Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. HPLC-High Resolution Mass Spectrometry (HPLC-HRMS)
2.3. HRMS Workflow Untargeted Metabolomics Approach
2.4. Evaluation of Antioxidant Properties (ABTS Assay)
2.5. Growth Inhibition Assay against Escherichia coli
2.6. Determination of Minimal Inhibitory Concentration (MICs)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Molecules with Antioxidant Properties
3.2. Evaluation of Functional Properties
3.2.1. Antioxidant Activity
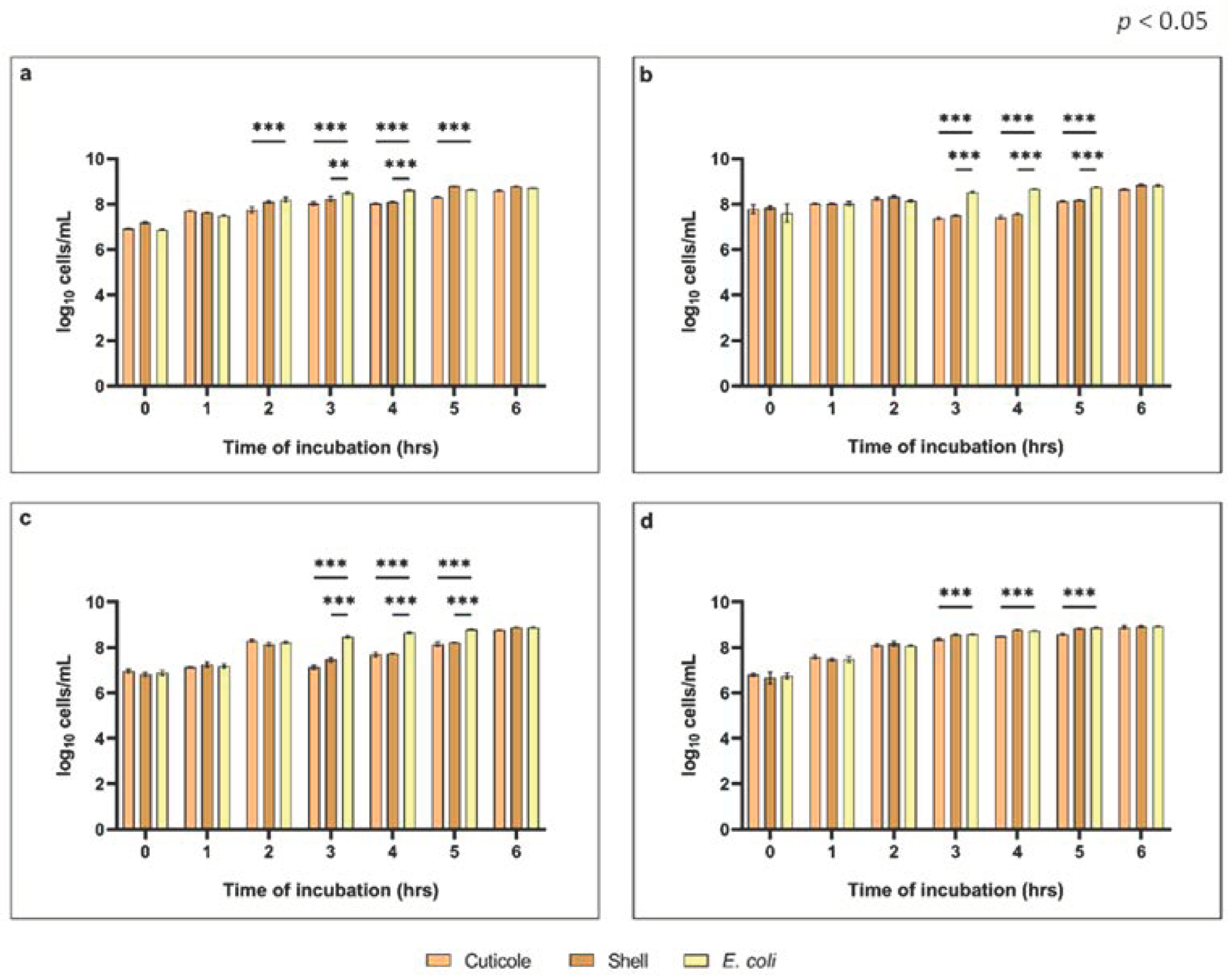
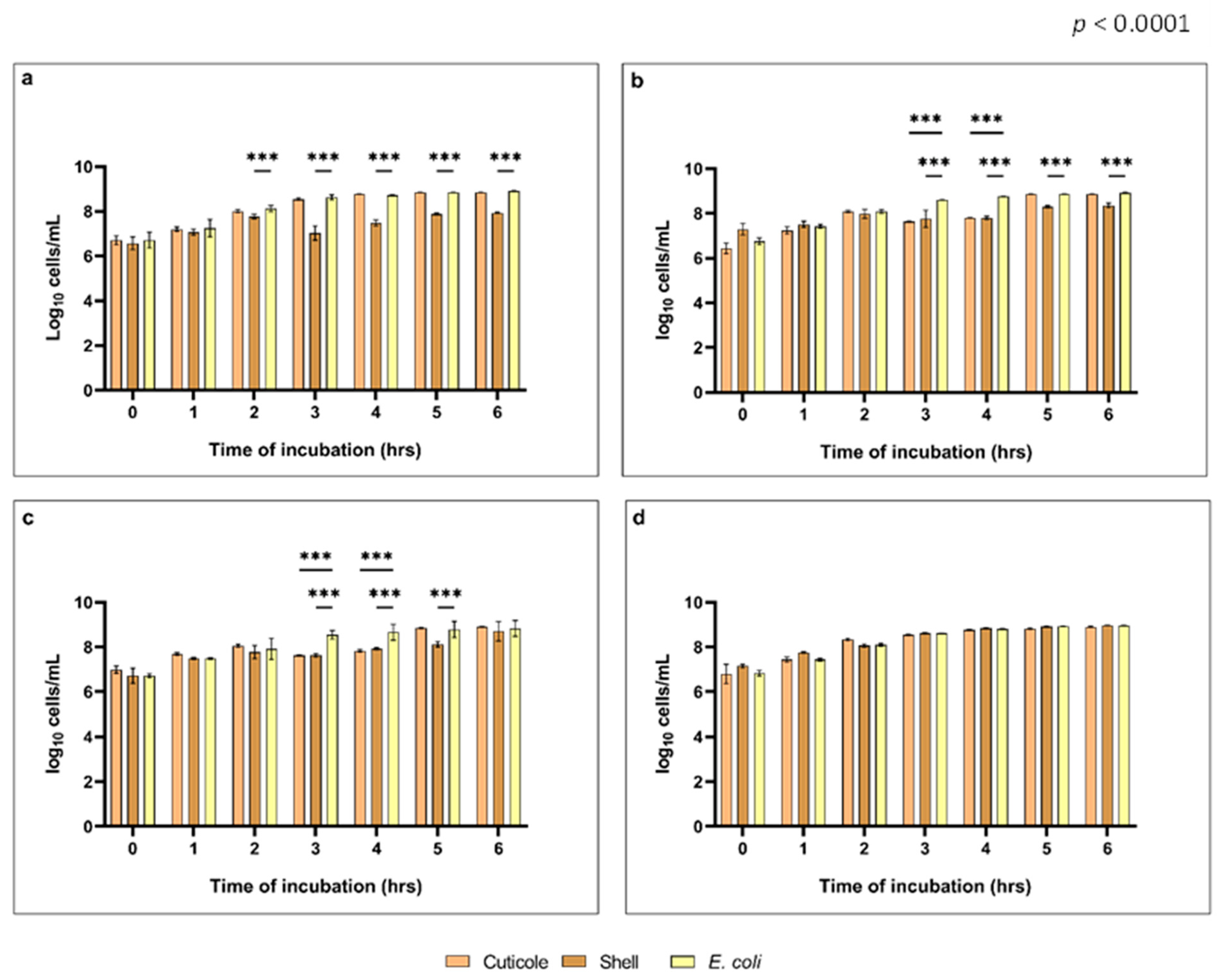
3.2.2. Evaluation of the Growth Inhibitory Activity against Verocytotoxic Escherichia coli and Determination of Minimal Inhibitory Concentrations (MICs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellen MacArthur Circular Economy. Available online: https://ellenmacarthurfoundation.org/topics/circular-economy-introduction/overview (accessed on 21 December 2022).
- McCarthy, B.; Kapetanaki, A.B.; Wang, P. Circular Agri-Food Approaches: Will Consumers Buy Novel Products Made from Vegetable Waste? Rural. Soc. 2019, 28, 91–107. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Melini, V.; Melini, F.; Luziatelli, F.; Ruzzi, M. Functional Ingredients from Agri-Food Waste: Effect of Inclusion Thereof on Phenolic Compound Content and Bioaccessibility in Bakery Products. Antioxidants 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of Feeding Plant By-Products: A Review of the Implications for Ruminant Meat Production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and Quebracho Tannins in Pig Nutrition: The Effects on Performance and Intestinal Health. Animal 2021, 15, 100064. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Reggi, S.; Caprarulo, V.; Hejna, M.; Sgoifo Rossi, C.; Callegari, M.; Baldi, A.; Rossi, L. Evaluation of Tannin Extracts, Leonardite and Tributyrin Supplementation on Diarrhoea Incidence and Gut Microbiota of Weaned Piglets. Animals 2021, 11, 1693. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural Food Additives: Quo Vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Nikmaram, N.; Budaraju, S.; Barba, F.J.; Lorenzo, J.M.; Cox, R.B.; Mallikarjunan, K.; Roohinejad, S. Application of Plant Extracts to Improve the Shelf-Life, Nutritional and Health-Related Properties of Ready-to-Eat Meat Products. Meat. Sci. 2018, 145, 245–255. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Santos, M.J.M.C.; Silva, L.K.R.; Pereira, L.C.L.; Santos, I.A.; da Silva Lannes, S.C.; da Silva, M.V. Natural Antioxidants Used in Meat Products: A Brief Review. Meat. Sci. 2019, 148, 181–188. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C. da S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780429211645. [Google Scholar]
- Lelli, V.; Molinari, R.; Merendino, N.; Timperio, A.M. Detection and Comparison of Bioactive Compounds in Different Extracts of Two Hazelnut Skin Varieties, Tonda Gentile Romana and Tonda Di Giffoni, Using a Metabolomics Approach. Metabolites 2021, 11, 296. [Google Scholar] [CrossRef]
- Bener, M.; Şen, F.B.; Önem, A.N.; Bekdeşer, B.; Çelik, S.E.; Lalikoglu, M.; Aşçı, Y.S.; Capanoglu, E.; Apak, R. Microwave-Assisted Extraction of Antioxidant Compounds from by-Products of Turkish Hazelnut (Corylus avellana L.) Using Natural Deep Eutectic Solvents: Modeling, Optimization and Phenolic Characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Aborehab, N.M.; Al-Karmalawy, A.A.; Fernie, A.R.; Alseekh, S.; Ezzat, S.M. Potential Valorization of Edible Nuts By-Products: Exploring the Immune-Modulatory and Antioxidants Effects of Selected Nut Shells Extracts in Relation to Their Metabolic Profiles. Antioxidants 2022, 11, 462. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yılmaz, C.; Durmaz, G.; Gökmen, V. Hazelnut Skin Powder: A New Brown Colored Functional Ingredient. Food Res. Int. 2014, 65, 291–297. [Google Scholar] [CrossRef]
- Demirbas, A. Furfural Production from Fruit Shells by Acid-Catalyzed Hydrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 157–165. [Google Scholar] [CrossRef]
- del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic Composition of Hazelnut Skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef]
- Yuan, B.; Lu, M.; Eskridge, K.M.; Hanna, M.A. Valorization of Hazelnut Shells into Natural Antioxidants by Ultrasound-Assisted Extraction: Process Optimization and Phenolic Composition Identification. J. Food Process Eng. 2018, 41, e12692. [Google Scholar] [CrossRef]
- Frazzini, S.; Scaglia, E.; Dell’Anno, M.; Reggi, S.; Panseri, S.; Giromini, C.; Lanzoni, D.; Sgoifo Rossi, C.A.; Rossi, L. Antioxidant and Antimicrobial Activity of Algal and Cyanobacterial Extracts: An In Vitro Study. Antioxidants 2022, 11, 992. [Google Scholar] [CrossRef]
- Leoni, V.; Giupponi, L.; Pavlovic, R.; Gianoncelli, C.; Cecati, F.; Ranzato, E.; Martinotti, S.; Pedrali, D.; Giorgi, A.; Panseri, S. Multidisciplinary Analysis of Italian Alpine Wildflower Honey Reveals Criticalities, Diversity and Value. Sci. Rep. 2021, 11, 19316. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut Shells (Italian Cultivar “Marrone Di Roccadaspide” PGI): Antioxidant Activity and Chemical Investigation with in Depth LC-HRMS/MSn Rationalization of Tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef] [PubMed]
- Dell’Anno, M.; Sotira, S.; Rebucci, R.; Reggi, S.; Castiglioni, B.; Rossi, L. In Vitro Evaluation of Antimicrobial and Antioxidant Activities of Algal Extracts. Ital. J. Anim. Sci. 2020, 19, 103–113. [Google Scholar] [CrossRef]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving Accuracy of Cell and Chromophore Concentration Measurements Using Optical Density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.H.; Lyne, P.M.; Grange, J. Collins and Lyne’s Microbiological Methods; Butterworth-Heinemann: Oxford, UK, 1995; Volume 493. [Google Scholar]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Bioactive Compounds in Different Hazelnut Varieties and Their Skins. J. Food Compos. Anal. 2015, 43, 203–208. [Google Scholar] [CrossRef]
- Masullo, M.; Cerulli, A.; Mari, A.; de Souza Santos, C.C.; Pizza, C.; Piacente, S. LC-MS Profiling Highlights Hazelnut (Nocciola Di Giffoni PGI) Shells as a Byproduct Rich in Antioxidant Phenolics. Food Res. Int. 2017, 101, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, M.; Valenti, B.; Luciano, G.; Priolo, A.; Rapisarda, T.; Belvedere, G.; Marino, V.M.; Esposto, S.; Taticchi, A.; Servili, M.; et al. Hazelnut as Ingredient in Dairy Sheep Diet: Effect on Sensory and Volatile Profile of Cheese. Front. Nutr. 2019, 6, 125. [Google Scholar] [CrossRef]
- Natalello, A.; Luciano, G.; Morbidini, L.; Valenti, B.; Pauselli, M.; Frutos, P.; Biondi, L.; Rufino-Moya, P.J.; Lanza, M.; Priolo, A. Effect of Feeding Pomegranate Byproduct on Fatty Acid Composition of Ruminal Digesta, Liver, and Muscle in Lambs. J. Agric. Food Chem. 2019, 67, 4472–4482. [Google Scholar] [CrossRef]
- Dong, J.-W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.-T. Re-Evaluation of ABTS•+ Assay for Total Antioxidant Capacity of Natural Products. Nat. Prod. Commun. 2015, 10, 1934578X1501001. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.-M.; Mocan, A.; Crișan, G.; Vlase, L.; Loghin, F.; Popa, D.-S.; Tomuta, I. Enhanced Recovery of Antioxidant Compounds from Hazelnut (Corylus avellana L.) Involucre Based on Extraction Optimization: Phytochemical Profile and Biological Activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Daniela, G.; Simona, P.; Giuseppe, Z.; Vincenzo, G. Phenolic Acid Profile and Antioxidant Capacity of Hazelnut (Corylus avellana L.) Kernels in Different Solvent Systems. J. Food Nutr. Res. 2010, 49, 195–205. [Google Scholar]
- Contini, M.; Baccelloni, S.; Massantini, R.; Anelli, G. Extraction of Natural Antioxidants from Hazelnut (Corylus avellana L.) Shell and Skin Wastes by Long Maceration at Room Temperature. Food Chem. 2008, 110, 659–669. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, Separation, and Detection Methods for Phenolic Acids and Flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Cacace, J.E.; Mazza, G. Extraction of Anthocyanins and Other Phenolics from Black Currants with Sulfured Water. J. Agric. Food Chem. 2002, 50, 5939–5946. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L. Bioactive Properties and Chemical Composition of Six Walnut (Juglans regia L.) Cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Smith, A.H.; Sundset, M.A.; Mackie, R.I. The BaeSR Two-Component Regulatory System Mediates Resistance to Condensed Tannins in Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 535–539. [Google Scholar] [CrossRef]
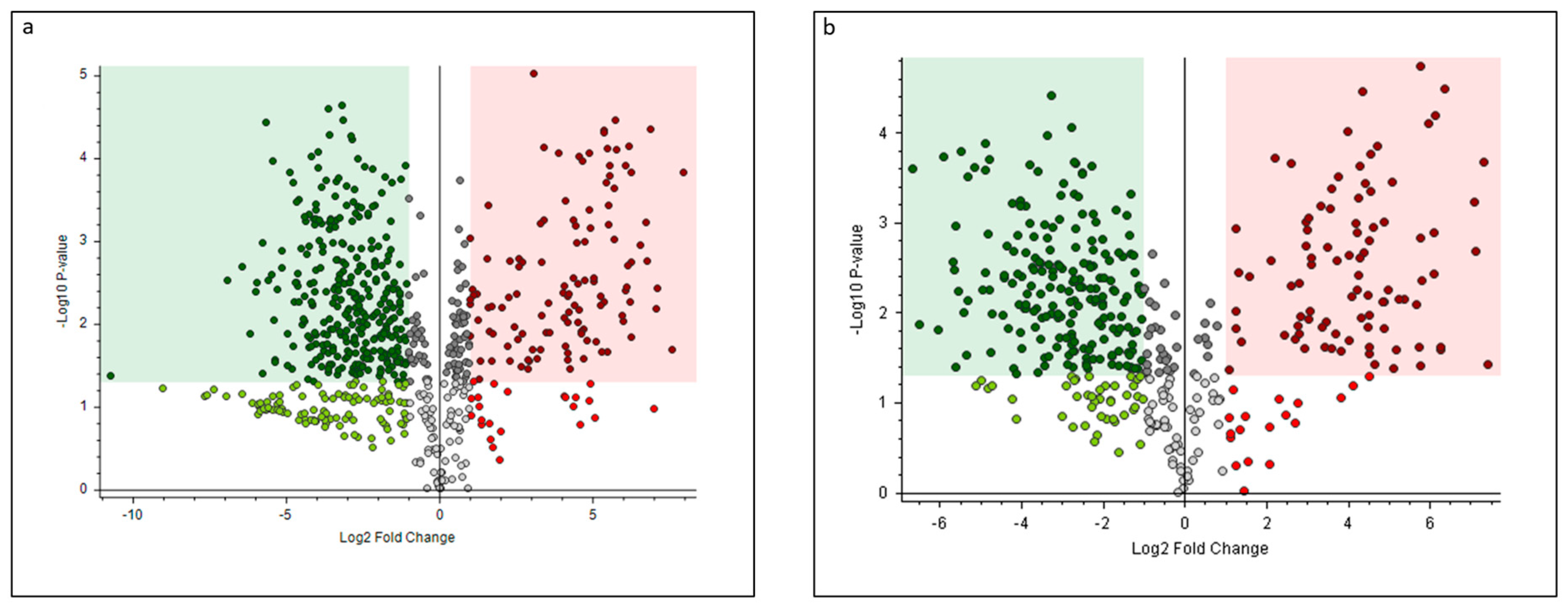
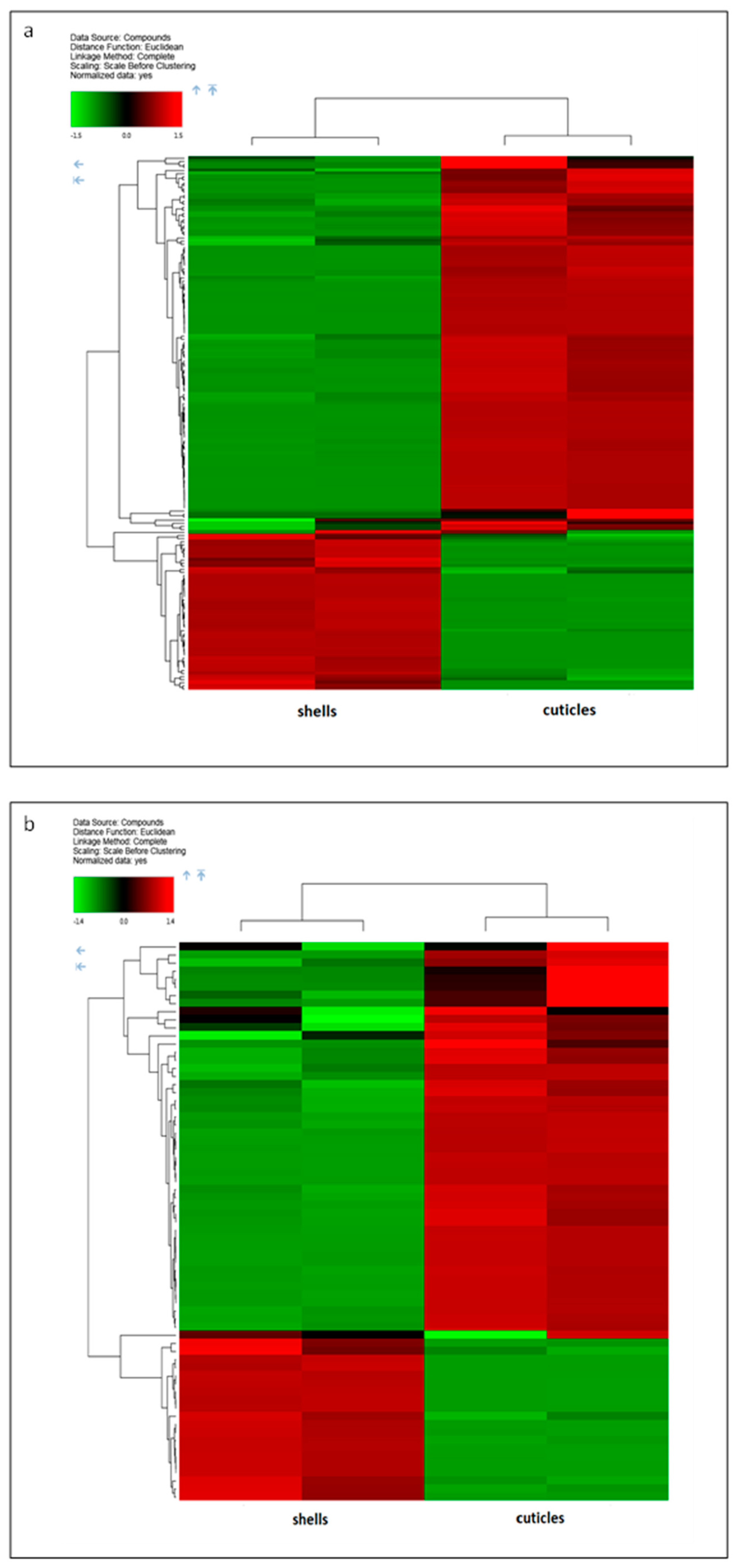

| Class | Name | Shells vs. Cuticles | ||
|---|---|---|---|---|
| Log2 Fold Change | p-Value | Regulation | ||
| Lignan | Lariciresinol 4-o-glucoside | −2.76 | 0.0002 | down |
| Cycloolivil | 5.55 | 0.0002 | up | |
| Dihydroconiferin | −2.91 | 0.0225 | down | |
| Pyranones | Dihydrokavain | 0.85 | 0.0032 | up |
| Amino acids and metabolites | Citrulline | −3.27 | 0.0034 | down |
| Glutamic acid | −2.66 | 0.0004 | down | |
| Isoleucine | −2.59 | 0.0020 | down | |
| Tryptophan | −4 | 0.0004 | down | |
| Arginine | −4.64 | 0.0549 | down | |
| Aspartic acid | −1.12 | 0.1216 | down | |
| Asparagine | −4.08 | 0.1099 | down | |
| Alpha-ketoglutaric acid | −0.3 | 0.1150 | ns | |
| O-heptanoylcarnitine | −4.38 | 0.1412 | down | |
| Propionylcarnitine | −4.56 | 0.1450 | down | |
| Kynurenic acid | −2.76 | 0.0398 | down | |
| Anisoles | 2-[4-(3-hydroxypropyl)-2-methoxyphenoxy]-1,3-propanediol | −0.22 | 0.1818 | ns |
| Vanillin | 4.21 | 0.0224 | up | |
| Homovanillic acid | 4.22 | 0.0073 | up | |
| Anthracenecarboxylic acids | Carminic acid | −6.92 | 0.0030 | down |
| Aurone flavonoids | Aureusidin 6-glucuronide | −5.77 | 0.0010 | down |
| Bractein | −2.77 | 0.0048 | down | |
| 2,6,3’,4’-Tetrahydroxy-2-benzylcoumaranone | −4.17 | 0.0185 | down | |
| Nigrescin | −1.9 | 0.0392 | down | |
| Castillene B | 2.7 | 0.0018 | up | |
| Amaronol A | 2.31 | 0.0017 | up | |
| Azaspirodecane derivatives | Petasitenine | 0.66 | 0.0502 | ns |
| Benzoquinones | 5-o-methylembelin | −0.75 | 0.4685 | ns |
| Carbohydrates and derivatives | Trehalose | −4.19 | 0.0310 | down |
| Hexose | 1.22 | 0.0043 | up | |
| Saccharic acid | −0.8 | 0.0117 | down | |
| Gluconic acid | −0.71 | 0.0098 | down | |
| Mannitol | −0.96 | 0.0133 | down | |
| Xylitol | −0.35 | 0.1614 | ns | |
| Paeonolide | −3.06 | 0.0144 | down | |
| Chalcones and dihydrochalcones | Pseudosindorin | −3.49 | 0.0007 | down |
| Lusianin | 2.25 | 0.0662 | ns | |
| Desmosdumotin C | −0.3 | 0.0740 | ns | |
| Hamilcone | 4.73 | 0.0264 | up | |
| 2’,6’-Dihydroxy-4’-methoxy-3’-prenyldihydrochalcone | −3.45 | 0.0057 | down | |
| 2’-Hydroxy-4’,6’-dimethoxy-3’-methyldihydrochalcone | 4.4 | 0.0029 | up | |
| 2’,4’,4-Trihydroxy-3’,3-methoxychalcone | 0.99 | 0.0303 | up | |
| Derricin | −3.61 | 0.0000 | down | |
| Pseudosindorin | −0.56 | 0.1130 | ns | |
| Brosimacutin H | 6.8 | 0.0017 | up | |
| Grandiflorone | −1.38 | 0.0280 | down | |
| Phloretin | −2.84 | 0.0006 | down | |
| Trilobatin | −2.73 | 0.0064 | down | |
| Kanzonol B | −5.66 | 0.0000 | down | |
| 4,2’,6’-Trihydroxy-4’-methoxy-3’,5’-dimethyldihydrochalcone | 4.75 | 0.0030 | up | |
| Isoneobavachalcone | −1.61 | 0.0056 | down | |
| 2’,3’,4’,6’-Tetramethoxychalcone | 1.66 | 0.0125 | up | |
| Okanin | −2.72 | 0.0174 | down | |
| Aspalathin | 0.25 | 0.0391 | up | |
| Okanin 3,4,3’,4’-tetramethyl ether | 4.11 | 0.0003 | up | |
| Chalconaringenin 2’-xyloside | 5.45 | 0.0002 | up | |
| 4-Hydroxy-2’,4’-dimethoxydihydrochalcone | 4.23 | 0.0044 | up | |
| Purpuritenin B | −3.08 | 0.0033 | down | |
| Phloretin 3’,5’-Di-C-glucoside | −1.36 | 0.0499 | down | |
| Chromenes | Sec-o-glucosylhamaudol | 2.47 | 0.0108 | up |
| 2-Methyl-5-acetonyl-7-hydroxychromone | 4.84 | 0.0161 | up | |
| Eugenitin | −2.25 | 0.0261 | down | |
| Coumarins | Scopoletin acetate | 6.9 | 0.0000 | up |
| 5,6,7-Trimethoxy-2H-chromen-2-one | 6.12 | 0.0037 | up | |
| Glabrocoumarone A | −3.91 | 0.0010 | down | |
| Diarylheptanoid | 1,7-bis(4-hydroxyphenyl)-3,5-heptanediol | −0.96 | 0.1334 | ns |
| Dipeptide | Leucylproline | 0.34 | 0.0195 | up |
| Flavanones and conjugates | Eriodictyol | −1.82 | 0.0257 | down |
| Pectolinarigenin 7-rhamnoside | −1.57 | 0.2553 | ns | |
| Protofarrerol | 5.55 | 0.0006 | up | |
| Naringenin | −3.39 | 0.0048 | down | |
| 5,6,7,3’,4’-Pentamethoxyflavanone | 7.6 | 0.0207 | up | |
| Fustin | −4.01 | 0.0136 | down | |
| Astilbin | −3.13 | 0.0018 | down | |
| Hesperetin | 5.37 | 0.0000 | up | |
| Brosimacutin A | 4.88 | 0.0004 | up | |
| 5,4’-Dihydroxy-6-C-prenylflavanone 4’-xylosyl-(1->2)-rhamnoside | 2.9 | 0.0276 | up | |
| Naringin dihydrochalcone | −2.32 | 0.0038 | down | |
| Flavanonol | Dihydromorin | −3.38 | 0.0007 | down |
| Flavans and conjugates | 3,4,7-Trihydroxy-5,4’-dimethoxy-6,8-dimethylflavan | 4.55 | 0.0069 | up |
| Epiafzelechin 3-O-gallate | −3.88 | 0.0021 | down | |
| Nitenin | −3.33 | 0.0251 | down | |
| Vitexin 2”-O-p-coumarate | −2.45 | 0.0075 | down | |
| Myricitrin | −2.57 | 0.0347 | down | |
| Tricetin | −6.41 | 0.0021 | down | |
| Tangeritin | 4.4 | 0.0061 | up | |
| Baohuoside 1 | 6.08 | 0.0001 | up | |
| Luteolin | −3.88 | 0.0249 | down | |
| 5,7,3’-Trihydroxy-6,4’,5’-trimethoxyflavanone | 5.7 | 0.0002 | up | |
| Barbatoflavan | −2.76 | 0.0153 | down | |
| 8-Hydroxytricetin 7-glucuronide | −1.76 | 0.0002 | down | |
| 6-Methoxyluteolin 7-glucuronide | −1.53 | 0.0212 | down | |
| Flavonols and conjugates | Kaempferol-3-glucoside | −3.2 | 0.0031 | down |
| Quercetin | −3.42 | 0.0039 | down | |
| Miquelianin | −4.81 | 0.0353 | down | |
| Myricetin | −4.32 | 0.0015 | down | |
| Ampelopsin 3’-methyl ether 4’-rhamnoside | −4.49 | 0.0012 | down | |
| Kaempferol 3-(2”-p-coumaryl-alpha-L-arabinopyranoside) | −1.89 | 0.0773 | down | |
| Quercetin 3-(2”-galloylgalactoside) | −2.35 | 0.0225 | down | |
| Diffutin | −2.22 | 0.0013 | down | |
| Glycinol derivate | (6αs,11αs)-4-dimethylallyl-3,6α,9-trihydroxypterocarpan | 5.79 | 0.0001 | down |
| Hydrolyzable tannins | 3,4-di-o-methylellagic acid | −3.12 | 0.0023 | down |
| Gallic acid | −0.47 | 0.0176 | down | |
| Robinetinidol 3-O-gallate | −6.16 | 0.0131 | down | |
| 3,4,3’-Tri-O-methylellagic acid | −1.27 | 0.0157 | down | |
| Propyl galiate | 4.09 | 0.0085 | up | |
| Epigallocatechin | −2.79 | 0.0165 | down | |
| Ellagic acid | −1.64 | 0.0746 | down | |
| Quinate | 2.03 | 0.1993 | ns | |
| Organic acid | 2-methylcitric acid | −1.15 | 0.0145 | down |
| Malic acid | −0.52 | 0.0249 | down | |
| 2,4-Dihydroxybenzoic acid | −1.92 | 0.0356 | down | |
| Citric acid | −0.43 | 0.1457 | down | |
| Isoflavone | Genistein | −1.79 | 0.0229 | down |
| Isoprenoid | Abscisic acid | −1.69 | 0.0018 | down |
| Lipids and derivates | Choline | −1.55 | 0.0037 | down |
| Monoolein | −3.08 | 0.0268 | down | |
| Oleic acid | −3.59 | 0.0312 | down | |
| 1-Linoleoyl glycerol | −2.58 | 0.0068 | down | |
| 9(Z),11(E)-Conjugated linoleic acid | −3.81 | 0.0694 | down | |
| 2-methoxyestrone 3-glucosiduronic acid | −2.06 | 0.0031 | down | |
| Palmitoleic Acid | 2.29 | 0.0049 | down | |
| Ethyl myristate | −1.09 | 0.1308 | ns | |
| Stearic acid | −0.22 | 0.3352 | down | |
| 5-Hydroxy-2-furoic acid | −0.39 | 0.0504 | down | |
| 2,3-Dihydroxypropyl stearate | 1.12 | 0.0500 | up | |
| Stearidonic acid | −1.15 | 0.0484 | down | |
| Nucleotide/nucleoside | Adenosine | −4.11 | 0.0059 | down |
| Cytidine | −3.45 | 0.0198 | down | |
| Phenolic acid and derivates | 4-Methoxycinnamic acid | 3.31 | 0.0205 | up |
| Coniferyl ferulate | 7.07 | 0.0066 | up | |
| 2-Protocatechuoyl phloroglucinol carboxylic acid | −4.37 | 0.0006 | down | |
| Chlorogenic acid | −5.13 | 0.0021 | down | |
| N-feruloylglycine | −4.94 | 0.0321 | down | |
| Sinapinic acid | −3.66 | 0.0134 | down | |
| Scutellarioside II | −4.63 | 0.0024 | down | |
| Phenolic glycosides | Avenein | −5.76 | 0.0393 | down |
| Piperazines | 2-piperazinecarboxamide | −4.47 | 0.0103 | down |
| Piperidinecarboxylic acid | Nipecotic acid | 1.78 | 0.0532 | up |
| Proanthocyanidins/ tannins | Catechin | −0.37 | 0.0471 | down |
| Epicatechin | −0.5 | 0.0025 | down | |
| Cinnamtannin A2 | −3.54 | 0.0117 | down | |
| Cinnamtannin D2 | −3.83 | 0.0325 | down | |
| Fisetinidol | −0.98 | 0.0234 | down | |
| 4’-methyl-epigallocatechin-3’-glucuronide | −1.58 | 0.0013 | down | |
| Gallocatechin gallate | −4.37 | 0.0005 | down | |
| Epicatechin 5-O-beta-D-glucopyranoside-3-benzoate | −2.56 | 0.0395 | down | |
| Pterocarpans | Phaseollin | −1.78 | 0.0025 | down |
| Quinazolines | Fumiquinazoline D | 3.59 | 0.0127 | up |
| Sesquiterpene | Cnicin | 5.13 | 0.0165 | up |
| Spicatin | 4.18 | 0.0047 | up | |
| Shikimic acid derivate | 4-coumaroylshikimic acid | −0.91 | 0.0266 | down |
| Chorismic acid | 4.65 | 0.0122 | up | |
| Stilbenoid | 4-prenyloxyresveratrol | 0.87 | 0.0135 | up |
| Vanillyl mandelic acid | −2.89 | 0.0001 | down | |
| Ampelopsin 3’-methyl ether 4’-rhamnoside | −1.8 | 0.0132 | down | |
| Terpenoids | Genipin | 1.32 | 0.0462 | up |
| Loganin | −4.01 | 0.0023 | down | |
| Triterpene | Bruceine D | 1.56 | 0.0016 | up |
| Unclassified | 5,6,7-Trimethoxy-2-(2,3,4-trimethoxybenzylidene)indan-1-one | 4.4 | 0.0006 | up |
| (4E)-1,7-Bis(4-hydroxyphenyl)-4-hepten-3-one | −4.11 | 0.0005 | down | |
| 3-[3-(beta-D-Glucopyranosyloxy)-2-methoxyphenyl]propanoic acid | −3.56 | 0.0007 | down | |
| 2-[(5Z)-5-tetradecenyl]cyclobutanone | 0.87 | 0.1403 | ns | |
| 1,7-bis(4-hydroxyphenyl)heptan-3-one | −3.74 | 0.0179 | down | |
| 4-(4-Hydroxyphenyl)-2-butanyl 6-O-[(4ξ)-α-L-threo-pentofuranosyl]-β-D-glucopyranoside | −3.48 | 0.0192 | down | |
| Class | Name | Shells vs. Cuticles | ||
|---|---|---|---|---|
| Log2 Fold Change | p-Value | Regulation | ||
| Lignan | Dihydroconiferin | 3.02 | 0.0012 | up |
| Amino acids and metabolites | Tryptophan | −2.07 | 0.0129 | down |
| Citrulline | −2.7 | 0.0266 | down | |
| Phenylalanine | −1.62 | 0.0387 | down | |
| Anisoles | Vanillin | −2.09 | 0.0041 | down |
| Anthracenecarboxylic acids | Carminic acid | −0.98 | 0.0054 | down |
| Aurone flavonoids | Castillene B | −1.37 | 0.0276 | down |
| Carbohydrates and derivatives | Saccharic acid | −1.3 | 0.0507 | ns |
| Raffinose | −5.12 | 0.0650 | ns | |
| Trehalose | −4.8 | 0.0706 | ns | |
| Chalcones and dihydrochalcones | Phlorizin | −3.35 | 0.0015 | down |
| Brosimacutin H | −3.52 | 0.0029 | down | |
| Phloretin | −3.32 | 0.0029 | down | |
| Okanin 3’-glucoside | −3.89 | 0.0081 | down | |
| 2’,3’,4’,6’-Tetramethoxychalcone | 0.5 | 0.0189 | up | |
| Okanin 3,4,3’,4’-tetramethyl ether | 6.28 | 0.0244 | up | |
| Lusianin | −4.97 | 0.0561 | ns | |
| 4-Hydroxy-2’,4’-dimethoxydihydrochalcone | −2.62 | 0.0848 | ns | |
| Okanin | −0.31 | 0.3281 | ns | |
| Coumarins | Glabrocoumarone A | 5.99 | 0.0001 | up |
| Scopoletin acetate | −1.84 | 0.0027 | down | |
| Diarylheptanoid | 1,7-Bis(4-hydroxyphenyl)-3,5-heptanediol | −1.43 | 0.0008 | down |
| Dipeptide | Leucylproline | −0.43 | 0.0322 | down |
| Flavanones and conjugates | Fustin | −2.77 | 0.0001 | down |
| Naringin dihydrochalcone | 5.78 | 0.0015 | up | |
| Naringenin | −3.88 | 0.0090 | down | |
| 5,6,7,3’,4’-Pentamethoxyflavanone | 7.44 | 0.0382 | up | |
| Flavans and conjugates | 5,7-Dihydroxy-3-methoxy-4’-prenyloxyflavone | 4.43 | 0.0004 | up |
| Myricitrin | −3.23 | 0.0011 | down | |
| 3,4,7-Trihydroxy-5,4’-dimethoxy-6,8-dimethylflavan | −2.18 | 0.0054 | down | |
| 6-Methoxyluteolin 7-glucuronide | 5.78 | 0.0387 | up | |
| 8-Hydroxytricetin 7-glucuronide | −2.33 | 0.0518 | ns | |
| 4’,5,6,7-Tetramethoxyflavanone | −0.08 | 0.3485 | ns | |
| Tricetin | 0.11 | 0.7318 | ns | |
| Myricetin | −3.09 | 0.0029 | down | |
| Quercitrin | −4.06 | 0.0082 | down | |
| Kaempferol-3-glucoside | 0.34 | 0.0199 | up | |
| Hydrolyzable tannins | 3,4,3’-Tri-O-methylellagic acid | 5 | 0.0057 | up |
| Quinate | 2.82 | 0.0175 | up | |
| Propyl galiate | −1.6 | 0.0270 | down | |
| Robinetinidol 3-O-gallate | −4.22 | 0.0914 | ns | |
| Gallic acid | −0.67 | 0.1800 | ns | |
| Isoflavone | Genistein | 0.3 | 0.0903 | up |
| Isoprenoid | Abscisic acid | −1.11 | 0.0051 | down |
| Lipids and derivates | 2-Arachidonoyl glycerol | −2.57 | 0.0051 | down |
| Choline | −2.19 | 0.0077 | down | |
| Oleic acid | −2.1 | 0.0921 | ns | |
| 9,12,13-Trihydroxy-15-octadecenoic acid | −1.1 | 0.2899 | ns | |
| 13-Hydroperoxylinoleic acid | −0.15 | 0.2955 | ns | |
| Nucleotide/nucleoside | Adenine | −1.73 | 0.0150 | down |
| Adenosine | −2.05 | 0.1434 | ns | |
| Organic acid | 2,4-Dihydroxybenzoic acid | −1.17 | 0.0160 | down |
| Malic acid | −1.13 | 0.0855 | ns | |
| Phenolic acid and derivates | 4-Methoxycinnamic acid | 7.11 | 0.0006 | up |
| Chlorogenic acid | −0.7 | 0.0145 | down | |
| Coniferyl ferulate | 0.57 | 0.0226 | up | |
| 2-Protocatechuoyl phloroglucinol carboxylic acid | 2.94 | 0.0253 | up | |
| Proanthocyanidins/ tannins | Catechin | −5.32 | 0.0073 | down |
| Epicatechin | −0.52 | 0.0937 | ns | |
| Pterocarpans | Medicocarpin | 1.58 | 0.0040 | up |
| Phaseollin | 0.74 | 0.0534 | ns | |
| Shikimic acid derivate | 4-coumaroylshikimic acid | −1.71 | 0.0660 | ns |
| Unclassified | 3-hydroxy-3,4-bis[(4-hydroxy-3-methoxyphenyl)methyl]oxolan-2-one | 7.32 | 0.0002 | up |
| 3-[3-(beta-D-Glucopyranosyloxy)-2-methoxyphenyl]propanoic acid | −3.76 | 0.0051 | down | |
| (4E)-1,7-Bis(4-hydroxyphenyl)-4-hepten-3-one | −3.84 | 0.0063 | down | |
| 5,6,7-Trimethoxy-2-(2,3,4-trimethoxybenzylidene)indan-1-one | 4.52 | 0.0108 | up | |
| Didodecyl-3,3-thiodipropionate (DLTDP) | −0.35 | 0.0455 | down | |
| Extraction Solvent | MIC (mg/mL) | |
|---|---|---|
| Cuticle | Shell | |
| Acetone (40/60) | 5 | 10 |
| Methanol (50/50) | 1 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazzini, S.; Zuorro, A.; Panseri, S.; Pavlovic, R.; Sgoifo Rossi, C.A.; Rossi, L. Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability 2023, 15, 3268. https://doi.org/10.3390/su15043268
Frazzini S, Zuorro A, Panseri S, Pavlovic R, Sgoifo Rossi CA, Rossi L. Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability. 2023; 15(4):3268. https://doi.org/10.3390/su15043268
Chicago/Turabian StyleFrazzini, Sara, Antonio Zuorro, Sara Panseri, Radmila Pavlovic, Carlo Angelo Sgoifo Rossi, and Luciana Rossi. 2023. "Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli" Sustainability 15, no. 4: 3268. https://doi.org/10.3390/su15043268
APA StyleFrazzini, S., Zuorro, A., Panseri, S., Pavlovic, R., Sgoifo Rossi, C. A., & Rossi, L. (2023). Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability, 15(4), 3268. https://doi.org/10.3390/su15043268







