A Comparative Study on the Crystalline and Surface Properties of Carbonized Mesoporous Coconut Shell Chars
Abstract
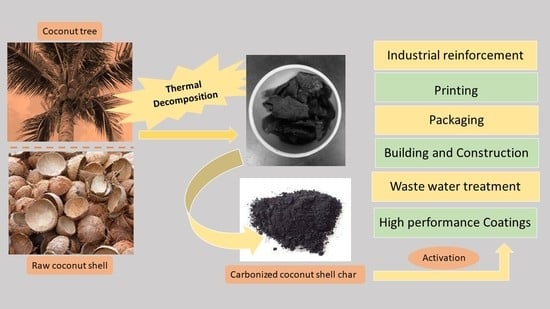
1. Introduction
2. Experimental
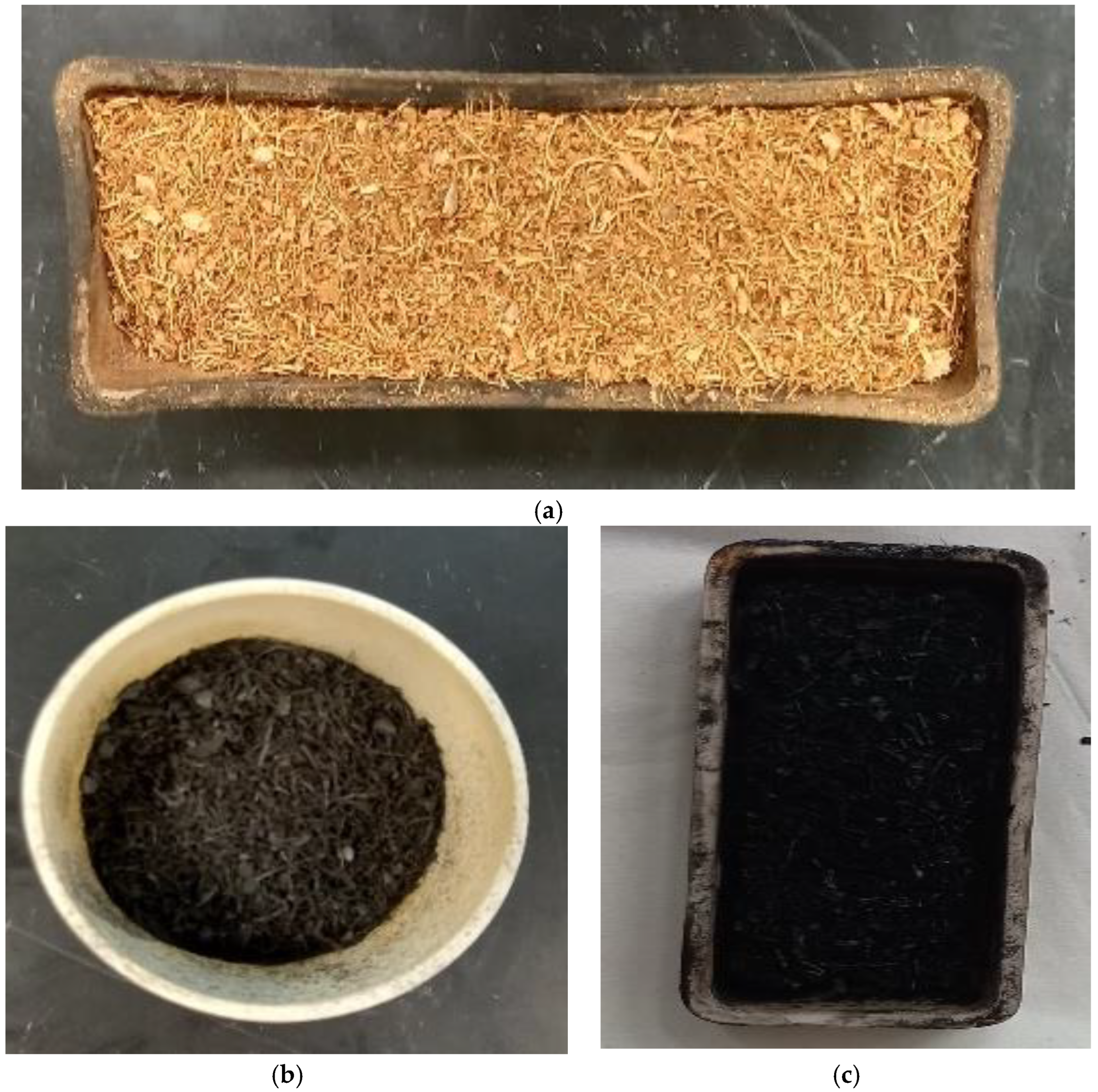
2.1. Raw Materials
2.2. Thermal Decomposition of Coconut Shells
- Muffle Furnace sample (MFCB)
- b.
- Tube Furnace sample (TFCB)
2.3. Characterization
3. Results and Discussion
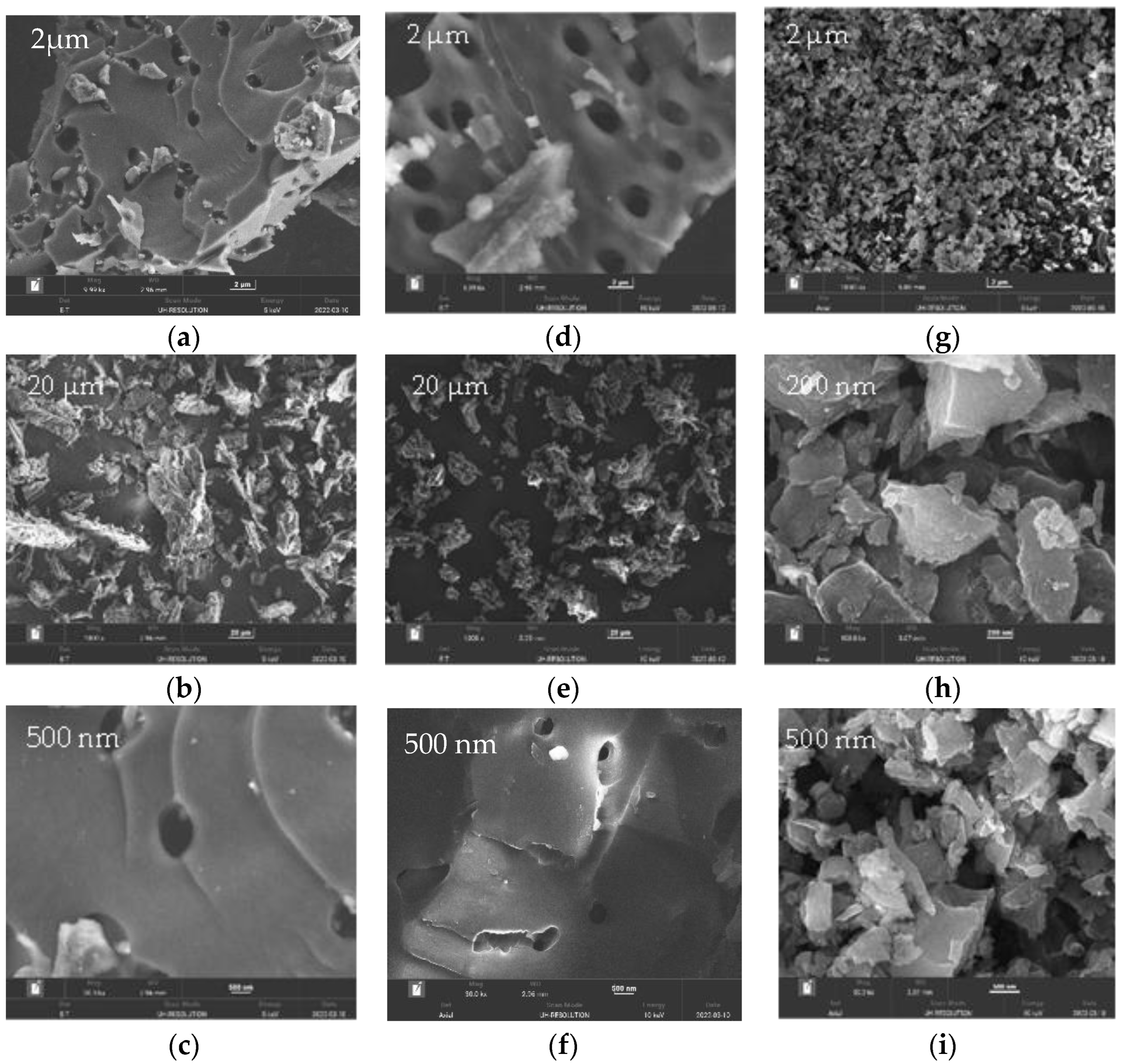
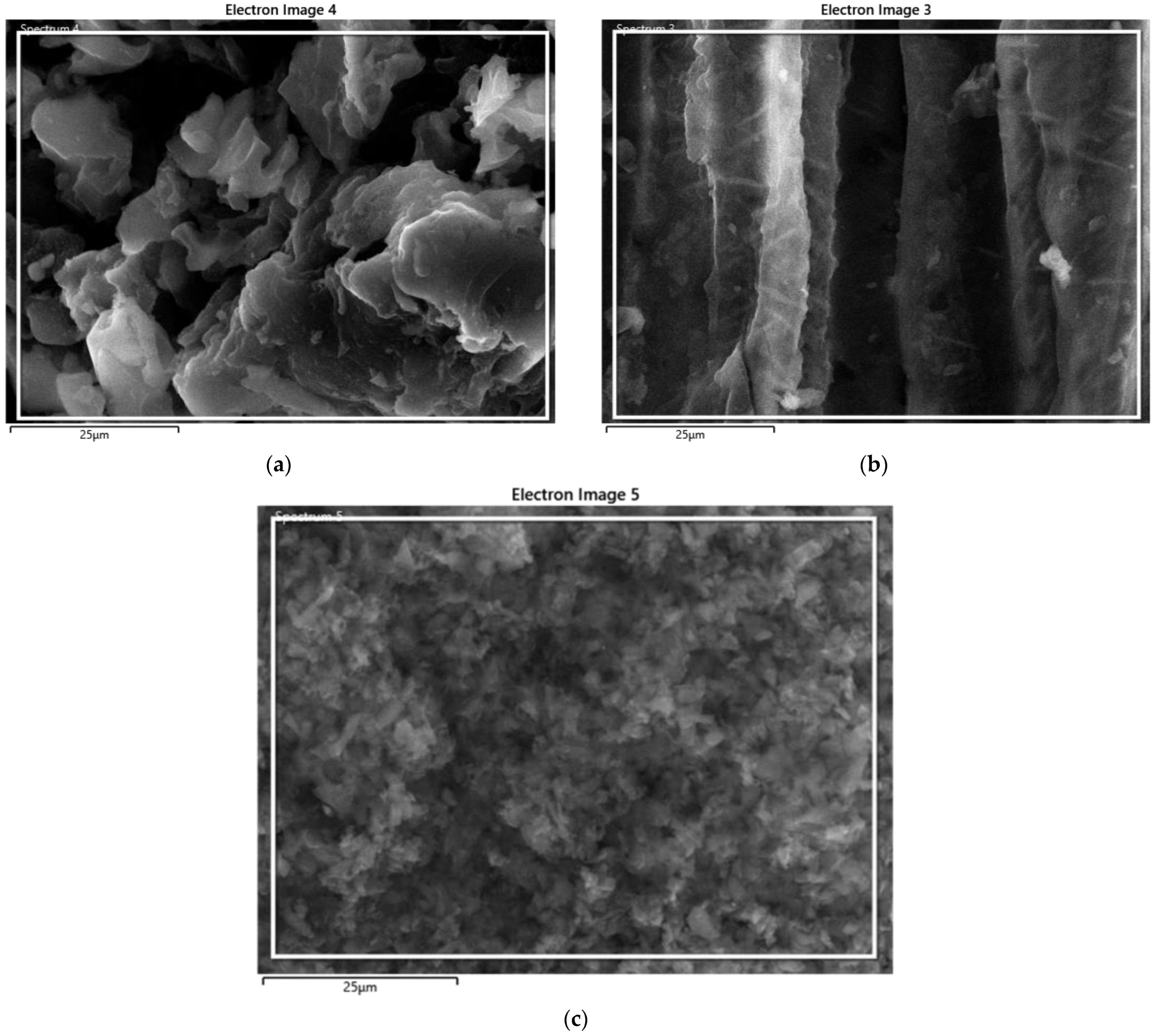
3.1. Surface Morphology and Composition
3.2. X-ray Diffraction Analysis
3.3. SAP Analysis
- Micropores; pore size less than 2 nm;
- Mesopores; pore size ranging from 2–50 nm;
- Macropores; pore size greater than 50 nm.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Carbon nanopowder |
| XRD | X-ray Powder Diffraction |
| MFCB | Muffle furnace sample |
| FESEM | Field-emission scanning electron microscope |
| SAP | Surface area and particle size |
| De | Desorption |
| BET | Brunauer, Emmet, and Teller |
| TFCB | Tube furnace sample |
| BSE | Back scattered-electron |
| IUPAC | International union of pure and applied chemistry |
| STP | Standard temperature pressure |
| Ad | Adsorption |
References
- Donyanavard, P. Production of Activated Carbon from Agricultural Waste and Investigation of Its Efficiency for Textile Wastewater Treatment. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2022. [Google Scholar]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.; Zhou, J.; Luo, Q.; Wu, W.; Mao, Z.; Ma, W. Screen-Printed Carbon Black/Recycled Sericin@Fabrics for Wearable Sensors to Monitor Sweat Loss. ACS Appl. Mater. Interfaces 2022, 14, 11813–11819. [Google Scholar] [CrossRef] [PubMed]
- Pagliero, M.; Alloisio, M.; Costa, C.; Firpo, R.; Mideksa, E.A.; Comite, A. Carbon Black/Polyvinylidene Fluoride Nanocomposite Membranes for Direct Solar Distillation. Energies 2022, 15, 740. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S.; You, C.; Tian, N.; Li, Y.; Chopra, N. Recent progress in morphological engineering of carbon materials for electromagnetic interference shielding. Carbon 2021, 172, 569–596. [Google Scholar] [CrossRef]
- Xia, G.; Ye, J.; Zheng, Z.; Li, X.; Chen, C.; Hu, C. Catalytic FeP decorated carbon black as a multifunctional conducting additive for high-performance lithium-sulfur batteries. Carbon 2021, 172, 96–105. [Google Scholar] [CrossRef]
- Roy, N.; Sengupta, R.; Bhowmick, A.K. Modifications of carbon for polymer composites and nanocomposites. Prog. Polym. Sci. 2012, 37, 781–819. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Pazhouhanfar, Y.; Delbari, S.A.; Shaddel, S.; Babapoor, A.; Mohammadpourderakhshi, Y.; Van Le, Q.; Shokouhimehr, M.; Asl, M.S.; Namini, A.S. Beneficial role of carbon black on the properties of TiC ceramics. Ceram. Int. 2020, 46, 23544–23555. [Google Scholar] [CrossRef]
- Fan, Y.; Fowler, G.D.; Zhao, M. The past, present and future of carbon black as a rubber reinforcing filler—A review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Priya, D.S.; Kennedy, L.J.; Anand, G.T. Emerging trends in biomass-derived porous carbon materials for energy storage application: A critical review. Mater. Today Sustain. 2023, 21, 100320. [Google Scholar] [CrossRef]
- da Silva, E.L.; Torres, M.; Portugau, P.; Cuña, A. High surface activated carbon obtained from Uruguayan rice husk wastes for supercapacitor electrode applications: Correlation between physicochemical and electrochemical properties. J. Energy Storage 2021, 44, 103494. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Munhoz, M.G.C.; Fonseca, B.C.D.S.; Boss, A.F.N.; de Almeida-Mattos, P.; Braghiroli, F.L.; Bouafif, H.; Koubaa, A.; e Silva, G.F.B.L.; Baldan, M.R. Xerogel-like Materials from Sustainable Sources: Properties and Electrochemical Performances. Energies 2021, 14, 7977. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules 2019, 9, 587. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; da Silva, E.L.; Cuña, A.; Malfatti, C.F.; Marcuzzo, J.S.; Baldan, M.R.; Celzard, A.; Fierro, V.; e Silva, G.F.B.L. A Sustainable Carbon Material from Kraft Black Liquor as Nickel-Based Electrocatalyst Support for Ethanol Electro-Oxidation. Waste Biomass-Valorization 2021, 12, 2507–2519. [Google Scholar] [CrossRef]
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural waste management strategies for environmental sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef]
- Zanzi, R.; Sjöström, K.; Björnbom, E. Rapid pyrolysis of agricultural residues at high temperature. Biomass-Bioenergy 2002, 23, 357–366. [Google Scholar] [CrossRef]
- Moayedi, H.; Aghel, B.; Abdullahi, M.M.; Nguyen, H.; Rashid, A.S.A. Applications of rice husk ash as green and sustainable biomass. J. Clean. Prod. 2019, 237, 117851. [Google Scholar] [CrossRef]
- Bhat, V.S.; Kanagavalli, P.; Sriram, G.; Prabhu, B.R.; John, N.S.; Veerapandian, M.; Kurkuri, M.; Hegde, G. Low cost, catalyst free, high performance supercapacitors based on porous nano carbon derived from agriculture waste. J. Energy Storage 2020, 32, 101829. [Google Scholar] [CrossRef]
- Li, Z.; Guo, D.; Liu, Y.; Wang, H.; Wang, L. Recent advances and challenges in biomass-derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Yan, B.; Feng, L.; Zheng, J.; Zhang, Q.; Jiang, S.; Zhang, C.; Ding, Y.; Han, J.; Chen, W.; He, S. High performance supercapacitors based on wood-derived thick carbon electrodes synthesized via green activation process. Inorg. Chem. Front. 2022, 9, 6108–6123. [Google Scholar] [CrossRef]
- Wei, L.; Deng, W.; Li, S.; Wu, Z.; Cai, J.; Luo, J. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J. Bioresour. Bioprod. 2022, 7, 63–72. [Google Scholar] [CrossRef]
- Boundzanga, H.M.; Cagnon, B.; Roulet, M.; de Persis, S.; Vautrin-Ul, C.; Bonnamy, S. Contributions of hemicellulose, cellulose, and lignin to the mass and the porous characteristics of activated carbons produced from biomass residues by phosphoric acid activation. Biomass-Convers. Biorefinery 2020, 12, 3081–3096. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Li, J.R.; Xu, J.R.; Zhang, M.Q.; Rong, M.Z. Carbon black/polystyrene composites as candidates for gas sensing materials. Carbon 2003, 41, 2353–2360. [Google Scholar] [CrossRef]
- Hindermann-Bischoff, M.; Ehrburger-Dolle, F. Electrical conductivity of carbon black–polyethylene composites: Experimental evidence of the change of cluster connectivity in the PTC effect. Carbon 2001, 39, 375–382. [Google Scholar] [CrossRef]
- Ayub, S.; Guan, B.; Ahmad, F.; Javed, M.; Mosavi, A.; Felde, I. Preparation Methods for Graphene Metal and Polymer Based Composites for EMI Shielding Materials: State of the Art Review of the Conventional and Machine Learning Methods. Metals 2021, 11, 1164. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Watson, A.Y.; Valberg, P.A. Carbon Black and Soot: Two Different Substances. AIHAJ-Am. Ind. Hyg. Assoc. J. 2001, 62, 218–228. [Google Scholar] [CrossRef]
- Gergova, K.; Petrov, N.; Eser, S. Adsorption properties and microstructure of activated carbons produced from agricultural by-products by steam pyrolysis. Carbon 1994, 32, 693–702. [Google Scholar] [CrossRef]
- McDougall, G.J. The physical nature and manufacture of activated carbon. J. S. Afr. Inst. Min. Metall. 1991, 91, 109–120. [Google Scholar]
- Daud, W.M.A.W.; Ali, W.S.W. Comparison on pore development of activated carbon produced from palm shell and coconut shell. Bioresour. Technol. 2004, 93, 63–69. [Google Scholar] [CrossRef]
- Dong, M.; Li, Q.; Liu, H.; Liu, C.; Wujcik, E.K.; Shao, Q.; Ding, T.; Mai, X.; Shen, C.; Guo, Z. Thermoplastic polyurethane-carbon black nanocomposite coating: Fabrication and solid particle erosion resistance. Polymer 2018, 158, 381–390. [Google Scholar] [CrossRef]
- Rampur, V.; Banagar, A.R.; Ganesh, U.L.; Srinivas, C.V.; Reur, S.C. Mechanical characterization of wood apple and coconut shell reinforced hybrid composites. AIP Conf. Proc. 2020, 2247, 040001. [Google Scholar]
- González, J.; Román, S.; Encinar, J.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Liyanage, C.D.; Pieris, M. A Physico-Chemical Analysis of Coconut Shell Powder. Procedia Chem. 2015, 16, 222–228. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Kaur, P.; Park, J.-S. From coconut shell biomass to oxygen reduction reaction catalyst: Tuning porosity and nitrogen doping. Renew. Sustain. Energy Rev. 2021, 147, 111173. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhang, L.; Tian, M. Mechanical and tribological properties of acrylonitrile–butadiene rubber filled with graphite and carbon black. Mater. Des. 2012, 39, 450–457. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Medalia, A.I. Electrical conduction in carbon black composites. Rubber Chem. Technol. 1986, 59, 432–454. [Google Scholar] [CrossRef]
- Dannenberg, E.M. Bound rubber and carbon black reinforcement. Rubber Chem. Technol. 1986, 59, 512–524. [Google Scholar] [CrossRef]
- Zbair, M.; Bottlinger, M.; Ainassaari, K.; Ojala, S.; Stein, O.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Hydrothermal Carbonization of Argan Nut Shell: Functional Mesoporous Carbon with Excellent Performance in the Adsorption of Bisphenol A and Diuron. Waste Biomass-Valorization 2020, 11, 1565–1584. [Google Scholar] [CrossRef]
- Huang, X.; Wu, S.; Du, X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon 2016, 101, 135–142. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Hong, Z.-Q.; Li, J.-X.; Zhang, F.; Zhou, L.-H. Synthesis of magnetically graphitic mesoporous carbon from hard templates and its application in the adsorption treatment of traditional Chinese medicine wastewater. Acta Phys.-Chim. Sin. 2013, 29, 590–596. [Google Scholar]
- Azimi, E.B.; Badiei, A.; Ghasemi, J.B. Efficient removal of malachite green from wastewater by using boron-doped mesoporous carbon nitride. Appl. Surf. Sci. 2019, 469, 236–245. [Google Scholar] [CrossRef]
- Ji, L.; Liu, F.; Xu, Z.; Zheng, S.; Zhu, D. Adsorption of Pharmaceutical Antibiotics on Template-Synthesized Ordered Micro- and Mesoporous Carbons. Environ. Sci. Technol. 2010, 44, 3116–3122. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Tang, H.; Mao, Y.; Chen, H.; Ji, F. Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon. Appl. Surf. Sci. 2020, 528, 147048. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y. Mesoporous carbons: Recent advances in synthesis and typical applications. RSC Adv. 2015, 5, 83239–83285. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Brouzgou, A.; Liang, Y.; Fu, R.; Wu, D.; Tsiakaras, P.; Song, S. A facile soft-template synthesis of ordered mesoporous carbon/tungsten carbide composites with high surface area for methanol electrooxidation. J. Power Sources 2012, 200, 8–13. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass-Convers. Biorefinery 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.; Peng, J.; Zhang, L.; Guo, S.; Xia, H. Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind. Crop. Prod. 2008, 28, 190–198. [Google Scholar] [CrossRef]
- Prauchner, M.J.; Rodríguez-Reinoso, F. Chemical versus physical activation of coconut shell: A comparative study. Microporous Mesoporous Mater. 2012, 152, 163–171. [Google Scholar] [CrossRef]
- Marsh, H.; Reinoso, F.R. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Wang, J.; Shen, X.; He, Z.; Jiang, E.; Xu, X. Effect of surfactant on hydrothermal carbonization of coconut shell. Bioresour. Technol. 2019, 284, 214–221. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 45–64. [Google Scholar]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Fan, H.; Wang, J. Wood carbonization as a protective treatment on resistance to wood destroying fungi. Int. Biodeterior. Biodegrad. 2018, 129, 42–49. [Google Scholar] [CrossRef]
- Warren, B.E. X-ray diffraction study of carbon black. J. Chem. Phys. 1934, 2, 551–555. [Google Scholar] [CrossRef]
- Heckman, F. Microstructure of carbon black. Rubber Chem. Technol. 1964, 37, 1245–1298. [Google Scholar] [CrossRef]
- Ungár, T.; Gubicza, J.; Ribárik, G.; Pantea, C.; Zerda, T. Microstructure of carbon blacks determined by X-ray diffraction profile analysis. Carbon 2002, 40, 929–937. [Google Scholar] [CrossRef]
- Kang, D.S.; Lee, S.M.; Lee, S.H.; Roh, J.S. X-ray diffraction analysis of the crystallinity of phenolic resin-derived carbon as a function of the heating rate during the carbonization process. Carbon Lett. 2018, 27, 108–111. [Google Scholar]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Daud, W.M.A.W.; Ali, W.S.W.; Sulaiman, M.Z. The effects of carbonization temperature on pore development in palm-shell-based activated carbon. Carbon 2000, 38, 1925–1932. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F. Microporous structure of activated carbons as revealed adsorption methods. Chem. Phys. Carbon 1989, 21, 1. [Google Scholar]
- Rodriguez-Reinoso, F. Controlled Gasification of Carbon and Pore Structure Development. In Fundamental Issues in Control of Carbon Gasification Reactivity; Springer: Berlin/Heidelberg, Germany, 1991; pp. 533–571. [Google Scholar]
- Marquez-Linares, F.; Roque-Malherbe, R.M.A. Synthesis and Characterization of Large Specific Surface Area Nanostructured Amorphous Silica Materials. J. Nanosci. Nanotechnol. 2006, 6, 1114–1118. [Google Scholar] [CrossRef]
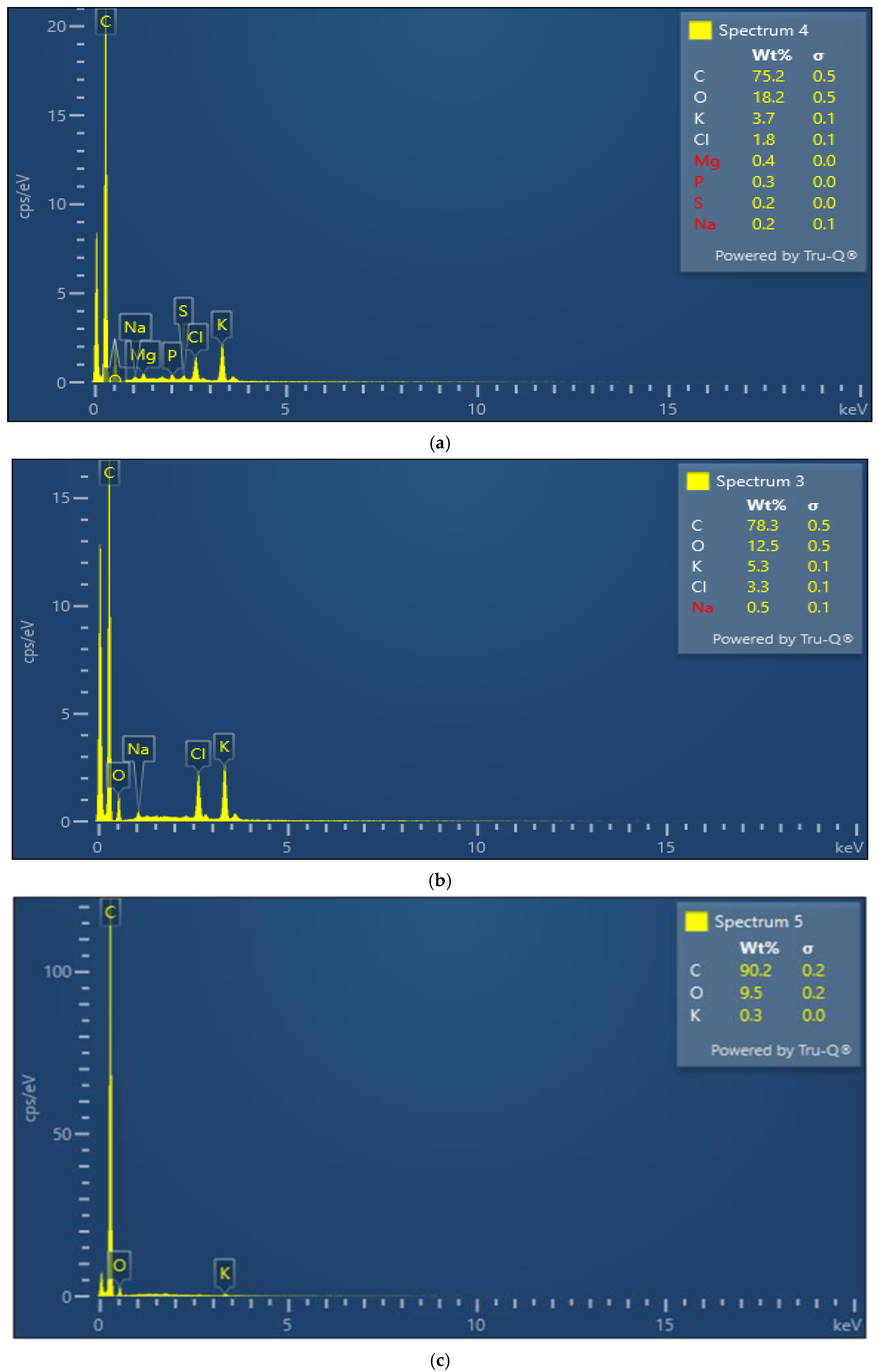
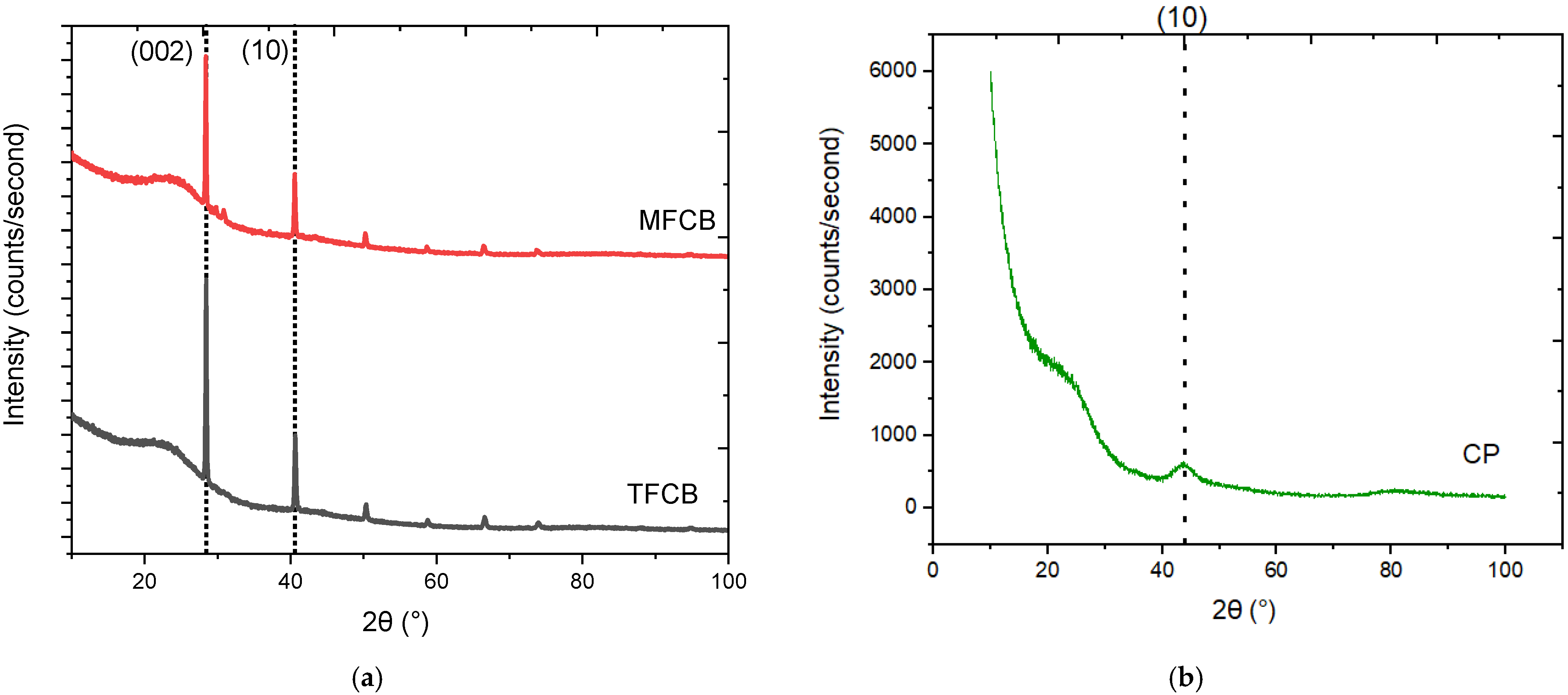
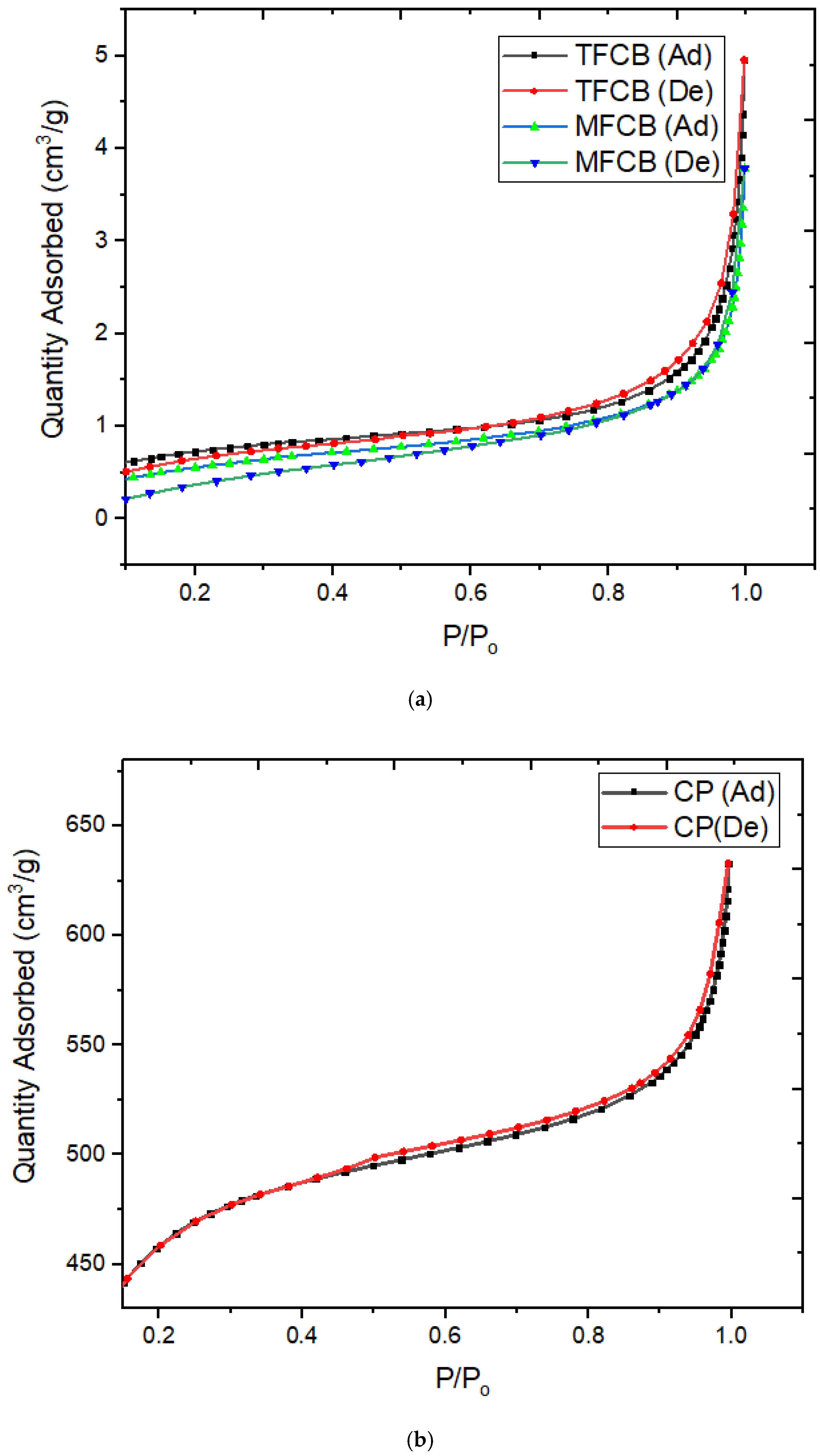
| Parameter | MFCB | TFCB | CP |
|---|---|---|---|
| d002 (Å) | 3.15 | 3.16 | - |
| d10 (Å) | 2.22 | 2.22 | 2.23 |
| FWHM | 0.21 | 0.22 | 7.2 |
| Lc (nm) | 37.93 | 36.63 | 1.17 |
| La (nm) | 81.77 | 78.97 | 2.53 |
| N (Items) | 12.03 | 12.79 | 0.53 |
| δ (nm−2) | 0.69 | 0.74 | 723 |
| ε (×10−3) | 3.73 | 3.88 | 85.6 |
| ρ (g/cm3) | 0.24 | 0.26 | 0.34 |
| Crystallinity Index (%) | 25.8 | 23.4 | 13 |
| Sample | Specific Surface Area (SBET) | Langmuir Surface Area (SL) | Average Pore Diameter (D) |
|---|---|---|---|
| MFCB | 2.0827 m²/g | 3.3269 m²/g | 10.0844 nm |
| TFCB | 2.5486 m²/g | 3.9141 m²/g | 9.4434 nm |
| CP | 1517 m²/g | 2175 m²/g | 2.51232 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, Z.U.; Chuan, L.K.; Guan, B.H.; Ahmad, F.; Ayub, S. A Comparative Study on the Crystalline and Surface Properties of Carbonized Mesoporous Coconut Shell Chars. Sustainability 2023, 15, 6464. https://doi.org/10.3390/su15086464
Nisa ZU, Chuan LK, Guan BH, Ahmad F, Ayub S. A Comparative Study on the Crystalline and Surface Properties of Carbonized Mesoporous Coconut Shell Chars. Sustainability. 2023; 15(8):6464. https://doi.org/10.3390/su15086464
Chicago/Turabian StyleNisa, Zaib Un, Lee Kean Chuan, Beh Hoe Guan, Faiz Ahmad, and Saba Ayub. 2023. "A Comparative Study on the Crystalline and Surface Properties of Carbonized Mesoporous Coconut Shell Chars" Sustainability 15, no. 8: 6464. https://doi.org/10.3390/su15086464
APA StyleNisa, Z. U., Chuan, L. K., Guan, B. H., Ahmad, F., & Ayub, S. (2023). A Comparative Study on the Crystalline and Surface Properties of Carbonized Mesoporous Coconut Shell Chars. Sustainability, 15(8), 6464. https://doi.org/10.3390/su15086464