Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Catalysts
2.3. HDO Experiments
2.4. HDO Products Analysis
2.5. Catalyst Characterization Methods
3. Results and Discussion
3.1. Fresh Catalyst Characterizations
3.2. HDO Products Distribution
3.3. Upgraded Oil Properties
3.4. GC-MS Analysis of the Oils
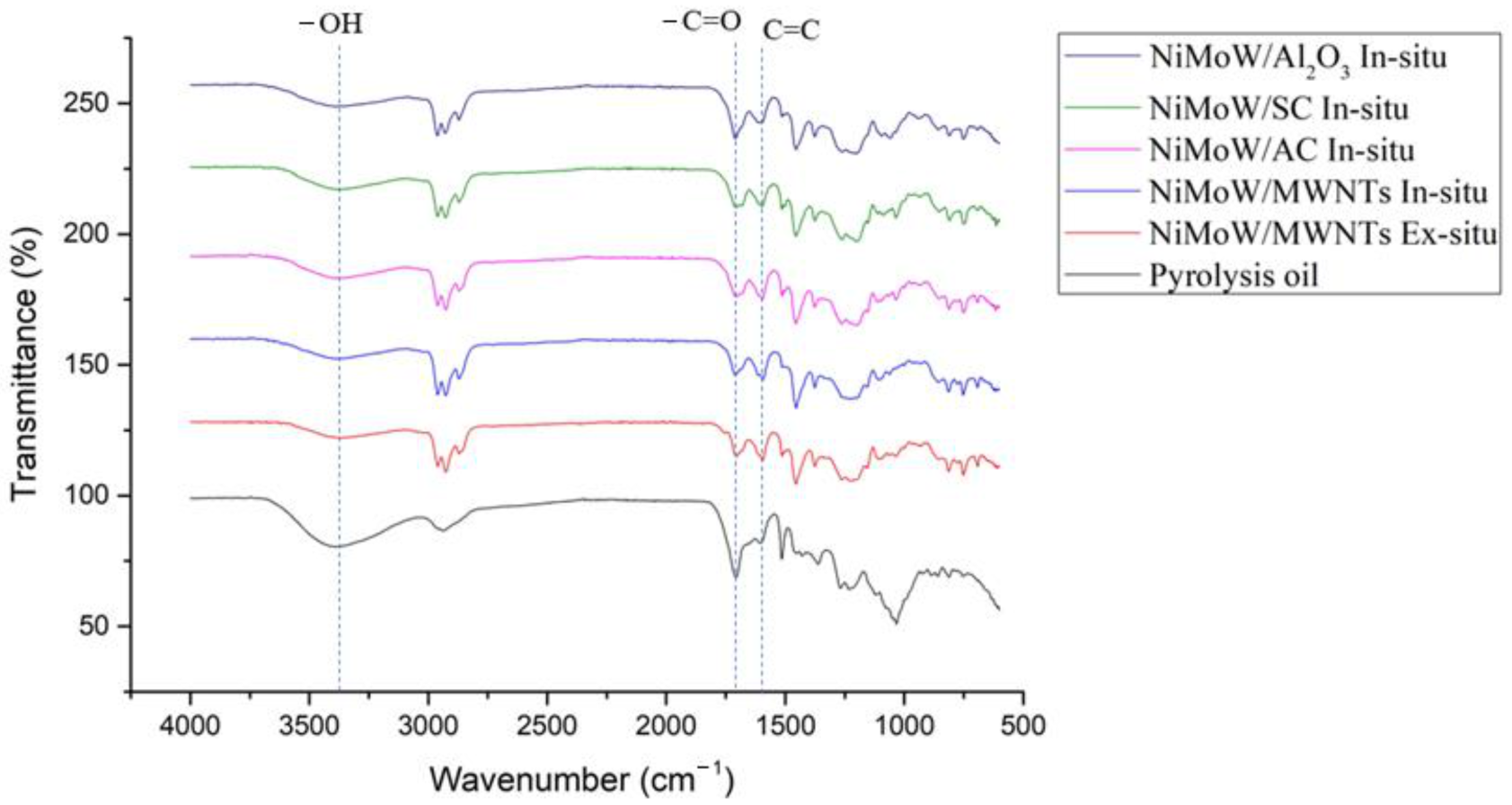
3.5. FT-IR Spectra of the Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nolte, M.W.; Shanks, B.H. A Perspective on Catalytic Strategies for Deoxygenation in Biomass Pyrolysis. Energy Technol. 2017, 5, 7–18. [Google Scholar] [CrossRef]
- Qin, Z.; Zhuang, Q.; Cai, X.; He, Y.; Huang, Y.; Jiang, D.; Lin, E.; Liu, Y.; Tang, Y.; Wang, M.Q. Biomass and Biofuels in China: Toward Bioenergy Resource Potentials and Their Impacts on the Environment. Renew. Sustain. Energy Rev. 2018, 82, 2387–2400. [Google Scholar] [CrossRef]
- Petrus, L.; Noordermeer, M.A. Biomass to Biofuels, a Chemical Perspective. Green Chem. 2006, 8, 861–867. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, Properties and Challenges of Algae Biomass for Biofuel Application: An Overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of Biomass and Upgrading of Bio-Oil: A Review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Joo, H.S.; Yang, Y.H. Biowaste-to-Bioenergy Using Biological Methods—A Mini-Review. Energy Convers Manag. 2018, 177, 640–660. [Google Scholar] [CrossRef]
- Schmitt, N.; Apfelbacher, A.; Jäger, N.; Daschner, R.; Stenzel, F.; Hornung, A. Thermo-Chemical Conversion of Biomass and Upgrading to Biofuel: The Thermo-Catalytic Reforming Process—A Review. Biofuels Bioprod. Biorefining 2019, 13, 822–837. [Google Scholar] [CrossRef]
- Ansari, K.B.; Kamal, B.; Beg, S.; Wakeel Khan, M.A.; Khan, M.S.; al Mesfer, M.K.; Danish, M. Recent Developments in Investigating Reaction Chemistry and Transport Effects in Biomass Fast Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2021, 150, 111454. [Google Scholar] [CrossRef]
- Perkins, G.; Bhaskar, T.; Konarova, M. Process Development Status of Fast Pyrolysis Technologies for the Manufacture of Renewable Transport Fuels from Biomass. Renew. Sustain. Energy Rev. 2018, 90, 292–315. [Google Scholar] [CrossRef]
- Panwar, N.L.; Paul, A.S. An Overview of Recent Development in Bio-Oil Upgrading and Separation Techniques. Environ. Eng. Res. 2020, 26, 200382. [Google Scholar] [CrossRef]
- Leng, L.; Li, H.; Yuan, X.; Zhou, W.; Huang, H. Bio-Oil Upgrading by Emulsification/Microemulsification: A Review. Energy 2018, 161, 214–232. [Google Scholar] [CrossRef]
- Gea, S.; Arafat, Y.; Averroes, H.; Rahman, F.; Ahmad, P.; Pulungan, N. A Comprehensive Review of Experimental Parameters in Bio–Oil Upgrading from Pyrolysis of Biomass to Biofuel Through Catalytic Hydrodeoxygenation. Bioenergy Res. 2022, 16, 325–347. [Google Scholar] [CrossRef]
- Pafili, A.; Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Wang, W.; Liu, G.; Papadakis, V.G.; Goula, M.A. Recent Progress in the Steam Reforming of Bio-Oil for Hydrogen Production: A Review of Operating Parameters, Catalytic Systems and Technological Innovations. Catalysts 2021, 11, 1526. [Google Scholar] [CrossRef]
- Shan Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of Bio-Oil from Thermochemical Conversion of Various Biomass–Mechanism, Challenges and Opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Omar, S.; Yang, Y.; Wang, J. A Review on Catalytic & Non-Catalytic Bio-Oil Upgrading in Supercritical Fluids. Front. Chem. Sci. Eng. 2021, 15, 4–17. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Pastor-Blas, M.M.; Odriozola, J.A.; Sepúlveda-Escribano, A.; Reina, T.R. In-Situ HDO of Guaiacol over Nitrogen-Doped Activated Carbon Supported Nickel Nanoparticles. Appl. Catal. A Gen. 2021, 620, 2–9. [Google Scholar] [CrossRef]
- Attia, M.; Farag, S.; Chaouki, J. Upgrading of Oils from Biomass and Waste: Catalytic Hydrodeoxygenation. Catalysts 2020, 10, 1381. [Google Scholar] [CrossRef]
- Guo, C.; Tirumala Venkateswara Rao, K.; Reyhanitash, E.; Yuan, Z.; Rohani, S.; Xu, C.; He, S. Novel Inexpensive Transition Metal Phosphide Catalysts for Upgrading of Pyrolysis Oil via Hydrodeoxygenation. AIChE J. 2016, 59, 215–228. [Google Scholar] [CrossRef]
- Valentini, F.; Kozell, V.; Petrucci, C.; Marrocchi, A.; Gu, Y.; Gelman, D.; Vaccaro, L. Formic Acid, a Biomass-Derived Source of Energy and Hydrogen for Biomass Upgrading. Energy Environ. Sci. 2019, 12, 2646–2664. [Google Scholar] [CrossRef]
- Park, J.Y.; Jeon, W.; Lee, J.H.; Nam, B.; Lee, I.G. Effects of Supercritical Fluids in Catalytic Upgrading of Biomass Pyrolysis Oil. Chem. Eng. J. 2019, 377, 120312. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, I.-G.; Park, J.-Y.; Lee, K.-Y. Efficient Upgrading of Pyrolysis Bio-Oil over Ni-Based Catalysts in Supercritical Ethanol. Fuel 2019, 241, 207–217. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, I.G.; Jeon, W.; Ha, J.H.; Lee, K.Y. Catalytic Upgrading of Bio-Tar over a MgNiMo/Activated Charcoal Catalyst under Supercritical Ethanol Conditions. Catal. Today 2018, 316, 237–243. [Google Scholar] [CrossRef]
- Jogi, R.; Mäki-Arvela, P.; Virtanen, P.; Kumar, N.; Hemming, J.; Smeds, A.; Lestander, T.A.; Mikkola, J.P. Biocrude Production through Hydro-Liquefaction of Wood Biomass in Supercritical Ethanol Using Iron Silica and Iron Beta Zeolite Catalysts. J. Chem. Technol. Biotechnol. 2019, 94, 3736–3744. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Huš, M.; Likozar, B. Hydrogenation and Hydrodeoxygenation of Aromatic Lignin Monomers over Cu/C, Ni/C, Pd/C, Pt/C, Rh/C and Ru/C Catalysts: Mechanisms, Reaction Micro-Kinetic Modelling and Quantitative Structure-Activity Relationships. Chem. Eng. J. 2019, 359, 305–320. [Google Scholar] [CrossRef]
- Oh, S.; Hwang, H.; Choi, H.S.; Choi, J.W. The Effects of Noble Metal Catalysts on the Bio-Oil Quality during the Hydrodeoxygenative Upgrading Process. Fuel 2015, 153, 535–543. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Xiao, L.P.; Xiao, W.Z.; Li, X.Y.; Wang, Q.; Sun, R.C. Nitrogen-Doped Carbon Anchored Ruthenium Nanoparticles for Biofuel Upgrade. Fuel 2022, 314, 123100. [Google Scholar] [CrossRef]
- He, Y.; Liu, R.; Yellezuome, D.; Peng, W.; Tabatabaei, M. Upgrading of Biomass-Derived Bio-Oil via Catalytic Hydrogenation with Rh and Pd Catalysts. Renew. Energy 2022, 184, 487–497. [Google Scholar] [CrossRef]
- Jahromi, H.; Adhikari, S.; Roy, P.; Hassani, E.; Pope, C.; Oh, T.; Karki, Y. Production of Green Transportation Fuels from Brassica Carinata Oil: A Comparative Study of Noble and Transition Metal Catalysts. Fuel Process. Technol. 2021, 215, 106737. [Google Scholar] [CrossRef]
- Cordero-lanzac, T.; Bilbao, J. Advances and Challenges in the Valorization of Bio-Oil: Hydrodeoxygenation Using Carbon-Supported Catalysts. Energy Fuels 2021, 35, 17008–17031. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Wang, H.; Li, H.; Han, X.; Zeng, Y.; Xu, C.C. A Review of Bio-Oil Upgrading by Catalytic Hydrotreatment: Advances, Challenges, and Prospects. Mol. Catal. 2021, 504, 111438. [Google Scholar] [CrossRef]
- Gutierrez, A.; Turpeinen, E.M.; Viljava, T.R.; Krause, O. Hydrodeoxygenation of Model Compounds on Sulfided CoMo/Γ-Al2O3 and NiMo/Γ-Al2O3 Catalysts; Role of Sulfur-Containing Groups in Reaction Networks. Catal. Today 2017, 285, 125–134. [Google Scholar] [CrossRef]
- Thongkumkoon, S.; Kiatkittipong, W.; Hartley, U.W.; Laosiripojana, N.; Daorattanachai, P. Catalytic Activity of Trimetallic Sulfided Re-Ni-Mo/Γ-Al2O3 toward Deoxygenation of Palm Feedstocks. Renew. Energy 2019, 140, 111–123. [Google Scholar] [CrossRef]
- Macedo, L.S.; da Silva, V.T.; Bitter, J.H. Activated Carbon, Carbon Nanofibers and Carbon-Covered Alumina as Support for W2C in Stearic Acid Hydrodeoxygenation. ChemEngineering 2019, 3, 24. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Wu, X.; Betancourt, L.E.; Tu, C.; Liao, M.; Cui, X.; Li, F.; Zheng, J.; Li, R. Ni/Hierarchical ZSM-5 Zeolites as Promising Systems for Phenolic Bio-Oil Upgrading: Guaiacol Hydrodeoxygenation. Fuel 2020, 274, 117859. [Google Scholar] [CrossRef]
- Conditions, P.; Shell, W.C.; Gas, C. Different Pyrolysis Process Conditions of South Asian Waste Coconut Shell and Characterization of Gas, Bio-Char, and Bio-Oil. Energies 2020, 8, 1970. [Google Scholar] [CrossRef]
- Mendes, F.L.; da Silva, V.T.; Pacheco, M.E.; Toniolo, F.S.; Henriques, C.A. Bio-Oil Hydrotreating Using Nickel Phosphides Supported on Carbon-Covered Alumina. Fuel 2019, 241, 686–694. [Google Scholar] [CrossRef]
- Yang, T.; Shi, L.; Li, R.; Li, B.; Kai, X. Hydrodeoxygenation of Crude Bio-Oil in Situ in the Bio-Oil Aqueous Phase with Addition of Zero-Valent Aluminum. Fuel Process. Technol. 2019, 184, 65–72. [Google Scholar] [CrossRef]
- Palizdar, A.; Sadrameli, S.M. Catalytic Upgrading of Biomass Pyrolysis Oil over Tailored Hierarchical MFI Zeolite: Effect of Porosity Enhancement and Porosity-Acidity Interaction on Deoxygenation Reactions. Renew. Energy 2020, 148, 674–688. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Julson, J.; Muthukumarappan, K.; Kharel, P.R. Upgrading Pyrolysis Bio-Oil to Hydrocarbon Enriched Biofuel over Bifunctional Fe-Ni/HZSM-5 Catalyst in Supercritical Methanol. Fuel Process. Technol. 2017, 167, 117–126. [Google Scholar] [CrossRef]
- Yang, Y.; Gilbert, A.; Xu, C. (Charles) Hydrodeoxygenation of Bio-Crude in Supercritical Hexane with Sulfided CoMo and CoMoP Catalysts Supported on MgO: A Model Compound Study Using Phenol. Appl. Catal. A Gen. 2009, 360, 242–249. [Google Scholar] [CrossRef]
- Xu, C.; Etcheverry, T. Hydro-Liquefaction of Woody Biomass in Sub- and Super-Critical Ethanol with Iron-Based Catalysts. Fuel 2008, 87, 335–345. [Google Scholar] [CrossRef]
- Valle, B.; Palos, R.; Bilbao, J.; Gayubo, A.G. Role of Zeolite Properties in Bio-Oil Deoxygenation and Hydrocarbons Production by Catalytic Cracking. Fuel Process. Technol. 2022, 227, 107130. [Google Scholar] [CrossRef]
- Suwanwong, S.; Hutem, A. Efficiency of Capacitor in Ccto Ceramics with Activated Carbon Derived from Tamarind Fruit shells. PSRU J. Sci. Technol. 2021, 6, 1–12. [Google Scholar]
- Tang, H.; Lin, J.; Cao, Y.; Jibran, K.; Li, J. Influence of NiMoP Phase on Hydrodeoxygenation Pathways of Jatropha Oil. Energy 2022, 243, 123048. [Google Scholar] [CrossRef]
- Ford, J.W.; Chaudhari, R.V.; Subramaniam, B. Supercritical Deoxygenation of a Model Bio-Oil Oxygenate. Ind. Eng. Chem. Res. 2010, 49, 10852–10858. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Valle, B.; Aguayo, A.T.; Olazar, M.; Bilbao, J. Pyrolytic Lignin Removal for the Valorization of Biomass Pyrolysis Crude Bio-Oil by Catalytic Transformation. J. Chem. Technol. Biotechnol. 2010, 85, 132–144. [Google Scholar] [CrossRef]
- Valle, B.; Castaño, P.; Olazar, M.; Bilbao, J.; Gayubo, A.G. Deactivating Species in the Transformation of Crude Bio-Oil with Methanol into Hydrocarbons on a HZSM-5 Catalyst. J. Catal. 2012, 285, 304–314. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Liu, Y.; Tang, S.; Chen, G.; Zhang, R.; Tang, X. Coke Formation on the Surface of Ni/HZSM-5 and Ni-Cu/HZSM-5 Catalysts during Bio-Oil Hydrodeoxygenation. Fuel 2017, 189, 23–31. [Google Scholar] [CrossRef]
- López, M.; Palacio, R.; Mamede, A.S.; Fernández, J.J.; Royer, S. Hydrodeoxygenation of Guaiacol into Cyclohexane over Mesoporous Silica Supported Ni–ZrO2 Catalyst. Microporous Mesoporous Mater. 2020, 309, 110452. [Google Scholar] [CrossRef]
- Duan, M.; Cheng, Q.; Wang, M.; Wang, Y. In Situ Hydrodeoxygenation of Vanillin over Ni-Co-P/HAP with Formic Acid as a Hydrogen Source. RSC Adv. 2021, 11, 10996–11003. [Google Scholar] [CrossRef]
- Sharma, V.; Getahun, T.; Verma, M.; Villa, A.; Gupta, N. Carbon Based Catalysts for the Hydrodeoxygenation of Lignin and Related Molecules: A Powerful Tool for the Generation of Non-Petroleum Chemical Products Including Hydrocarbons. Renew. Sustain. Energy Rev. 2020, 133, 110280. [Google Scholar] [CrossRef]
- Nie, R.; Tao, Y.; Nie, Y.; Lu, T.; Wang, J.; Zhang, Y.; Lu, X.; Xu, C.C. Recent Advances in Catalytic Transfer Hydrogenation with Formic Acid over Heterogeneous Transition Metal Catalysts. ACS Catal. 2021, 11, 1071–1095. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Han, X.; Zeng, Y.; Xu, C.C. Upgrading Pyrolysis Oil by Catalytic Hydrodeoxygenation Reaction in Supercritical Ethanol with Different Hydrogen Sources. Chem. Eng. J. 2022, 446, 136952. [Google Scholar] [CrossRef]
- Gazsi, A.; Bánsági, T.; Solymosi, F. Decomposition and Reforming of Formic Acid on Supported Au Catalysts: Production of CO-Free H2. J. Phys. Chem. C 2011, 115, 15459–15466. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
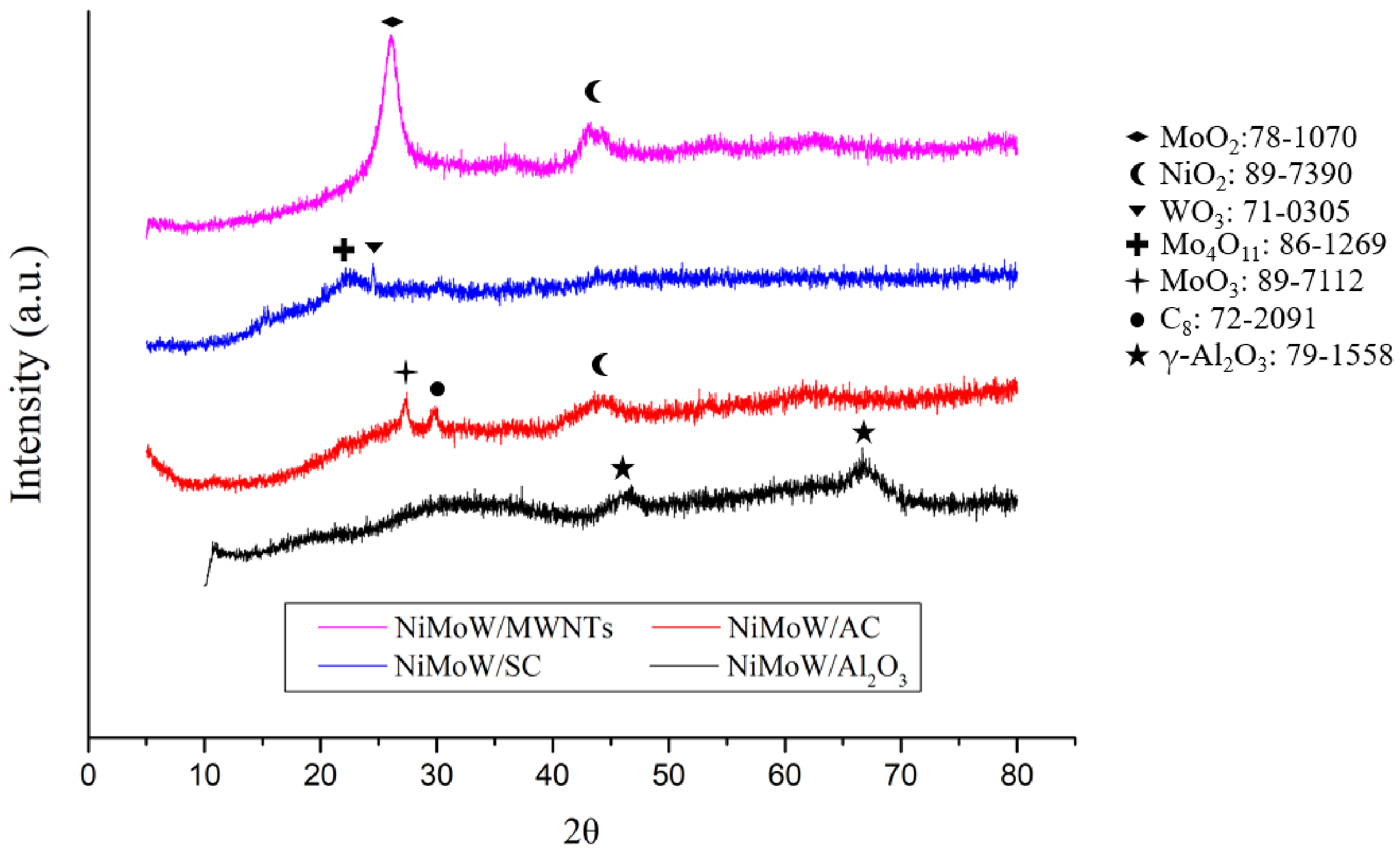
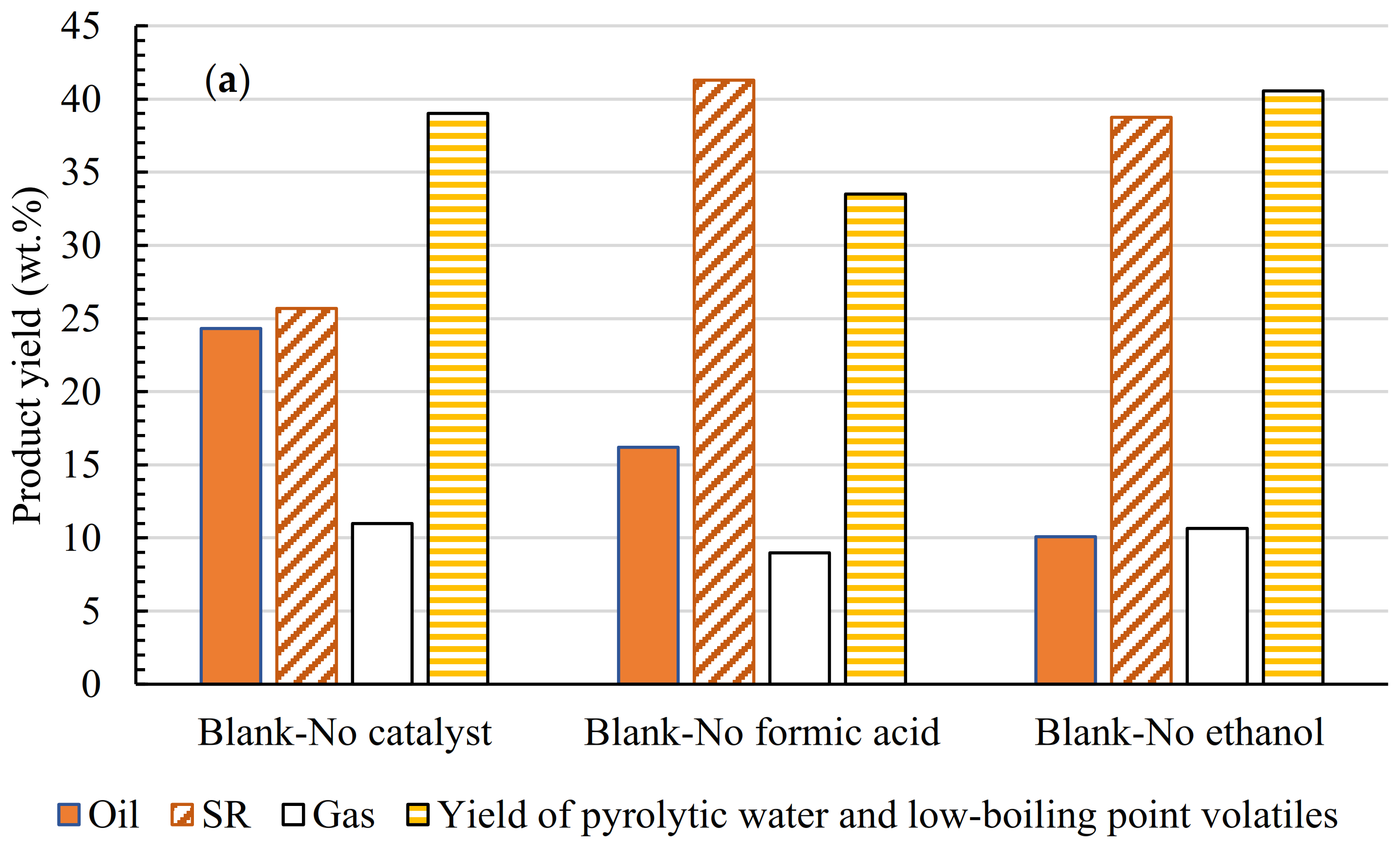
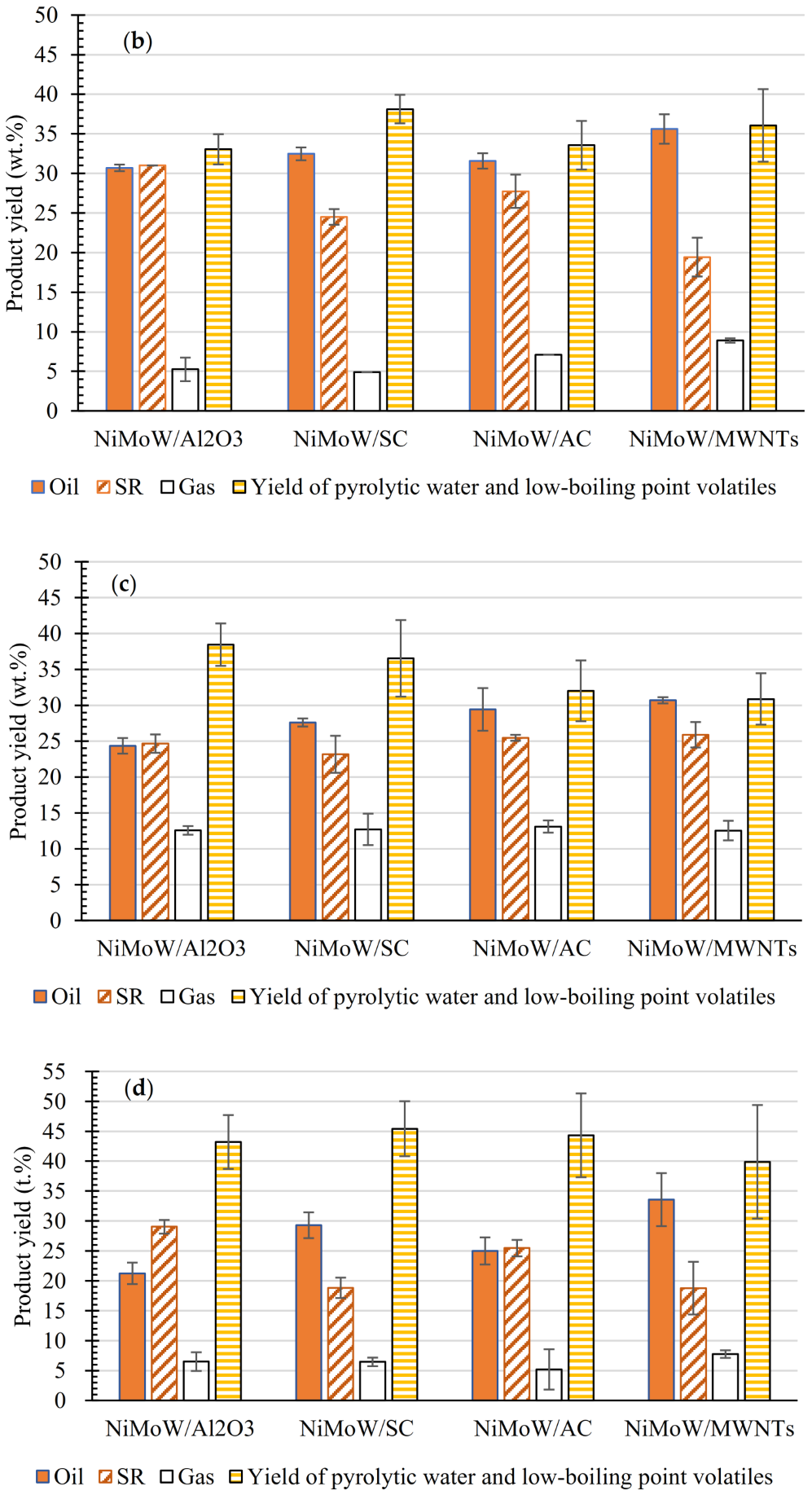
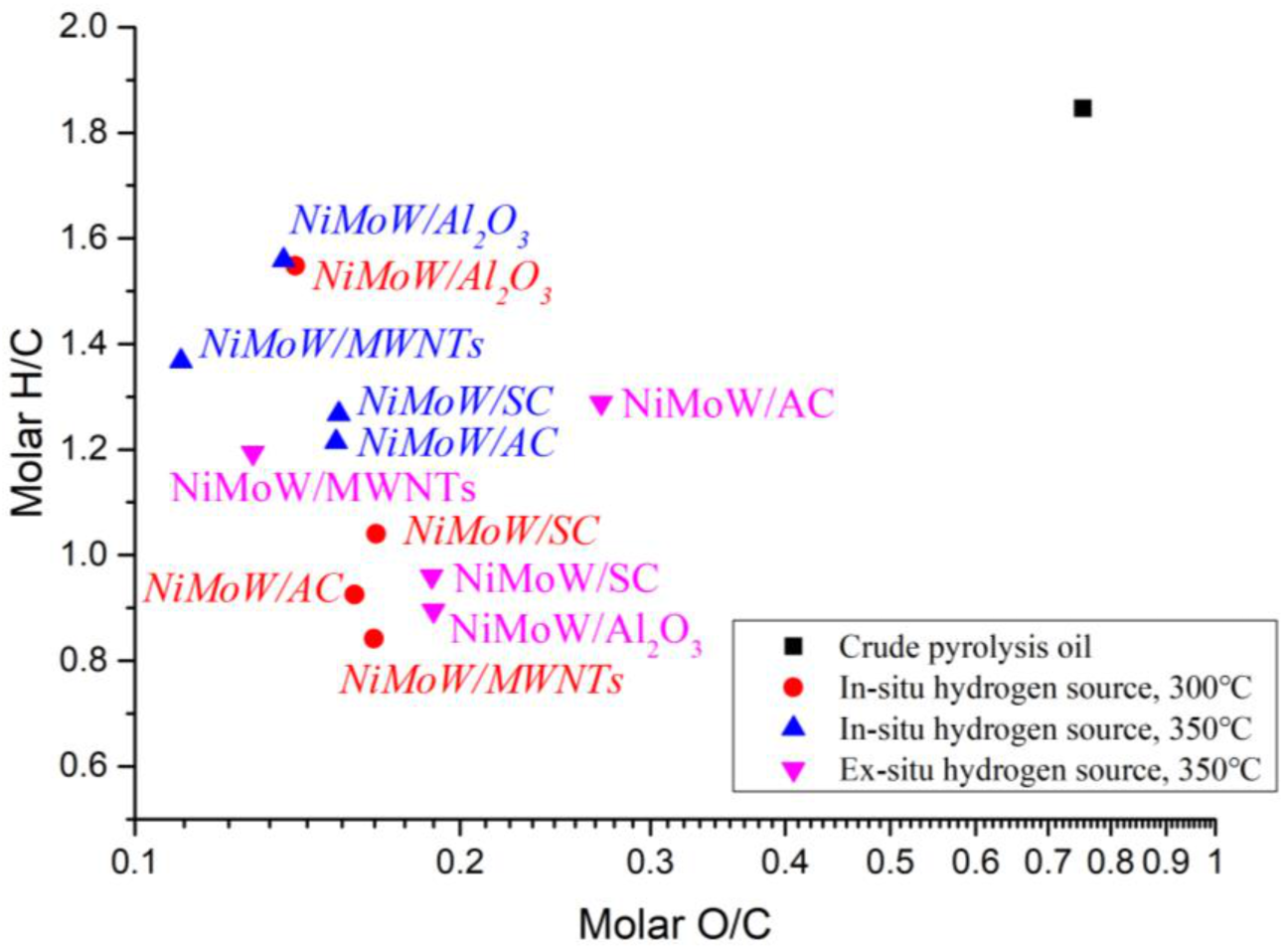
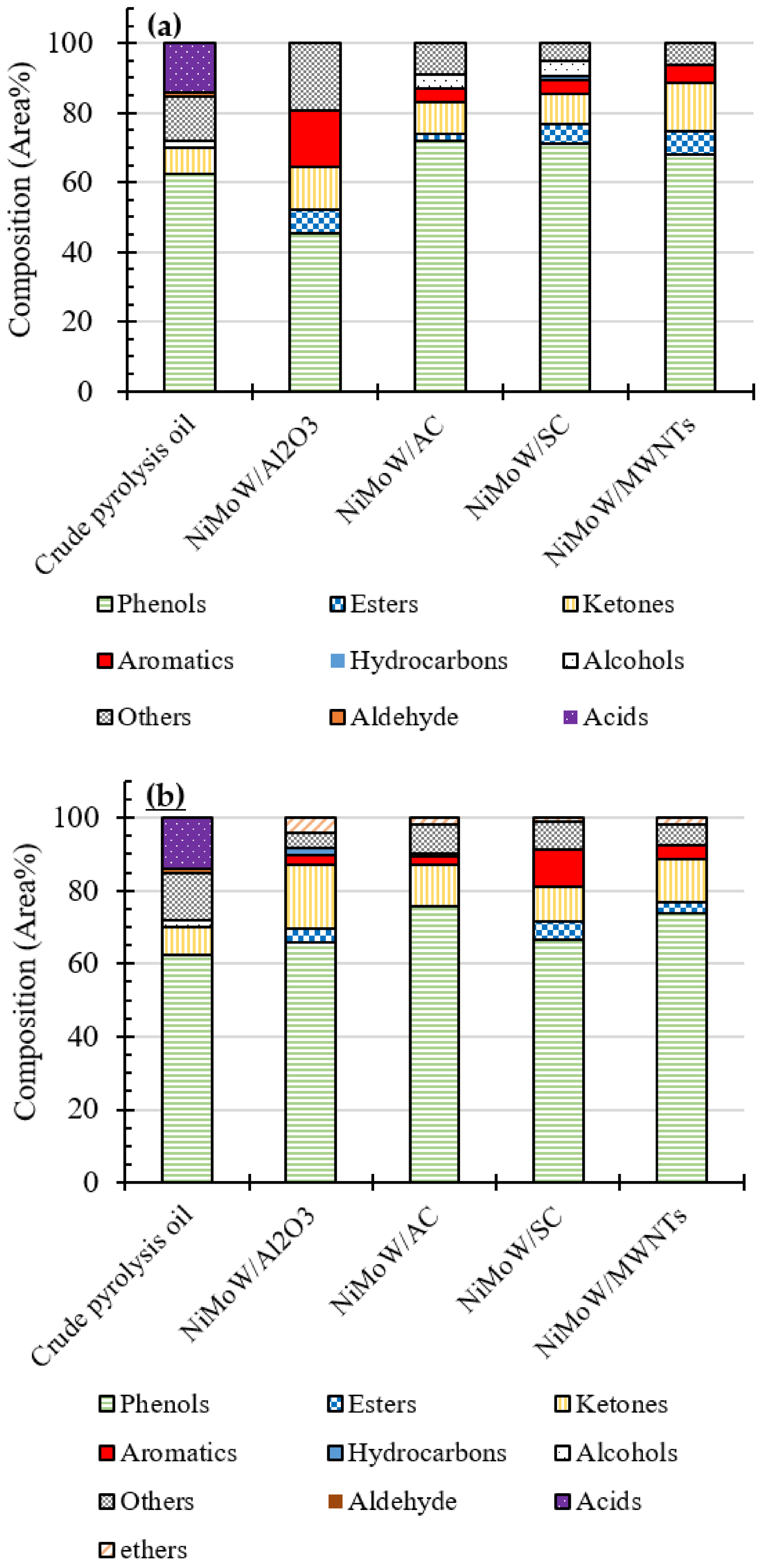
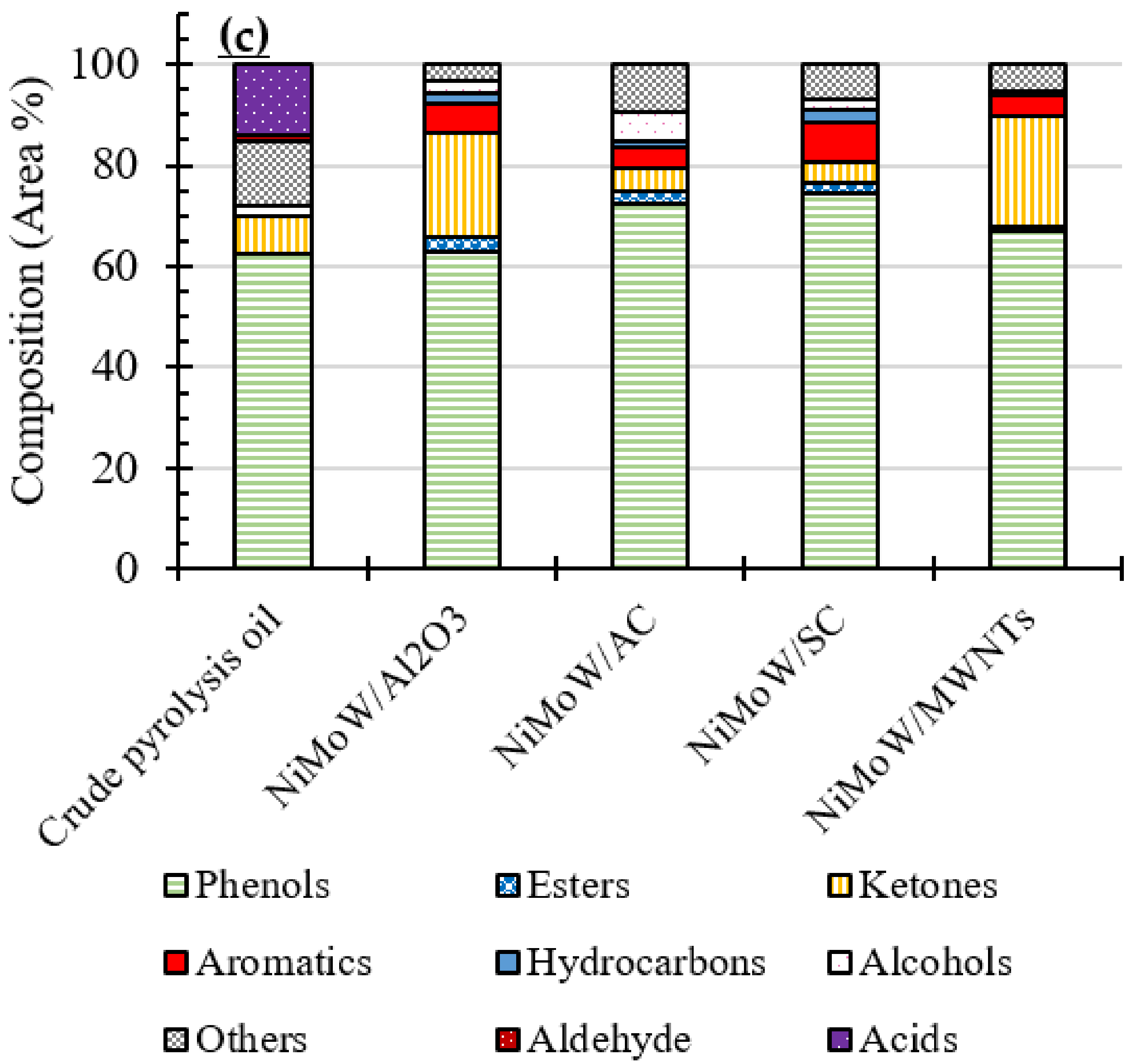
| Total Acid Number (TAN) (mgKOH/g) | Viscosity (mPa·s) | Elemental Analysis a | H/C (−) | O/C (−) | HHV c (MJ/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C (wt.%) | H (wt.%) | O b (wt.%) | N (wt.%) | S (wt.%) | |||||
| 136.0 | 105.3 | 46.21 | 7.11 | 46.45 | 0.21 | 0.02 | 1.85 | 0.75 | 17.49 |
| Proximate Analysis (wt.%) a | |
|---|---|
| Volatile matter | 77.62 |
| Ash | 0.44 |
| Fixed carbon | 21.94 |
| Elemental analysis (wt.%) a | |
| C | 50.65 |
| H | 5.47 |
| O b | 43.35 |
| N | 0.09 |
| S | - |
| NiMoW/ Al2O3 | NiMoW/ AC | NiMoW/ SC | NiMoW/ MWNTs | |
|---|---|---|---|---|
| Ni (wt.%) | 10 | 5.19 | 3.99 | 5.15 |
| Mo (wt.%) | 6 | 2.4 | 1.98 | 2.8 |
| W (wt.%) | 5 | - | - | - |
| Surface area (m2/g) | 131.9 | 706.8 | 7.7 | 86.9 |
| Average pore diameter (nm) | 5.8 | 2.3 | 5.7 | 17.4 |
| Total pore volume (cm3/g) | 0.19 | 0.41 | 0.011 | 0.72 |
| Sample | TAN (mgKOH/g) | Viscosity (mPa⋅s) | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | HHV (MJ/Kg) | DOD (%) |
|---|---|---|---|---|---|---|---|---|---|
| In situ hydrogen source at 300 °C for 2 h | |||||||||
| NiMoW/Al2O3 | 52.4 | 23.4 | 75.66 | 9.76 | 0.38 | 0.00 | 14.20 | 36.98 | 69.27 |
| NiMoW/SC | 69.3 | 16.2 | 76.13 | 6.60 | 0.30 | 0.00 | 16.97 | 32.12 | 63.27 |
| NiMoW/AC | 56.1 | 16.7 | 77.34 | 5.96 | 0.23 | 0.00 | 16.47 | 31.71 | 64.36 |
| NiMoW/MWNTs | 68.8 | 18.5 | 77.17 | 5.41 | 0.29 | 0.00 | 17.13 | 30.76 | 62.93 |
| In situ hydrogen source at 350 °C for 2 h | |||||||||
| NiMoW/Al2O3 | 55.6 | 20.9 | 75.81 | 9.85 | 0.46 | 0.00 | 13.88 | 37.21 | 69.96 |
| NiMoW/SC | 59.6 | 25.1 | 76.01 | 8.03 | 0.31 | 0.00 | 15.65 | 34.36 | 66.13 |
| NiMoW/AC | 62.7 | 32 | 76.38 | 7.73 | 0.25 | 0.00 | 15.64 | 34.06 | 66.15 |
| NiMoW/MWNTs | 47.2 | 21.2 | 79.09 | 9.01 | 0.26 | 0.00 | 11.64 | 37.53 | 74.81 |
| Ex situ hydrogen source at 350 °C for 2 h | |||||||||
| NiMoW/Al2O3 | 56.40 | 13.60 | 75.13 | 5.60 | 0.34 | 0.00 | 18.93 | 30.01 | 59.04 |
| NiMoW/SC | 57.30 | 18.60 | 74.89 | 5.99 | 0.33 | 0.00 | 18.79 | 30.51 | 59.34 |
| NiMoW/AC | 65.50 | 13.00 | 68.00 | 7.30 | 0.21 | 0.00 | 24.49 | 29.04 | 47.01 |
| NiMoW/MWNTs | 59.10 | 20.60 | 78.46 | 7.81 | 0.28 | 0.00 | 13.45 | 35.27 | 70.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Han, X.; Wang, H.; Zeng, Y.; Xu, C.C. Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials. Sustainability 2023, 15, 7768. https://doi.org/10.3390/su15107768
Zhang M, Han X, Wang H, Zeng Y, Xu CC. Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials. Sustainability. 2023; 15(10):7768. https://doi.org/10.3390/su15107768
Chicago/Turabian StyleZhang, Mingyuan, Xue Han, Huanang Wang, Yimin Zeng, and Chunbao Charles Xu. 2023. "Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials" Sustainability 15, no. 10: 7768. https://doi.org/10.3390/su15107768
APA StyleZhang, M., Han, X., Wang, H., Zeng, Y., & Xu, C. C. (2023). Hydrodeoxygenation of Pyrolysis Oil in Supercritical Ethanol with Formic Acid as an In Situ Hydrogen Source over NiMoW Catalysts Supported on Different Materials. Sustainability, 15(10), 7768. https://doi.org/10.3390/su15107768





_Xu.png)

