Comparison of Vegetables of Ecological and Commercial Production: Physicochemical and Antioxidant Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Vegetable Material
2.2. Physical Properties
2.2.1. Color Measurement
2.2.2. Texture, Moisture, and Ashes
2.3. Bioactive Compounds Analysis
2.3.1. Extraction of Antioxidant Compounds
2.3.2. Total Phenolic Compounds
2.3.3. Ascorbic Acid
2.3.4. Total Anthocyanins Content (TAC)
2.3.5. Total Betalain Content (TBC)
2.3.6. β-Carotene, Chlorophylls, and Lycopene
Extraction Method
2.4. Antioxidant Activity
2.4.1. 2,2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS•+)
2.4.2. 2,2′-Diphenyl-1-picrylhydrazyl (DPPH)
2.4.3. Ferric-Reducing Antioxidant Power (FRAP)
2.4.4. The Chelating Activity of Ferrous Ions
2.5. Statistical Analysis
3. Result and Discussion
3.1. Physical Properties
3.1.1. Color
3.1.2. Texture, Moisture, and Ashes
3.2. Bioactive Compounds
3.2.1. Total Phenolics Compounds (TPC)
3.2.2. Ascorbic Acid
3.2.3. β-Carotene and Chlorophyll
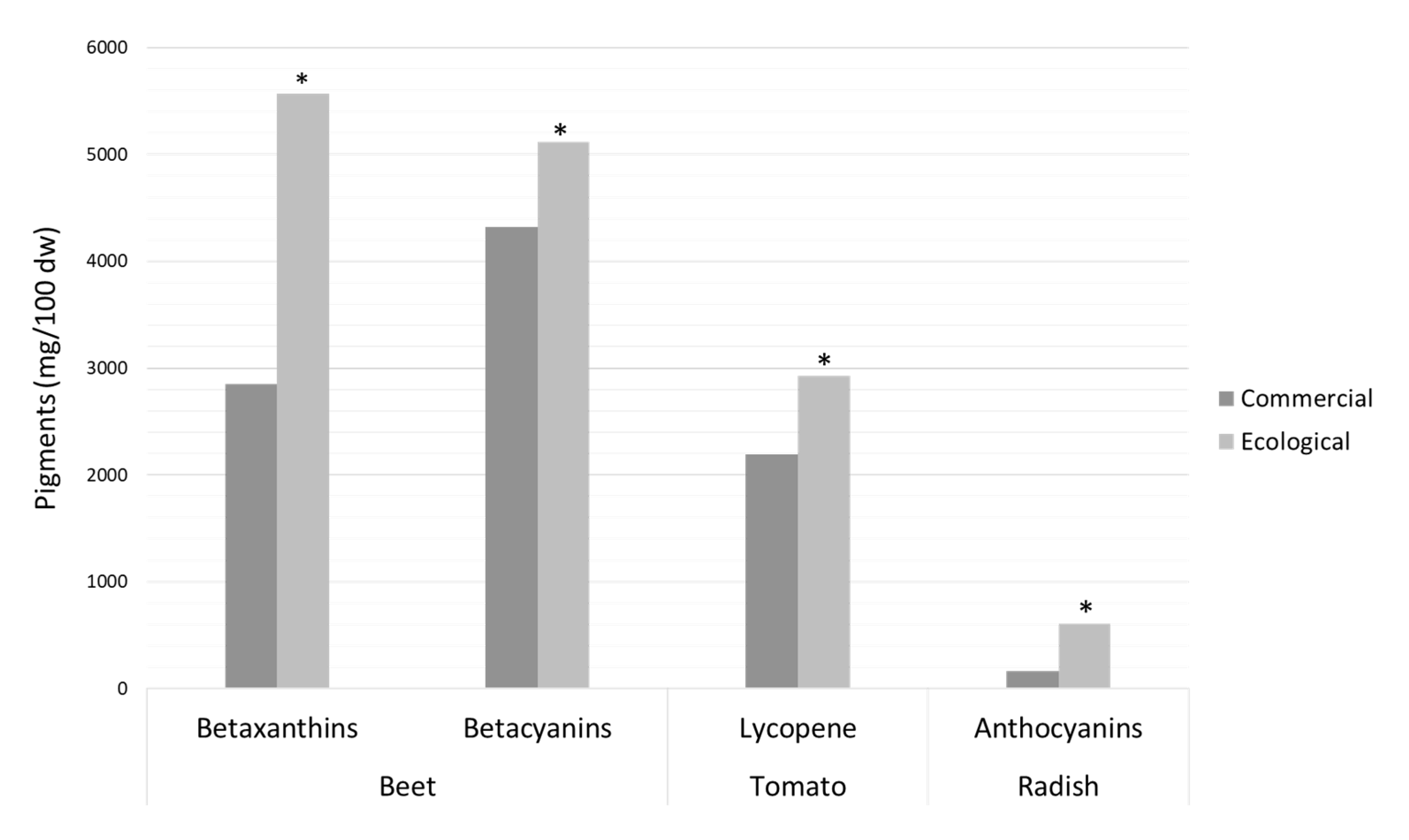
3.2.4. Betalains, Lycopene, and Anthocyanins
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Sustainable Food Systems, Concept and Framework; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- De Brito, C.; Gonçalves, Q.; Schlindwein, M.M. Agroforestry Systems: A Systematic Review Focusing on Traditional Indigenous Practices, Food and Nutrition Security, Economic Viability, and the Role of Women. Sustainability 2021, 13, 11397. [Google Scholar]
- Brandt, K.; Leifert, C.; Sanderson, R.; Seal, C.J. Agroecosystem management and nutritional quality of plant foods: The case of organic fruits and vegetables. CRC. Crit. Rev. Plant Sci. 2011, 30, 177–197. [Google Scholar] [CrossRef]
- Lairon, D. Nutritional quality and safety of organic food. A review. Agron. Sustain. Dev. 2010, 30, 33–41. [Google Scholar] [CrossRef]
- Browne, H.R.B.A.W.; Harris, P.J.C.; Cadoret, K. Smallholder Farmers and Organic Certification: Accessing the EU Market from the Developing World. Biol. Agric. Hortic. 2001, 19, 183–199. [Google Scholar] [CrossRef]
- Olesen, J.E.; Bindi, M. Consequences of climate change for European agricultural productivity, land use and policy. Eur. J. Agron. 2002, 16, 239–262. [Google Scholar] [CrossRef]
- Willer, H.; Yussefi-Menzler, M.; Sorensen, N. The World of Organic Agriculture: Statistics and Emerging Trends 2008; IFOAN: Bonn, Germany, 2008; ISBN 9781849775991. [Google Scholar]
- Williams, D.R.; Dixon, P.S. Impact of Garden-Based Learning on Academic Outcomes in Schools: Synthesis of Research between 1990 and 2010. Rev. Educ. Res. 2013, 83, 211–235. [Google Scholar] [CrossRef]
- Amaya-Castellanos, C.; Shamah-Levy, T.; Escalante-Izeta, E.; del Morales-Ruán, C.M.; Jiménez-Aguilar, A.; Salazar-Coronel, A.; Uribe-Carvajal, R.; Amaya-Castellanos, A. Development of an educational intervention to promote healthy eating and physical activity in Mexican school-age children. Eval. Program Plann. 2015, 52, 159–168. [Google Scholar] [CrossRef]
- Luciana, N.G.; da Cassiano, S.O.; Lorena, G.S.; Renata, M.T.D.; Cristiane, B.; Erika, M.M.T. Nutritional composition of vegetables grown in organic and conventional cultivation systems in Uberlndia, MG. Afr. J. Agric. Res. 2017, 12, 1848–1851. [Google Scholar] [CrossRef]
- Schifferstein, H.N.J.; Wehrle, T.; Carbon, C. Consumer expectations for vegetables with typical and atypical colors: The case of carrots. Food Qual. Prefer. 2018, 72, 98–108. [Google Scholar] [CrossRef]
- Arce-lopera, C.; Masuda, T.; Kimura, A.; Wada, Y.; Okajima, K. Model of vegetable freshness perception using luminance cues. Food Qual. Prefer. 2015, 40, 279–286. [Google Scholar] [CrossRef]
- Thomsen, M.G.; Riley, H.; Borge, G.I.A.; Lea, P.; Gunnar, B.; Division, A.C. Effects of soil type and fertilization on yield, chemical parameters, sensory quality and consumer preference of swede (Brassica napus L. ssp. rapifera). Eur. J. Hortic. Sci. 2018, 82, 1611–4434. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Schreiner, M. Quality Dynamics and Quality Assurance of Fresh Fruits and Vegetables in Pre- and Postharvest. Prod. Pract. Qual. Assess. Food Crop. 2006, 3, 401–449. [Google Scholar] [CrossRef]
- Tena, N.; Mart, J. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic E ff ect in Human Health. Antopxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Divya, P.; Puthusseri, B.; Neelwarne, B. Carotenoid content, its stability during drying and the antioxidant activity of commercial coriander (Coriandrum sativum L.) varieties. Food Res. Int. 2012, 45, 342–350. [Google Scholar] [CrossRef]
- Oliveira, F.; Dos Santos, R.; De Souza Rosa, L.; Anderson-Junger, T. Organic and conventional vegetables: Comparison of the physical and chemical characteristics and antioxidant activity. Afr. J. Biotechnol. 2016, 15, 1746–1755. [Google Scholar] [CrossRef]
- Hattab, S.; Bougattass, I.; Hassine, R.; Dridi-Al-Mohandes, B. Metals and micronutrients in some edible crops and their cultivation soils in eastern-central region of Tunisia: A comparison between organic and conventional farming. Food Chem. 2019, 270, 293–298. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Critical Reviews in Biotechnology Valorization of fruits and vegetable wastes and by- products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- INEGI. Compendio de Información Geográfica Municipal 2010 Pachuca de Soto Hidalgo; INEGI: Aguascalientes, Mexico, 2010. [Google Scholar]
- Johnson, G.M.; Fairchild, M.D. A Top Down Description of S-CIELAB and CIEDE2000. Color Res. Appl. 2003, 28, 425–435. [Google Scholar] [CrossRef]
- Medina, M.S.; Tudela, J.A.; Marín, A.; Allende, A.; Gil, M.I. Short postharvest storage under low relative humidity improves quality and shelf life of minimally processed baby spinach (Spinacia oleracea L.). Postharvest. Biol. Technol. 2012, 67, 1–9. [Google Scholar] [CrossRef]
- Viñas, P.; Campillo, N. Gas Chromatography: Mass Spectrometry Analysis of Polyphenols in Foods; 2019; ISBN 9780128137680. [Google Scholar]
- Muñoz-Bernal, Ó.A.; Torres-Aguirre, G.A.; Núñez-Gastélum, J.A.; de la Rosa, L.A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Álvarez-Parrilla, E. Nuevo Acercamiento a La Interacción Del Reactivo De Folin-Ciocalteu Con Azúcares Durante La Cuantificación De Polifenoles Totales. Tip 2017, 20, 23–28. [Google Scholar] [CrossRef]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic Acid and Element Contents of Foods of Trabzon (Turkey). J. Agric. Food Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Giustu, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–13. [Google Scholar] [CrossRef]
- Ferndndez-López, J.A.; Castellar, R.; Obón, J.M.; Alrnela, L. Screening and Mass-Spectral Confirmation of Betalains in Cactus Pears. Chromatographia 2002, 55, 591–595. [Google Scholar]
- Righi, H.; Camila, S.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Braniša, J.; Jenisová, Z.; Porubská, M.; Jomová, K.; Valko, M.; Klaudia, R. Spectrophotometric Determination of Chlorophylls and Carotenoids. An Effect of Sonication and Sample Processing. J. Microbiol. Biotechnol. Food Sci. 2014, 3, 61–64. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple method simultaneous determination of Chrorophyll and carotenoids in tomato fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Ciência Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Gulcin, I.; Sat, I.; Beydemir, S.; Kufrevioglu, O.I. Evaluation of the in vitro antioxidant properties of broccoli extracts (Brassica oleracea L.). Ital. J. Food Sci. 2004, 16, 17–30. [Google Scholar]
- Kamga, R.T.; Kouamé, C.; Atangana, A.R.; Chagomoka, T.; Ndango, R. Nutritional Evaluation of Five African Indigenous Vegetables. J. Hortic. Res. 2013, 21, 99–106. [Google Scholar] [CrossRef]
- Simunovic, M.P. Acquired color vision deficiency. Surv. Ophthalmol. 2016, 61, 132–155. [Google Scholar] [CrossRef]
- Moreira, M.D.R.; Roura, S.I.; Del Valle, C.E. Quality of Swiss chard produced by conventional and organic methods. LWT—Food Sci. Technol. 2003, 36, 135–141. [Google Scholar] [CrossRef]
- Chen, X. Main Factors Affecting Post-Harvest Grain Loss during the Sales Process: A Survey in Nine Provinces of China. Sustainability 2018, 10, 661. [Google Scholar] [CrossRef]
- Rajapaksha, L.; Gunathilake, D.M.C.C.; Pathirana, S.M.; Fernando, T.N. Reducing post-harvest losses in fruits and vegetables for ensuring food security—Case of Sri Lanka. MOJ Food Process Technols 2021, 9, 7–16. [Google Scholar] [CrossRef]
- Jayalath, M.M.; Perera, H.N. Mapping Post-Harvest Waste in Perishable Supply Chains through System Dynamics: A Sri Lankan Case Study. J. Agric. Sci. Sri Lanka 2021, 16, 526–543. [Google Scholar] [CrossRef]
- Masarirambi, M.T.; Mavuso, V.; Songwe, V.D.; Nkambule, T.P. Indigenous post-harvest handling and processing of traditional vegetables in Swaziland: A review. Afr. J. Agric. Res. 2010, 5, 3333–3341. [Google Scholar] [CrossRef]
- Butnariu, M.; Butu, A. Chemical Composition of Vegetables and their Products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783642416095. [Google Scholar]
- Kapusta-duch, J.; Leszczy, T.; Florkiewicz, A.; Filipiak-florkiewicz, A. Ecology of Food and Nutrition Comparison of Calcium and Magnesium Contents in Cruciferous Vegetables Grown in Areas around Steelworks, on Organic Farms, and Those Available in Retail. Ecol. Food Nutr. 2011, 50, 37–41. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- González-Barraza, L.; Díaz-Godínez, R.; Castillo-Guevara, C.; Nieto-Camacho, A.; Méndez-Iturbide, D. Phenolic compounds: Presence, identification and antioxidant activity in plants and fruits Compuestos fenólicos: Presencia, identificación y propiedades antioxidantes en plantas y frutos. Mex. J. Biotechnol. 2017, 2017, 46–64. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef]
- Pinheiro, J.; Alegria, C.; Abreu, M.; Gonçalves, E.M.; Silva, C.L.M. Kinetics of changes in the physical quality parameters of fresh tomato fruits (Solanum lycopersicum, cv. ‘Zinac’) during storage. J. Food Eng. 2013, 114, 338–345. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 83. [Google Scholar]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of organic and conventional cropping systems on ascorbic acid, vitamin C, flavonoids, nitrate, and oxalate in 27 varieties of spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Adalid, A.M.; Roselló, S.; Nuez, F. Evaluation and selection of tomato accessions (Solanum section Lycopersicon) for content of lycopene, β-carotene and ascorbic acid. J. Food Compos. Anal. 2010, 23, 613–618. [Google Scholar] [CrossRef]
- 50. Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Review, Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer US Preventive Services Task Force Recommendation Statement. JAMA 2023, 23, 2326–2333. [Google Scholar] [CrossRef]
- Pintea, A.; Andrei, S.; Bunea, C.; Pop, R.; Bele, C. Carotenoid and fatty acid profiles of bilberries and cultivated blueberries from Romania. Chem.Pap. 2012, 66, 935–939. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef]
- Walsh, R.; Bartlett, H.; Eperjesi, F. Variation in carotenoid content of kale and other vegetables: A review of pre and post-harvest effects. J. Agric. Food Chem. 2015, 63, 9677–9682. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef]
- Nemzer, B. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Habibi, F.; García-Pastor, M.E.; Puente-Moreno, J.; Garrido-Auñón, F.; Serrano, M.; Valero, D. Anthocyanin in blood oranges: A review on postharvest approaches for its enhancement and preservation. Crit. Rev. Food Sci. Nutr. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Benítez-Estrada, A.; Villanueva-Sánchez, J.; González-Rosendo, G.; Alcántar-Rodríguez, V.E.; Puga-Díaz, R.; Quintero-Gutiérrez, A.G. Determinación de la capacidad antioxidante total de alimentos y plasma humano por fotoquimioluminiscencia: Correlación con ensayos fluorométricos (ORAC) y espectrofotométricos (FRAP). TIP Rev. Espec. Cienc. Químico Biológ. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Gandía-herrero, F.; Escribano, J.; García-carmona, F. Biological Activities of Plant Pigments Betalains. Crit. Rev. Food Sci. Nutr. 2014, 56, 37–41. [Google Scholar] [CrossRef]
- Rodriguez-amaya, D.B. Natural Food Pigments and Colorants; Springer: Cham, Switzerland, 2019; ISBN 9783319780306. [Google Scholar]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Merzlyak, M.N. Screening of Visible and UV Radiation as a Photoprotective Mechanism in Plants. Russ. J. Plant Physiol. 2008, 55, 719–737. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Moon, J.-K.; Shibamoto, T. Antioxidant assays for plant and food components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Bouaziz, A.; Abu, M.; Abdalla, S.; Baghiani, A.; Charef, N. Phytochemical analysis, hypotensive effect and antioxidant properties of Myrtus communis L. growing in Algeria. Asian Pac. J. Trop. Biomed. 2015, 5, 19–28. [Google Scholar] [CrossRef]
- Djenidi, H.; Khennouf, S.; Bouaziz, A. Antioxidant activity and phenolic content of commonly consumed fruits and vegetables in Algeria. Prog. Nutr. 2020, 22, 224–235. [Google Scholar] [CrossRef]
| Vegetable | Type | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| Chard | Com | 36.32 ± 3.64 | −6.32 ± 0.81 | 46.32 ± 3.48 | 23.49 ± 2.20 |
| Eco | 54.88 ± 3.62 * | −9.52 ± 0.81 * | 53.16 ± 4.32 * | ||
| Coriander | Com | 34.60 ± 1.64 | −7.75 ± 0.88 | 48.82 ± 4.27 | 13.08 ± 0.92 |
| Eco | 46.69 ± 3.52 * | −10.65 ± 0.75 * | 56.55 ± 3.03 * | ||
| Spinach | Com | 30.25 ± 2.91 | −6.72 ± 1.25 | 42.45 ± 4.47 | 11.81 ± 0.79 |
| Eco | 37.08 ± 2.58 * | −10.25 ± 0.42 * | 50.19 ± 3.18 * | ||
| Lettuce | Com | 53.76 ± 3.70 | −5.46 ± 1.05 | 22.89 ± 2.21 * | 9.57 ± 0.32 |
| Eco | 59.00 ± 5.32 * | −5.88 ± 0.81 | 21.37 ± 2.17 | ||
| Beet | Com | 28.71 ± 2.87 | 5.58 ± 0.87 | 23.77 ± 2.24 | 12.91 ± 1.10 |
| Eco | 34.73 ± 2.65 * | 16.77 ± 4.86 * | 28.15 ± 5.85 | ||
| Carrot | Com | 48.64 ± 2.38 | 33.33 ± 1.36 | 59.99 ± 4.31 | 10.49 ± 1.09 |
| Eco | 52.92 ± 2.48 * | 27.35 ± 3.75 * | 62.50 ± 3.77 | ||
| Tomato | Com | 36.27 ± 1.54 | 23.72 ± 2.30 | 45.61 ± 1.21 | 2.79 ± 0.40 |
| Eco | 34.48 ± 2.09 * | 26.97 ± 3.62 | 45.42 ± 5.32 | ||
| Radish | Com | 29.07 ± 2.09 | 39.19 ± 3.61 | 24.98 ± 3.21 | 4.67 ± 0.03 |
| Eco | 34.47 ± 1.25 * | 51.81 ± 2.74 * | 47.16 ± 1.98 * |
| Vegetable | Type | L* | a* | b* | ΔE |
|---|---|---|---|---|---|
| Chard | Com | 36.32 ± 2.46 | −6.32 ± 0.92 | 39.32 ± 2.24 | 5.7 ± 0.62 |
| Eco | 44.50 ± 2.87 * | −9.91 ± 0.63 * | 48.09 ± 2.67 * | ||
| Coriander | Com | 33.24 ± 3.20 | −8.60 ± 0.54 | 40.50 ± 4.03 | 8.64 ± 0.19 |
| Eco | 47.94 ± 3.85 * | −10.14 ± 0.26 * | 45.75 ± 4.52 * | ||
| Spinach | Com | 37.72 ± 2.21 | −7.15 ± 0.96 | 47.78 ± 3.78 | 12.70 ± 0.27 |
| Eco | 44.97 ± 3.08 * | −8.39 ± 5.71 | 50.34 ± 4.82 | ||
| Lettuce | Com | 61.16 ± 4.76 | −7.00 ± 1.15 | 33.28 ± 3.40 * | 8.76 ± 0.69 |
| Eco | 65.33 ± 3.47 * | −7.00 ± 0.66 | 27.19 ± 2.63 | ||
| Beet | Com | 11.25 ± 0.75 | 36.58 ± 2.59 | 22.53 ± 3.88 | 4.63 ± 0.40 |
| Eco | 15.09 ± 1.44 * | 38.92 ± 1.69 | 23.14 ± 2.38 | ||
| Carrot | Com | 53.80 ± 3.80 | 34.00 ± 5.67 | 45.91 ± 2.96 | 12.7 ± 1.06 |
| Eco | 59.06 ± 2.24 * | 35.85 ± 2.32 | 58.95 ± 6.24 * | ||
| Tomato | Com | 50.15 ± 6.50 | 15.77 ± 2.79 | 23.27 ± 2.60 | 8.56 ± 0.67 |
| Eco | 55.11 ± 9.90 | 17.68 ± 1.20 * | 23.19 ± 2.64 | ||
| Radish | Com | 74.36 ± 2.36 | 0.59 ± 0.21 | 6.66 ± 0.97 | 2.8 ± 0.49 |
| Eco | 75.77 ± 1.57 | −0.44 ± 0.14 * | 7.92 ± 0.67 * |
| Vegetable | Type | Texture (N/100 g o Piece) | Moisture (%) | Ashes (%) |
|---|---|---|---|---|
| Chard | Com | 3684 ± 312 | 88 ± 0.04 | 0.11 ± 0.001 |
| Eco | 3146 ± 289 * | 91 ± 0.03 | 0.12 ± 0.001 | |
| Coriander | Com | 4825 ± 228 | 87 ± 0.01 | 0.10 ± 0.01 |
| Eco | 3537 ± 228 * | 87 ± 0.01 | 0.22 ± 0.01 * | |
| Spinach | Com | 4884 ± 343 | 89 ± 0.01 * | 0.018 ± 0.03 |
| Eco | 2878 ± 171 * | 84 ± 0.02 | 0.10 ±0.01 | |
| Lettuce | Com | 2510 ± 241 | 93 ± 0.01 | 0.03 ± 0.00 |
| Eco | 2210 ± 213 * | 91 ± 0.01 | 0.08 ± 0.02 * | |
| Beet | Com | 15.85 ± 0.84 | 86 ± 0.02 | 0.08 ± 0.002 |
| Eco | 15.08 ± 0.66 * | 85 ± 0.04 | 0.13 ± 0.023 | |
| Carrot | Com | 18.99 ± 1.72 | 97 ± 0.45 | 0.09 ± 0.019 |
| Eco | 16.51 ± 0.73 * | 89 ± 0.03 | 0.97 ± 0.017 | |
| Tomato | Com | 1.66 ± 0.08 | 93 ± 0.0 | 0.02 ± 0.00 |
| Eco | 0.60 ± 0.09 * | 94 ± 0.0 * | 0.06 ± 0.02 * | |
| Radish | Com | 6.85 ± 0.70 | 94 ± 0.01 * | 0.03 ± 0.00 |
| Eco | 3.69 ± 0.47 * | 91 ± 0.06 | 0.02 ± 0.01 |
| Vegetable | Type | TPC (GAE) | Ascobic Acid (AA) | β-Carotene | Chlorophyll a | Chlorophyll b |
|---|---|---|---|---|---|---|
| Chard | Com | 426.76 ± 6.68 | 1219.37 ± 7.27 | 50.68 ± 0.27 | 509.24 ± 8.21 | 1286.05 ± 8.27 |
| Eco | 485.25 ± 2.38 * | 1276.51 ± 7.27 * | 50.34 ± 0.13 | 525.10 ± 3.06 * | 1302.07 ± 0.81 * | |
| Coriander | Com | 1151.11 ± 27.74 | 1035.24 ± 8.25 | 50.71 ± 0.08 | 524.33 ± 2.39 | 1233.34 ± 1.94 |
| Eco | 1429.35 ± 10.61 * | 1070.16 ± 7.21 * | 50.61 ± 0.20 | 529.14 ± 0.83 * | 1306.03 ± 1.31 * | |
| Spinach | Com | 443.73 ± 14.82 | 1208.41 ± 40.50 | 49.21 ± 0.29 | 508.18 ± 3.03 | 1261.31 ± 3.19 |
| Eco | 592.50 ± 5.46 * | 1222.71 ± 90.02 | 49.47 ± 0.14 | 508.48 ± 6.92 | 1271.62 ± 8.08 | |
| Lettuce | Com | 205.93 ± 3.67 | 1262.38 ± 17.17 | 11.61 ± 0.29 | 79.57 ± 5.53 | 128.39 ± 4.69 |
| Eco | 754.23 ± 44.41 * | 1310.00 ± 12.60 * | 47.20 ± 0.28 * | 508.64 ± 3.29 * | 854.94 ± 12.76 * | |
| Beet | Com | 277.99 ± 8.29 | 1284.44 ± 7.27 * | 1.44 ± 0.04 | 4.47 ± 0.36 | 11.08 ± 0.86 |
| Eco | 553.15 ± 33.22 * | 1043.17 ± 11.68 | 2.55 ± 0.16 * | 44.13 ± 0.27 * | 68.44 ± 1.65 * | |
| Carrot | Com | 88.18 ± 7.77 | 1278.25 ± 13.75 | 52.05 ± 0.09 | 12.71 ± 2.40 | 34.7 ± 1.65 |
| Eco | 102.22 ± 3.96 * | 1310 ± 26.51 | 53.23 ± 0.04 * | 40.05 ± 5.38 * | 140.16 ± 5.11 * | |
| Tomato | Com | 873.59 ± 2.56 * | 940.16 ± 11.98 | 18.04 ± 0.04 | 6.53 ± 0.30 | 21.49 ± 1.01 |
| Eco | 506.89 ± 1.31 | 1038.57 ± 20.76 * | 19.33 ± 0.16 * | 12.61 ± 0.53 * | 13.39 ± 2.71 * | |
| Radish | Com | 596.64 ± 4.26 | 932.22 ± 11.00 | 14.69 ± 0.59 | 19.99 ± 0.91 | 46.46 ± 0.45 |
| Eco | 1120.26 ± 7.57 * | 1105.24 ± 19.05 * | 16.71 ± 0.19 * | 21.45 ± 0.16 | 45.74 ± 0.80 |
| Vegetable | Type | ABTS•+ (µmol TE) | DPPH (µmol TE) | FRAP (μmol Fe (II)) | Chelating Activity (%) |
|---|---|---|---|---|---|
| Chard | Com | 3961.95 ± 342.37 | 55.44 ± 3.11 | 1056.35 ± 1.24 | 85.57 ± 1.87 |
| Eco | 3737.05 ± 159.54 | 108.52 ± 2.00 * | 3446.17 ± 207.77 * | 90.19 ± 0.03 * | |
| Coriander | Com | 3596.63 ± 235.40 | 27.54 ± 1.07 | 1063.44 ± 374.00 | 79.34 ± 0.14 |
| Eco | 3560.29 ± 226.21 | 73.00 ± 1.28 * | 1793.67 ± 15.48 * | 89.73 ± 0.78 * | |
| Spinach | Com | 2465.32 ± 181.42 | 0.12 ± 0.00 | 124.03 ± 3.23 | 81.50 ± 0.42 |
| Eco | 3395.38 ± 52.81 * | 0.14 ± 0.01 * | 176.94 ± 3.15 * | 92.75 ± 0.13 * | |
| Lettuce | Com | 4951 ± 194 | 6390.2 ± 268.7 | 263.49 ± 1.22 | 21:01 ± 0.74 |
| Eco | 6623 ± 639 * | 7600.32 ± 97.28 * | 865.05 ± 8.77 * | 49.56 ± 0.13 * | |
| Beet | Com | 4362.63 ± 382.41 | 30.03 ± 2.78 | 197.49 ± 3.73 | 44.45 ± 1.00 |
| Eco | 4231.84 ± 132.64 | 58.23 ± 1.93 * | 259.38 ± 6.12 * | 52.12 ± 0.09 * | |
| Carrot | Com | 89 ± 6.01 | ---- | 62.28 ± 4.61 | 23.86 ± 0.30 |
| Eco | 120.12 ± 8.88 * | ---- | 94.15 ± 3.49 * | 53.01 ± 4.50 * | |
| Tomato | Com | 41.28 ± 1.62 * | 0.04 ± 0.0 | 37.99 ± 0.92 | 83.98 ± 0.15 |
| Eco | 23.29 ± 1.17 | 0.07 ± 0.0 * | 57.27 ± 1.15 * | 92.35 ± 0.09 * | |
| Radish | Com | 4137.38 ± 302.41 | 275 ± 14 | 135.79 ± 5.71 | 21.27 ± 0.85 |
| Eco | 6565.53 ± 56.29 * | 1857 ± 101 * | 684.75 ± 13.79 * | 32.14 ± 2.56 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olguín-Hernández, Z.; Zafra-Rojas, Q.Y.; Cruz-Cansino, N.d.S.; Ariza-Ortega, J.A.; Añorve-Morga, J.; Ojeda-Ramírez, D.; Falfan-Cortes, R.N.; Arias-Rico, J.; Ramírez-Moreno, E. Comparison of Vegetables of Ecological and Commercial Production: Physicochemical and Antioxidant Properties. Sustainability 2023, 15, 5117. https://doi.org/10.3390/su15065117
Olguín-Hernández Z, Zafra-Rojas QY, Cruz-Cansino NdS, Ariza-Ortega JA, Añorve-Morga J, Ojeda-Ramírez D, Falfan-Cortes RN, Arias-Rico J, Ramírez-Moreno E. Comparison of Vegetables of Ecological and Commercial Production: Physicochemical and Antioxidant Properties. Sustainability. 2023; 15(6):5117. https://doi.org/10.3390/su15065117
Chicago/Turabian StyleOlguín-Hernández, Zacnicté, Quinatzin Yadira Zafra-Rojas, Nelly del Socorro Cruz-Cansino, Jose Alberto Ariza-Ortega, Javier Añorve-Morga, Deyanira Ojeda-Ramírez, Reyna Nallely Falfan-Cortes, Jose Arias-Rico, and Esther Ramírez-Moreno. 2023. "Comparison of Vegetables of Ecological and Commercial Production: Physicochemical and Antioxidant Properties" Sustainability 15, no. 6: 5117. https://doi.org/10.3390/su15065117
APA StyleOlguín-Hernández, Z., Zafra-Rojas, Q. Y., Cruz-Cansino, N. d. S., Ariza-Ortega, J. A., Añorve-Morga, J., Ojeda-Ramírez, D., Falfan-Cortes, R. N., Arias-Rico, J., & Ramírez-Moreno, E. (2023). Comparison of Vegetables of Ecological and Commercial Production: Physicochemical and Antioxidant Properties. Sustainability, 15(6), 5117. https://doi.org/10.3390/su15065117











