Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil
Abstract
1. Introduction
2. Materials and Methods
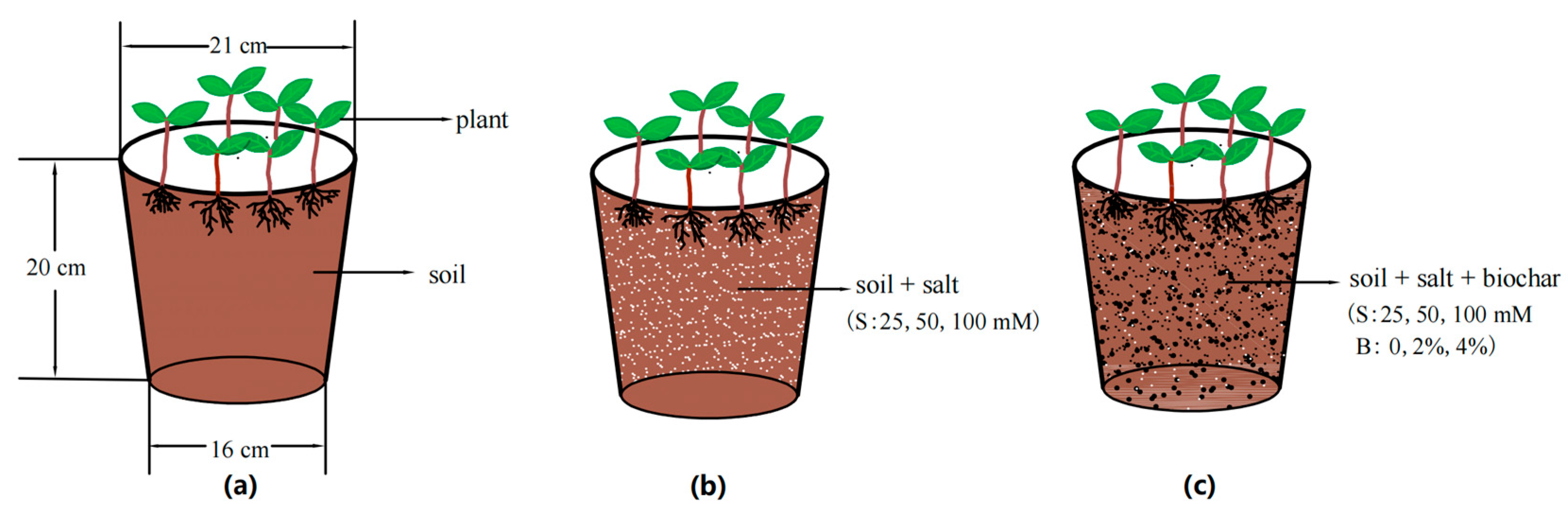
2.1. Experimental Materials and Experimental Designs
2.2. Data Recorded
2.2.1. Determination of Physical and Chemical Properties of the Soil
2.2.2. Determination of Stomatal Indicators in Chinese Cabbage
2.2.3. Measurement of Photosynthetic Indicators in Chinese Cabbage
2.2.4. Yield Measurement
2.3. Statistical Analysis
3. Results
3.1. Na+ and K+ Concentrations in Soil and Plant
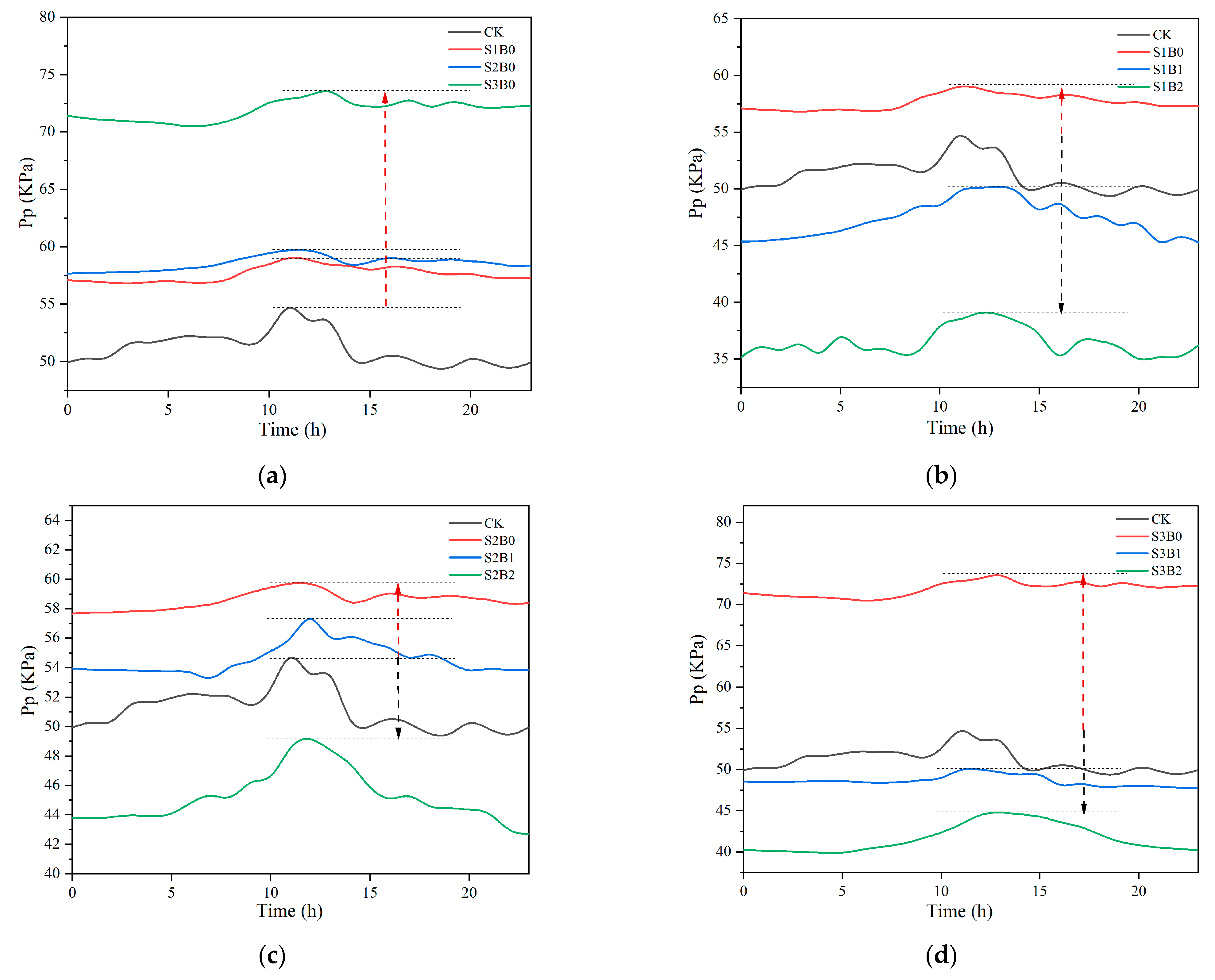
3.2. Soil Water Content and Leaf Pp Value
3.3. Leaf ABA Concentrations
3.4. Leaf Gas Exchange
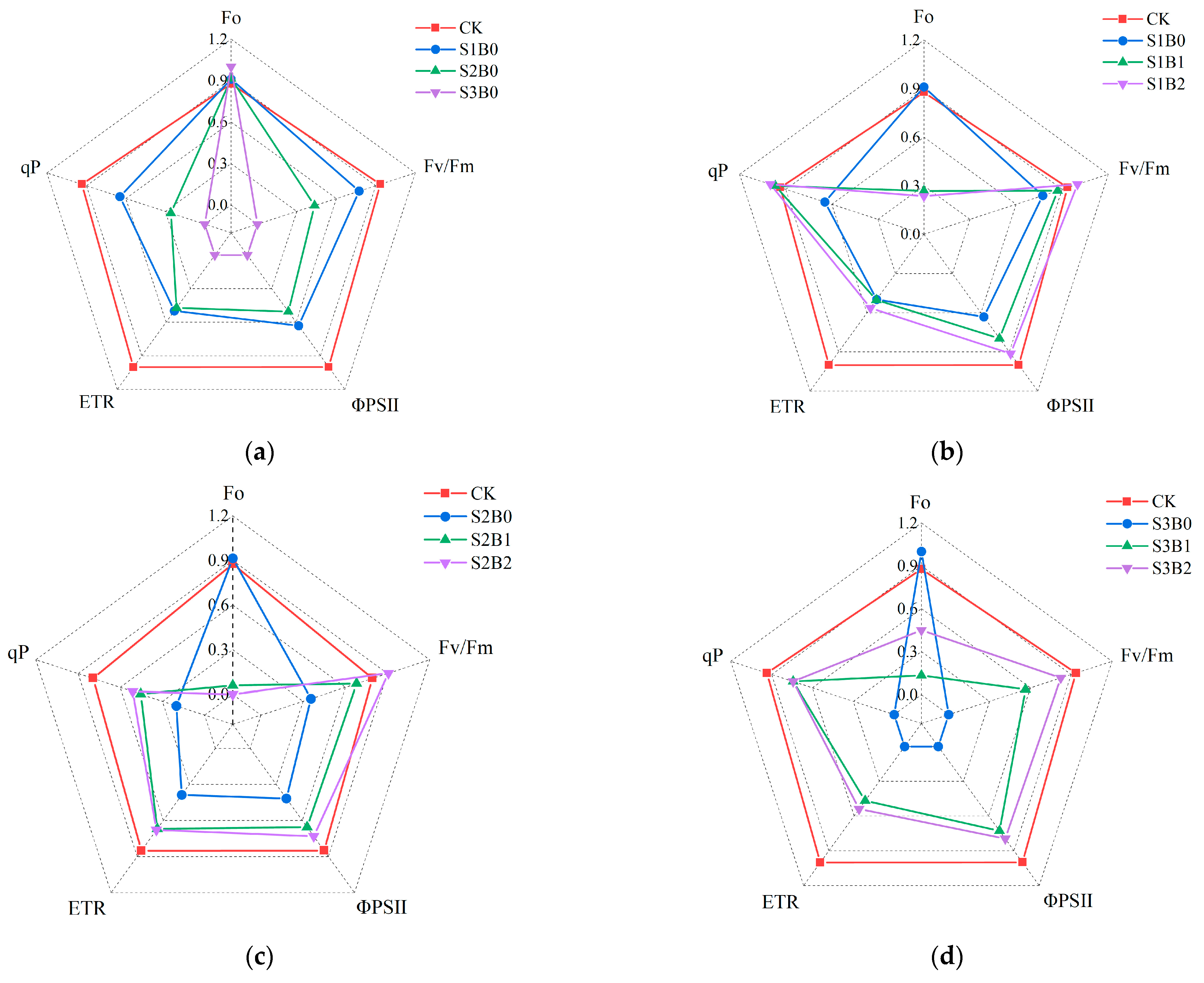
3.5. Chlorophyll Fluorescence
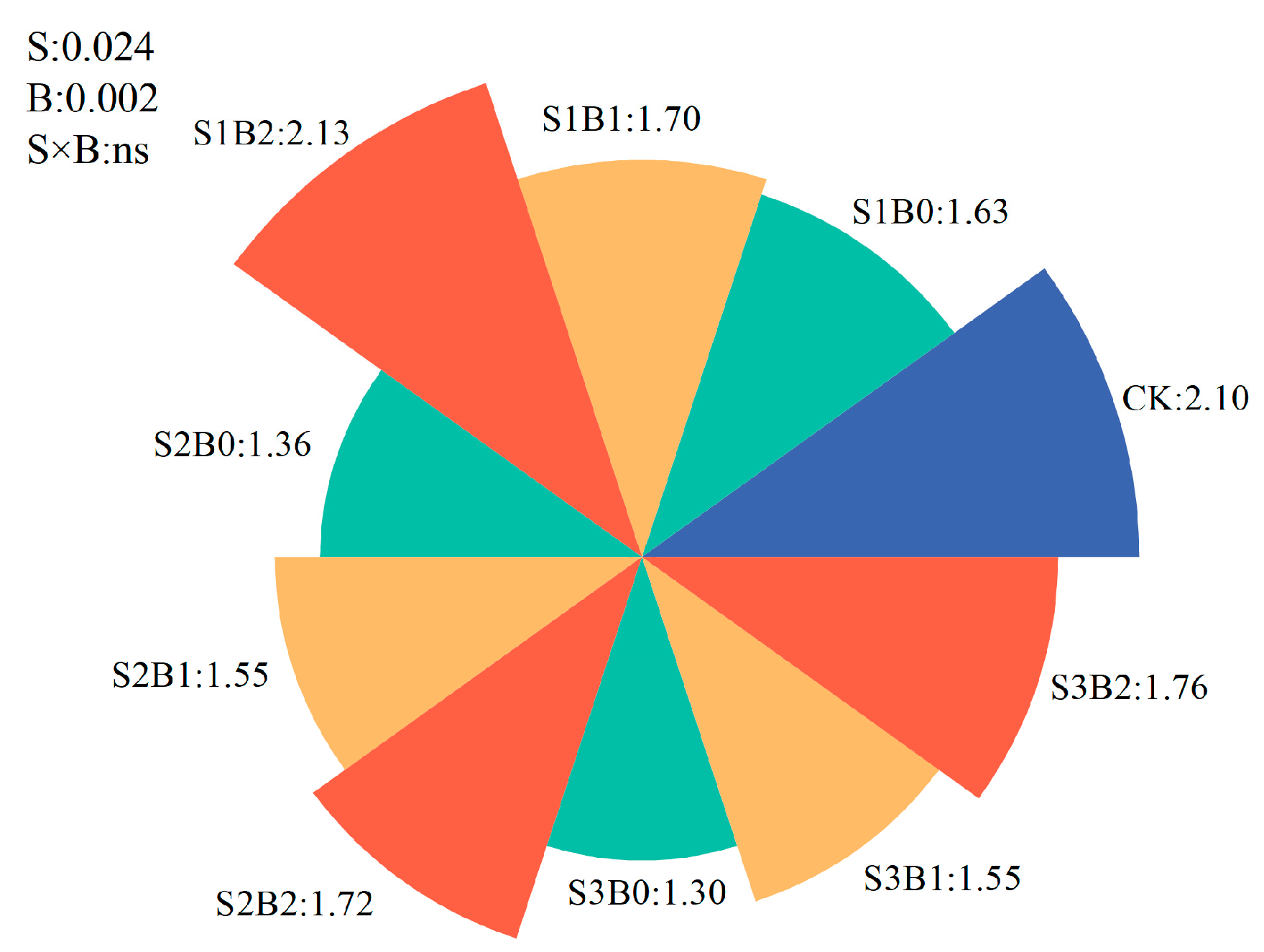
3.6. Plant Yield
3.7. PCA and Multiple Regression Analysis
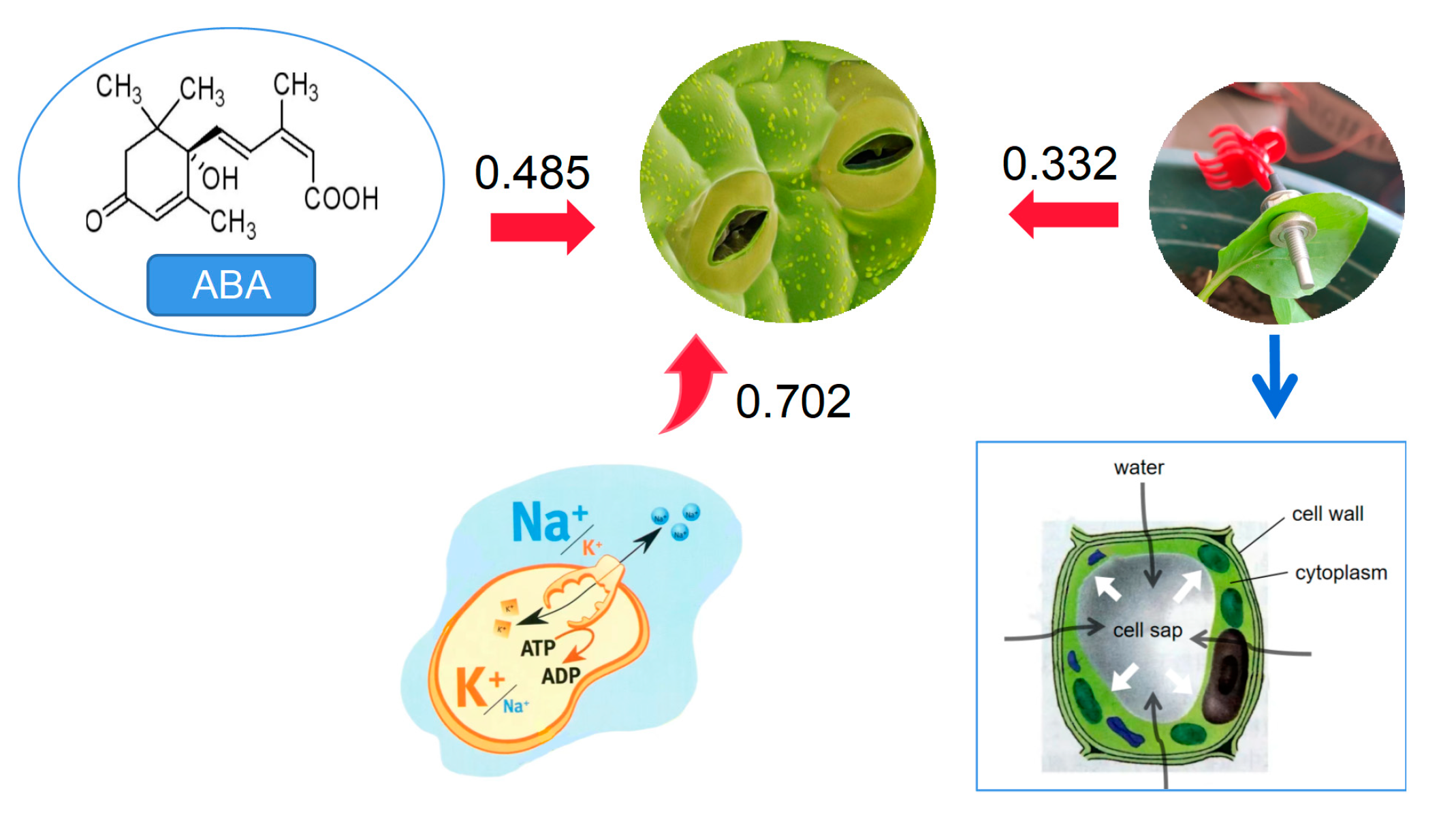
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amami, R.; Ibrahimi, K.; Sher, F.; Milham, P.J.; Khriji, D.; Annabi, H.A.; Abrougui, K.; Chehaibi, S. Effects of conservation and standard tillage on soil physico-chemical properties and overall quality in a semi-arid agrosystem. Soil Res. 2021, 60, 485–496. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Y.; Zhu, X.; Wang, W. Effects of saline-alkali stress on photosynthesis characteristics and antioxidant system in quinoa seedlings leaf. Acta Agrestia Sin. 2021, 29, 1689–1696. [Google Scholar]
- Mancarella, S.; Orsini, F.; Van Oosten, M.J.; Sanoubar, R.; Stanghellini, C.; Kondo, S.; Gianquinto, G.; Maggio, A. Leaf sodium accumulation facilitates salt stress adaptation and reserves photosystem functionality in salt stressed Ocimum basilicum. Environ. Exp. Bot. 2016, 130, 162–173. [Google Scholar] [CrossRef]
- Soothar, M.K.; Mounkaila Hamani, A.K.; Kumar Sootahar, M.; Sun, J.; Yang, G.; Bhatti, S.M.; Traore, A. Assessment of acidic biochar on the growth, physiology and nutrients uptake of maize (Zea mays L.) seedlings under salinity stress. Sustainability 2021, 13, 3150. [Google Scholar] [CrossRef]
- Lamnai, K.; Anaya, F.; Fghire, R.; Zine, H.; Wahbi, S.; Loutfi, K. Impact of Exogenous Application of Salicylic Acid on Growth, Water Status and Antioxidant Enzyme Activity of Strawberry Plants (Fragaria vesca L.) Under Salt Stress Conditions. Gesunde Pflanz. 2021, 73, 465–478. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.; Khojah, E.; Samra, B.N.; Fujita, M.; Nahar, K. Biochar and chitosan regulate antioxidant defense and methylglyoxal detoxification systems and enhance salt tolerance in Jute (Corchorus olitorius L.). Antioxidants 2021, 10, 2017. [Google Scholar] [CrossRef]
- Zhang, N.; Berman, S.R.; Joubert, D.; Vialet-Chabrand, S.; Marcelis, L.; Kaiser, E. Variation of photosynthetic induction in major horticultural crops is mostly driven by differences in stomatal traits. Front. Plant Sci. 2022, 13, 860229. [Google Scholar] [CrossRef]
- Stitt, M.; Borghi, G.L.; Arrivault, S. Targeted metabolite profiling as a top-down approach to uncover interspecies diversity and identify key conserved operational features in the Calvin-Benson cycle. J. Exp. Bot. 2021, 72, 5961–5986. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.; Sher, F.; Jahan, Z.; Malik, U.S.; Khan, M.D.; Americo-Pinheiro, J.H.; Vo, D.N. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ. Chem. Lett. 2022, 20, 2709–2726. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Al-Wabel, M.I.; Ok, Y.S.; Al-Harbi, A.; Wahb-Allah, M.; El-Naggar, A.H.; Ahmad, M.; Al-Faraj, A.; Al-Omran, A. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Parkash, V.; Singh, S. Potential of biochar application to mitigate salinity stress in eggplant. Hortscience 2020, 55, 1946–1955. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, H.; Qiu, R.; Gao, Y.; Duan, A. Role of hydraulic signal and ABA in decrease of leaf stomatal and mesophyll conductance in soil drought-stressed tomato. Front. Plant Sci. 2021, 12, 653186. [Google Scholar] [CrossRef] [PubMed]
- Walter, Z.; Andrea, M. Deep root growth, ABA adjustments and root water uptake response to soil water deficit in giant reed. Ann. Bot. 2019, 124, 605–615. [Google Scholar] [CrossRef]
- Xue, F.; Liu, W.; Cao, H.; Song, L.; Ji, S.; Tong, L.; Ding, R. Stomatal conductance of tomato leaves is regulated by both abscisic acid and leaf water potential under combined water and salt stress. Physiol. Plantarum. 2021, 172, 2070–2078. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Buckley, T.N.; Egea, G.; de Cires, A.; Hernandez-Santana, V.; Martorell, S.; Diaz-Espejo, A. Most stomatal closure in woody species under moderate drought can be explained by stomatal responses to leaf turgor. Plant Cell Environ. 2016, 39, 2014–2026. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment, an adaptive mechanism for coping with plant water deficits. Plant Cell Environ. 2017, 40, 1–3. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The physiological basis of drought tolerance in crop plants: A scenario-dependent probabilistic approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Lee, K.M.; Driever, S.M.; Heuvelink, E.; Rüger, S.; Zimmermann, U.; de Gelder, A.; Marcelis, L.M. Evaluation of diel patterns of relative changes in cell turgor of tomato plants using leaf patch clamp pressure probes. Physiol. Plant. 2012, 146, 439–447. [Google Scholar] [CrossRef]
- Jones, M.M.; Truner, N.C. Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol. 1978, 61, 122–126. [Google Scholar] [CrossRef]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.; Sinclair, G.; Duong, F.; Kluepfel, D.; McElrone, A. Predicting Stomatal closure and turgor loss in woody plants using predawn and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef]
- Bramley, H.; Ehrenberger, W.; Zimmermann, U.; Palta, J.A.; Rueger, S.; Siddique, K. Non-invasive pressure probes magnetically clamped to leaves to monitor the water status of wheat. Plant Soil 2013, 369, 257–268. [Google Scholar] [CrossRef]
- Aissaoui, F.; Chehab, H.; Bader, B.; Ben Salem, A.; M’barki, N.; Laamari, S.; Chihaoui, B.; Mahjoub, Z.; Boujnah, D. Early water stress detection on olive trees (Olea europaea L. cvs ‘chemlali’and ‘Chetoui’) using the leaf patch clamp pressure probe. Comput. Electron. Agr. 2016, 131, 20–28. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Aziz, M.M.; Siddique, K.H.M.; Flower, K.C. Film antitranspirants increase yield in drought stressed wheat plants by maintaining high grain number. Agr. Water Manage. 2015, 159, 11–18. [Google Scholar] [CrossRef]
- Zeiner, M.; Cindric, I.J.; Nemet, I.; Franjkovic, K.; Sondi, B.S. Influence of Soil Salinity on Selected Element Contents in Different Brassica Species. Molecules 2022, 27, 1878. [Google Scholar] [CrossRef]
- Linic, I.; Mlinaric, S.; Brkljacic, L.; Pavlovic, I.; Smikle, A.; Salopek-Sondi, B. Ferulic acid and salicylic acid foliar treatments reduce short-term salt stress in Chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 2022, 10, 2346. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Gao, S.; Lv, Y.; Chen, Z.; Cao, B. Different responses of two Chinese cabbage (Brassica rapa L. ssp. pekinensis) cultivars in photosynthetic characteristics and chloroplast ultrastructure to salt and alkali stress. Planta 2021, 254, 102. [Google Scholar] [CrossRef] [PubMed]
- Leila, M.; Mohammad, M.; Amir, L. Alleviating negative effects of salinity stress in summer savory (Satureja hortensis L.) by biochar application. Acta Physiol. Plant 2019, 41, 98. [Google Scholar] [CrossRef]
- Khadiga, A.; Abdullah, A. Alleviation of salinity induced growth and photosynthetic decline in wheat due to biochar and jasmonic acid application involves up-regulation of ascorbate-glutathione pathway, glyoxylase system and secondary metabolite accumulation. Rhizosphere 2022, 24, 100603. [Google Scholar] [CrossRef]
- Alfadil, A.A.; Xia, J.; Hiba, S.; Yousef, A.H.; Jamal, N.I.; Amar, A.A.H. Wheat straw biochar application improves the morphological, physiological, and yield attributes of maize and the physicochemical properties of soil under deficit irrigation and salinity stress. J. Plant Nutr. 2021, 44, 2399–2420. [Google Scholar] [CrossRef]
- Gong, Z.; Xin, J.; Shang, X.; Guo, X. Photosynthetic tolerance physiology of Toona sinensis seedlings under saline-alkali stress. Acta Bot. Boreal.-Occident. Sin. 2021, 41, 1199–1209. [Google Scholar]
- Liu, M.; Zhao, Y.; Liu, X.; Korpelainen, H.; Li, C. Ammonium and nitrate affect sexually different responses to salt stress in Populus cathayana. Physiol. Plant. 2022, 174, e13626. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Bitter, R.; Marchiori, P.; Ruger, S. A non-invasive plant-based probe for continuous monitoring of water stress in real time: A new tool for irrigation scheduling and deeper insight into drought and salinity stress physiology. Theor. Exp. Plant Phys. 2013, 25, 2–11. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Li, Y.; Quan, R.; Wang, J.; Huang, R.; Qin, H. Salt stress promotes abscisic acid accumulation to affect cell proliferation and expansion of primary roots in rice. Int. J. Mol. Sci. 2021, 22, 10892. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, nanosilicon, and biochar applications improve potato salt tolerance by modulating photosynthesis, water status, and biochemical constituents. Sustainability 2022, 14, 723. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. R. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.D.F.; Abdelaal, K.A.A.; Al Husnain, L.; AlGwaiz, H.I.M.; Hafez, Y.M. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Bioch. 2021, 166, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Lashari, M.S.; Ye, Y.; Ji, H.; Li, L.; Kibue, G.W.; Lu, H.; Zhang, J. Biochar–manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. J. Sci. Food Agric. 2015, 95, 1321–1327. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Daniel, M.; Sergio, T. Abscisic acid mediates drought and salt stress responses in Vitis vinifera—A review. Int. J. Mol. Sci. 2020, 21, 8648. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Huber, A.E.; Melcher, P.J.; Piñeros, M.A.; Setter, T.L.; Bauerle, T.L. Signal coordination before, during, and after stomatal closure in response to drought stress. New Phytol. 2019, 224, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.; AL-Huqail, A.A. Sustainable biochar and/or melatonin improve salinity tolerance in borage plants by modulating osmotic adjustment, antioxidants, and ion homeostasis. Plants 2022, 11, 765. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Fariba, R.; Hamidreza, O.; Mahdi, N.; Ebrahim, A. Effect of biochar on potassium fractions and plant-available P, Fe, Zn, Mn and Cu concentrations of calcareous soils. Arid Land Res. Manag. 2021, 36, 1–26. [Google Scholar] [CrossRef]
- Bilias, F.; Kalderis, D.; Richardson, C.; Barbayiannis, N.; Gasparatos, D. Biochar application as a soil potassium management strategy: A review. Sci. Total Environ. 2023, 858, 159782. [Google Scholar] [CrossRef] [PubMed]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sanchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Ag 2020, 7, 15. [Google Scholar] [CrossRef]
- Nong, W.; Shao, G.; Wu, S.; Cui, J. Effects of biochar on soil salt distribution and aggregate structure under Brackish water irrigation. China Rural. Water Hydropower 2022, 6, 154–161+168. [Google Scholar]
- Nguyen, B.T.; Dinh, G.D.; Nguyen, T.X.; Nguyen, D.T.P.; Vu, T.N.; Do, D.D. The potential of biochar to ameliorate the major constraints of acidic and salt-affected soils. J. Soil Sci. Plant Nut. 2022, 22, 1340–1350. [Google Scholar] [CrossRef]
- Yan, S.; Gao, Y.; Tian, M.; Tian, Y.; Li, J. Comprehensive evaluation of effects of various carbon-rich amendments on tomato production under continuous saline water irrigation: Overall soil quality, plant nutrient uptake, crop yields and fruit quality. Agr. Water Manag. 2021, 255, 106995. [Google Scholar] [CrossRef]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial effects of biochar and chitosan on antioxidative capacity, osmolytes accumulation, and anatomical characters of water-stressed barley plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotox. Environ. Safe. 2017, 137, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W. The γ-Aminobutyric Acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Dourado, P.R.M.; de Souza, E.R.; Santos, M.A.d.; Lins, C.M.T.; Monteiro, D.R.; Paulino, M.K.S.S.; Schaffer, B. Stomatal Regulation and Osmotic Adjustment in Sorghum in Response to Salinity. Agriculture 2022, 12, 658. [Google Scholar] [CrossRef]
- Farooq, M.; Rehman, A.; Al Alawi, A.K.M.; Al-Busaidi, W.M.; Lee, D.J. Integrated use of seed priming and biochar improves salt tolerance in cowpea. Sci. Hortic. 2020, 272, 109507. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef]
- Peccoux, A.; Loveys, B.; Zhu, J.; Gambetta, G.A.; Delrot, S.; Vivin, P.; Schultz, H.P.; Ollat, N.; Dai, Z. Dissecting the rootstock control of scion transpiration using model-assisted analyses in grapevine. Tree Physiol. 2018, 38, 1026–1040. [Google Scholar] [CrossRef]
- Ehrenberger, W.; Rüger, S.; Fitzke, R.; Vollenweider, P.; Guenthardt-Goerg, M.; Kuster, T.; Zimmermann, U.; Arend, M. Concomitant dendrometer and leaf patch pressure probe measurements reveal the effect of microclimate and soil moisture on diurnal stem water and leaf turgor variations in young oak trees. Funct. Plant Biol. 2012, 39, 297–305. [Google Scholar] [CrossRef]
- Ache, P.; Bauer, H.; Kollist, H.; Al-Rasheid, K.; Lautner, S.; Hartung, W.; Hedrich, R. Stomatal action directly feeds back on leaf turgor: New insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 2010, 62, 1072–1082. [Google Scholar] [CrossRef]
- Liao, Q.; Gu, S.; Kang, S.; Du, T.; Tong, L.; Wood, J.D.; Ding, R. Mild water and salt stress improve water use efficiency by decreasing stomatal conductance via osmotic adjustment in field maize. Sci. Total Environ. 2021, 805, 150364. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 360, 114055. [Google Scholar] [CrossRef]
- Ibrahimi, K.; Attia, K.B.; Amami, R.; Américo-Pinheiro, J.H.P.; Sher, F. Assessment of three decades treated wastewater impact on soil quality in semi-arid agroecosystem. J. Saudi Soc. Agric. Sci. 2022, 21, 525–535. [Google Scholar] [CrossRef]


| Soil Parameters | Value | |
|---|---|---|
| Volume weight (g·cm−3) | 1.35 | |
| Field water holding capacity (%) | 28.8 | |
| Total porosity (%) | 16.78 | |
| Organic matter (g·kg−1) | 13.42 | |
| pH | 8.15 | |
| Soil machinery composition | Grit 2 to 0.02 mm | 84.05 |
| Powdered sand grains 0.02 to 0.002 mm | 14.20 | |
| Viscous particles < 0.002 mm | 1.75 | |
| Treatments | Soil | Plant | |||
|---|---|---|---|---|---|
| Na+ (mg·g–1) | K+ (mg·g–1) | Na+ (mg·g–1) | K+ (mg·g–1) | Na+/K+ | |
| CK | 11.68 ± 1.73 bc | 22.52 ± 3.17 a | 17.20 ± 3.44 c | 38.13 ± 9.60 a | 0.47 ± 0.03 d |
| S1 B0 | 13.43 ± 1.49 abc | 23.31 ± 2.65 a | 30.39 ± 7.14 bc | 35.95 ± 0.54 ab | 0.85 ± 0.21 cd |
| S1 B1 | 13.21 ± 1.00 bc | 23.44 ± 3.57 a | 30.91 ± 7.77 bc | 41.37 ± 7.28 a | 0.75 ± 0.13 d |
| S1 B2 | 14.35 ± 0.40 abc | 24.52 ± 0.33 a | 25.09 ± 3.13 c | 43.74 ± 4.17 a | 0.59 ± 0.11 d |
| S2 B0 | 13.21 ± 0.75 abc | 23.66 ± 2.73 a | 62.57 ± 0.05 a | 36.54 ± 0.76 ab | 1.71 ± 0.04 bc |
| S2 B1 | 11.70 ± 1.11 bc | 23.95 ± 3.38 a | 62.51 ± 8.66 a | 38.18 ± 4.65 a | 1.73 ± 0.41 bc |
| S2 B2 | 16.26 ± 1.02 a | 24.29 ± 0.97 a | 61.26 ± 5.74 a | 43.63 ± 2.13 a | 1.41 ± 0.14 bcd |
| S3 B0 | 13.53 ± 0.15 abc | 23.39 ± 4.09 a | 64.61 ± 3.67 a | 23.65 ± 4.79 b | 2.96 ± 0.58 a |
| S3 B1 | 10.75 ± 1.30 c | 23.67 ± 0.75 a | 62.35 ± 5.55 a | 31.57 ± 1.11 ab | 2.05 ± 0.24 ab |
| S3 B2 | 15.13 ± 2.54 ab | 23.71 ± 3.92 a | 47.64 ± 8.92 ab | 33.10 ± 3.68 ab | 1.42 ± 0.15 bcd |
| p values | |||||
| S | ns | ns | 0.00 | 0.03 | 0.00 |
| B | 0.01 | ns | ns | ns | 0.04 |
| S × B | ns | ns | ns | ns | ns |
| Principal Components | PC1 | PC2 | PC3 | PC4 | ||||
|---|---|---|---|---|---|---|---|---|
| Biochar | N | Y | N | Y | N | Y | N | Y |
| Total eigenvalue | 3.034 | 2.412 | 1.733 | 1.888 | 1.458 | 1.398 | 1.063 | 1.009 |
| Percent (%) of variance | 37.927 | 30.145 | 21.662 | 23.594 | 18.221 | 17.471 | 13.282 | 12.612 |
| Cumulative (%) | 37.927 | 30.145 | 59.589 | 53.739 | 77.811 | 71.211 | 91.012 | 83.822 |
| Eigen vectors | ||||||||
| ΦPSⅡ | −0.862 | −0.448 | 0.278 | −0.561 | −0.256 | 0.406 | 0.030 | 0.341 |
| gs | −0.907 | 0.596 | 0.042 | 0.481 | −0.160 | −0.160 | 0.083 | 0.432 |
| Pp | 0.732 | 0.520 | −0.473 | −0.593 | 0.032 | −0.466 | 0.104 | −0.124 |
| ABA | 0.518 | 0.506 | −0.290 | 0.323 | −0.602 | 0.225 | −0.055 | 0.590 |
| Na+/K+ | −0.040 | −0.357 | −0.118 | 0.538 | 0.725 | 0.663 | −0.593 | −0.221 |
| Na+ (s) | 0.527 | 0.618 | 0.791 | −0.452 | −0.269 | 0.545 | −0.04 | −0.228 |
| K+ (s) | 0.476 | 0.782 | 0.840 | −0.238 | 0.242 | 0.442 | 0.006 | −0.069 |
| SWC | 0.045 | 0.457 | −0.027 | 0.578 | 0.479 | −0.086 | 0.829 | −0.487 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Zheng, L.; Zhao, J.; Ma, J.; Li, X. Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil. Sustainability 2023, 15, 4206. https://doi.org/10.3390/su15054206
Chen R, Zheng L, Zhao J, Ma J, Li X. Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil. Sustainability. 2023; 15(5):4206. https://doi.org/10.3390/su15054206
Chicago/Turabian StyleChen, Ruixia, Lijian Zheng, Jinjiang Zhao, Juanjuan Ma, and Xufeng Li. 2023. "Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil" Sustainability 15, no. 5: 4206. https://doi.org/10.3390/su15054206
APA StyleChen, R., Zheng, L., Zhao, J., Ma, J., & Li, X. (2023). Biochar Application Maintains Photosynthesis of Cabbage by Regulating Stomatal Parameters in Salt-Stressed Soil. Sustainability, 15(5), 4206. https://doi.org/10.3390/su15054206







