Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
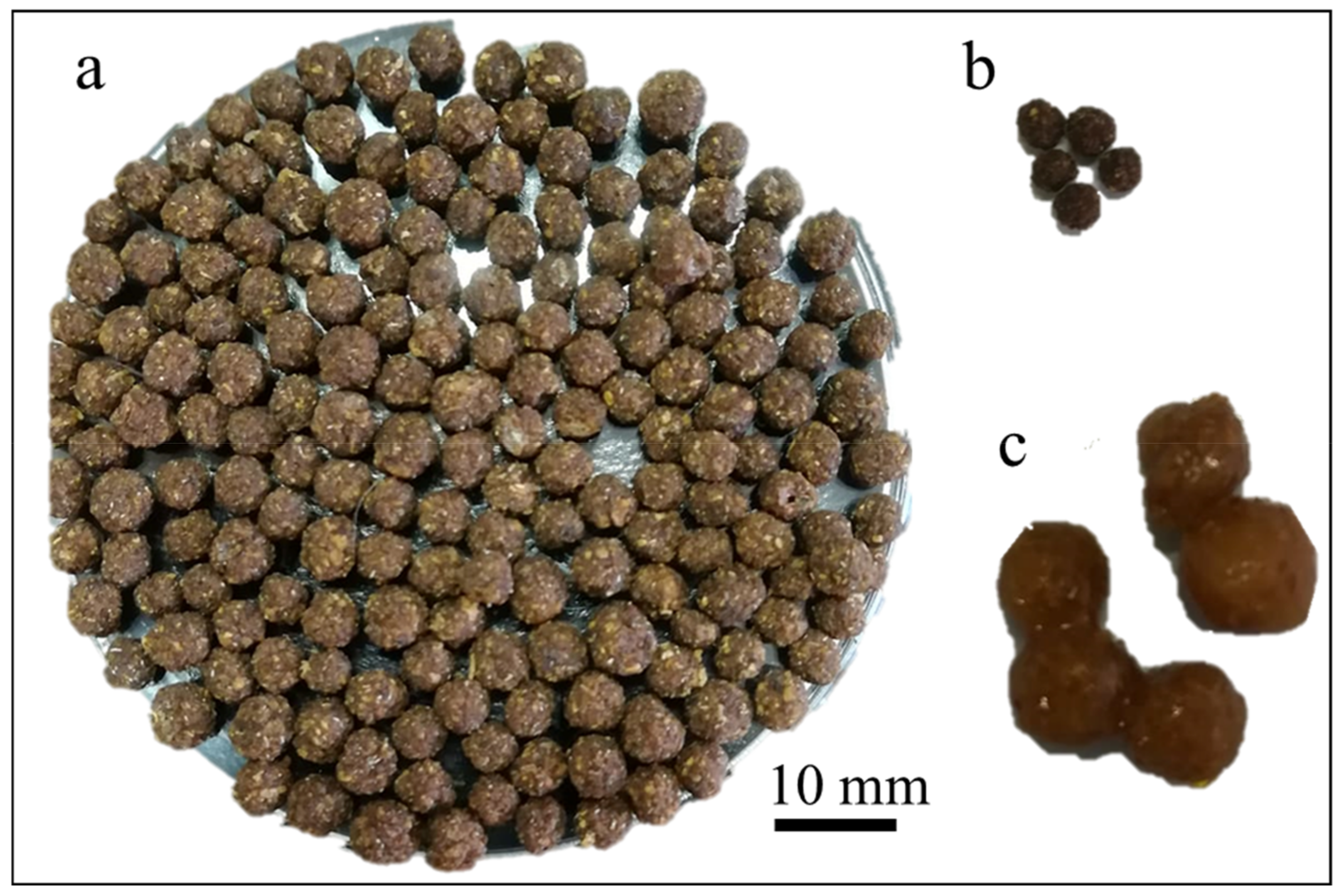
2.2. Extraction of Cellulose from Orange Fruit Peels
2.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
2.5. Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) Analyses
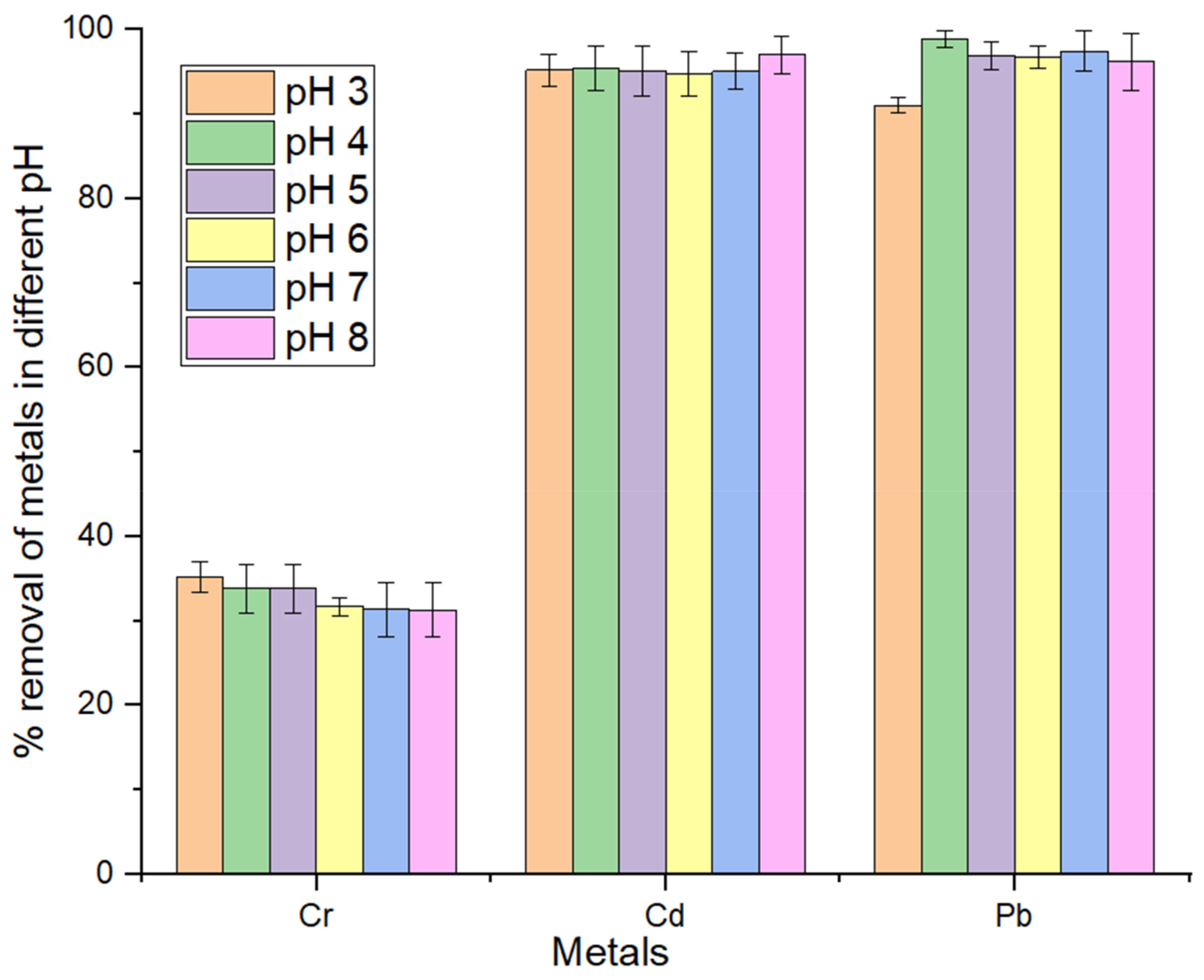
2.6. Effect of pH on Adsorption
2.7. Effect of Time on Adsorption
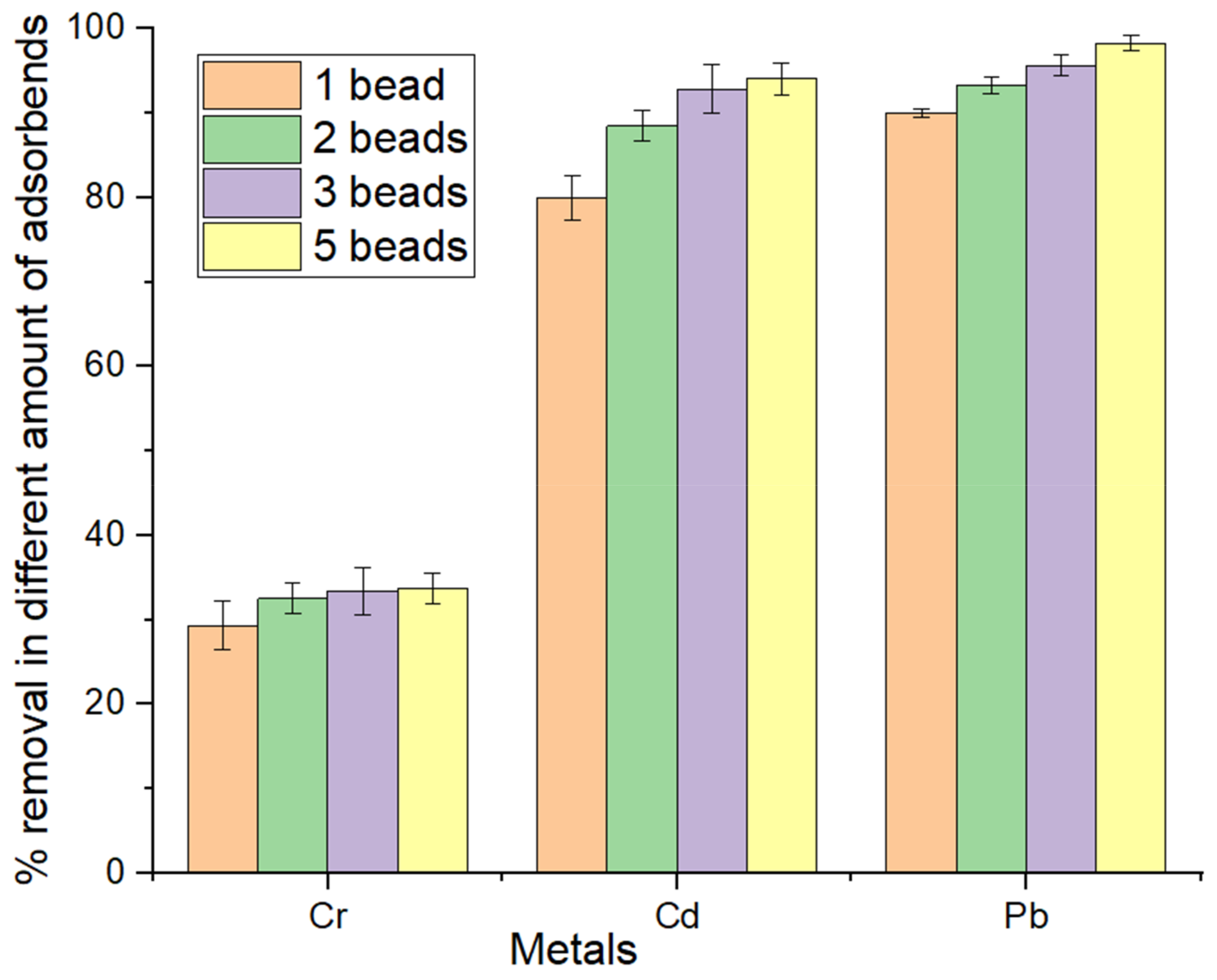
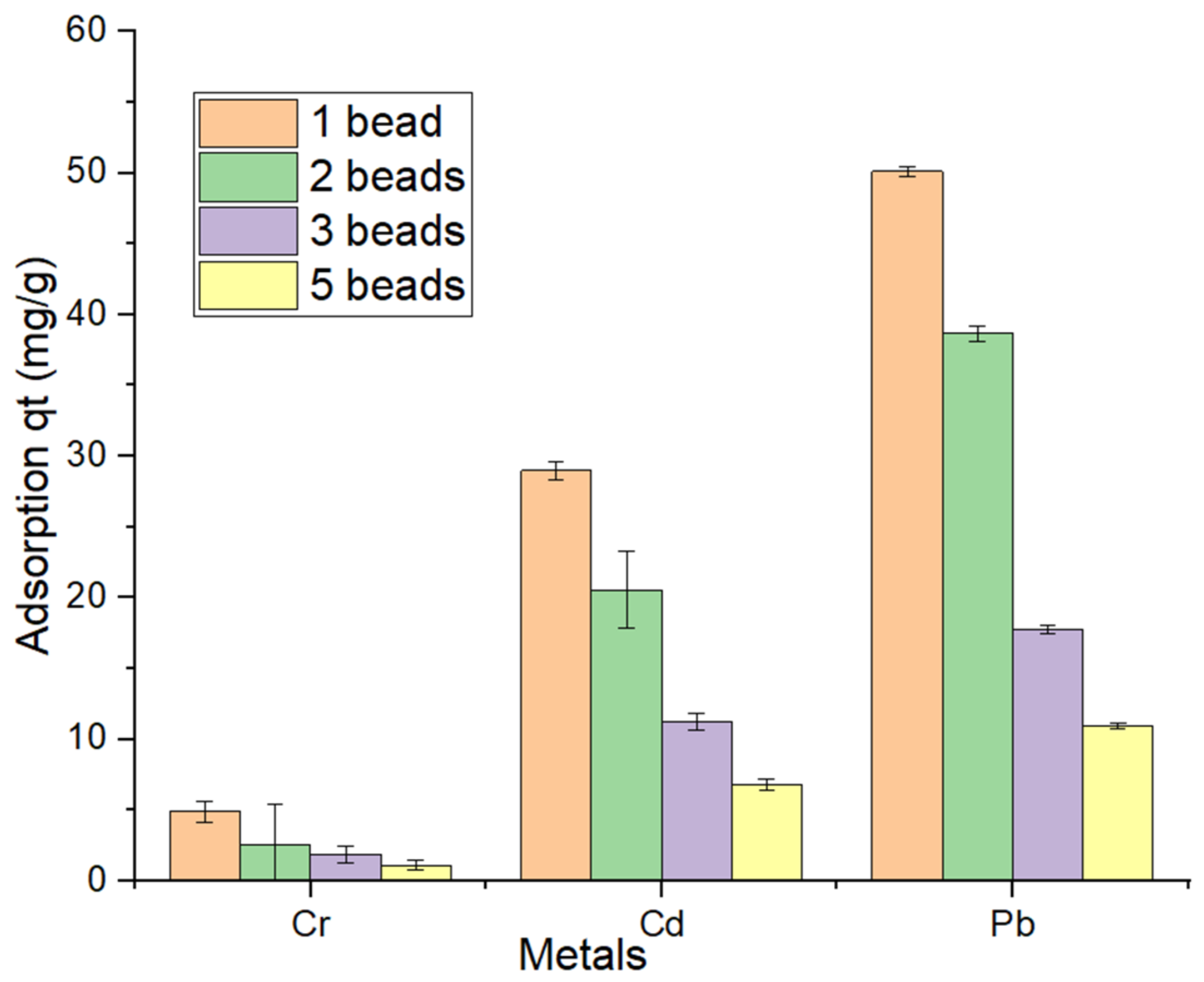
2.8. Bioadsorbent Dosages
2.9. Statistical Analysis
3. Results and Discussion
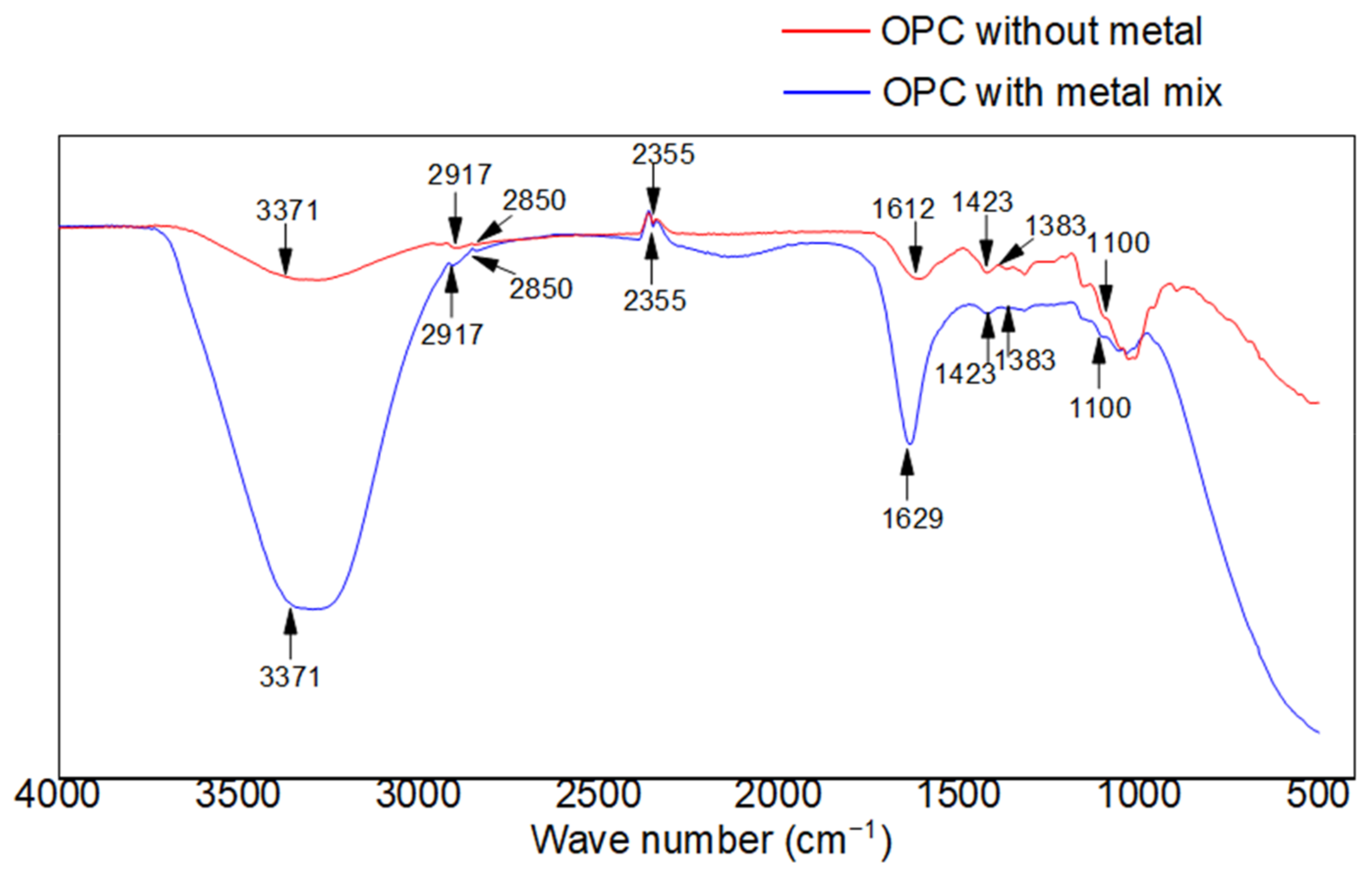
3.1. FT-IR Analysis
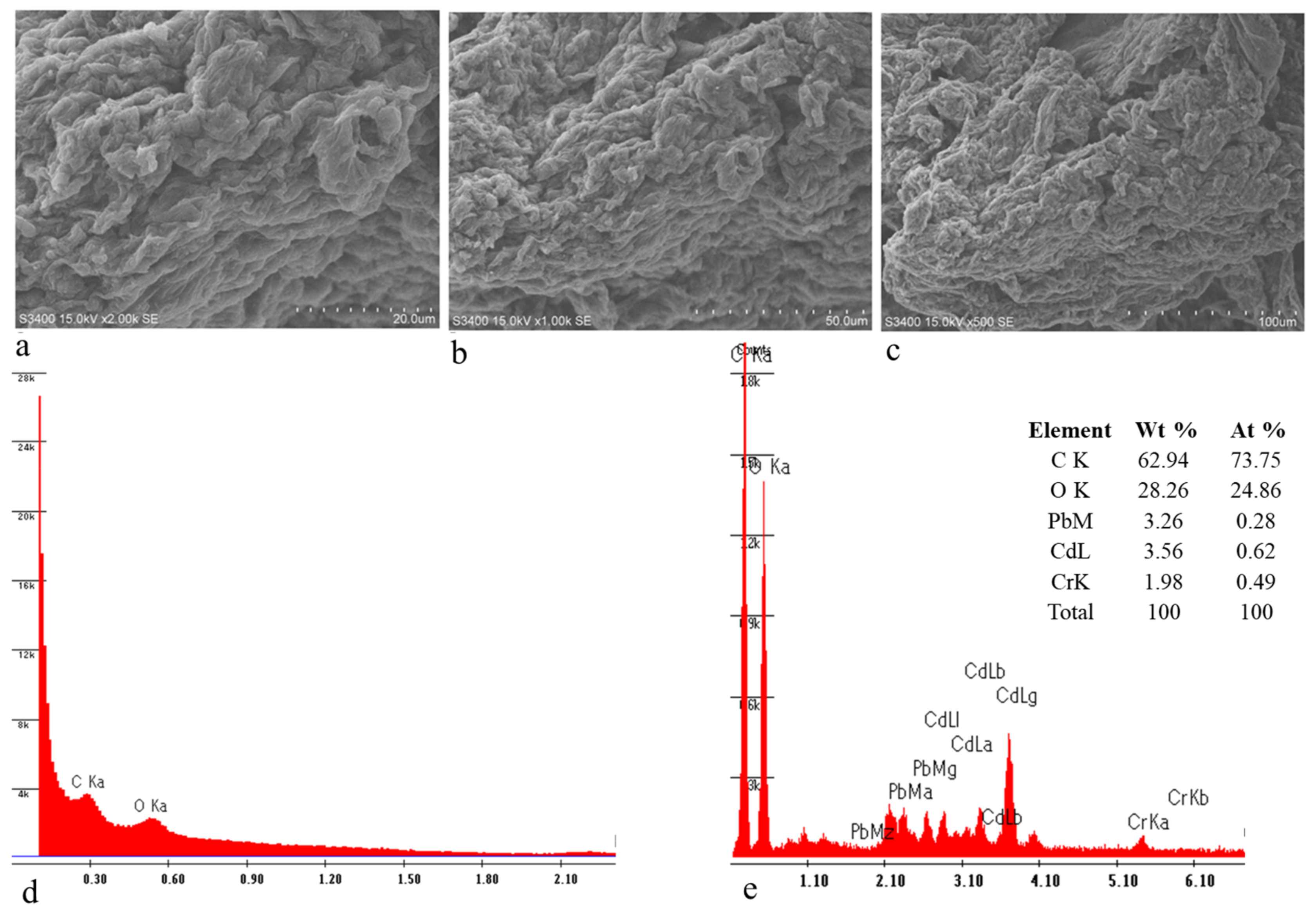
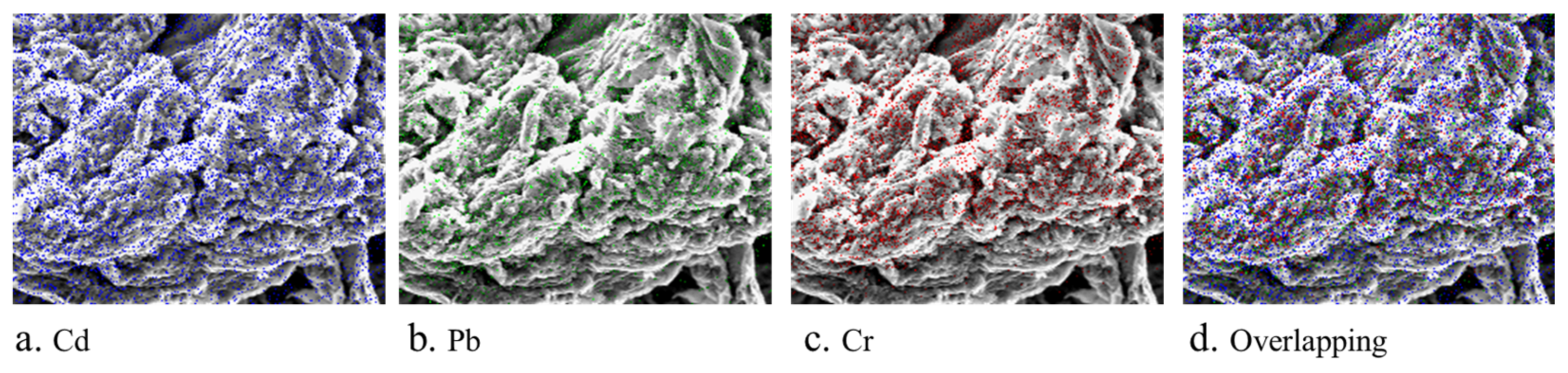
3.2. SEM-EDS Analysis
3.3. ICP-MS Analyses
3.4. Effect of pH
3.5. Effect of Adsorbent Dosage
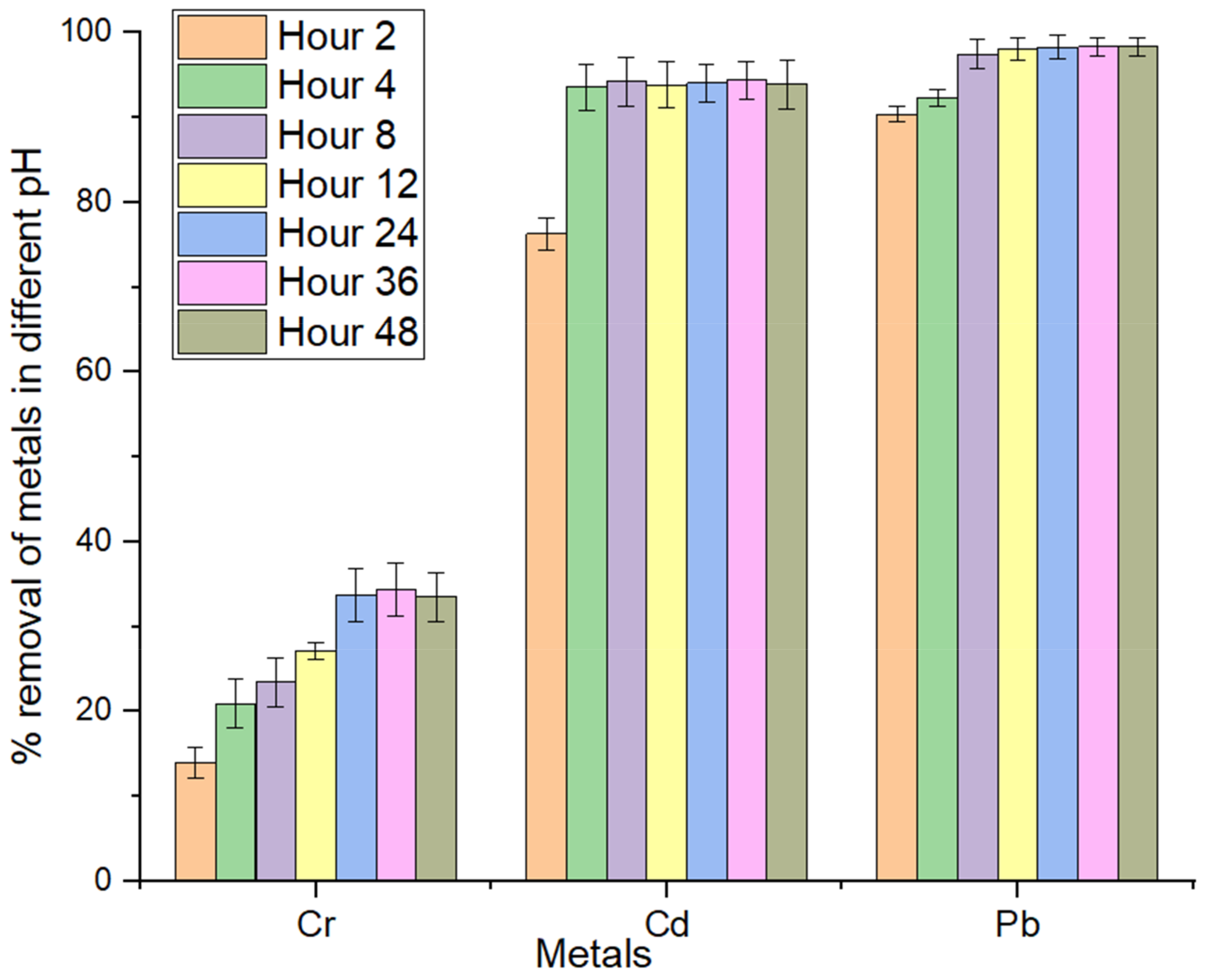
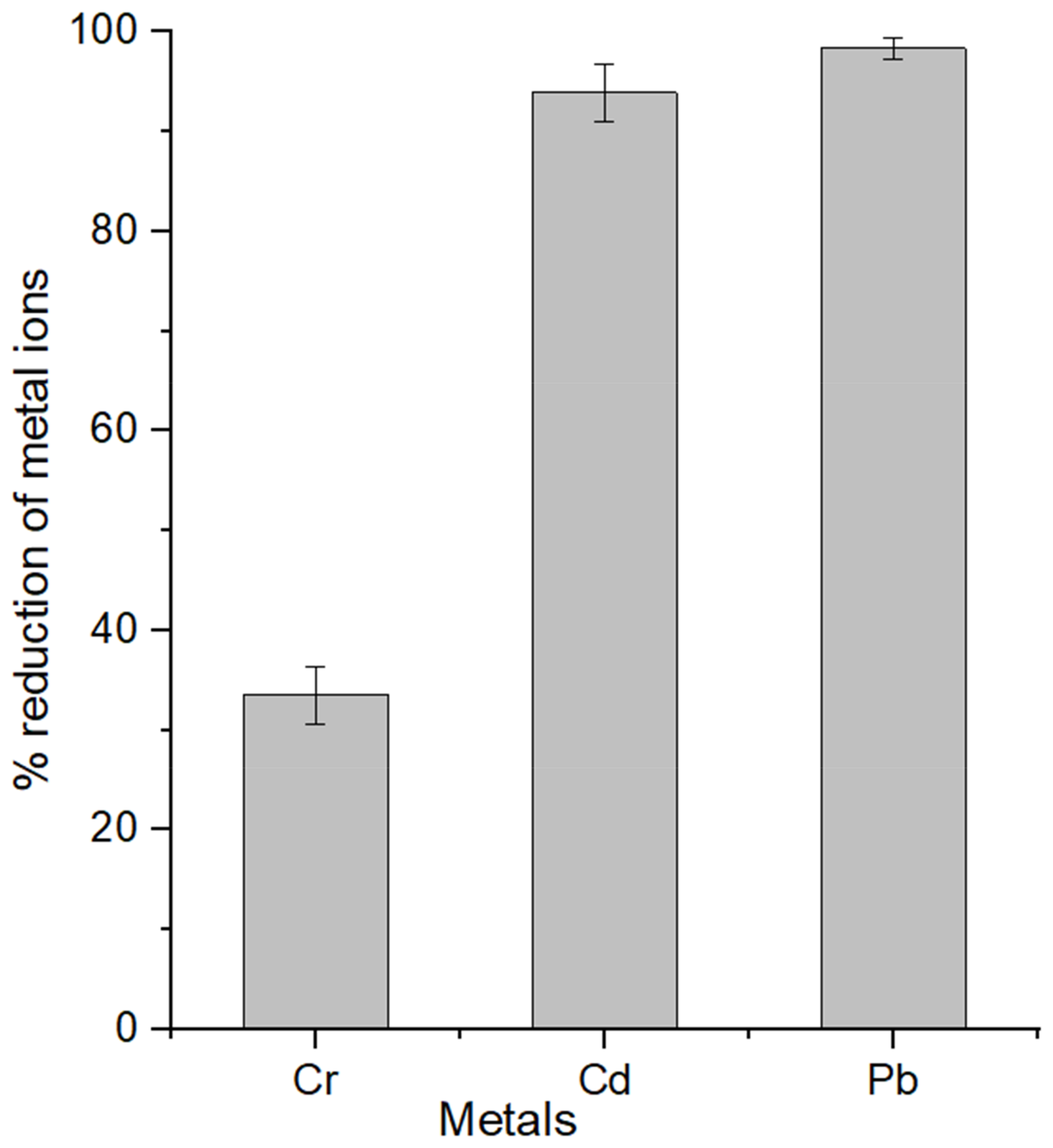
3.6. Influence of Contact Time
3.7. Future Research, Practice, and Policy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babu, D.J.; Prasanna, P.K.Y. Optimization of Cu (II) Biosorption onto Sea Urchin Test Using Response Surface Methodology and Artificial Neural Networks. Int. J. Environ. Sci. Technol. 2019, 16, 1885–1896. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Desale, P.; Kapadnis, B.P.; Hossain, K.; Saha, A.K.; Ghosh, S.; Olsson, B.; et al. Isolation and Characterization of a Lysinibacillus Strain B1-CDA Showing Potential for Bioremediation of Arsenics from Contaminated Water. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 1349–1360. [Google Scholar] [CrossRef]
- Asokan, N.M.R.S.; Sundari, N.S. Bioremediation of Chromium (VI) by Stenotrophomonas Maltophilia Isolated from Tannery Effluent. Int. J. Environ. Sci. Technol. 2018, 15, 207–216. [Google Scholar] [CrossRef]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Rahman, A. Bioremediation of Toxic Metals for Protecting Human Health and the Ecosystem; Örebro University: Örebro, Sweden, 2016; ISBN 9789175291468. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Moraes, R.R.L.V.J.S. Removal of Organic Pollutants from Wastewater Using Chitosan: A Literature Review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Ghori, N.H.G.T.; Imadi, M.Q.H.S.R.; Altay, A.G.V. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Lupa, L.; Maranescu, B.; Visa, A. Equilibrium and Kinetic Studies of Chromium Ions Adsorption on Co (II)-Based Phosphonate Metal Organic Frameworks. Sep. Sci. Technol. 2018, 53, 1017–1026. [Google Scholar] [CrossRef]
- Khan, A.; Khan, S.K.M.A.; Ullah, M.A.H.; Rehman, J.N.I.U. Heavy Metals Effects on Plant Growth and Dietary Intake of Trace Metals in Vegetables Cultivated in Contaminated Soil. Int. J. Environ. Sci. Technol. 2019, 16, 2295–2304. [Google Scholar] [CrossRef]
- Krstić, V.; Urošević, T.; Pešovski, B. A Review on Adsorbents for Treatment of Water and Wastewaters Containing Copper Ions. Chem. Eng. Sci. 2018, 192, 273–287. [Google Scholar] [CrossRef]
- Lim, J.T.; Tan, Y.Q.; Valeri, L.; Lee, J.; Geok, P.P.; Chia, S.E.; Ong, C.N.; Seow, W.J. Association between Serum Heavy Metals and Prostate Cancer Risk–A Multiple Metal Analysis. Environ. Int. 2019, 132, 105109. [Google Scholar] [CrossRef]
- Gupta, V.K.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L.; Suhas. Low-Cost Adsorbents: Growing Approach to Wastewater Treatmenta Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 783–842. [Google Scholar] [CrossRef]
- Genchi, G.; Graziantono, L.; Carocci, A.; Catalano, A. The Effects of Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Tripathi, M.; Upadhyay, S.K.; Kaur, M.; Kaur, K. Toxicity Concerns of Hexavalent Chromium from Tannery Waste. J. Biotechnol. Bioeng. 2018, 2, 40–44. [Google Scholar]
- Prithviraj, D.; Deboleena, K.; Neelu, N.; Noor, N.; Aminur, R.; Balasaheb, K.; Abul, M. Biosorption of Nickel by Lysinibacillus Sp. BA2 Native to Bauxite Mine. Ecotoxicol. Environ. Saf. 2014, 107, 260–268. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Hossain, K.; Saud, Z.A.; Saha, A.K.; Ghosh, S.; Olsson, B.; Mandal, A. Bioremediation of Hexavalent Chromium (VI) by a Soil-Borne Bacterium, Enterobacter Cloacae B2-DHA. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1136–1147. [Google Scholar] [CrossRef]
- Nawani, N.; Rahman, A.; Nahar, N.; Saha, A.; Kapadnis, B.; Mandal, A. Status of Metal Pollution in Rivers Flowing through Urban Settlements at Pune and Its Effect on Resident Microflora. Biologia 2016, 71, 494–507. [Google Scholar] [CrossRef]
- Yewale, P.P.; Rahman, A.; Nahar, N.; Saha, A.; Jass, J.; Mandal, A.; Nawani, N.N. Sources of Metal Pollution, Global Status, and Conventional Bioremediation Practices. Handb. Met. Interact. Bioremediation 2017, 25–40. [Google Scholar] [CrossRef]
- Acharya, J.; Kumar, U.; Rafi, P.M. International Journal of Current Engineering and Technology Removal of Heavy Metal Ions from Wastewater by Chemically Modified Agricultural Waste Material as Potential Adsorbent-A Review. Int. J. Curr. Eng. Technol. 2018, 8, 526–530. [Google Scholar]
- Yelebe, E.O.Z.R.; Nelson, B.O.E.S. Clean-up of Crude Oil-Contaminated Soils: Bioremediation Option. Int. J. Environ. Sci. Technol. 2020, 17, 1185–1198. [Google Scholar] [CrossRef]
- Nawani, N.; Rahman, A.; Mandal, A. Microbial Biomass for Sustainable Remediation of Wastewater. Biomass Biofuels Biochem. 2022, 12, 271–292. [Google Scholar]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar Derived from Non-Customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Nabipour, H.; Rohani, S.; Batool, S.; Yusuff, A.S. An Overview of the Use of Water-Stable Metal-Organic Frameworks in the Removal of Cadmium Ion. J. Environ. Chem. Eng. 2023, 11, 109131. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Agunwamba, J.C. A Review on the Applicability of Activated Carbon Derived from Plant Biomass in Adsorption of Chromium, Copper, and Zinc from Industrial Wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef]
- Popa, A.; Visa, A.; Maranescu, B.; Hulka, I.; Lupa, L. Chemical Modification of Chitosan for Removal of Pb(II) Ions from Aqueous Solutions. Materials 2021, 14, 7894. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Haque, A.; Ghosh, S.; Shinu, P.; Attimarad, M. Modified Shrimp-Based Chitosan as an Emerging Adsorbent Removing Heavy Metals (Chromium, Nickel, Arsenic, and Cobalt) from Polluted Water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Kanamarlapudi, S.L.R.K.; Chintalpudi, V.K.; Muddada, S. Application of Biosorption for Removal of Heavy Metals from Wastewater. Biosorption 2018. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of Cadmium on Cucumber Peel: Kinetics, Isotherm and Co-Ion Effect. Indian Chem. Eng. 2018, 60, 179–195. [Google Scholar] [CrossRef]
- Abdullah-Al-Mamun, M.; Hossain, M.S.; Debnath, G.C.; Sultana, S.; Rahman, A.; Hasan, Z.; Das, S.R.; Ashik, M.A.; Prodhan, M.Y.; Aktar, S.; et al. Unveiling Lignocellulolytic Trait of a Goat Omasum Inhabitant Klebsiella Variicola Strain HSTU-AAM51 in Light of Biochemical and Genome Analyses; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; ISBN 0123456789. [Google Scholar]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption.Pdf. Water Air Soil Pollut. 2018, 229, 1–50. [Google Scholar] [CrossRef]
- Thakur, V.; Sharma, E.; Guleria, A.; Sangar, S.; Singh, K. Modification and Management of Lignocellulosic Waste as an Ecofriendly Biosorbent for the Application of Heavy Metal Ions Sorption. Mater. Today Proc. 2020, 32, 608–619. [Google Scholar] [CrossRef]
- Nathan, R.J.; Barr, D.; Rosengren, R.J. Six Fruit and Vegetable Peel Beads for the Simultaneous Removal of Heavy Metals by Biosorption. Environ. Technol. 2022, 43, 1935–1952. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Guo, X.; Feng, N.; Tian, Q. Application of Orange Peel Xanthate for the Adsorption of Pb2+ from Aqueous Solutions. J. Hazard. Mater. 2009, 170, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Ravindran, L.; Sreekala, M.S.; Thomas, S. Novel Processing Parameters for the Extraction of Cellulose Nanofibres (CNF) from Environmentally Benign Pineapple Leaf Fibres (PALF): Structure-Property Relationships. Int. J. Biol. Macromol. 2019, 131, 858–870. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.; Macena, M.; Esteves, B.; Santos-Vieira, I. Lignocellulosic Materials Used as Biosorbents for the Capture of Nickel (II) in Aqueous Solution. Appl. Sci. 2022, 12, 933. [Google Scholar] [CrossRef]
- Wang, N.; Jin, R.N.; Omer, A.M.; Ouyang, X. kun Adsorption of Pb(II) from Fish Sauce Using Carboxylated Cellulose Nanocrystal: Isotherm, Kinetics, and Thermodynamic Studies. Int. J. Biol. Macromol. 2017, 102, 232–240. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A Review on Modification Methods to Cellulose-Based Adsorbents to Improve Adsorption Capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Liu, J.; Chen, T.W.; Yang, Y.L.; Bai, Z.C.; Xia, L.R.; Wang, M.; Lv, X.L.; Li, L. Removal of Heavy Metal Ions and Anionic Dyes from Aqueous Solutions Using Amide-Functionalized Cellulose-Based Adsorbents. Carbohydr. Polym. 2020, 230, 115619. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A Review on Agro-Industrial Waste (AIW) Derived Adsorbents for Water and Wastewater Treatment. J. Environ. Manage. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial Application of Cellulose Nano-Composites–A Review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Feng, N.C.; Guo, X.Y. Characterization of Adsorptive Capacity and Mechanisms on Adsorption of Copper, Lead and Zinc by Modified Orange Peel. Trans. Nonferrous Met. Soc. China 2012, 22, 1224–1231. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications-A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Ayub, A.; Irfan, M.; Rizwan, M.; Irfan, A. International Journal of Biological Macromolecules Development of Sustainable Magnetic Chitosan Biosorbent Beads for Kinetic Remediation of Arsenic Contaminated Water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Nadeem, R.; Bibi, S.; Rashid, U.; Hanif, A.; Jahan, N.; Ashfaq, Z.; Ahmed, Z.; Adil, M.; Naz, M. Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water 2021, 13, 3344. [Google Scholar] [CrossRef]
- He, C.; Lin, H.; Dai, L.; Qiu, R.; Tang, Y.; Wang, Y.; Duan, P.G.; Ok, Y.S. Waste Shrimp Shell-Derived Hydrochar as an Emergent Material for Methyl Orange Removal in Aqueous Solutions. Environ. Int. 2020, 134, 105340. [Google Scholar] [CrossRef]
- Sohail, A.; Javed, S.; Khan, M.U.; Umar, A. Biosorption of Heavy Metals onto the Bark of Prosopis Spicigira: A Kinetic Study for the Removal of Water Toxicity. Am. Eurasian J. Toxicol. Sci. 2015, 7, 300–315. [Google Scholar] [CrossRef]
- Reddy, N.A.; Lakshmipathy, R.; Sarada, N.C. Application of Citrullus Lanatus Rind as Biosorbent for Removal of Trivalent Chromium from Aqueous Solution. Alex. Eng. J. 2014, 53, 969–975. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR Spectrophotometry, Kinetics and Adsorption Isotherms Modeling, Ion Exchange, and EDX Analysis for Understanding the Mechanism of Cd2+ and Pb2+ Removal by Mango Peel Waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Zhang, L.; Huang, J.; Chen, F.; Yang, Z.; Yao, J.; Zhang, Z. Controlled Assembly of Fe3O4 Magnetic Nanoparticles on Graphene Oxide. Nanoscale 2011, 3, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- D’Halluin, M.; Rull-Barrull, J.; Bretel, G.; Labrugère, C.; Le Grognec, E.; Felpin, F.X. Chemically Modified Cellulose Filter Paper for Heavy Metal Remediation in Water. ACS Sustain. Chem. Eng. 2017, 5, 1965–1973. [Google Scholar] [CrossRef]
- Abd-Talib, N.; Chuong, C.S.; Mohd-Setapar, S.H.; Asli, U.A.; Pa’ee, K.F.; Len, K.Y.T. Trends in Adsorption Mechanisms of Fruit Peel Adsorbents to Remove Wastewater Pollutants (Cu (II), Cd (II) and Pb (II)). J. Water Environ. Technol. 2020, 18, 290–313. [Google Scholar] [CrossRef]
- Chen, S.; Xue, C.; Wang, J.; Feng, H.; Wang, Y.; Ma, Q.; Wang, D. Adsorption of Pb (II) and Cd (II) by Squid Ommastrephes Bartrami Melanin. Bioinorg. Chem. Appl. 2009, 2009, 901563. [Google Scholar] [CrossRef]
- Yang, X.; Cui, X. Adsorption Characteristics of Pb (II) on Alkali Treated Tea Residue. Water Resour. Ind. 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Lo, S.F.; Wang, S.Y.; Tsai, M.J.; Lin, L.D. Adsorption Capacity and Removal Efficiency of Heavy Metal Ions by Moso and Ma Bamboo Activated Carbons. Chem. Eng. Res. Des. 2012, 90, 1397–1406. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption Using Modified Orange Peel as Adsorbent. Adv. Mater. Res. 2011, 236, 237–240. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Pam, A.A.; Ikudayisi, A.V. Thermodynamic Properties of Chromium (III) Ion Adsorption by Sweet Orange (Citrus sinensis) Peels. Am. J. Anal. Chem. 2014, 05, 666–673. [Google Scholar] [CrossRef]
- Jisha, T.J.; Lubna, C.H.; Habeeba, V. Removal of Cr (VI) Using Orange Peel as an Adsorbent. Int. J. Adv. Res. Innov. Ideas Educ. 2017, 3, 276–283. [Google Scholar]
- Tejada-Tovar, C.; Gonzalez-Delgado, A.D.; Villabona-Ortiz, A. Removal of Cr (VI) from Aqueous Solution Using Orange Peel-Based Biosorbents. Indian J. Sci. Technol. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Gönen, F.; Serin, D.S. Adsorption Study on Orange Peel: Removal of Ni(II) Ions from Aqueous Solution. African J. Biotechnol. 2012, 11, 1250–1258. [Google Scholar] [CrossRef]
- Sakulthaew, C.; Chokejaroenrat, C.; Poapolathep, A.; Satapanajaru, T.; Poapolathep, S. Hexavalent Chromium Adsorption from Aqueous Solution Using Carbon Nano-Onions (CNOs). Chemosphere 2017, 184, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, H.; Liu, Y.; Song, S. A Review on Heavy Metal Ions Adsorption from Water by Graphene Oxide and Its Composites. J. Mol. Liq. 2017, 230, 496–504. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of PH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef]
- Singh, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Immobilised Apple Peel Bead Biosorbent for the Simultaneous Removal of Heavy Metals from Cocktail Solution. Cogent Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange Peel as Low-Cost Adsorbent in the Elimination of Cd(II) Ion: Kinetics, Isotherm, Thermodynamic and Optimization Evaluations. Bioresour. Bioprocess. 2020, 7, 34. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Abdel-Satar, A.M. Removal of Some Heavy Metals from Aqueous Solutions Using Natural Wastes Orange Peel Activated Carbon. IJRDO J. Appl. Sci. 2017, 3, 13–30. [Google Scholar]
- Memić, Š.A.M.; Sulejmanović, E.Š.J. Adsorptive Removal of Eight Heavy Metals from Aqueous Solution by Unmodified and Modified Agricultural Waste: Tangerine Peel. Int. J. Environ. Sci. Technol. 2018, 15, 2511–2518. [Google Scholar] [CrossRef]
- Al-Qahtani, K.M. Water Purification Using Different Waste Fruit Cortexes for the Removal of Heavy Metals. J. Taibah Univ. Sci. 2016, 10, 700–708. [Google Scholar] [CrossRef]
- Mallampati, R.; Xuanjun, L.; Adin, A.; Valiyaveettil, S. Fruit Peels as Efficient Renewable Adsorbents for Removal of Dissolved Heavy Metals and Dyes from Water. ACS Sustain. Chem. Eng. 2015, 3, 1117–1124. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, S.; Cao, Y.; Zhong, Q.; Wang, G.; Li, T.; Xu, X. Remediation of Cadmium, Lead and Zinc in Contaminated Soil with CETSA and MA/AA. J. Hazard. Mater. 2019, 366, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.M.S.; de Souza, H.D.P.; Cunha, J.R.M.S. Use of Orange Peel (Citrus sinensis) in the Bioabsorption of Potentially Toxic Metals from Water Resources through ICP-OES. Ciência e Nat. 2020, 42, e16. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Amin, M.N. Absorption Behaviours of Copper, Lead, and Arsenic in Aqueous Solution Using Date Palm Fibres and Orange Peel: Kinetics and Thermodynamics. Polish J. Environ. Stud. 2017, 26, 543–557. [Google Scholar] [CrossRef]
- Lahieb Faisal, M.; Al-Najjar, S.Z.; Al-Sharify, Z.T. Modified Orange Peel as Sorbent in Removing of Heavy Metals from Aqueous Solution. J. Green Eng. 2020, 10, 10600–10615. [Google Scholar]
- Yirga, A.; Werede, Y.; Nigussie, G.; Ibrahim, F. Dried Orange Peel: A Potential Bio Sorbent for Removal of Cu (II) and Cd (II) Ions from Aqueous Solution. Chem. J. 2020, 7, 2581–7507. [Google Scholar]
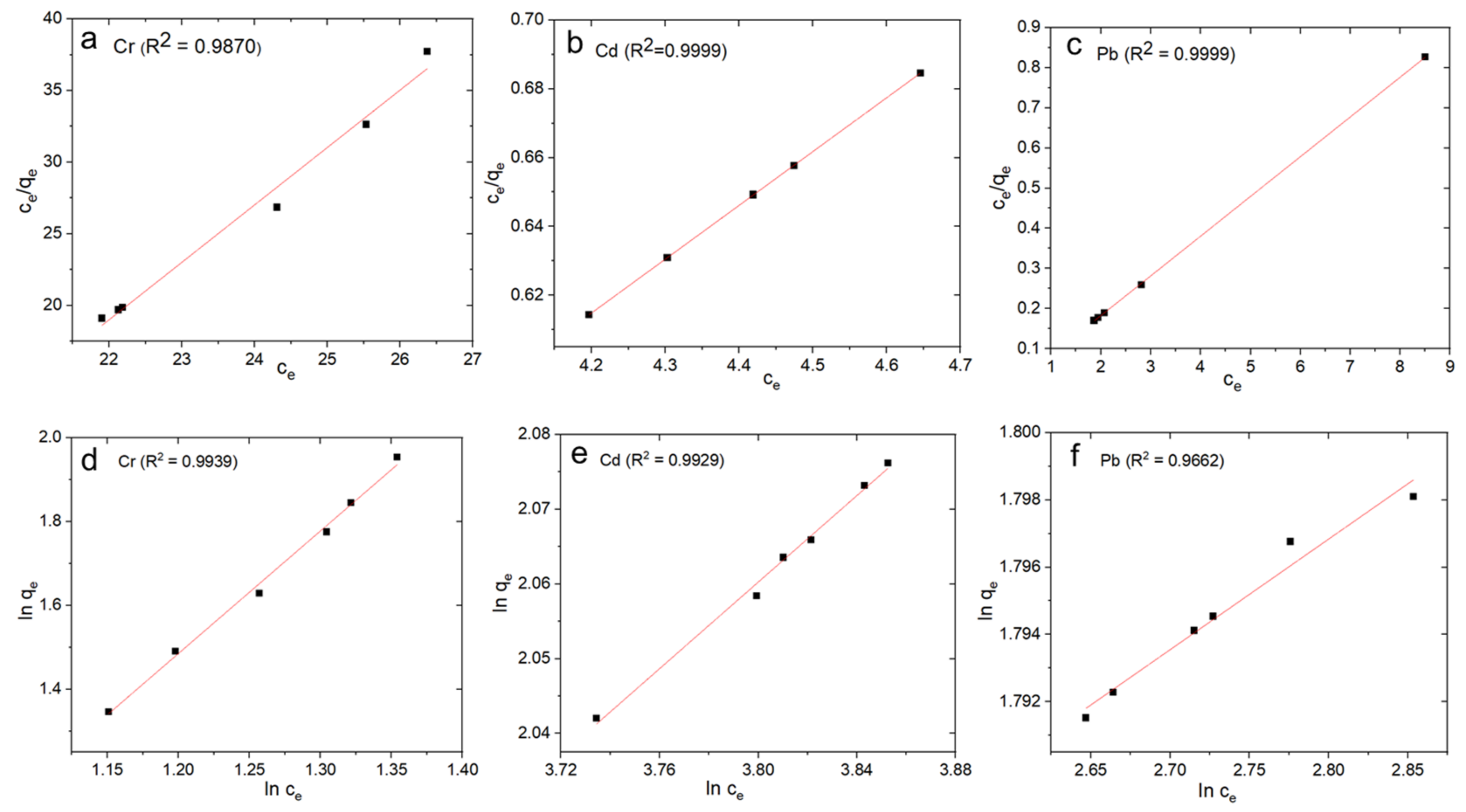
| Models | Parameters | Cr6+ | Pb2+ | Cd2+ |
|---|---|---|---|---|
| Langmuir | qmax (mg g−1) | 4.90 | 50.10 | 29.00 |
| KL(L/mg) | 0.0509 | 0.2015 | 0.2204 | |
| RL | 0.6574 | 0.0013 | 0.0037 | |
| R2 | 0.9879 | 0.9999 | 0.9999 | |
| Freundlich | KF (mg g−1) | 4.18 | 47.46 | 25.79 |
| 1/n | 0.2892 | 0.3291 | 0.2896 | |
| R2 | 0.9939 | 0.9662 | 0.9929 |
| Biosorbents | Cr % | Cd % | Pb % | Reference |
|---|---|---|---|---|
| Orange peel | 66.8 | [62] | ||
| Orange peel | 95.1 | [74] | ||
| Orange peel | 89.6 | 77.2 | 80 | [69] |
| Orange peel | 80 | [60] | ||
| Modified orange peel | 90 | 99 | [59] | |
| Orange peel | 44.42 | [68] | ||
| Orange peel | 85 | [75] | ||
| Orange peel | 64.3 | [45] | ||
| Orange peel | 91 | 98 | [36] | |
| Orange peel | 48.4 | [68] | ||
| Modified orange peel | 91 | [76] | ||
| Dried orange peel | 97.75 | [77] | ||
| Orange peel cellulose | 33.50 | 93.91 | 98.33 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Yoshida, K.; Islam, M.M.; Kobayashi, G. Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent. Sustainability 2023, 15, 4470. https://doi.org/10.3390/su15054470
Rahman A, Yoshida K, Islam MM, Kobayashi G. Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent. Sustainability. 2023; 15(5):4470. https://doi.org/10.3390/su15054470
Chicago/Turabian StyleRahman, Aminur, Kazuhiro Yoshida, Mohammed Monirul Islam, and Genta Kobayashi. 2023. "Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent" Sustainability 15, no. 5: 4470. https://doi.org/10.3390/su15054470
APA StyleRahman, A., Yoshida, K., Islam, M. M., & Kobayashi, G. (2023). Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent. Sustainability, 15(5), 4470. https://doi.org/10.3390/su15054470






