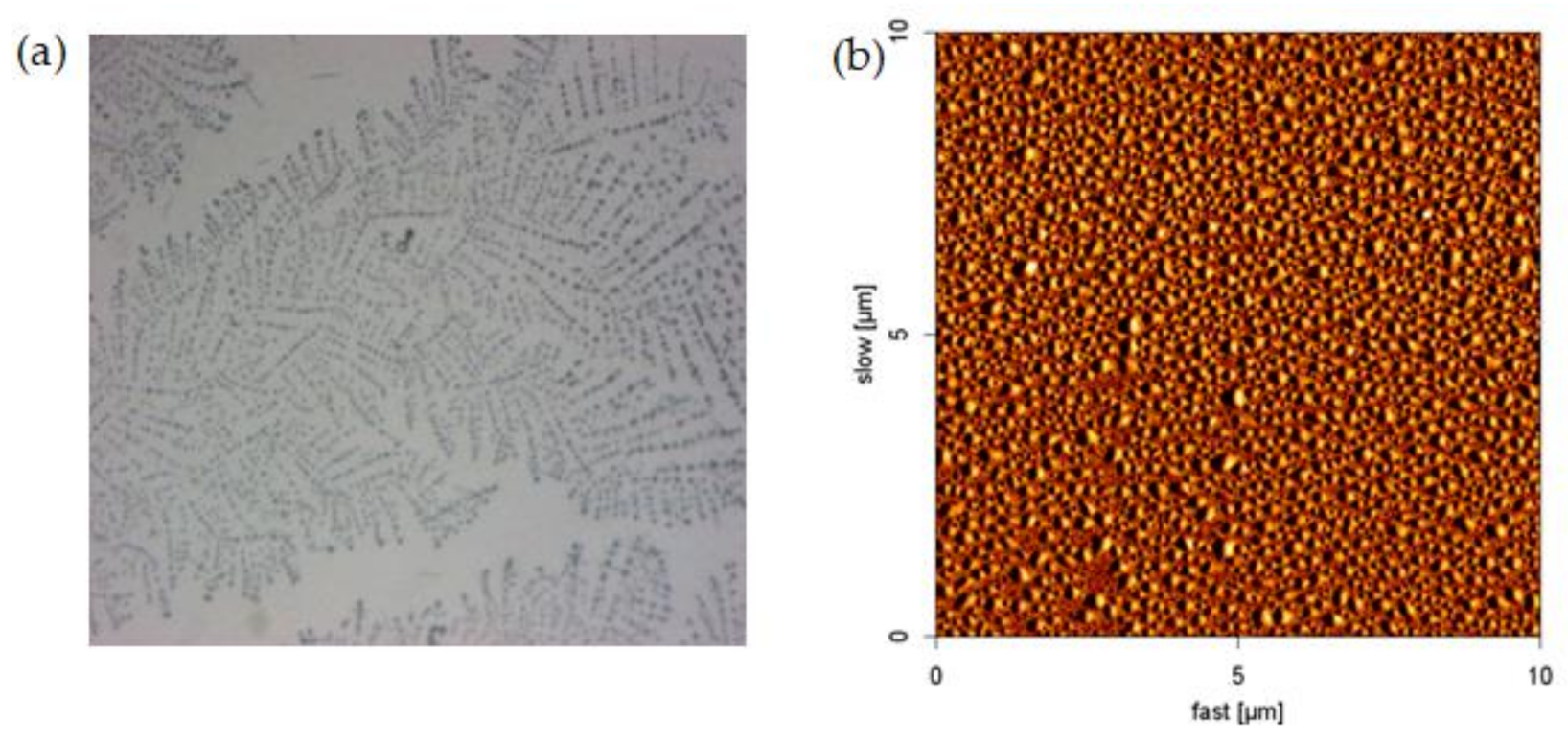
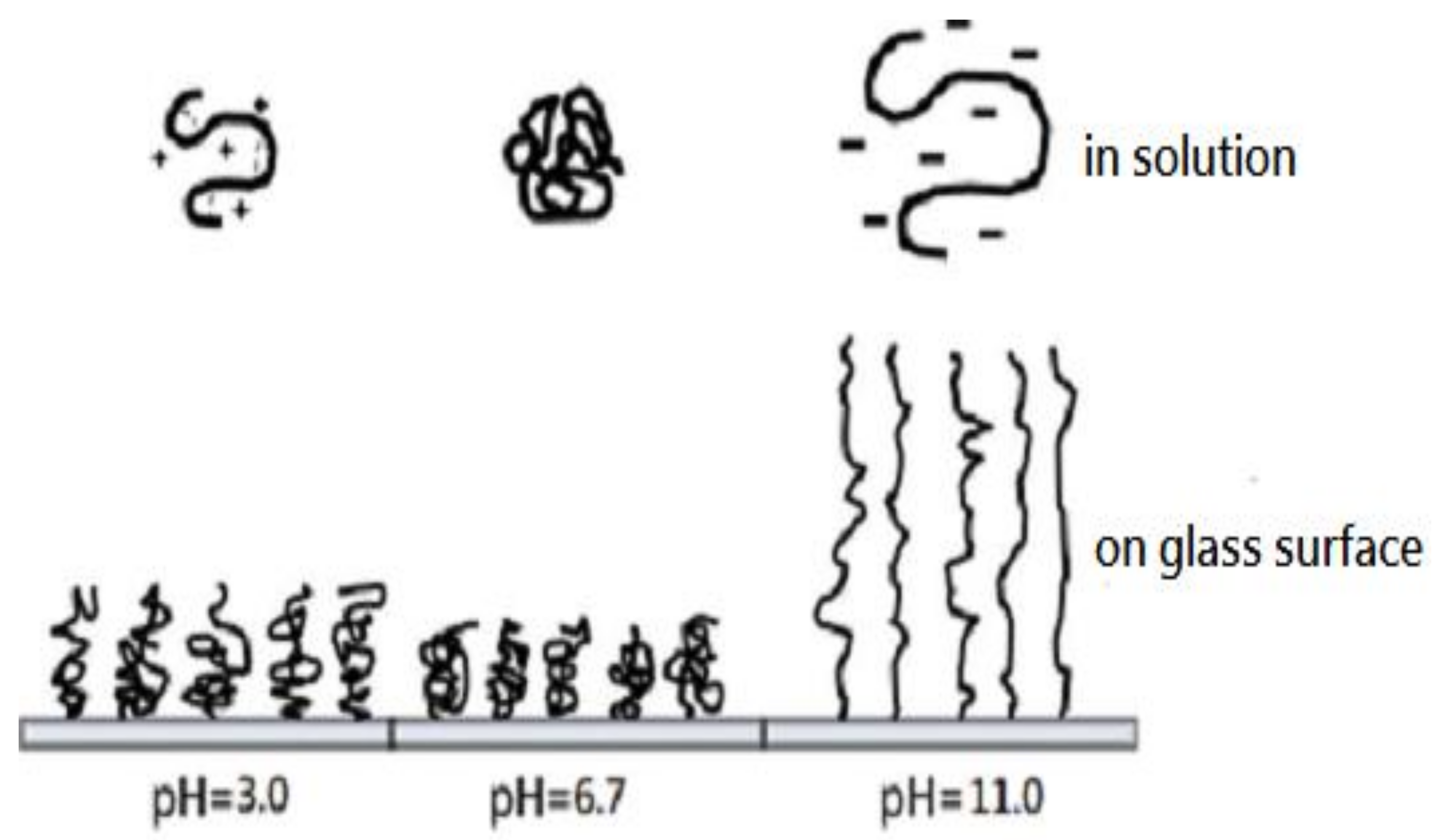
3.2. Effect of pH on the Morphology of NPAM
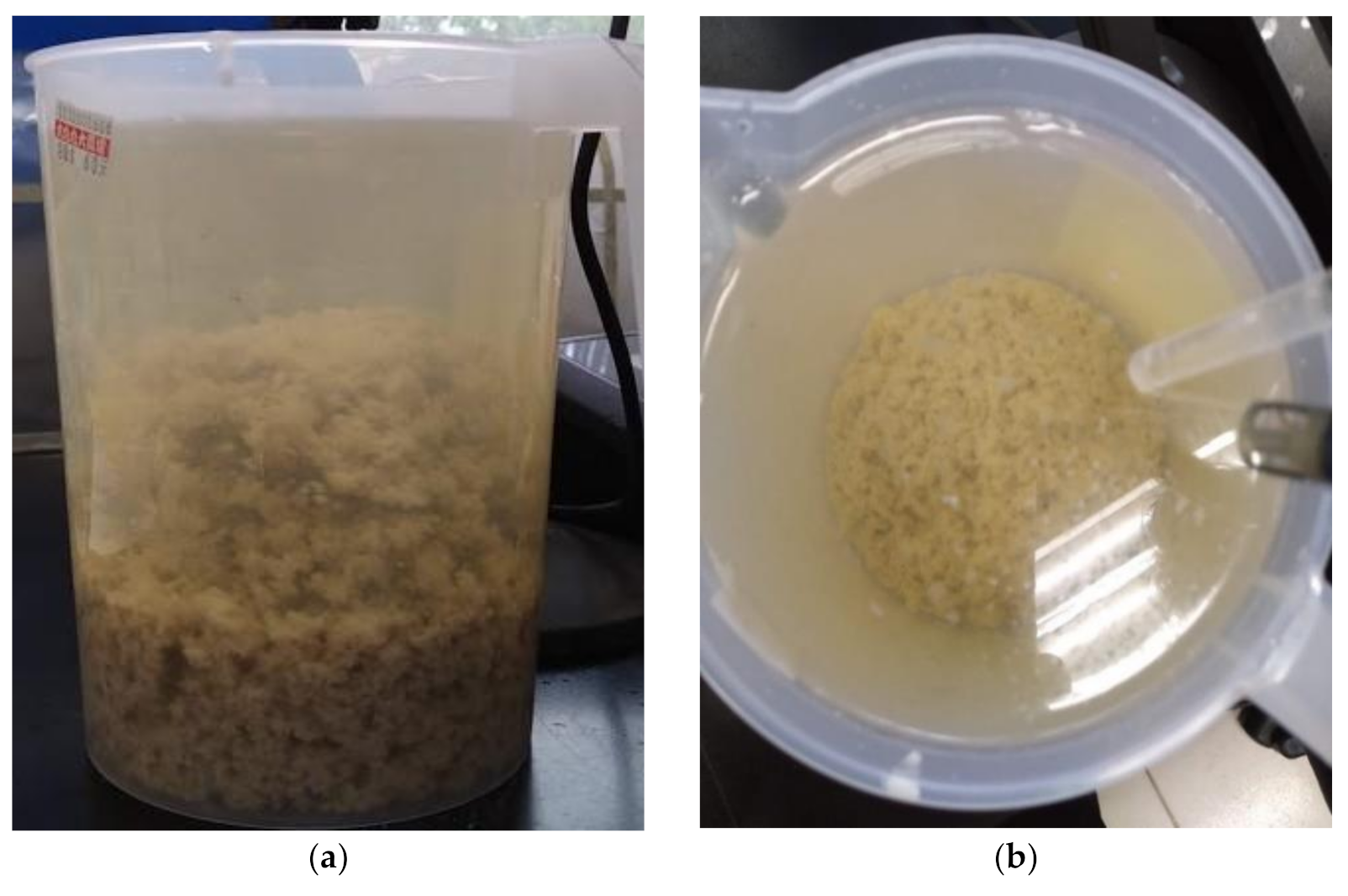
3.3. Wastewater Treatment: Coagulation–Flocculation
3.4. Wastewater Analysis before and after NPAM Coagulation–Flocculation
3.5. Economic Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ikenganyia, E.; Onyeonagu, C.; Mbah, C.; Azuka, C.; Aneke, I. Evaluation of the agronomic potentials of swine waste as a soil amendment. Afr. J. Agric. Res. 2014, 9, 3761–3765. [Google Scholar]
- Zaleski, H.M.; Paquin, D.G. Composted Swine Manure for Vegetable Crop Application; CTAHR: Honolulu, HI, USA, 2005. [Google Scholar]
- Bilotta, P.; Steinmetz, R.L.R.; Kunz, A.; Mores, R. Swine effluent post-treatment by alkaline control and UV radiation combined for water reuse. J. Clean. Prod. 2017, 140, 1247–1254. [Google Scholar] [CrossRef]
- Cattaneo, M.; Finzi, A.; Guido, V.; Riva, E.; Provolo, G. Effect of ammonia stripping and use of additives on separation of solids, phosphorus, copper and zinc from liquid fractions of animal slurries. Sci. Total Environ. 2019, 672, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Fongaro, G.; Viancelli, A.; Magri, M.; Elmahdy, E.; Biesus, L.; Kich, J.; Kunz, A.; Barardi, C. Utility of specific biomarkers to assess safety of swine manure for biofertilizing purposes. Sci. Total Environ. 2014, 479, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, L.B.; Halkides, C.J.; Song, B.; Williams, C.M.; Dubay, G.R.; Fries, A.; Farmer, J.; Fridrich, W.; Brookshire, C. Swine waste as a source of natural products: A carotenoid antioxidant. Agric. Sci. 2012, 3, 806. [Google Scholar] [CrossRef]
- Leite, S.; Leite, B.; Isola, A.; Freitas, L.; Souza, J. Application of Cleaner Production Methodology to Evaluate the Generation of Bioenergy in a Small Swine Farm. Chem. Eng. Trans. 2014, 39, 589–594. [Google Scholar]
- Shih, M.F.; Lay, C.H.; Lin, C.Y.; Chang, S.H. Exploring the environmental and economic potential for biogas production from swine manure wastewater by life cycle assessment. Clean Technol. Environ. Policy 2021. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chai, W.S.; Lay, C.H.; Chen, C.C.; Lee, C.Y.; Show, P.L. Optimization of hydrolysis-acidogenesis phase of swine manure for biogas production using two-stage anaerobic fermentation. Processes 2021, 9, 1324. [Google Scholar] [CrossRef]
- Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Fernández Echeverria, E.; Fernández-Lambert, G.; Sandoval Herazo, L.C. Plant biomass production in constructed wetlands treating swine wastewater in tropical climates. Fermentation 2021, 9, 296. [Google Scholar] [CrossRef]
- Muamar, A.; Tijane, M.; Shawqi, E.; El Housni, A.; Zouahri, A.; Bouksaim, M. Assessment of the impact of wastewater use on soil properties. J. Mater. Environ. Sci. 2014, 5, 961–966. [Google Scholar]
- Lenghan-Sauriol, M.-È.; Leduc, S.; Raghavan, G. An Integrated System Approach for Swine Manure Management at the Farm Level; Unpublished Work; McGill University: Montréal, QC, Canada, 2009; Available online: https://escholarship.mcgill.ca/concern/reports/6w924b947?locale=en (accessed on 28 September 2021).
- Morris, T.F.; Murrell, T.S.; Beegle, D.B.; Camberato, J.J.; Ferguson, R.B.; Grove, J.; Ketterings, Q.; Kyveryga, P.M.; Laboski, C.A.; McGrath, J.M. Strengths and limitations of nitrogen rate recommendations for corn and opportunities for improvement. Agron. J. 2018, 110, 1–37. [Google Scholar] [CrossRef]
- Malakar, A.; Snow, D.D.; Ray, C. Irrigation Water Quality—A Contemporary Perspective. Water 2019, 11, 1482. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Ariel Szogi, A.A.; Hunt, P.G.; Millner, P.D.; Humenik, F.J. Development of environmentally superior treatment system to replace anaerobic swine lagoons in the USA. Bioresour. Technol. 2007, 98, 3184–3194. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Liu, B. Swine wastewater treatment in anaerobic digesters with floating medium. Trans. ASAE 2002, 45, 799–805. [Google Scholar] [CrossRef][Green Version]
- Makara, A.; Kowalski, Z. Pig manure treatment and purification by filtration. J. Environ. Manag. 2015, 161, 317–324. [Google Scholar] [CrossRef]
- Viancelli, A.; Kunz, A.; Steinmetz, R.; Kich, J.; Souza, C.; Canal, C.; Coldebella, A.; Esteves, P.; Barardi, C. Performance of two swine manure treatment systems on chemical composition and on the reduction of pathogens. Chemosphere 2013, 90, 1539–1544. [Google Scholar] [CrossRef]
- Dosta, J.; Rovira, J.; Galí, A.; Macé, S.; Mata-Alvarez, J. Integration of a Coagulation/Flocculation step in a biological sequencing batch reactor for COD and nitrogen removal of supernatant of anaerobically digested piggery wastewater. Bioresour. Technol. 2008, 99, 5722–5730. [Google Scholar] [CrossRef]
- De Godos, I.; Guzman, H.O.; Soto, R.; García-Encina, P.A.; Becares, E.; Muñoz, R.; Vargas, V.A. Coagulation/flocculation-based removal of algal–bacterial biomass from piggery wastewater treatment. Bioresour. Technol. 2011, 102, 923–927. [Google Scholar] [CrossRef]
- Garcia, M.; Vanotti, M.; Szogi, A. Simultaneous separation of phosphorus sludge and manure solids with polymers. Trans. ASABE 2007, 50, 2205–2215. [Google Scholar] [CrossRef]
- Vanotti, M.; Szogi, A.; Hunt, P.; Ellison, A.; Millner, P.; Humenik, F. Development of an environmentally superior treatment system for replacing anaerobic swine waste lagoons. Proc. Water Environ. Fed. 2005, 2005, 4073–4092. [Google Scholar] [CrossRef][Green Version]
- Vanotti, M.; Rice, J.; Ellison, A.; Hunt, P.; Humenik, F.; Baird, C. Solid-liquid separation of swine manure with polymer treatment and sand filtration. Trans. ASAE 2005, 48, 1567–1574. [Google Scholar] [CrossRef]
- Haveroen, M.E.; MacKinnon, M.D.; Fedorak, P.M. Polyacrylamide added as a nitrogen source stimulates methanogenesis in consortia from various wastewaters. Water Res. 2005, 39, 3333–3341. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, Z.; Zhao, Y.; Zhang, H.; Feng, Y. Biodegradation of polyacrylamide by bacteria isolated from activated sludge and oil-contaminated soil. J. Hazard. Mater. 2010, 175, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Sang, G.; Pi, Y.; Bao, M.; Li, Y.; Lu, J. Biodegradation for hydrolyzed polyacrylamide in the anaerobic baffled reactor combined aeration tank. Ecol. Eng. 2015, 84, 121–127. [Google Scholar] [CrossRef]
- Zhao, L.; Bao, M.; Yan, M.; Lu, J. Kinetics and thermodynamics of biodegradation of hydrolyzed polyacrylamide under anaerobic and aerobic conditions. Bioresour. Technol. 2016, 216, 95–104. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Smith, E.A.; Prues, S.L.; Oehme, F.W. Environmental degradation of polyacrylamides. Ecotoxicol. Environ. Saf. 1997, 37, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part I. Linear polyacrylamide. Polymer 2003, 44, 1331–1337. [Google Scholar] [CrossRef]
- Caulfield, M.J.; Hao, X.; Qiao, G.G.; Solomon, D.H. Degradation on polyacrylamides. Part II. Polyacrylamide gels. Polymer 2003, 44, 3817–3826. [Google Scholar] [CrossRef]
- Holliman, P.J.; Clark, J.A.; Williamson, J.C.; Jones, D.L. Model and field studies of the degradation of cross-linked polyacrylamide gels used during the revegetation of slate waste. Sci. Total Environ. 2005, 336, 13–24. [Google Scholar] [CrossRef]
- Kuboi, T.; Fujii, K. Toxicity of cationic polymer flocculants to higher plants: I. Seedling assay. Soil Sci. Plant Nutr. 1984, 30, 311–320. [Google Scholar] [CrossRef]
- Chou, L.H.; Tsai, R.I.; Chang, J.R.; Lee, M.T. Regenerable adsorbent for removing ammonia evolved from anaerobic reaction of animal urine. J. Environ. Sci. 2006, 18, 1176–1181. [Google Scholar] [CrossRef]
- Entry, J.A.; Sojka, R. The efficacy of polyacrylamide and related compounds to remove microorganisms and nutrients from animal wastewater. J. Environ. Qual. 2000, 29, 1905–1914. [Google Scholar] [CrossRef]
- Entry, J.A.; Sojka, R.; Watwood, M.; Ross, C. Polyacrylamide preparations for protection of water quality threatened by agricultural runoff contaminants. Environ. Pollut. 2002, 120, 191–200. [Google Scholar] [CrossRef]
- Gorham, J.M.; Wnuk, J.D.; Shin, M.; Fairbrother, H. Adsorption of natural organic matter onto carbonaceous surfaces: Atomic force microscopy study. Environ. Sci. Technol. 2007, 41, 1238–1244. [Google Scholar] [CrossRef]
- Sastry, N.; Dave, P.; Valand, M. Dilute solution behaviour of polyacrylamides in aqueous media. Eur. Polym. J. 1999, 35, 517–525. [Google Scholar] [CrossRef]
- Uranta, K.G.; Gomari, S.R.; Russell, P.; Hamad, F. Determining safe maximum temperature point (SMTP) for polyacrylamide polymer (PAM) in saline solutions. J. Oil Gas Petrochem. Sci. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Kramer, G.; Somasundaran, P. Conformational behavior of polyelectrolyte complexes at the solid/liquid interface. Langmuir 2002, 18, 9357–9361. [Google Scholar] [CrossRef]
- Yu, X.; Somasundaran, P. Kinetics of polymer conformational changes and its role in flocculation. J. Colloid Interface Sci. 1996, 178, 770–774. [Google Scholar] [CrossRef]
- Besra, L.; Sengupta, D.; Roy, S.; Ay, P. Influence of polymer adsorption and conformation on flocculation and dewatering of kaolin suspension. Sep. Purif. Technol. 2004, 37, 231–246. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Israelachvili, J. Effect of pH and salt on the adsorption and interactions of an amphoteric polyelectrolyte. Macromolecules 1992, 25, 5081–5088. [Google Scholar] [CrossRef]
- Song, X.; Cao, M.; Han, Y.; Wang, Y.; Kwak, J.C. Adsorption of hydrophobically modified poly (acrylamide)-co-(acrylic acid) on an amino-functionalized surface and its response to the external solvent environment. Langmuir 2007, 23, 4279–4285. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.-T.; Somasundaran, P. Adsorption of polyacrylamide on oxide minerals. Langmuir 1989, 5, 854–860. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Doumenc, F.; Guerrier, B. Drying of a solution in a meniscus: A model coupling the liquid and the gas phases. Langmuir 2010, 26, 13959–13967. [Google Scholar] [CrossRef]
- Kaya, D.; Belyi, V.; Muthukumar, M. Pattern formation in drying droplets of polyelectrolyte and salt. J. Chem. Phys. 2010, 133, 114905. [Google Scholar] [CrossRef]

| pH | Viscosity (mPa·s) (c.p) | |
|---|---|---|
| Pure water | 6.37 | 0.99 |
| PAM | 6.7 | 1.15 |
| PAM in acid | 3.0 | 1.21 |
| PAM in base | 11.0 | 8.35 |
| Unit | Wastewater | Treated Water | Removal Rate (%) | Method Code | Method Detection Limit | |
|---|---|---|---|---|---|---|
| COD | mg/L | 1214 | 303 | 75.0 | NIEA W515 54A | 1.3276 |
| Cu2+ | mg/L | 0.09 | 0.0033 | 96.3 | NIEA W306 54A | 0.01373 |
| Zn2+ | mg/L | 0.8 | 0.0173 | 97.8 | NIEA W306 54A | 0.0132 |
| NH4+-N | mg/L | 204 | 1.68 | 99.2 | NIEA W448 51B | 0.045 |
| TP | mg/L | 2.16 | 0.109 | 94.9 | NIEA W427 53B | 0.033 |
| TN | mg/L | 492 | 4.55 | 99.1 | NIEA W423 52C | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, W.-C.; Chang, C.-C. Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide. Sustainability 2022, 14, 1742. https://doi.org/10.3390/su14031742
Lee W-C, Chang C-C. Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide. Sustainability. 2022; 14(3):1742. https://doi.org/10.3390/su14031742
Chicago/Turabian StyleLee, Wan-Chen, and Chih-Cheng Chang. 2022. "Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide" Sustainability 14, no. 3: 1742. https://doi.org/10.3390/su14031742
APA StyleLee, W.-C., & Chang, C.-C. (2022). Effectively Recycling Swine Wastewater by Coagulation–Flocculation of Nonionic Polyacrylamide. Sustainability, 14(3), 1742. https://doi.org/10.3390/su14031742






