Abstract
Hydrothermal liquefaction (HTL) is a thermochemical process for production of biocrude oils, commonly from wet biomass under inert atmosphere (N2). Influence of reaction atmosphere on HTL of pinewood sawdust was investigated in this work, at 300 °C for 60 min with the presence of KOH or H2SO4 catalyst under N2, H2, and O2 atmosphere, respectively. Very interestingly, the reaction atmosphere showed significant influence on both products distribution and properties of the biocrude oils. Generally, H2 atmosphere enhanced biomass degradation in the presence of either KOH or H2SO4 catalyst, producing the highest biocrude oil yield, lowest solid residue yield, and the best oil quality in terms of total acid number (TAN), viscosity and average molecular weights (Mn, Mw). Whereas the HTL in O2 atmosphere showed the poorest performance in terms of yields and properties of biocrude oils. The highest quality of biocrude oil was produced using KOH catalyst in H2 atmosphere with the maximum biocrude yield (approx. 34 wt.%) and the highest energy recovery (ER) in biocrude (ER = 73.14%). The measured properties of the oil are as follows: TAN = 40.2 mg KOH/g, viscosity = 51.2 cp, Mn = 470 g/mol, Mw = 767 g/mol. In addition, the biocrude oils produced in H2 atmosphere contain more light oil (naphtha) fraction (23.9 wt.% with KOH and 16.5 wt.% with H2SO4) with lower boiling points, while those generated in O2 atmosphere have more carboxylic acid compounds.
1. Introduction
The growing energy demand has led to extensive use and overexploitation of fossil resources, resulting in an energy crisis and severe environmental issues such as climate changes [1,2,3]. To mitigate the pressure from the global energy shortage and global warming due to fast increased CO2 emission, as well as to satisfy the clean energy demand, sustainable development and carbon neutrality, biomass has gained immense interest for the production of energy, fuels, chemicals and materials [4,5]. Biomass is a renewable and abundantly available resource from dedicated energy crops or various organic wastes, such as wood and forestry residues, agricultural crops and crop residues, marine products, wastewater sludge, microalgae, etc. [6,7,8].
Biomass can be converted into energy, fuels and chemicals via biochemical and thermochemical technologies. Biochemical treatments mainly refer to anaerobic digestion, fermentation, and photobiological hydrogen production. The objective of these treatments was to utilize various microorganisms or enzymes to convert the biomass into a variety of products and intermediates. This process offers an opportunity to produce an assortment of fuels and chemicals, including biogas, hydrogen, ethanol, butanol, acetone, and various organic acids [9]. However, biochemical processes are very sensitive to operating conditions (such as pH, temperature, and residence time) and quite slow (usually requiring days or weeks to complete). [10,11,12]. Thermochemical processes include gasification, hydrothermal liquefaction (HTL), pyrolysis, and combustion [13,14]. Pyrolysis and HTL are two main thermochemical pathways developed for the transformation of raw biomass materials into liquid products that can be further processed to produce biofuels or bio-based chemicals. Compared with pyrolysis, HTL has gained increasing attention because it can produce high quality biocrude oils (5–20% oxygen) as the primary products with little gas generated [15]. More advantageously, HTL can handle wet feedstocks (with >70% water content) without the requirement for drying [13,16], and it typically operates in an inert atmosphere (N2) at milder temperatures (250–400 °C), though under high pressure (50–250 bar) [6,17,18].
Many studies focus on the effects of HTL process parameters, e.g., reaction temperature, pressure, residence time, and types of solvent. Generally, operations at a moderate temperature between 250–350 °C obtain a higher biocrude yield; too high temperature would reduce bio-crude yield and increase the amount of solid residue due to bio-oil cracking and the repolymerization/condensation of cracking intermediates [16,19,20,21]. Shortening residence time can prevent these reactions happening to a certain extent, resulting in an increased biocrude yield. However, a too-short duration would cause incomplete biomass degradation [22]. A lower biomass-to-solvent ratio increased the yield of biocrude and inhibited the formation of solid residues and gases owing to better dissolution of reaction intermediates/products in the solvent (water or a mixture of water and organic solvent) and the enhanced hydrolysis reactions of the biomass [23]. Catalyst is another critical factor that affects HTL biocrude yield and properties. Homogeneous catalysts, including soluble acids, bases, and alkali salts, are commonly used in biomass HTL. Strong acids (sulfuric acid and hydrochloric acid) exhibited great performance in biomass conversion. However, strong acids could corrode the experimental equipment, and the biomass conversion might decrease when increasing the acid content over a certain level [24,25]. In addition, acidic condition facilitates the condensation and re-polymerization reactions of the reaction intermediates, thereby reducing the yield of biocrude. Alkaline catalysts such as NaOH, K2CO3, KOH, and Na2CO3 have been widely used in HTL processes [14]. Alkaline catalysts can neutralize the acid compounds in the hydrothermal products, which prevents the repolymerization/condensation of the reaction intermediates, thus reducing the formation of solid residue/char [26]. Recently, researchers have employed mathematical models to optimize the parameters of HTL of biomass. The goal of this optimization is to obtain biocrude with high yield, high carbon and hydrogen content, and low heteroatom content, along with the corresponding reaction conditions and material composition. The primary methods include empirical summaries based on experimental laws, response surface method (RSM), kinetics modeling, and machine learning (ML). For example, Zhu et al. [27] utilized RSM to optimize reaction temperature, reaction time, catalyst dosage and biomass/water ratio for highest bio-oil yield. Obeid et al. [28] employed kinetics modeling to predict the distribution of biocrude yield. Cheng et al. [29] used multiple linear regression (MLR), regression tree (RT) and random forest (RF) to predict the quantity and quality of biomass HTL products.
However, few studies reported the effects of the reaction atmosphere on biomass HTL with respect to the products distribution and properties of the biocrude. HTL of biomass is typically operated in an inert atmosphere, usually pressurized nitrogen, to avoid boiling of the reaction mixtures. Zhang et al. found that using a reducing gas (CO, H2) as the HTL reactor pressurizing gas could inhibit condensation, cyclisation, and re-polymerization of free radicals of the reaction intermediates, and hence stabilize the depolymerized lignocellulose fragments and reduce char formation [30]. Yin et.al reported that using CO or H2 as the reaction atmosphere in HTL of cattle manure led to increased biocrude production by 5–15 wt.% [31]. Interestingly, Yin et al. also tested oxygen as an oxidative atmosphere for cattle manure HTL, resulting in much lower biocrude yield compared with those obtained under N2 or CO atmosphere, due to the oxidation of feedstocks/products in the presence of an excess of oxygen in the reactor [31]. In another research by Rahimi et al. in HTL conversion of lignin, oxygen atmosphere was found to promote the cleavage of alkyl aryl ether units in lignin to yield low molecular weight compounds such as vanillin and benzylic/aliphatic alcohols [32,33].
Lignocellulosic biomass is one of the most commonly used feedstocks for HTL process. However, no research has indicated which atmosphere (inert, oxidative, or reductive) works best for the HTL conversion of lignocellulosic biomass. In this study we for the first time compared the oil product yield and properties from HTL of a typical lignocellulosic biomass-pinewood sawdust—under different reaction atmospheres (N2, H2, and O2) on HTL of a typical lignocellulosic biomass-pinewood sawdust. The tests were conducted with a biomass-to-water ratio of 1:15 (w/w) at 300 °C for 60 min under N2, H2, or O2 atmosphere in the presence of a homogeneous catalyst: KOH or H2SO4. The biocrude products were comprehensively characterized for their physicochemical properties, i.e., viscosity, total acid number (TAN), functional groups by Fourier-transform infrared spectroscopy (FT-IR), volatile compositions by gas chromatography-mass spectrometry (GC-MS), volatility by thermogravimetry analysis (TGA), and average molecular weights and distributions by gel permeation chromatography (GPC).
2. Materials and Methods
2.1. Materials
Pinewood sawdust was collected from a local sawmill (London, ON, Canada). Prior to the HTL tests, it was crushed and sieved to reduce particle size below 0.3 mm, followed by pre-drying at 105 °C in an oven for 24 h. It contains 40.9 wt.% cellulose, 28.5 wt.% hemicellulose and 28.4 wt.% lignin. Its proximate analysis and ultimate analysis, were obtained from our previous work [34], as shown in Table 1. Potassium hydroxide (KOH, 99 wt.%) and sulfuric acid (H2SO4, 98 wt.%) were purchased from Sigma-Aldrich (Oakville, ON, Canada), and VWR International (Mississauga, ON, Canada), respectively. Reagent-grade acetone (99.5 wt.%) was purchased from Fisher Scientific (Ottawa, BC, Canada). De-ionized water was used as the solvent in HTL experiments.

Table 1.
Proximate analysis and ultimate analysis of the pinewood sawdust feedstock.
2.2. Apparatus and Methods
2.2.1. HTL Experiments
The HTL experiments were conducted in a 100 mL Parr 4590 autoclave batch reactor (made of SS 316L) equipped with a stirrer and temperature controller. In a typical HTL run, 2 g of pinewood sawdust together with 30 g de-ionized water (solid-to-liquid ratio is fixed as 1:15 w/w) were loaded into the reactor. The catalyst (KOH or H2SO4) dosage was set to be 5 wt.% with respect to the mass of dry biomass feedstock. The reactor was sealed, and the air inside was displaced by vacuuming and purging for three times with N2, H2, or O2, followed by pressurizing the reactor to 2 MPa using N2, H2, or O2. Then the reactor was heated under 100 rpm stirring at approx. 10 °C/min to 300 °C and maintained at the temperature for 60 min for reaction, followed by quenching in a water bath.
2.2.2. Products Separation
The products from the HTL experiments include gaseous products, biocrude oil, aqueous-phase (AP) products, and solid residues (SR). After the reactor was quenched to room temperature, the gas products from HTL were vented into a gas bag and analyzed by Micro-GC-TCD (Agilent 3000). The gas products composition (mainly CO2) was used for calculation of mass of gas products, Mgas. Then the reactor was opened and the solid/liquid products inside the reactor were first washed out with distilled water. The resulting suspension was filtered under vacuum through a pre-weighted VWR No. 413 filter paper. The reactor was then further washed with reagent-grade acetone to collect the water-insoluble oil components. The mixture was filtered under vacuum through the same filter paper (VWR No. 413) retaining the solid residue on it. The solid residue or char was rinsed with additional acetone until the filtrate became colorless, followed by oven dried at 105 °C until attaining a constant weight to determine the mass of solid residue (MSR). The filtrate was evaporated under reduced pressure at 50 °C until bio-crude oil was precipitated on the flask inner wall. The remaining aqueous solution was decanted and collected as the aqueous product (AP). The oily product sticking on the flask, designated as biocrude oil or simply bio-oil, was weighed to determine the mass of biocrude, Mbiocrude. The yields of the HTL products were calculated based on dry and ash-free mass of biomass feedstock, MBiomass,daf, as follows:
2.2.3. Products Analysis
The composition of the collected gaseous products in the gas bag was analyzed with a Micro-GC-TCD (Agilent Micro-GC 3000) by injecting 50 mL of air as the internal standard. A PerkinElmer Fourier transform infrared spectrometer (FT-IR, MA, USA) was used to determine the functional groups of the biocrude products. The biocrude viscosity at 80 °C was measured using a Brookfield CAP 2000 + Viscometer. The weight/number average molecular weights (Mw, Mn), polydispersity index (PDI, =Mw/Mn), of the obtained biocrude products were determined by Gel Permeation Chromatography (GPC-UV, Waters Breeze). Volatile compositions of the biocrude products were analyzed by Gas Chromatograph-Mass Spectrometer (GC–MS, Agilent Technologies, 5977A MSD, Santa Clara, CA, USA) equipped with an HP-5MS column (30 m × 250 μm × 0.25 μm). The GC temperature program was set as: held at 40 °C for 5 min, then increased at 10 °C/min to 150 °C and held for 2 min, increased at 10 °C/min to 290 °C and held for 5 min. The elemental compositions (C, H, N, and S) of the biomass feedstock and biocrude products were analyzed on an elemental analyzer (Vario EL Cube). The O content was calculated by difference on a dry basis (%O = 100% − %Ash − %C − %H − %N − %S) where the ash content was determined by ashing at 700 °C for 4 h in a muffle furnace. The higher heating values (HHV) of the biomass feedstock, bio-crude oils, and solid residue were calculated by Dulong equation [HHV (MJ/kg) = 0.338C + 1.428(H-O/8) + 0.095S]. The proximate analysis (volatile matters and fixed carbon contents) of biomass feedstock and biocrude products was determined on a thermogravimetric analyzer (Pris 1 TGA, Waltham, MA, USA), where the sample was heated in 30 mL/min N2 flow from 25 °C to 800 °C at 10 °C /min, followed by soaking at this temperature for 15 min in 30 mL/min air flow for ashing. The total acid number (TAN) of biocrude was determined by titration on a pH meter (Titroline 7000) using 0.01 N KOH and phenolphthalein as the titration solution and indicator, respectively. TAN was calculated in milligrams of KOH/gram of the biocrude sample as follows:
where:
A = KOH solution required for titration of the sample, mL,
B = KOH solution required for titration of the blank, mL,
N = Normality of the KOH solution,
W = Mass of the biocrude sample, g.
3. Results and Discussions
3.1. HTL Products Distribution
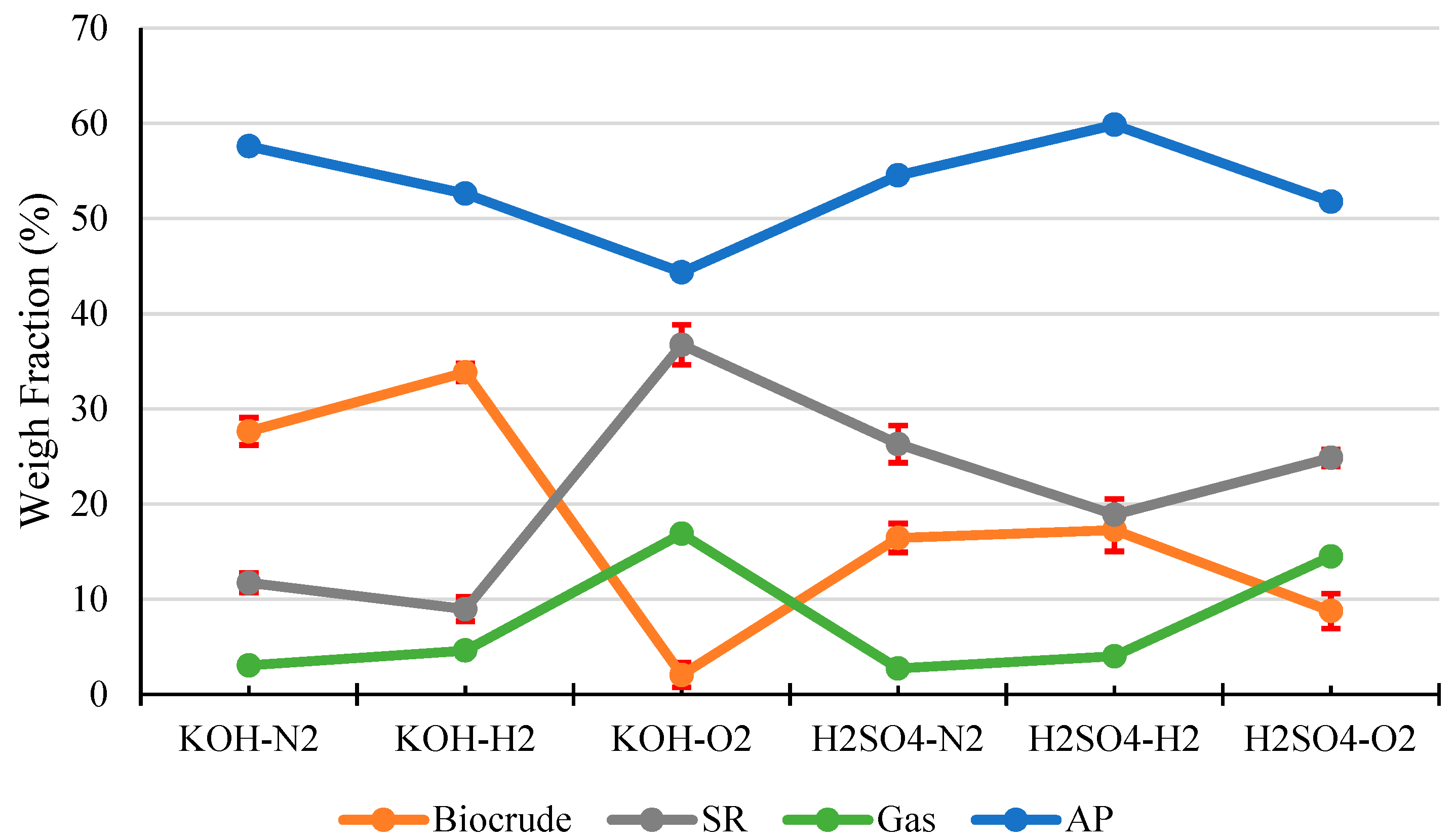
Figure 1 shows the products distribution in HTL of pinewood sawdust under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst. As clearly shown in the Figure, reaction atmosphere and catalyst both have significant influence on the products distribution in HTL of the woody biomass. In terms of biocrude yield and SR yield, the alkali catalyst (KOH) outperformed the acid catalyst (H2SO4), and H2 atmosphere was superior to both N2 and O2. The highest biocrude yield (approx. 34 wt.%.) and maximum biomass conversion (approx. 91%, or SR~9 wt.%) was achieved under the H2 atmosphere with KOH catalyst. In contrast, the presence of O2 atmosphere with KOH catalyst produced the lowest biocrude yield (approx. 2 wt.%) and minimum biomass conversion (approx. 63%, or SR~37 wt.%).
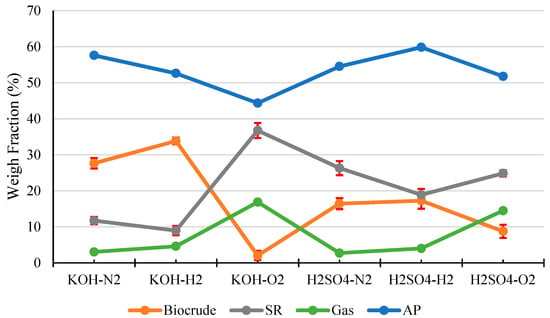
Figure 1.
Products distribution from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
Pinewood sawdust is a typical woody biomass contains 60–70 wt.% holocellulose (cellulose and hemicellulose), 20–30 wt.% lignin, and 5–10 wt.% others (extractives and ash) [35]. Holocellulose includes polysaccharides with β(1→4) linkage [36], and lignin is a well-known polymer of phenylpropane linked mainly by β-O-4 ether linkage. During HTL, the β(1→4) linkage in holocellulose, and β-O-4 ether linkage in lignin can be broken via hydrolytic depolymerization, forming carbohydrate/phenolic monomers and oligomers as HTL reaction intermediates, which would further undergo recombination reactions to form biocrude components including organic acids and phenolics [37]. The hydrolytic depolymerization process could be catalyzed by acid catalyst or self-catalyzed by the organic acids formed in the biomass HTL process. However, the acid catalyst could catalyze the condensation/repolymerization, decarboxylation and dehydration reactions of the HTL reaction intermediates to form SR product [38,39], whereas an alkaline catalyst, e.g., KOH can neutralize the organic acids produced and inhibit condensation/repolymerization reactions [15], hence restricting SR yield and improving biocrude yield, which explains the results in Figure 1.
On the other hand, the reaction atmosphere exhibits a significant influence on the HTL products distribution. First of all, the H2 atmosphere could play positive roles in breaking of the β(1→4) linkage in holocellulose and β-O-4 ether linkage in lignin via reductive depolymerization and stabilizing the reaction intermediates [40], which would hence suppress the condensation/repolymerization reactions, restrict SR formation and improve biocrude yield, as evidenced by the results of this study (Figure 1). In contrast, under O2 atmosphere, the reaction intermediates are converted into more carboxylic compounds that promote the formation of SR product, accompanied by significantly reduced yields of biocrude (Figure 1). More gaseous products were generated under O2 atmosphere. Further analysis of the gas products indicates that the amount of produced CO2 in O2 atmosphere is much higher than those in N2 or H2 (Table 2), as a result of the enhanced decarboxylation and deep oxidation of the intermediates by O2. In addition, the reductive environment (H2) somewhat limits the production of CO2. In a summary, among all three types of gas atmosphere examined, biomass HTL under H2 atmosphere showed the highest biocrude yields and the lowest SR yields. It is worth noting that although an inert atmosphere (N2) exhibited an average performance in terms of biocrude yield and biomass conversion, N2 is more economical and safer than H2 and O2, hence has been employed most often for biomass HTL.

Table 2.
Yield of gas products obtained from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
3.2. Properties of Biocrude Products
Table 3 presents the results of the elemental compositions, HHV, viscosity, average molecular weights, and TAN of the obtained biocrude oils from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst. In terms of elemental compositions of the biocrude products, the effects of catalyst type were minimal, but the atmosphere showed significant influence on elemental compositions and all other oil properties. Generally, the biocrude oils obtained under N2 or H2 atmosphere have a better quality than those obtained under O2 atmosphere: a higher H/C ratio, lower O/C ratio, higher HHV, lower TAN, much lower viscosity and much smaller Mn and Mw. HTL operations under N2 or H2 atmosphere also led to much higher energy recovery (65–73% with KOH catalyst and 35–42% with H2SO4 catalyst) than those under O2 atmosphere (approx. 3% with KOH catalyst and 16% with H2SO4 catalyst). The HTL experiment using KOH catalyst under H2 achieved the best energy recovery (ER~73%) owing to the highest biocrude production yield (~34 wt.%) obtained under these conditions. Although surprisingly the H/C ratios of the biocrude oils obtained under H2 atmosphere are smaller than those of the oils obtained under N2 (suggesting more condensed structure of the oils, whose causes require future research), the oils obtained under H2 atmosphere have better properties: lower TAN, much lower viscosity and smaller Mn and Mw. Apparently, the biocrude oils from the HTL experiments under H2 atmosphere are much less viscous and have smaller molecular mass than the oils obtained under N2 or O2 atmosphere, which could be attributed to reductive depolymerization of holocellulose and lignin, as well as hydrocracking reactions. These reactions would result in breakage of the C-O-C and C–C bonds of the complex macromolecular structure of biomass substrate/intermediates into simpler small-molecule substances [41], and hence a reduced viscosity of the biocrude products. Compared with the average molecular weight of the Illinois shale oil (Mw = 670 g/mol, Mn = 270 g/mol) [42], the biocrude oils from the HTL still have larger average molecular weights (Mw = 654–1347 g/mol, Mn = 417–753 g/mol), because deep-depolymerization of lignocellulose is very difficult under testing conditions due to the unavoidable occurrence of self-condensation and repolymerization reactions during the conversion process [43].

Table 3.
Properties of biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
The Mw and Mn of the biocrude oils from the experiments with KOH catalyst are slightly higher than those with H2SO4 catalyst. This result might be owing to the acid-catalyzed cleavage of alkyl-aryl ether linkages, e.g., β-O-4 linkage, in lignin, as well as the acid-catalyzed breakage of the 1,4′-β-glycosidic bonds (β(1→4) linkage) in holocellulose [44]. Moreover, compared with oils obtained with KOH catalyst, the oils obtained with H2SO4 catalyst are more acidic, especially for those obtained under O2 atmosphere (with a greater TAN), suggesting the presence of more organic acids, which can be confirmed by the GC-MS analysis results as discussed below.
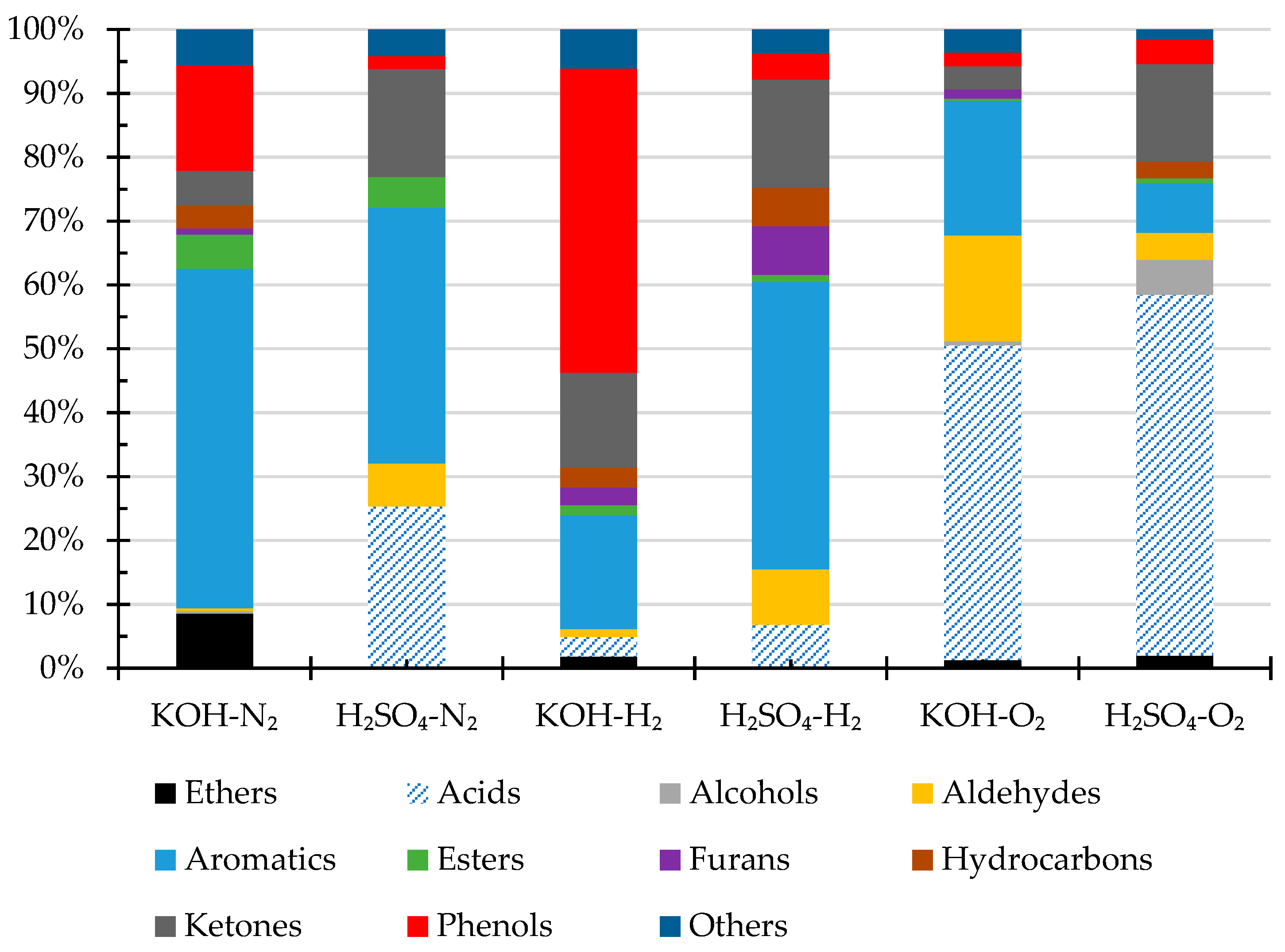
The volatile compounds of the bio-crude oils were analyzed by GC-MS. The results are shown in Figure 2. The identified compounds include carboxylic acids, alcohols, aldehydes, aromatics, esters, ethers, hydrocarbons, ketones, nitrogenous compounds, and phenols. The main volatile compounds in the oils obtained under N2 or H2 atmosphere with KOH catalysts are phenols and aromatic compounds, and the oils obtained under H2 atmosphere contain more phenols than those obtained under N2 atmosphere, likely owing to the reductive depolymerization of lignin [40,45]. In comparison to oils obtained under N2 or H2 atmosphere with KOH catalyst, the biocrudes obtained with H2SO4 catalyst contain much more carboxylic acids and ketones, suggesting acid-catalyzed degradation of cellulose and hemicellulose structures [46].
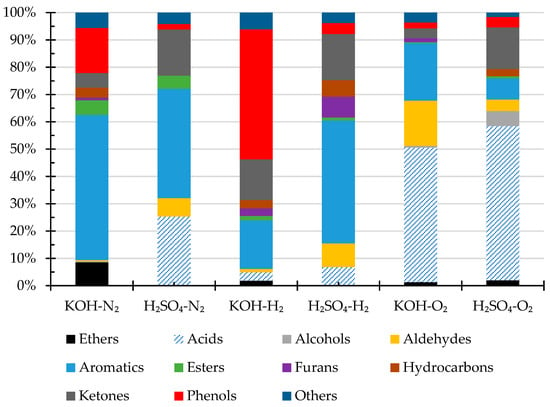
Figure 2.
Volatile compositions (area% by GC-MS) of the biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
More interestingly, the oils from the HTL experiments under O2 atmosphere contain much more acids than those obtained under H2 or N2. Estimated by the area %, the oils obtained under O2 atmosphere with KOH or H2SO4 catalyst contain approx. 41% and 56% of acids, respectively, corresponding to much higher TAN as evidenced in the results presented previously in Table 3. Under the oxygen atmosphere, phenols could deprotonate to form phenoxy radicals by electron transfer from oxygen and be stabilized by the resonance, resulting in the formation of carbonyl and carboxylic acid, and H2SO4 acid could also catalyze aromatic ring opening, leading to formation of pentatonic acids [47]. The detailed compounds in bio-crude oils were summarized in Table A1.
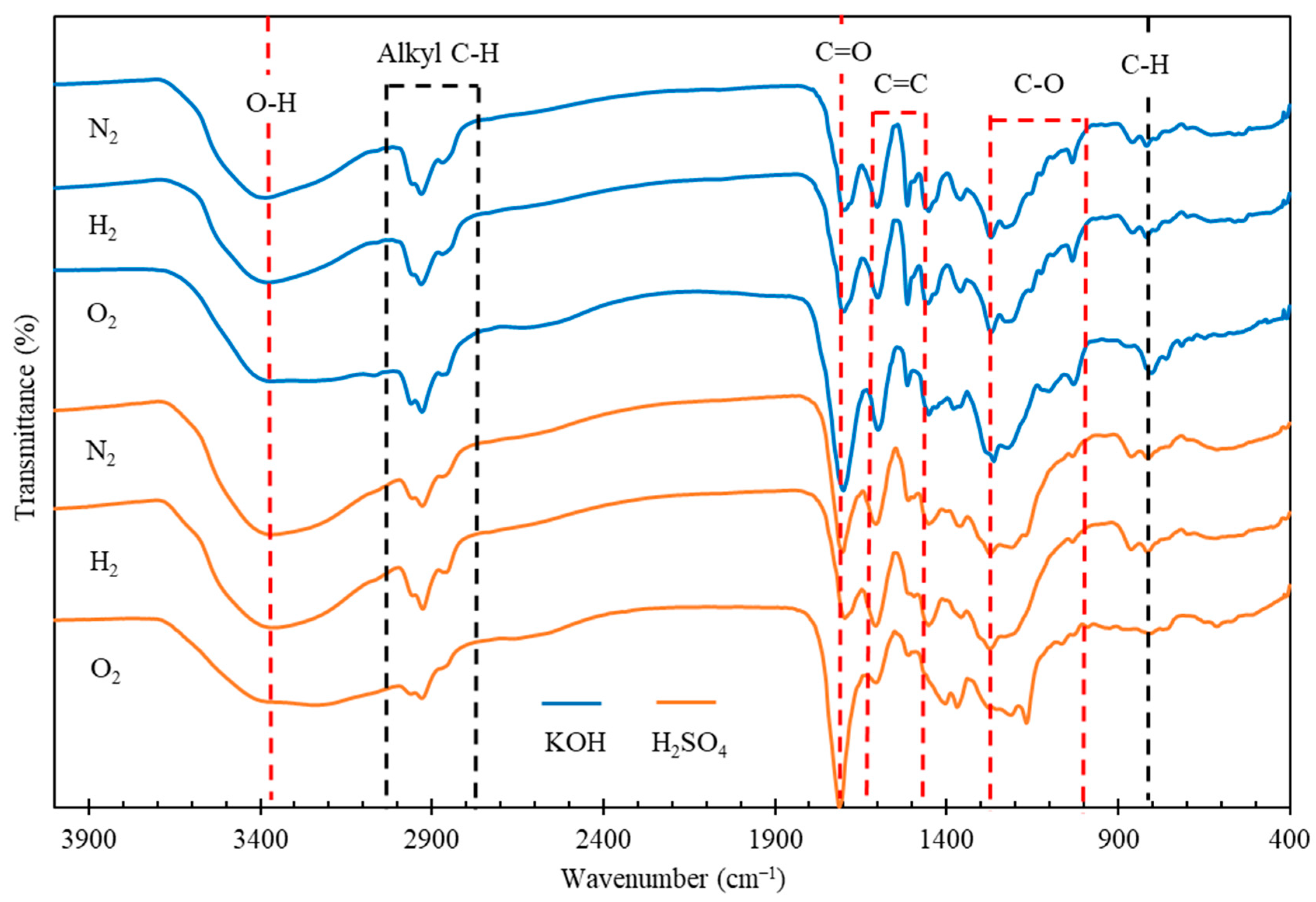
The functional groups of the biocrude oils were identified by FT-IR and the spectra are illustrated in Figure 3. The broad IR absorption at 3350 cm−1 is typical of O-H stretching, suggesting the presence of alcohols, phenols, carboxylic acids, or water residues in the oils. The absorbance at 1700 cm−1, representing the C=O stretching vibration of carbonyl groups, indicates the presence of ketones, aldehydes, esters or carboxylic acids in the oils. The relatively medium-intense peaks at 1611 and 1495 cm−1 represent aromatic nuclei, indicating the presence of aromatic rings and their derivatives. The IR absorption bands between 3000 and 2840 cm−1 are attributed to C-H stretching vibrations, indicating the presence of alkyl C-H in the oils. The two absorption peaks at 1370 and 1456 cm−1 are attributed to the bending vibrations of methyl (-CH3) and methylene (-CH2) groups, respectively. The presence of C-H bonds indicates the alkane groups in the biocrude oils. The bands between 1280 and 1000 cm−1 could be related to C-O vibrations, suggesting that the oils may contain acids, phenols, furans, or alcohols. The presence of the peak at 860 cm−1, attributed to C-H bending, suggests the possible presence of single, polycyclic, and substituted aromatics. In the oils obtained under O2 atmosphere, more oxygen containing compounds were produced in the biocrude oils, evidenced by the intensive peaks representing O-H, C=O and C-O bonds, compared to those of the oils obtained under N2 or H2 atmosphere. In addition, the intensities of aromatic absorptions at 1611 and 1495 cm−1 are weaker in the biocrudes obtained when using H2SO4 catalyst, especially under O2 atmosphere, suggesting that the presence of oxidative agents (i.e., H2SO4 and O2) could possibly restrict the aromaticity of reaction intermediates derived from lignin in pinewood.
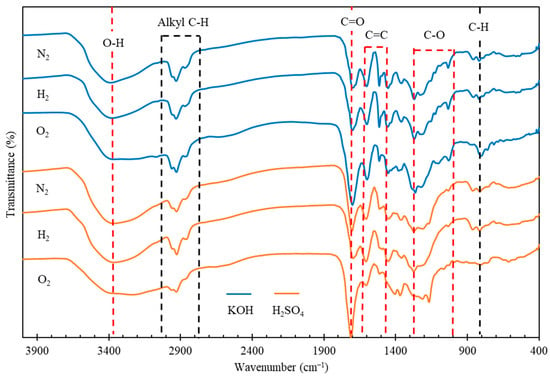
Figure 3.
FT-IR spectra of biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
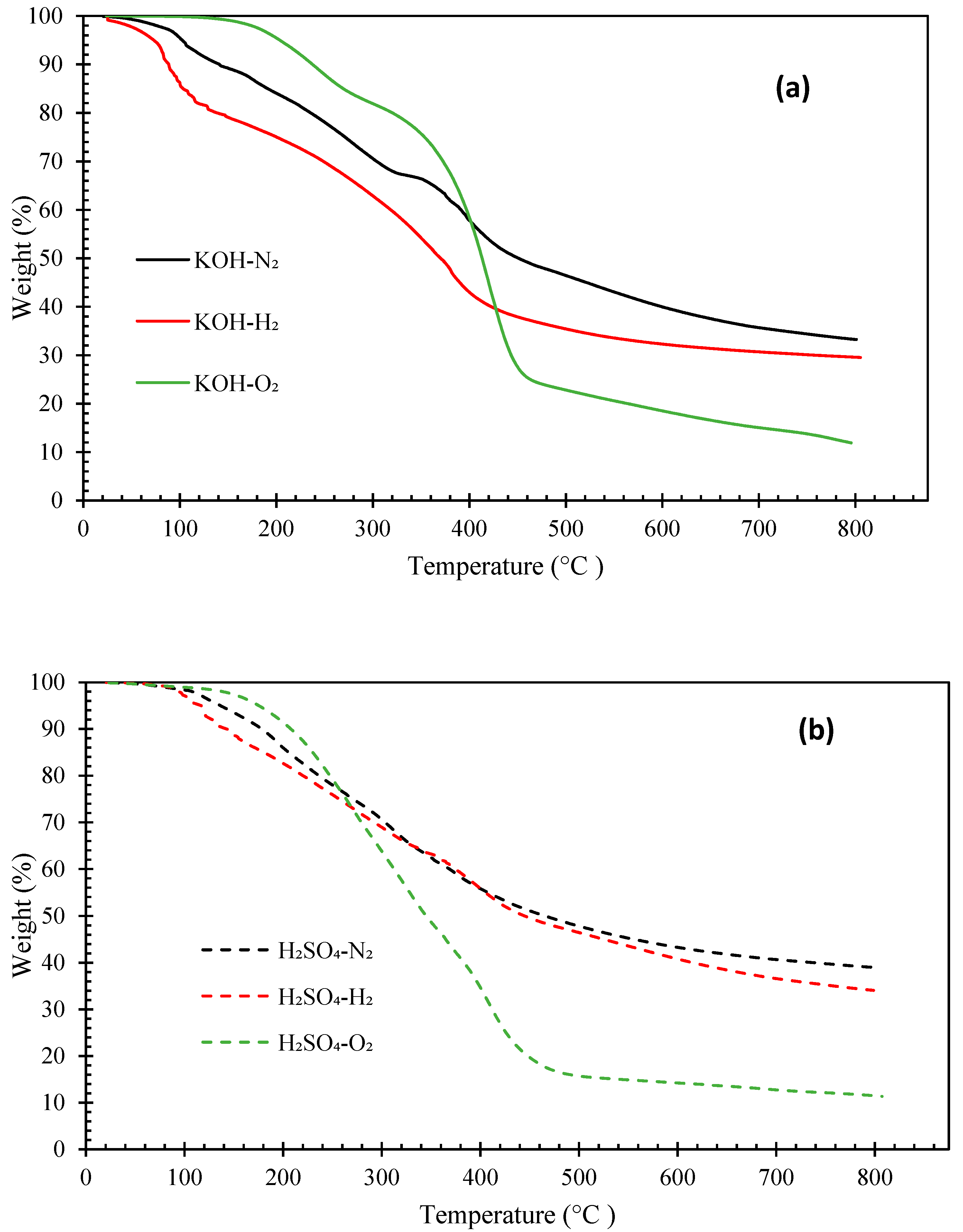
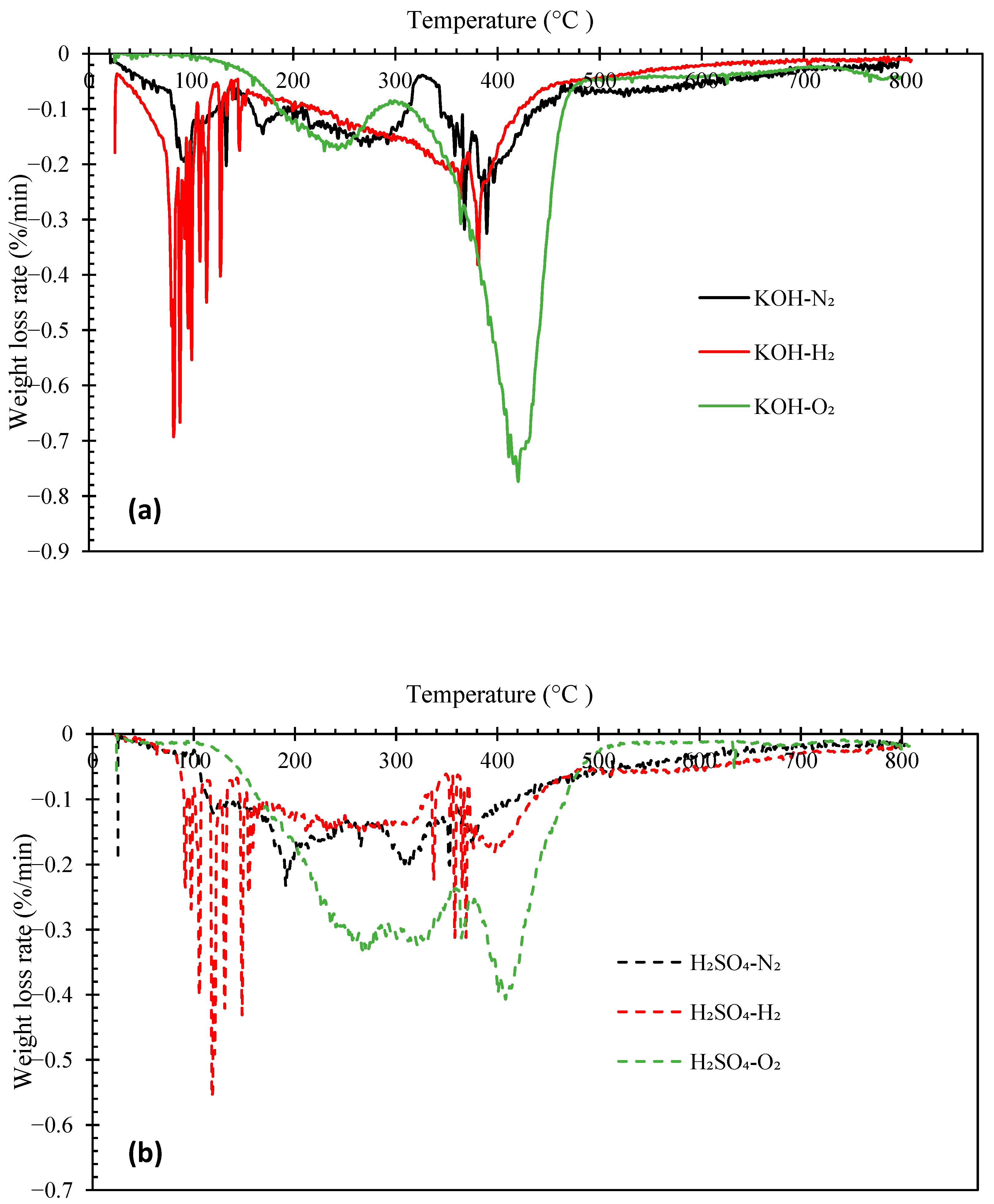
Figure 4 and Figure 5 illustrate the thermogravimetric (TG) and derivative thermogravimetric (DTG) curves, respectively, of the biocrude oils obtained under N2, H2, and O2 with KOH or H2SO4 catalyst. The boiling point distributions of the oils, estimated from the TG curves, are presented in Table 4. The biocrudes obtained under N2 and H2 atmosphere had similar decomposition curves (TG). The initial decomposition of these biocrudes occurred at around 100–140 °C. Whereas, the decomposition of bio-oils from HTL of pinewood under O2 started at around 160–180 °C. The distillate or volatile fractions of the oils (with a boiling point below 600 °C) obtained under N2 and H2 atmosphere vary from 59.21 wt.% to 67.26 wt.%, which fell in the range between North American tar sand bitumen (44–65 wt.% distillate) and the Venezuelan crude oil (66 wt.% distillate) [31,38]. However, the biocrudes produced under O2 atmosphere are more thermally unstable with over 80 wt.% of volatile matters. Generally, the boiling point distributions of the oils obtained under N2 and H2 with KOH catalyst are similar, but the oil obtained under H2 has a higher light fraction (<193 °C) (23.89 wt.%). The oils obtained under O2 have more mild boiling fractions (343–538 °C) (55.85 wt.% with KOH catalyst, 35.43 wt.% with H2SO4 catalyst) and the least heavy residues fraction (>538 °C) (21.07 wt.% with KOH catalyst and 15.10 wt.% with H2SO4 catalyst).
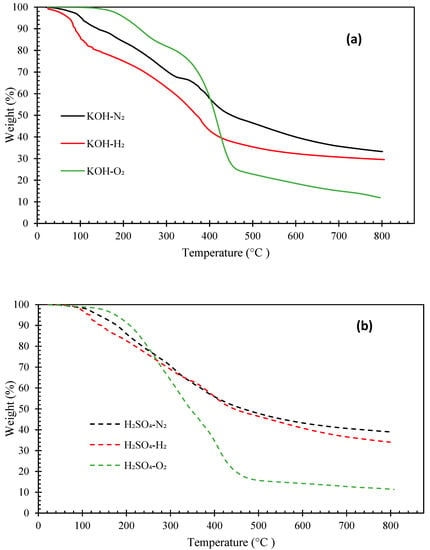
Figure 4.
TG curves of biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, with KOH (a) or H2SO4 (b) catalyst.
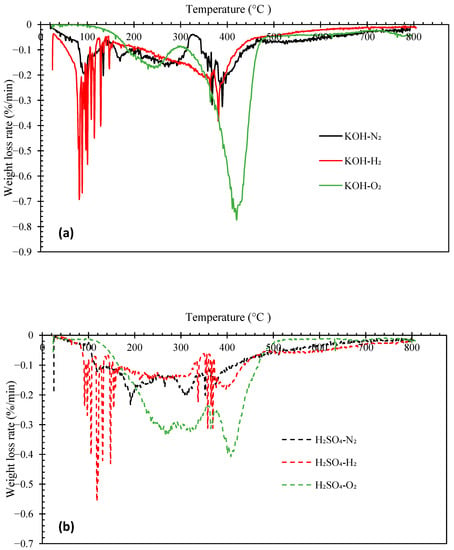
Figure 5.
DTG curves of biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, with KOH (a) or H2SO4 (b) catalyst.

Table 4.
Boiling point distributions of the biocrude products from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
The DTG peaks of the biocrude oils locate at two main temperatures ranges: from room temperature to 300 °C and from 300 °C to 450 °C (Figure 5). The weight loss from room temperature to 300 °C could be due to the evaporation of low molecular weight fractions in the oil samples. The major weight loss of all biocrude oil samples occurred between 300 °C and 450 °C. Interestingly, intensive weight loss peaks were observed between 100 °C and 200 °C in the oils obtained under the H2 atmosphere, likely owing to the presence of more low molecular weight compounds (e.g., phenols) that evaporate in this temperature range, as confirmed by the GC-MS and GPC results discussed previously. The oils obtained under O2 atmosphere are more thermally stable than those obtained under N2 or H2, with intensive weight loss peaks from 300 °C to 450 °C. In contrast, the DTG curves of the oils obtained under N2 have flat and broad peaks across the entire temperature range.
3.3. Economic Analysis
To perform a cost-benefit analysis of the HTL process, the costs and benefits associated with the process need to be considered, including capital investment (e.g., equipment and infrastructure, site development, engineering, etc.), operating costs (e.g., feedstock, labor, post-treatment, energy, maintenance, etc.), value of the produced oils, and environmental benefits. As mentioned above, the bio-oil produced under O2 atmosphere has a higher oxygen content and average molecular weight compared to those obtained under N2 and H2 atmosphere and thus requires a much higher upgrading cost, including additional equipment, energy, and labor cost, to convert the biocrude into gasoline-like products. In addition, the significant lower bio-oil yield from pinewood HTL process under O2 atmosphere will increase the feedstock and labor cost as well due to the more raw materials consumed and longer operating time required to achieve the aimed producing capacity. Besides, the higher TAN value of bio-oil obtained under O2 will cause corrosion problem on core HTL equipment, which will increase the maintenance costs. Although biomass HTL under H2 atmosphere showed the best biocrude yields and chemical properties, the safety issues accompanying H2 will possibly cause additional operating cost (e.g., insurance, training, etc.). Thus, N2 has been employed most often for biomass HTL. A comprehensive and detailed techno-economic assessment will be carried out in the future research.
4. Conclusions
The study investigated the effect of reaction atmosphere on hydrothermal liquefaction (HTL) of pinewood sawdust using KOH or H2SO4 catalyst at 300 °C, 20 bar for 60 min under N2, H2, and O2 atmospheres. The results showed that HTL under H2 atmosphere with KOH catalyst led to the highest biocrude yield (approx. 34 wt.%) and the lowest solid residue (SR) yield (approx. 9 wt.%). Biocrude oils obtained under N2 or H2 exhibited higher energy recovery and better quality (higher H/C ratio, lower O/C ratio, higher HHV, lower TAN, and much lower viscosity and molecular weight) than those obtained under O2. The oils produced in N2 or H2 atmosphere mainly contained phenols and aromatic compounds and showed similar boiling point distributions, while more acids were detected in the oils produced in O2, which presented the highest mild boiling fractions and the least heavy residue fraction.
Author Contributions
Conceptualization, H.W., X.H., Y.Z. and C.X.; methodology, H.W., Y.J., Y.Z. and C.X.; formal analysis, H.W., Y.J., E.P., X.H., Y.Z. and C.X.; investigation, H.W., Y.J. and E.P.; resources, X.H., Y.Z. and C.X.; writing—original draft preparation, H.W. and Y.J.; writing—review and editing, X.H., Y.Z. and C.X.; supervision, Y.Z. and C.X.; project administration, C.X.; funding acquisition, Y.Z. and C.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Canadian NRCan Forest Innovation and OERD Clean Energy programs, as well as by Natural Science and Engineering Research Council of Canada (NSERC).The Funding number of NSERC is RGPIN-2019-05159.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available upon request.
Acknowledgments
The authors gratefully acknowledge the technical support from NRCan CanmetMATERIALS corrosion and microscopy labs, and the facilities and support offered by Institute for Chemicals and Fuels from Alternative Resources (ICFAR) at Western University.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Detailed chemical compounds identified in the biocrude oil obtained from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
Table A1.
Detailed chemical compounds identified in the biocrude oil obtained from HTL of pinewood sawdust at 300 °C for 60 min under N2, H2, and O2, respectively, with KOH or H2SO4 catalyst.
| RT a (min) | Compounds Name | Relative Composition by Percent Area | |||||
|---|---|---|---|---|---|---|---|
| KOH-N2 | KOH-H2 | KOH-O2 | H2SO4-N2 | H2SO4-H2 | H2SO4-O2 | ||
| 3.780 | Acetic acid | 3.036 | |||||
| 5.967 | 2-Pentanone, 4-hydroxy-4-methyl- | 1.409 | 1.025 | 2.917 | 2.731 | 0.824 | |
| 7.700 | 2-Cyclopenten-1-one, 2-methyl- | 0.435 | |||||
| 8.517 | 1,3-Dioxolane-4-methanol, 2,2-dimethyl-, (S)- | 0.468 | |||||
| 8.948 | Benzaldehyde | 0.640 | |||||
| 9.142 | 2-Cyclopenten-1-one, 3-methyl- | 1.036 | |||||
| 9.527 | Phenol | 0.658 | |||||
| 9.908 | Cyclotetrasiloxane, octamethyl- | 0.789 | |||||
| 10.288 | 2,5-Furandione, dihydro-3-methyl- | 0.900 | |||||
| 10.555 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 1.264 | 0.569 | ||||
| 10.868 | DL-Norvaline, N-[(phenylmethoxy)carbonyl]- | 0.838 | |||||
| 11.054 | Acetophenone | 1.284 | 3.519 | 1.320 | |||
| 11.109 | Pentanoic acid, 4-oxo- | 0.927 | 25.374 | 6.830 | 51.470 | ||
| 11.464 | Phenol, 2-methoxy- | 4.110 | 42.040 | 1.627 | |||
| 11.959 | 2-Isopropylbenzenethiol, S-methyl- | 2.366 | |||||
| 12.204 | N-Chlorocarbonyl-N-methoxy-N-isopropylamine | 2.131 | |||||
| 12.284 | Pentanoic acid, 2-methyl-4-oxo- | 1.155 | |||||
| 12.454 | Phenol, 2,6-dimethyl- | 0.902 | |||||
| 12.919 | 2-Cyclopenten-1-one, 2,3,4,5-tetramethyl- | 0.935 | |||||
| 13.028 | Benzoic acid | 11.585 | 1.545 | ||||
| 13.143 | Creosol | 2.549 | 1.818 | 0.656 | |||
| 13.232 | Catechol | 2.648 | 1.136 | ||||
| 13.341 | Butanedioic acid, monopropargyl ester | 0.400 | |||||
| 13.460 | 1H-Benzimidazole, 2-ethyl- | 0.723 | |||||
| 13.895 | Butanedioic acid, methyl- | 0.780 | |||||
| 14.022 | 4-Nonanol, 4-methyl- | 0.615 | |||||
| 14.200 | Cyclohexene, 1-methyl-4-(1-methylethylidene)- | 0.926 | 1.700 | 0.966 | |||
| 14.230 | Naphthalene, 2,6-bis(1,1-dimethylethyl)- | 1.404 | 0.734 | ||||
| 14.327 | Benzoic acid, 3-methyl- | 2.079 | |||||
| 14.420 | Phenol, 4-ethyl-2-methoxy- | 4.622 | 2.253 | 1.428 | |||
| 14.445 | 1H-Inden-1-one, 2,3-dihydro- | 1.314 | 1.479 | ||||
| 14.805 | 1-Methylindan-2-one | 1.250 | 0.869 | ||||
| 15.067 | 2-Cyclopenten-1-one, 2,3-dimethyl- | 0.733 | 1.958 | 1.482 | |||
| 15.113 | 3-Cyclohexen-1-one, 2-isopropyl-5-methyl- | 0.834 | 0.445 | ||||
| 15.210 | Hydrocinnamic acid | 1.012 | 0.170 | ||||
| 15.325 | 1,4-Benzenediol, 2-methyl- | 0.516 | |||||
| 15.405 | 1(3H)-Isobenzofuranone | 0.891 | |||||
| 15.418 | 7-Methylindan-1-one | 0.370 | 1.520 | 1.395 | 0.484 | ||
| 15.553 | Benzaldehyde, 3-hydroxy- | 2.111 | |||||
| 15.629 | Phenol, 2-methoxy-4-propyl- | 3.286 | 1.121 | ||||
| 15.963 | Benzene, 1-ethenyl-4-methyl- | 1.105 | 0.812 | 2.971 | |||
| 16.001 | Ethanone, 1-(3-hydroxyphenyl)- | 1.363 | 0.865 | ||||
| 16.048 | Vanillin | 0.578 | 0.260 | 5.944 | 0.508 | 1.040 | |
| 16.149 | Benzoic acid, 3-(1-methylethyl)- | 0.882 | |||||
| 16.293 | 1H-Inden-1-one, 2,3-dihydro-3,3-dimethyl- | 1.203 | |||||
| 16.331 | 1,2-Diethoxybenzene | 0.809 | 2.494 | 1.059 | 0.900 | ||
| 16.412 | 2-(Cyclohex-1-enyl)-furan | 1.896 | |||||
| 16.623 | Acetophenone, 4′-hydroxy- | 1.674 | |||||
| 16.754 | Phenol, 2-methoxy-4-(1-propenyl)- | 1.309 | |||||
| 16.792 | Benzoic acid, 3-formyl- | 1.281 | |||||
| 16.953 | 3-Ethenylheptan-2,6-dione | 0.932 | |||||
| 16.961 | Phenol, 2-(1,1-dimethylethyl)- | 0.531 | |||||
| 17.067 | 4-Methylphthalaldehyde | 0.556 | |||||
| 17.071 | 1,4-Benzenediamine, N,N,N′,N′-tetramethyl- | 1.014 | |||||
| 17.169 | 1-Tetralone, 8-hydroxy- | 0.267 | |||||
| 17.287 | Benzoic acid, 4-hydroxy- | 0.342 | |||||
| 17.355 | Apocynin | 1.549 | 10.221 | 0.470 | |||
| 17.384 | Ethyl 3-(2-furyl)propenoate | 0.615 | |||||
| 17.782 | 4-Hydroxy-3-methylacetophenone | 0.602 | |||||
| 17.883 | 2-Cyclopentenecarboxylic acid, 5-hydroxy-5-methyl-2-(1-methylethyl)-, methyl ester, trans- | 0.681 | |||||
| 18.175 | 2-Propanone, 1-(4-hydroxy-3-methoxyphenyl)- | 1.637 | 2.935 | 0.868 | |||
| 18.196 | 1-(4-methylthiophenyl)-2-propanone | 1.716 | |||||
| 18.234 | Benzenamine, 4-methyl-3-nitro- | 0.930 | |||||
| 18.462 | 4-Acetylbenzoic acid | 4.376 | |||||
| 18.648 | Phenol, 2-(1,1-dimethylethyl)-4-methyl- | 0.509 | |||||
| 18.813 | Benzene, 1-methyl-3,5-bis[(trimethylsilyl)oxy]- | 2.952 | 0.223 | ||||
| 18.890 | 4-Hexylphenol, trimethylsilyl ether | 1.816 | |||||
| 19.109 | 4-Ethoxy-3-anisaldehyde | 3.261 | |||||
| 19.460 | Phthalan | 0.563 | |||||
| 19.562 | Phenol, 2,6-dimethyl-4-nitro- | 0.261 | |||||
| 19.630 | Benzofuran, 2,3-dihydro- | 0.558 | 0.477 | ||||
| 19.659 | 1,4-Benzenedicarboxaldehyde, 2-methyl- | 0.405 | |||||
| 19.828 | Aminosalicylic Acid | 0.487 | 0.218 | ||||
| 19.951 | Benzene, 1-methoxy-4-(1-methyl-2-propenyl)- | 1.820 | 0.183 | ||||
| 19.968 | Benzaldehyde, 3-methyl- | 1.708 | |||||
| 20.146 | Homovanillic acid | 1.719 | |||||
| 20.213 | Naphthalene, 2-ethoxy- | 0.689 | |||||
| 20.315 | Ether, bis(p-tert-butylphenyl) | 1.287 | |||||
| 20.365 | 2-Naphthalenol, 3-methoxy- | 1.046 | |||||
| 20.391 | 9-Methyl-3,4-dihydro-2H-pyrido(1,2-a)pyrimidin-2-one | 0.465 | |||||
| 20.446 | 4-Hydroxy-3,5-dimethylbenzoic acid | 0.375 | |||||
| 20.496 | 4,6-Dimethoxy-1-naphthaldehyde | 2.105 | |||||
| 20.539 | Terephthalic acid | 0.226 | |||||
| 20.547 | Benzofuran, 2,3-dihydro-2,2,4,6-tetramethyl- | 0.965 | 1.511 | ||||
| 20.695 | 1,2-Dihydropyrido(3,2,1-kl)phenothiazin-3-one | 1.450 | |||||
| 20.704 | Butan-1-one, 1-(2,3-dihydro-7,8-dinitro-1,4-benzodioxin-6-yl)- | 0.984 | |||||
| 20.962 | 10H-Phenothiazine, 2-(trifluoromethyl)- | 1.202 | |||||
| 20.983 | 2-Propenoic acid, 3-(3-hydroxyphenyl)-, methyl ester | 0.736 | |||||
| 21.122 | 5-Hydroxy-1-tetralone | 0.553 | |||||
| 21.148 | Salicylhydroxamic acid | 0.692 | |||||
| 21.258 | 1,4-Benzenediamine, N,N′-diethyl- | 2.026 | |||||
| 21.346 | Benzaldehyde, 2-nitro-, diaminomethylidenhydrazone | 1.575 | 1.229 | ||||
| 21.363 | Methyleugenol | 0.777 | 1.482 | ||||
| 21.389 | 2-Methyl-5-nitrobenzoic acid | 1.085 | |||||
| 21.397 | 1H-Benzotriazole-5,6-dicarbonitrile | 0.481 | |||||
| 21.592 | Pyridine, 4-(5-benzo[1,3]dioxol-5-yl-[1,2,4]oxadiazol-3-yl)- | 1.139 | |||||
| 21.735 | Benzenesulfonic acid, 4-methyl- | 0.628 | |||||
| 21.820 | 4-Methoxycinnamaldehyde | 1.680 | |||||
| 21.854 | Dihydrofuranno(3,2-f)coumaran | 2.134 | 1.056 | 1.447 | 0.584 | ||
| 22.006 | 4,4′-Stilbenedicarbonitrile | 1.748 | |||||
| 22.010 | (6,7-Dimethoxy-3,4-dihydro-1-isoquinolinyl)acetonitrile | 0.941 | 1.290 | 1.385 | 0.363 | ||
| 22.137 | 1-[2,2′:5′,2″]Terthiophen-5-yl-ethanone | 1.232 | |||||
| 22.209 | Benzene, 1-phenyl-4-(2,2-dicyanoethenyl) | 1.378 | |||||
| 22.213 | Benzo(a)phenazine | 1.615 | |||||
| 22.222 | Dibenzo[c,h][2,6]naphthyridine | 0.481 | |||||
| 22.230 | 2,2′-Bi-1H-indene | 1.321 | |||||
| 22.340 | 1,4-Dimethyl-4,5,7,8-tetrahydroimidazo-[4,5-E]-1,4-diazepin-5,8(6H)-dione | 1.538 | 2.477 | 2.927 | 1.088 | ||
| 22.509 | 2-Ethoxyphenyl isocyanate | 1.220 | 1.912 | ||||
| 22.535 | Asarone | 0.785 | 0.977 | 1.374 | 0.789 | ||
| 22.683 | Tricyclo[4.3.0.0(7,9)]nonane, 2,2,5,5,8,8-hexamethyl-, (1.alpha.,6.beta.,7.alpha.,9.alpha.)- | 1.683 | 0.934 | ||||
| 22.924 | Benzaldehyde, 2-methoxy-4-methyl- | 0.944 | |||||
| 23.106 | 6-Acetyl-5-hydroxy-2,7-dimethyl-1,4-naphthoguinone | 1.211 | |||||
| 23.135 | Benzoic acid, 3-methoxy- | 0.643 | 0.495 | ||||
| 23.321 | As-Indacene, 1,2,3,6,7,8-hexahydro-1,1,6,6-tetramethyl-4-(1-methylethyl)- | 2.247 | |||||
| 23.385 | (+)-3-Carene, 2-.alpha.-isopropenyl- | 1.063 | |||||
| 23.402 | 2,2′-Isopropylidenebis(3-methylbenzofuran) | 1.837 | 1.728 | ||||
| 23.579 | 2,4,5,6-Tetrachloro-nicotinamide | 0.587 | |||||
| 23.588 | 9,10-Dihydro-12H-5-oxabenzocyclodecene-6,11-dione | 1.082 | |||||
| 23.596 | Fluorene, 2,7-bis(1-hydroxyethyl)- | 3.234 | |||||
| 23.681 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | 0.287 | |||||
| 23.824 | [1,2,4]Triazolo[1,5-a]pyrimidine, 6-chloro-2-(2-furanyl)-5,7-dimethyl- | 1.299 | |||||
| 23.884 | Benzene, 2-ethyl-1,3-dimethyl- | 1.862 | |||||
| 23.968 | Dithiocarbonic acid, O-ethyl ester, methylene-S(IV)-trifluoromethyl ester | 0.689 | |||||
| 24.006 | Estra-1,3,5(10)-trien-3-ol | 0.564 | |||||
| 24.019 | n-Hexadecanoic acid | 1.996 | 1.063 | ||||
| 24.159 | 7-Oxabicyclo[4.1.0]heptane, 1-(2,3-dimethyl-1,3-butadienyl)-2,2,6-trimethyl-, (E)- | 0.419 | |||||
| 24.180 | 1,2-Dimethyl-3-nitro-4-nitroso-benzene | 0.532 | |||||
| 24.302 | Benzenemethanol, 2-methyl-.alpha.-phenyl- | 0.595 | |||||
| 24.311 | 1-Phenanthrenecarboxaldehyde, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, [1S-(1.alpha.,4a.alpha.,10a.beta.)]- | 2.870 | |||||
| 24.412 | Methyl 2-hydroxy-4-methoxybenzoate, trimethylsilyl ether | 1.238 | |||||
| 24.488 | Phenol, 4,4′-methylenebis- | 0.412 | |||||
| 24.531 | 7,8-Dihydro-9H-cyclopenta[a]pyren-9-one | 1.256 | 1.563 | 0.684 | |||
| 24.552 | 1,4-Cyclohexanedicarboxylic acid, 2,5-dioxo-, diethyl ester | 0.877 | |||||
| 24.704 | Ketone, 7-methoxy-2-benzofuranyl methyl | 1.641 | |||||
| 24.729 | 2-Acetyl-3-methylbenzo[b]thiophene | 1.523 | |||||
| 24.742 | Benzo[b]thiophene, 2,3-diethyl- | 0.969 | |||||
| 24.801 | 2,4(1H,3H)-Quinazolinedione, 1,3-diethyl- | 0.811 | |||||
| 24.818 | 1-METHOXY-2-TERT.-BUTYL-4,6-DINITROBENZENE | 1.304 | |||||
| 24.962 | di-p-Tolylacetylene | 0.863 | |||||
| 25.118 | 5-Methoxy-2-naphthalen-2-yl-2H-indazole | 0.659 | |||||
| 25.127 | Silane, dimethyl(2-naphthoxy)isobutoxy- | 0.598 | |||||
| 25.199 | Homovanillic acid | 4.555 | |||||
| 25.211 | 2,3,6-Trimethoxybenzoic acid | 0.723 | |||||
| 25.216 | Imidazo[1,2-b]-1,2,4-triazine, 6-(3-methoxyphenyl)-2,3-dimethyl- | 1.817 | |||||
| 25.372 | Androstane-3,17-dione, (5.alpha.)- | 0.398 | 2.705 | 1.271 | 1.073 | ||
| 25.385 | 2,5-Diethyl-3,4-diphenyl cyclopentadienone | 1.263 | 0.974 | ||||
| 25.478 | 1,2,3,4-Tetrahydrobenz[a]anthracene | 1.459 | 1.355 | ||||
| 25.512 | 9H-Xanthen-9-one, 1,3-dihydroxy-2-methyl- | 0.807 | |||||
| 25.541 | 3,5-di-tert-Butyl-4-hydroxybenzyl alcohol | 1.207 | |||||
| 25.736 | Naphthalene, 1-(2-naphthalenyloxy)- | 3.021 | 1.884 | 4.552 | 2.427 | ||
| 25.833 | cis-Vaccenic acid | 9.907 | |||||
| 25.842 | 4-Methoxyphenol, pentafluoropropionate | 2.017 | |||||
| 25.871 | 2-Hexanone, phenyl(2-propenyl)hydrazone | 0.664 | 0.925 | ||||
| 26.066 | Ethyl Oleate | 3.889 | 3.408 | 4.803 | 1.827 | 1.477 | |
| 26.192 | 1,2-Epoxy-3,4-dihydroxycyclohexano[a]pyrene, (3s,4s-) | 1.911 | |||||
| 26.205 | 2H-1-Benzopyran-3(4H)-one, 8-methoxy-2-(1-naphthalenyl)-, oxime | 1.142 | 0.851 | ||||
| 26.273 | 3H-Benzo[f]chromen-3-one, 2-(4-methoxyphenyl)- | 0.812 | |||||
| 26.328 | 3,4-Dimethoxychalcone | 1.625 | |||||
| 26.450 | Benzoic acid, 4,5-dimethoxy-2-(2-phenylethenyl)- | 1.794 | |||||
| 26.455 | Estra-1,3,5(10)-triene-6,17-dione, 3-hydroxy- | 1.055 | |||||
| 26.543 | 1,3-Cyclohexanedione, 2-[4-(4-methoxyphenylamino)-2-thiazolyl]- | 1.047 | 1.159 | ||||
| 26.590 | Retene | 2.830 | 2.305 | ||||
| 26.708 | Benzene, 1,3-dimethoxy-5-[(1E)-2-phenylethenyl]- | 1.780 | 1.156 | ||||
| 26.713 | p-Bis(p-methoxyphenyliminomethyl)benzene | 2.831 | 0.880 | ||||
| 26.806 | trans-3′,4′,5′-Trimethoxy-4-(methylthio)chalcone | 0.696 | 2.653 | 1.466 | |||
| 26.899 | 1-Benzhydrylazetidin-3-ol | 0.574 | |||||
| 26.966 | 2-(((6-Fluoro-4H-1,3-benzodioxin-8-yl)methyl)sulfanyl)-1H-benzimidazole | 1.289 | |||||
| 26.975 | Benzoic acid, 4,5-dimethoxy-2-(2-phenylethenyl)- | 0.877 | |||||
| 26.983 | 2-Amino-4-azido-5-[3,4,5-trimethoxybenzyl]pyrimidine | 0.965 | |||||
| 26.987 | 3Alpha,5-cyclo-6beta,19-epoxy-5alpha-androstan-17-one | 0.663 | |||||
| 27.148 | Acridin-9-yl-(2,4-dimethoxy-phenyl)-amine | 0.979 | |||||
| 27.203 | 13-(2-Methoxyphenyl)tricyclo[8.2.2.24,7] hexadeca-1(13),4,6,10(14),11,15-hexaene-5-carbaldehyde | 2.588 | |||||
| 27.254 | Estra-1,3,5(10)-trien-17-one, 3-methoxy- | 1.301 | |||||
| 27.296 | (.+/−.)-Uleine | 1.801 | |||||
| 27.322 | N,N-Dimethylindoaniline | 4.244 | |||||
| 27.457 | 2-[4-(1,2-Diphenyl-but-1-enyl)-phenoxy]-ethylamine | 1.827 | |||||
| 27.461 | Benzofuran-5-ol, 3-(2-furanoyl)-4-dimethylaminomethyl- | 1.432 | |||||
| 27.567 | Homovanillic acid | 1.146 | |||||
| 27.571 | Ethyl homovanillate | 5.919 | |||||
| 27.732 | Benzeneacetic acid, 4-hydroxy-3-methoxy-, methyl ester | 0.713 | 2.750 | 2.782 | |||
| 27.740 | 3-Penten-2-one, 4-(2,2,6-trimethyl-7-oxabicyclo[4.1.0]hept-1-yl)-, (E)- | 4.038 | 3.109 | ||||
| 27.829 | 2(1H)-Pyrazinone, 3,5,6-tris(1,1-dimethylethyl)- | 0.788 | |||||
| 27.931 | Pyridine, 2-(phenylethynyl)- | 0.556 | |||||
| 27.969 | 8H-5,12b-(Iminoethano)-1H-phenanthro[3,2-d][1,3]dioxin, 2,3,4,4a,5,6-hexahydro-15-methyl-, [4aR-(4a.alpha.,5.alpha.,12b.alpha.)]- | 1.431 | |||||
| 28.146 | 1-(10-Methylanthracen-9-yl)ethanone | 0.522 | 3.722 | ||||
| 28.239 | 1H-1,2,3,4-Tetrazole-1,5-diamine, N(1)-[(2-ethoxy-3-methoxyphenyl)methyl]- | 0.385 | |||||
| 28.243 | 3-(3-Hydroxy-4-methoxyphenyl)-l-alanine | 0.769 | |||||
| 28.315 | Benzaldehyde, 2-nitro-, diaminomethylidenhydrazone | 1.978 | |||||
| 28.556 | Methyl p-(trans-styryl)-trans-cinnamate | 1.551 | |||||
| 28.772 | 1-.beta.-d-Ribofuranosylpyrazolo[3,4-d]pyrimidin-4(5H)-one | 1.052 | |||||
| 29.229 | Benzene, 1-methoxy-4-(2-cyano-2-phenylethenyl) | 0.661 | 1.046 | ||||
| 29.415 | 2-(E)-Heptenoic acid, (4S)-4-[(t-butoxycarbonyl-(R)-alanyl)amino]-6-methyl-, ethyl ester | 1.102 | |||||
| 29.664 | 4H-1-Benzopyran-4-one, 5-hydroxy-7-methoxy-2-phenyl- | 2.369 | 1.245 | ||||
| 29.685 | Dinaphtho[2,1-b:1′,2′-d]furan | 2.566 | |||||
| 30.096 | 4-Methoxy-4′,5′-methylenedioxybiphenyl-2-carboxylic acid | 2.098 | |||||
| 30.282 | i-Propyl nonadecanoate | 1.419 | |||||
| 30.336 | 2,5-Cyclohexadien-1-one, 4-[[4-(dimethylamino)phenyl]imino]-2,5-dimethyl- | 0.674 | |||||
| 30.425 | Dibenz[a,h]anthracene, 1,2,3,4-tetrahydro- | 0.801 | 0.612 | ||||
| 30.455 | 4H-1-Benzopyran-4-one, 5,7-dimethoxy-2-phenyl- | 1.208 | |||||
| 30.540 | Benzene, 1-(1,1-dimethylethyl)-3,5-dimethyl-2,4,6-trinitro- | 0.547 | |||||
| 30.722 | 4H-1-Benzopyran-4-one, 5,7-dimethoxy-2-phenyl- | 2.505 | 4.742 | 2.323 | |||
| 31.309 | Dimethyldaidzin | 0.944 | |||||
| 31.318 | 3H-1,3,4-Benzotriazepine, 7-chloro-2-(methylamino)-5-phenyl- | 3.414 | |||||
| 31.457 | 4-(1,1-Dimethylallyl)-9-methoxy-7H-furo[3,2-g][1]benzopyran-7-one | 2.442 | |||||
| 31.690 | Benzothiophen-3(2H)-one, 2-(4-ethoxy-3-methoxybenzylideno)- | 0.889 | |||||
| 31.952 | Benzaldehyde, 2,4-dihydroxy- | 0.633 | 0.807 | 4.897 | 2.024 | ||
| 31.969 | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, O-methyloxime, (+)- | 8.108 | |||||
| 32.942 | Silane, [[(16.alpha.,17.alpha.)-16,17-epoxyestra-1,3,5(10)-trien-3-yl]oxy]trimethyl- | 1.385 | |||||
a Retention time.
References
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Jäger, N.; Apfelbacher, A.; Daschner, R.; Hornung, A.; Pant, K. Integrated thermo-catalytic reforming of residual sugarcane bagasse in a laboratory scale reactor. Fuel Process. Technol. 2018, 171, 277–286. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Abdel Maksoud, M.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilisation technologies: A review. Environ. Chem. Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of biomass and upgrading of bio-oil: A review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef] [PubMed]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Nazari, L.; Xu, C.C.; Ray, M.B. Advanced Technologies (Biological and Thermochemical) for Waste-to-Energy Conversion. In Green Chemistry and Sustainable Technology Advanced and Emerging Technologies for Resource Recovery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 55–95. [Google Scholar]
- Demirkaya, E.; Dal, O.; Yüksel, A. Liquefaction of waste hazelnut shell by using sub-and supercritical solvents as a reaction medium. J. Supercrit. Fluids 2019, 150, 11–20. [Google Scholar] [CrossRef]
- Cao, L.; Iris, K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, W. Key technologies for bioethanol production from lignocellulose. Biotechnol. Adv. 2010, 28, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Krishna, B.B.; Mishra, G.; Kumar, J.; Bhaskar, T. Strategies for selection of thermo-chemical processes for the valorisation of biomass. Renew. Energy 2016, 98, 226–237. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.S.; Sharma, K.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L.A. Influence of process conditions on hydrothermal liquefaction of eucalyptus biomass for biocrude production and investigation of the inorganics distribution. Sustain. Energy Fuels 2021, 5, 1477–1487. [Google Scholar] [CrossRef]
- Weir, A.; del Barco Carrión, A.J.; Queffélec, C.; Bujoli, B.; Chailleux, E.; Uguna, C.; Snape, C.; Airey, G. Renewable binders from waste biomass for road construction: A review on thermochemical conversion technologies and current developments. Constrcution Build. Mater. 2022, 330, 127076. [Google Scholar] [CrossRef]
- Singh, R.; Balagurumurthy, B.; Prakash, A.; Bhaskar, T. Catalytic hydrothermal liquefaction of water hyacinth. Bioresour. Technol. 2015, 178, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, M. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Kumar, P.; Mohanty, K. Hydrothermal liquefaction of biomass for bio-crude production: A review on feedstocks, chemical compositions, operating parameters, reaction kinetics, techno-economic study, and life cycle assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Nagappan, S.; Bhosale, R.R.; Nguyen, D.D.; Chi, N.T.L.; Ponnusamy, V.K.; Woong, C.S.; Kumar, G. Catalytic hydrothermal liquefaction of biomass into bio-oils and other value-added products–A review. Fuel 2021, 285, 119053. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Hao, S.; Luo, G.; Zhang, S.; Chen, J. Effect of glycerol as co-solvent on yields of bio-oil from rice straw through hydrothermal liquefaction. Bioresour. Technol. 2016, 220, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Jindal, M.K.; Jha, M.K. Effect of process parameters on hydrothermal liquefaction of waste furniture sawdust for bio-oil production. RSC Adv. 2016, 6, 41772–41780. [Google Scholar] [CrossRef]
- Cheng, S.; D’cruz, I.; Wang, M.; Leitch, M.; Xu, C. Highly efficient liquefaction of woody biomass in hot-compressed alcohol− water co-solvents. Energy Fuels 2010, 24, 4659–4667. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, J.; Zhao, J.; Tu, S. Liquefaction of bamboo shoot shell for the production of polyols. Bioresour. Technol. 2014, 153, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Chen, G. Optimizing the conditions for hydrothermal liquefaction of barley straw for bio-crude oil production using response surface methodology. Sci. Total Environ. 2018, 630, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Smith, N.; Lewis, D.M.; Hall, T.; van Eyk, P. A kinetic model for the hydrothermal liquefaction of microalgae, sewage sludge and pine wood with product characterisation of renewable crude. Chem. Eng. J. 2022, 428, 131228. [Google Scholar] [CrossRef]
- Cheng, F.; Porter, M.D.; Colosi, L.M. Is hydrothermal treatment coupled with carbon capture and storage an energy-producing negative emissions technology? Energy Convers. Manag. 2020, 203, 112252. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.-T. Hydrothermal liquefaction of protein-containing feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–168. [Google Scholar]
- Yin, S.; Dolan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhao, B.; Wang, H.; Bao, G.; Hu, Y.; Xu, C.C.; Long, H. Valorization of the spent catalyst from flue gas denitrogenation by improving bio-oil production from hydrothermal liquefaction of pinewood sawdust. Fuel 2022, 312, 122804. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; Li, W.; Xu, C.C. Alkali-catalyzed liquefaction of pinewood sawdust in ethanol/water co-solvents. Biomass Bioenergy 2020, 134, 105485. [Google Scholar] [CrossRef]
- Santos, R.V.; Mendes, M.A.; Alexandre, C.; Carrott, M.R.; Rodrigues, A.; Ferreira, A.F. Assessment of Biomass and Biochar of Maritime Pine as a Porous Medium for Water Retention in Soils. Energies 2022, 15, 5882. [Google Scholar] [CrossRef]
- Ravichandran, S.R.; Venkatachalam, C.D.; Sengottian, M.; Sekar, S.; Kandasamy, S.; Subramanian, K.P.R.; Purushothaman, K.; Chandrasekaran, A.L.; Narayanan, M. A review on hydrothermal liquefaction of algal biomass on process parameters, purification and applications. Fuel 2022, 313, 122679. [Google Scholar] [CrossRef]
- Yin, S.; Tan, Z. Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Appl. Energy 2012, 92, 234–239. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Zhang, D.; Feng, L. Catalytic upgrading of bio-oil in hydrothermal liquefaction of algae major model components over liquid acids. Energy Convers. Manag. 2017, 154, 336–343. [Google Scholar] [CrossRef]
- Huang, S.; Mahmood, N.; Tymchyshyn, M.; Yuan, Z.; Xu, C.C. Reductive de-polymerization of kraft lignin for chemicals and fuels using formic acid as an in-situ hydrogen source. Bioresour. Technol. 2014, 171, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A review of production and upgrading of algal bio-oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Blazina, G.V.; Rajagopalan, K.; Strathmann, T.J. Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour. Technol. 2012, 109, 178–187. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Yuan, Z.; Schmidt, J.; Xu, C.C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. [Google Scholar] [CrossRef]
- Hao, B.; Xu, D.; Jiang, G.; Sabri, T.A.; Jing, Z.; Guo, Y. Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. Green Chem. 2021, 23, 1562–1583. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Y.; Zhang, J.; Schideman, L.; Yu, G.; Zhang, P.; Minarick, M. Co-liquefaction of swine manure and mixed-culture algal biomass from a wastewater treatment system to produce bio-crude oil. Appl. Energy 2014, 128, 209–216. [Google Scholar] [CrossRef]
- Camus, M.; Condassamy, O.; Ham-Pichavant, F.; Michaud, C.; Mastroianni, S.; Mignani, G.; Grau, E.; Cramail, H.; Grelier, S. Oxidative Depolymerization of Alkaline Lignin from Pinus Pinaster by Oxygen and Air for Value-Added Bio-Sourced Synthons. Polymers 2021, 13, 3725. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).