Abstract
Microfibers (MFs) are one of the most prevalent microplastic (MP) sub-groups found in the aquatic environment released from many sources, including household laundry. MPs pose risks to the growth rate of terrestrial/aquatic biota and through biomagnification. Although MFs can be ingested by humans, their toxic effects and potential impact on public health are not yet clearly understood. Moreover, the removal of MPs, including MFs, during wastewater treatment is a challenge, since treatment plants are not designed to collect them. Therefore, this work aims to study the potential of the in situ phytoremediation of microfibers from a domestic washing machine effluent by growing barley in a vertical hydroponic system. The temporal variation in barley growth, water quality parameters, length distribution of MFs, and their removal were evaluated over 4 weeks. We investigated the MFs’ interaction with two systems: without barley (System NP) (used as a control) and with barley (System P). The results show the barley growth is negatively affected at the end of 4 weeks, mainly by the accumulation of phosphate and the presence of fungi. However, the level of dissolved oxygen in System P is satisfactory and the presence of MFs decreases considerably (mainly for MFs > 600 µm) from different interactions with the barley roots. These interactions were corroborated by microscopy images. The total removal of MFs through the hydroponic system was 52% in week 2, decreasing to 42%. This is the first time that the removal of MFs has been evaluated using vertical hydroponics, which demonstrates that this phytoremediation system can be used at the household level. It also shows that vertical hydroponics, as an experimental methodology, for the analyses of MFs’ impacts on plant health has merit. It is expected that this study will contribute to new investigations of MF removal by green technologies.
Keywords:
barley; green technology; hydroponics; Hordeum vulgare; macrophytes; microfiber; roots; washing wastewater 1. Introduction
Plastic plays an important role in our lives; they are in a majority of consumer products due to being lighter or more economical than alternative materials. However, due to their linear and high usage, they end up in the environment where they can persist for decades and, depending on the environmental conditions, degrade into microscopic particles known as microplastics (MPs). MPs, a concept first explored in the early 2000s [1], are defined by their size < 5 mm [2,3] and composition.
MPs have recently attracted more attention owing to their potential impact on ecosystems, particularly the risks they pose to terrestrial/aquatic biota, where biomagnification can occur [4]. MPs can have detrimental effects on aquatic and terrestrial biota’s ingestion and growth rate [5,6,7]. Additionally, long-term exposure to MPs could be related to diseases, such as hormonal and liver and kidney disorders [8,9].
Generally, MPs can be divided into fibres, fragments, films, foam, and beads. The properties of MPs’ composition vary widely, such as (i) polyvinyl chloride (PVC), a polymer with properties, such as a robust, rigid, solid structure. It can be applied in building and construction, medical tubing, and water pipes, among others. (ii) Polyethylene terephthalate (PET), related to high rigidity and a very-low moisture absorption capacity. Its sources of water contamination usually originate from the disposal of fibres, bottles, jars, and plastic containers in surface waters. (iii) Polyamide (nylon), which contains properties, such as high temperature and electrical resistance, and is applied, e.g., in synthetic textile fibres and carpets [10,11,12,13,14,15]. As a subcategory of MPs, microfibers (MFs) are threads mostly derived from synthetic fabrics during clothes washing [16,17]. It has been estimated that ~35% of MPs found in the aquatic system are from washing textiles [18]. Moreover, the removal of MPs, including MFs, during wastewater treatment is a challenge [19,20]. MFs in laundry wastewater are identified as the greatest source of MFs pollution [21]. MPs/MFs not removed during conventional wastewater treatment are discharged into the water, soil, and atmosphere through their effluents and sludge. Sludge is used as an agricultural fertilizer that can lead to the accumulation of MPs/MFs in the soil [22]. The quantitative analysis of MFs released from the laundry is complex. Several studies indicated that the effect of pipeline residuals, washing machine performance, and cloth dryers are underestimated [23,24].
Factors determining MF loss from domestic washing are still being studied. There is research aiming to obtain an efficient and reliable way to reduce MF release at the household level through changing washing habits, additives, washing machine performance, and potentially incorporating water treatment at the source of the pollution. The advantage of having treatment at the source level is that water reuse can be adopted by households to reuse the water in gardening, for instance.
Recent research has focused on MPs’ impact on aquatic systems. While the effects on the soil’s physical properties and biota [25,26] are starting to become a focal point, there is still limited data on the impact of MPs on plant growth [27,28,29,30]. There are two main methods for culturing plants: soil and soilless (hydroponics). Liao et al. (2019) [30] demonstrated that the impact of MPs on plants in hydroponic conditions was greater than those under soil culture conditions. This is because hydroponics increases the rhizosphere and water interface. In addition, MPs are more uniformly dispersed and thus more mobile in a fluid environment than in soil [29]. Therefore, the increase in the probability of MP contact with the root matt, i.e., the MPs being filtered, makes hydroponics an excellent method to study the impact of MFs on plants, but it could also act as a biofilter (Figure 1). There is a consensus on the need for more sustainable technologies, such as phytoremediation, for the possible delivery of lower net carbon levels. From this perspective, nature-based systems, such as hydroponic systems, represent an opportunity to reduce carbon emissions from treatment plants [31], including MP biofiltration. In general, this technology does not require chemical additions, requires natural materials (plants), and demands lower operational costs compared to conventional wastewater treatment processes, as only a periodic inspection is required [31,32]. However, despite hydroponic technology being applied to remove some nutrients (nitrogen and phosphorus), faecal coliforms, and some pollutants (pharmaceuticals) [33,34,35], there is a gap in the studies addressing the removal of MPs (including MFs) by a vertical hydroponic system.
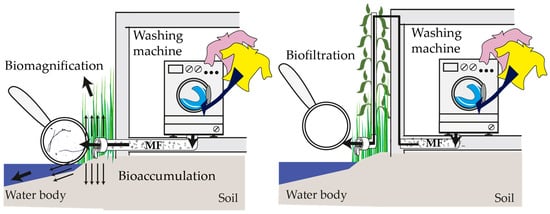
Figure 1.
Current practice in discharge leads to significant bioaccumulation and biomagnification of MPs/MFs in aquatic and terrestrial ecosystems. A vertical hydroponic system could be used as a biofilter for the removal of MPs/MFs.
This study aims to (1) investigate whether a vertical hydroponic system using Hordeum vulgare (barley) can be used to reduce the abundance of MFs, and (2) explore how a vertical hydroponic system can be used as an experimental method to investigate the impact of MPs on plant health. This is the first study reported in the literature on MF removal using barley vertical hydroponics. Due to the significant gap in this topic to date, it is expected that this work will contribute to new studies and the optimization of existing ones in the green technology sector for MP removal.
2. Materials and Methods
2.1. Washing Wastewater Collection
The effluent containing MFs was generated by washing a 100% polyester blanket (pink colour, size 1.40 × 2.00 m) for 1.5 h at 30 °C. Two types of effluents were sampled from different washing modes. One included detergent (1 washing capsule) and the other did not. With the aim to dilute the detergent concentration to avoid a higher phosphate concentration in the effluent [36], 11 L of washing effluent, including detergent, and 9 L of effluent without detergent were mixed into a large bucket, and the content was shaken for a minute to homogenize the solution.
The initial characteristics of the diluted, washing wastewater are listed in Table 1.

Table 1.
Initial parameters of washing wastewater after dilution.
2.2. Experimental Set-Up
Water filtration and MF sorption experiments using barley were conducted in two hydroponic systems, as illustrated in Figure 2. System NP was a controlled system (without plants) investigating whether MFs could be adhered to the hydroponic system’s walls, pump, and tubing. System P (containing 5 rows by 3 columns of barley, a total of 15 plants) was used to investigate the hydroponic plants’ potential for filtrating wastewater and their ability to retain the MFs contained in the wastewater. For System P, a 140 W LED grow light was added to produce an average photosynthetically active radiation (PAR) value of 200 µmol/m2·s. The light was switched on for a period of 10 h a day (8 a.m.–6 p.m.) to simulate the diurnal cycle. Both systems had a water pump connected to a timer with eight cycles a day, which controlled the water circulation. Each cycle consisted of a 1 h running period followed by a 2 h cut-off. Then, the water flow of each dripper was 3.3 mL/s. Aliquots were obtained from the tap, bottom, and pump for quantifying the MFs. All experiments were run for four weeks. Additionally, a pre-test using the same apparatus in different conditions was run with 1 barley grown for 3 weeks with LED timing set for 12 h.
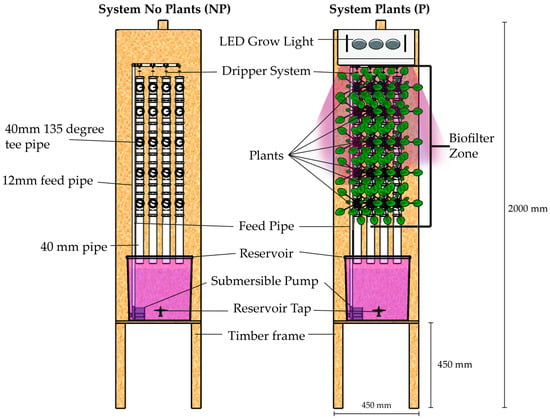
Figure 2.
Designed experiment of hydroponic phytoremediation system: on the left is the system with no plants (NP) and on the right is the system with plants (P).
Figures presented in the Supplementary Materials show the barley cultivation step (Figure S1) and the experimental apparatus of vertical hydroponics (Figure S2).
2.3. Plant Propagation Stage
Hordeum vulgare (barley) was used for the vertical hydroponic system due to its strong roots and rapid growth [37]. Brand Natural VITA barley seeds were purchased (UPM Trade Limited) and 1% concentration of Formulex nutrient solution was added to the inner mesh tray every week to provide essential macro- and micronutrients. After two weeks, 15 plants with similar root lengths longer than ~5 cm were placed into System P. The barley growth was estimated from their root lengths during different growth stages. Barley plants from the top, middle, and bottom rows were taken out of the pot and recorded for 4 weeks.
2.4. Analytical Procedures
The daily measurements for pH, electric conductivity, dissolved oxygen (DO), and turbidity parameters were measured in triplicate, according to the standard method for the examination of water and wastewater (23rd edition). For extracting the aliquots, the wastewater in the tank was first stirred uniformly. Then, 30 mL of sample from each system was extracted by a syringe from the tank. The pH value and electrical conductivity were analysed with an S47 SevenMultiTM duel pH/conductivity meter. Turbidity was measured using HACH 2100An turbidity and dissolved oxygen, and its corresponding temperature was analysed using a Jenway 9200 dissolved oxygen meter. Total suspended solids (TSSs) were measured according to the standard method (Method 2540D). Total organic carbon (TOC) was measured with a total organic carbon analyser (TOC-L CPH 179 model, Shimadzu, Milton Keynes, UK). The samples were previously filtrated with a syringe filter (pore size: 45 μm) and analysed based on auto-generating a calibration curve using standard methods. Ion concentration was analysed with an ion chromatography system (DIONEX ICS-1100, Thermo Fisher Scientific, Sunnyvale, CA, USA) [38] every four days. For this, the samples were filtered in triplicate using 0.45 μm cellulose acetate membrane before injection. For anion phosphate (PO43−), nitrate (NO3−), sulphate (SO42−), chloride (Cl−), nitrite (NO2−), and bromide (Br−), the analytical and guard columns were IonPac AS23 4 mm and IonPac AG23, respectively. Eluent solution consisted of 4.5 mmol/L Na2CO3 with 0.8 mmol/L NaHCO3 at the flow rate of 1mL/min. For cation calcium (Ca), magnesium (Mg), potassium (K), sodium (Na), lithium (Li), and ammonium (NH4+), the analytical column was IonPac CS12A 4 mm and guard column was IonPac CG12A 4 mm. Eluent was 20 mmol/L of methane sulfonic acid at the flow rate of 1 mL/min. For both cation and anion determinations, the column temperature was set at 30 ℃. Microbial growth was assessed using the membrane filtration method [39] (Millipore, pore size: 45 μm) for a comparison of bacterial colonies between Escherichia coli (E. coli) and total coliform. The investigated samples were diluted with the following dilutions: 1:104, 1:105, and 1:106. Following filtration, the membrane filter was removed and placed in a plate containing agar substrate, incubated for 24 h at 37 °C. Bacterial colonies were counted once a week in duplicate from the water reservoir of both systems. The assessment of bacterial growth was conducted for 4 weeks.
All equipment calibrations were performed before the tests and according to the manufacturer’s guidelines.
2.5. Microfiber Adherence and Count
The MFs adhering to the plant roots were analysed by an endoscope stereomicroscope (Bysameyee USB Digital Microscope, China) with a three-dimensional view, a stereo microscope Carl Zeiss (Discovery V.8, Gottingen, Germany) recorded in an Axiocam 305 Colour Digital Camera, and scanning electron microscope—SEM (Zeiss Evo50 from Oxford Instruments, Cambridge, UK, 20 kV). For the SEM, the samples were pre-treated with gold/palladium (20 nm). Barley roots from the top, middle, and bottom rows were evaluated.
MFs were manually counted from 0.1 mL samples obtained from the tank’s bottom, water pump area, and pipe outlet (Figure 2) to compare the MF abundance between the different positions. The samples were divided into 10 × 10 grids (2 mm squares) and placed in a lamina to be counted with the stereo microscope.
2.6. Data Analysis
Data from the MF counting and length distribution of different sampling positions were analysed with variance (ANOVA) tests with Tukey HSD. All the data were processed with Origin 2021b software.
3. Results
3.1. Ion and Water Parameters
Variations in anion concentrations (n = 3) in the water reservoir of Systems NP and P are shown in Figure 3. The concentration of PO43− in System NP (without plants) did not change considerably over the weeks, showing variations ranging from 2.8 to 8.5 mg/L. On the other hand, in System P (with plants), PO43− increased from 0.64 mg/L in week 1 to 139.98 mg/L in week 3. Then, PO43− decreased considerably to 45.9 mg/L at the end of the long-term interactions (4 weeks). The increasing PO43− levels may be related to lower PO43− absorption by the barley since the maximum PO43− uptake rate occurs when the pH is 5–6 [40]. The pH gradually increased in both systems (Figure S3, Supplementary Materials), and this may have influenced the abnormally high accumulation of PO43− in week 2. In this way, the root activities were hindered, with an inhibition of rhizospheric activities. Furthermore, the inherent detergent present in washing wastewater contributed to the increasing trend of pH in both systems [41]. NO3− increased to 29.5 mg/L in System NP and 44.3 mg/L in System P from week 3. This increased concentration of NO3− in System P may be due to the breakdown of nitrogen-containing substances found in nitrogen fertilizers and plant manures, resulting in the formation of NO3− [42]. Similar trends can be observed for the levels of SO42− and Cl−. SO42− gradually increased from 16.6 to 67.4 mg/L in System NP and from 16.2 to 77.2 mg/L in System P. The overall accumulation of Cl− and SO42− indicates that plants are unable to absorb them. Moreover, Cl− toxicity can play a significant role in the reduction in barley growth [43]. Additionally, Br− and NO2− were analysed because some detergents used for washing/cleaning have straight hydrocarbon chains of cetyltrimethylammonium bromide (CTAB) [44], and nitrite may be contained in laundry water in a concentration range of 1 to 3 mg/L [45]. However, the values were <1 mg/L for both anions and there were no significant changes in either system (ANOVA, p > 0.05).
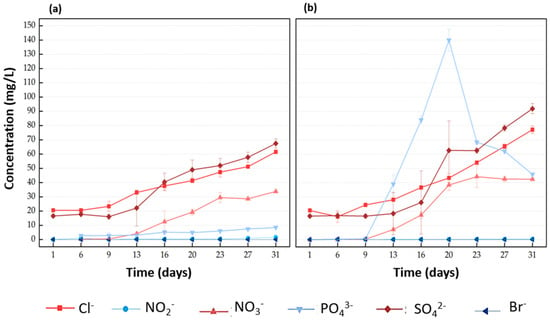
Figure 3.
Variation in anions’ concentrations (n = 3): chloride (Cl−), nitrite (NO2−), nitrate (NO3−), phosphate (PO43−), sulphate (SO42−), and bromide (Br−) in the water reservoir of (a) System NP and (b) System P.
A similar ion variation was also observed for the concentration of the cations, mainly for Ca, Na, and K, from the third week (Figure S4). Ca is an important macronutrient and its concentration in hydroponic systems can significantly influence plant growth [46]. In both systems (no plants—NP, and with plants—P), there was an increase in Ca concentration between weeks 1 and 2. However, System P showed a progressive reduction in week 3, with stability in calcium concentrations at the end of the long-term interactions. From these results, it can be suggested that calcium was absorbed by the barley plants mainly from week 3. Additionally, increasing potassium concentrations in the solution generally decreased Ca concentrations in both the tops and roots of the barley plants [47]. Considering that there was an increase in the potassium concentration in the water reservoir from week 3 onwards in System P, concomitant with the reduction in calcium levels, this fact can also be considered to have occurred in the investigated hydroponic system. In addition, the potassium concentration’s increase may be related to the lower absorption capacity of the nutrient by the plant, since signs of the reduced growth of barley roots from week 3 onwards were observed in this study (Table S1). Regarding sodium concentration, there was an increase over the weeks in both systems, with relatively higher concentrations in the last week of phytoremediation in the system with plants. The non-intake of sodium in this case by the barley could be due to the plant’s tolerance to Na accumulation and its ion-exclusion (Na) mechanisms. This is a recognized physiological mechanism to aid salinity tolerance in plant species, such as barley [48,49].
Previous studies reported that aquatic vegetation could reduce water turbidity, stabilizing sludge and reducing sediment suspension [50,51]. However, the increase in turbidity in System P was the opposite (Figure 4a). In this vertical hydroponic system, the water flow was periodically circulated with the sediments settled at the bottom of the tank, which is a factor contributing to turbidity increase. Total suspended solid (TSS) variations in System P (Figure 4b) corroborate the high turbidity identified in the same system. There was a notable TSS increase in System P from week 3, compared to System NP, which might be related to the increased turbidity [52]. Despite E. coli not being detected in the two systems, there were high quantities of coliform (Figure 4c), mainly in System P, in the final week, peaking at 123,333 CFU/mL coliform colonies. It is suggested that a small-scale ecological system feeding the growth of microbes and zooplankton formed in the rhizosphere of the barley plants [53,54]. Additionally, in the bottom rows of the hydroponic system, worms and fungi were observed among the roots (Figure S5), which may also explain the turbidity increase and bacteria present in the water tank at the late stage. In addition, the present study showed promising results regarding dissolved oxygen (DO). The concentration in System P was higher than in System NP (Figure 4d); this is likely due to the water flow over the route matt having a stronger water/atmosphere interaction, due to the turbulent flow and greater surface area [55].
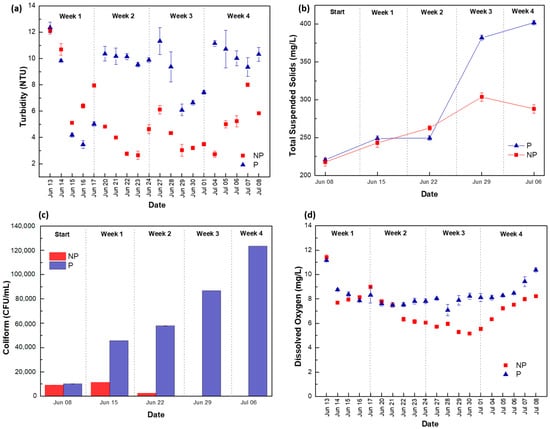
Figure 4.
Variation in water parameters (n = 3) in Systems NP and P, (a) turbidity, (b) total suspended solids (TSSs), (c) total coliform concentration, and (d) dissolved oxygen (DO).
Other parameters, such as EC and TOC variations, are shown in Figures S6 and S7, respectively. In general, the EC showed a tendency to increase over the weeks (in both systems), as observed in the other parameters previously discussed. This increase in conductivity may be associated with an increase in TSSs [56] and an increase in the levels of phosphates, sulphates, and nitrogen in the system [57]. Despite these results, System P showed a reduction in EC values, mainly in the third week. While TOC levels progressively increased over the weeks in System NP, in System P, a reduction in and the stability of the levels were verified. This indicates the efficiency and TOC control capability of this hydroponic system [58].
3.2. Growth and Length of Roots
Root length was reduced from week 3, decreasing 2–4 cm in week 4, in addition to the leaf upwards, as shown in Table S1. Additionally, from week 3, the barley roots were affected by black spots (Figure S5f), indicating fungi presence [59]. Thus, these results suggest that the treatment was affecting the barley negatively (e.g., vigour and root length were not optimal) by the presence of fungi on the roots and phosphate accumulation (as discussed in Section 3.1). Furthermore, a negative interference of MFs in the plant’s health could be considered. A relevant observation is that there is a possibility of the cross-contamination of fungi in the laboratory environment, which would justify its presence in the barley roots during the experiments. In relation to MFs’ negative effect, when in contact with MPs in high concentrations, plant height and root vigour can be considerably affected [60]. On the other hand, recent studies investigating the effect of MPs on plants showed that MPs generally exhibited non-significant effects on root morphology [61], or further exposure resulted in a decrease in the effects on the root, indicating the ability to tolerate MPs for an extended period [62]. However, despite the results, further investigations are needed to understand the effect of different types and shapes of MPs on the health of plants’ roots [61].
3.3. Adherence of Microfibers to Barley Roots
Figure 5 shows microscope images of barley roots exposure to (diluted) washing wastewater containing MFs. During the 4 weeks, MFs transported by water flow were entangled (Figure 5a–d) and adhered (Figure 5e,f) vertically by the intensive root network of barley, as indicated by red arrows. As for the longer MFs, they were more likely to be entangled around the roots (Figure S5a,b). According to Mateos-Cárdenas [63], different mechanisms of interaction between MPs and plants can occur, such as adsorption and/or entrapment on plant structures. The adherence level depends on the hydrophobic or hydrophilic attractions of the MPs, electrostatic forces, and interaction with the root hairs and root surface layer, for example [63,64,65]. In this study, no MFs were detected among the lateral roots, despite the hydroponic barley developing a strong filtration network with lateral roots (Figure S5c). Among some interaction mechanisms, adhesion has been proposed as one of the initial interactions between MPs and roots [62], and different plant species have been reported to be in contact with MFs by adsorption [66]. According to Huang et al., 2021 [67], adsorption may be more effective on rough-surface plants, mainly for aged MPs. According to the images shown in Figure 5e,f, we can suggest that, in this study, the adsorption mechanism may have occurred for some fibres, mainly those of a shorter length.
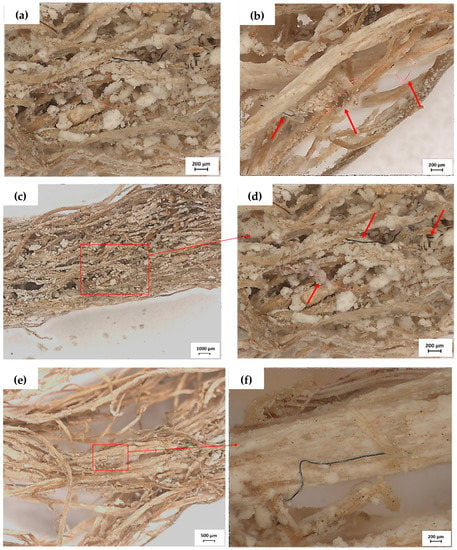
Figure 5.
Barley roots after 4 weeks of exposure to (diluted) washing wastewater containing MFs. The roots were examined under a stereo microscope. The red arrows show MFs: (a) Under 200 μm scale: A long dark fibre entangled to the roots’ surface, and (b) A long pink fibre and a small dark fibre entangled to the roots’ surface; (c) Under 1000 μm scale: A region of the roots with some MFs; (d) Under 200 μm scale: Long fibres dark and pink entangled between the roots; (e) Under 500 μm scale: A region of the roots with a dark fibre adhered; and (f) Under 200 μm scale: fibre adhered on the root surface.
The SEM micrographs (Figure 6) show specific locations of MFs in the roots. In the phytoremediation process, only some ends of the MFs were attached to the root surface, while the other end remained stretched and unadhered (Figure 6a,b). The morphological characteristics of MPs and surface loads are factors that may affect the adherence of MFs to plants [63]. Despite MFs being prone to adhering to the roots, in the present study, no evidence showed the uptake of MFs in the internal root structure, which was previously reported [68,69,70]. Furthermore, the potential for MF intrusion on the root surface is difficult to confirm, as differentiating natural root fibres from MFs on SEM images is a challenge (Figure 6c,d).
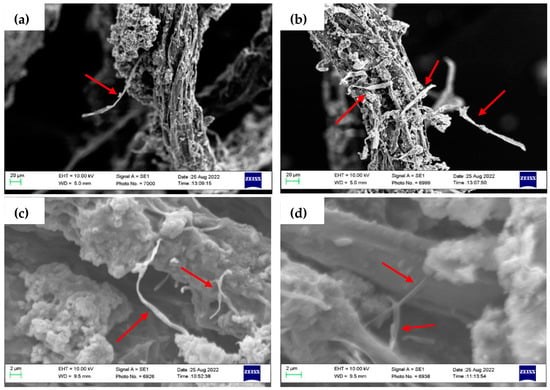
Figure 6.
SEM images of barley roots. The red arrows show potential MFs. Under 20 μm scale: (a) a long fibre attached to the surface; (b) several fibres adhere to the root surface. Under 2 μm scale: (c) fibres are trapped between floccule; (d) potential MF intrusion into the root.
3.4. MF Length Distribution and Count
In general, shorter MFs (<400 μm) were predominant in the pump, while MFs with longer lengths (>400 μm) were generally concentrated in both systems at the bottom of the water reservoir over the weeks (Figure 7). Thus, fibres with a length that did not adhere to the barley structure were re-circulated and/or remained at the bottom of the reservoir during the experiment. Regarding the MFs identified in the tap, from week 1 (in System P), there was a relevant decay of MFs > 600 μm to a distribution of MFs with length < 300 μm. A quantitative assessment of the distribution of MFs in the System P tap shown in Figure 8b supports this result. In this case, the distribution of fibres (600–800 μm) in week 1 was 31%, decreasing to 14% in week 4. Furthermore, MFs in the range of 200–400 μm in the System P tap showed a relevant difference in distribution, with 16% in week 1 and 37% in week 4. In addition, at the pump, there was less fluctuation in the length distribution as a function of temporal variation. For both systems, the greatest distribution of MFs was concentrated at <400 μm over the weeks. At the bottom, the greatest distribution of MFs in System P was concentrated around 400–600 μm in weeks 1 and 4 (33% for both), in addition to MFs between 200–400 μm in week 4 (33%). In System NP (no barley), the greatest distribution of MFs in the bottom remained constant in the range of 600–800 μm over the weeks. These results indicate the lower efficiency of the P hydroponic system in removing fibres of a smaller length and/or lower adherence capacity on the roots and greater efficiency in adhering longer MFs on the barley roots (mainly by entanglement—see Section 3.3). In addition, previous investigations reported that small sizes of MPs (mainly nanosized) tended to be removed by some constructed wetlands [71]. It is worth noting that one of the challenges in removing MPs, including MFs in water treatment plants, is their high quantity and relatively low density, such as polyethylene terephthalate (PET)/polyester (PEST), common polymers in MFs [72,73,74]. As the number of studies for the removal of MFs by phytoremediation (by vertical hydroponics) is still scarce, there is a significant difficulty to compare the results obtained in the present study.
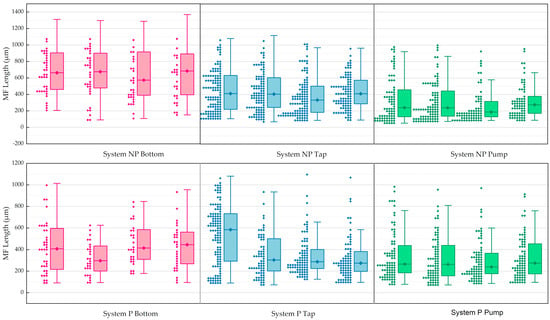
Figure 7.
MFs’ length distributions (n = 3) in System NP (first row) and System P (second row).
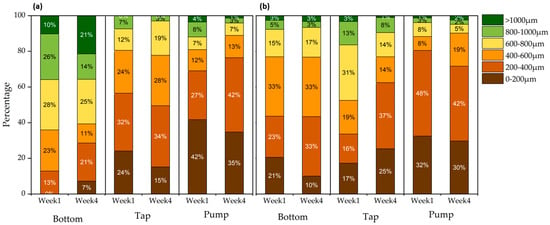
Figure 8.
MFs’ length distributions in percentage in (a) System NP and (b) System P.
Systems NP and P contain the least MFs at the bottom compared to the tap and pump (Figure 9), indicating that MFs undergo continuous circulation under the strong hydrodynamic conditions of the hydroponic apparatus, which is not favourable for MF deposition. MFs in the System P tap significantly decreased from the initial MF level of 144 MFs/100 µL in incoming wastewater. Tap sampling contained 69 ± 3 MFs/100 µL and 83 ± 4 MFs/100 µL at weeks 2 and 4, respectively, with temporal variations being significantly different (ANOVA, p < 0.05). The latter result may be due to the reduced root growth from week 2 onwards (as discussed in Section 3.2). Most studies report on MF abundance in the sea, lake, and reservoirs [75,76], which restrict comparisons with the results of this study, since the scales evaluated are larger and from different matrices. Moreover, the abundance of MF pollution changes over time, is particularly dependent on the health of plants [68], and the randomness of sampling and potential effect of the pump can interfere with the analysis, which can suck tiny fibres. It is worth mentioning that despite the positive correlation between the abundance of MFs in the tap and pump with the growth of barley, no significant difference was observed in the abundance of MFs between the tap and pump in System P (ANOVA, p > 0.05) (Figure S8).
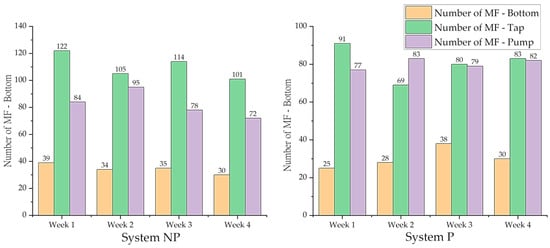
Figure 9.
MF count and removal rate (n = 3) in System NP (left) and System P (right) in the bottom, tap, and pump of the systems.
3.5. MF Removal
The MF removal percentage was calculated based on the initial count of 144 MFs/100 µL in the raw washing wastewater (diluted washing wastewater). Due to the vertical structure of the apparatus, the removal percentage at the bottom does not reflect the overall removal of MFs. Therefore, the removal rate at the bottom was excluded from the comparison to elicit a bias. Compared to the initial MF count, MF removal in the System P tap increased from 37% (week 1) to 52% (week 2), which indicated that MFs could adhere to the hydroponic apparatus under the effect of hydrodynamic circulation (Figure 10). However, from week 3, the growth of the roots showed signs of reduction (Table S1) concomitant with lower nutrient absorption (Section 3.1), where MF removal decreased at around 42.4–44.4%. Apart from that, the MF removal in System NP might have been due to the retention of some MFs in the system apparatus during wastewater circulation. A recent investigation reported that the removal efficiency of MPs by constructed wetlands varies significantly, with lower removal rates of roughly 20–30% and higher removal rates of 100% [77]. One of the factors associated with this wide-ranging removal efficiency is that different characteristics of MPs, such as their shape and size, have an impact on the phytoremediation process [71]. Previous studies reported that duckweed (L. minor) was capable of absorbing 10–45 μm polyethylene MPs under a microcosm investigation [78], and penny grass (Hydrocotyle vulgaris) could bioaccumulate nanoscale polymer debris [79]. However, these studies focused on the effect of MP debris on plants’ growth and photosynthesis.
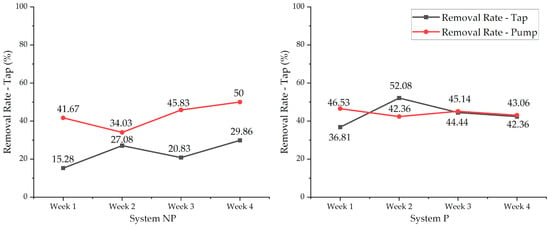
Figure 10.
MF removal rate (n = 3) in System NP (left) and System P (right) in the tap and pump of the systems.
Based on the experimental results, the barley-free system (System NP) showed a higher level of MF pollution than the barley system (System P), conclusively indicating that the barley plant can retain MFs. However, despite the present study indicating MFs can be efficiently removed with the barley roots’ involvement, it is difficult to compare with other studies, as the vertical hydroponics system’s ability to remove MPs has been insufficiently explored to date. Additionally, due to resource constraints, the experiments could not be repeated; hence, we recommend to repeat the experiments.
4. Conclusions
To date, the removal of MFs using wastewater treatment plant (WWTP) is a challenge. Additionally, MF numbers released during laundry are underestimated, as the effect of pipeline residuals and washing machine performance are not considered. In this sense, phytoremediation could be considered a nature-based technology for the in situ removal of MFs from wastewater. However, there is still a limited number of studies on the interactions between plants and MPs, mainly for the potential of MP removal by hydroponic systems.
The present investigation is the first to observe a vertical hydroponic system as a mitigation system for MFs. Additionally, it is a study of the impact of waterborne MF contamination on plants. In this sense, this study showed that the vertical hydroponic system using barley emerges as a promising technology for MF removal and an experiment methodology for the study of MF impacts on the health of plants. Based on the results, it can be concluded that barley can survive in directly emitted diluted washing wastewater in the hydroponic system, but is highly sensitive to water flow and fungus contamination. Additionally, the accumulation of PO43− plays an important role in barley health. Regarding water parameters, there was a significant increase in all factors (pH, EC, turbidity, DO, TSS, bacterial concentration, and TOC) in the hydroponic apparatus filled with barley.
The system with barley attained a maximum MF removal rate of 52% in week 2 (concentration of 91 MFs/100 µL in week 1) and a lower distribution of MFs > 600 µm after 4 weeks, indicating a higher removal efficiency for MFs of a larger size. Several mechanisms might be involved in this removal, such as adsorption and entanglement. However, further studies are needed to identify these mechanisms. In-depth studies on how the flow from the drip system can affect plants and how the adherence of the MPs to plants during phytoremediation can disturb the structure and growth of the plants are also needed. Furthermore, as our results are based on short-term observations (4 weeks), longer interaction experiments are needed to elucidate the MFs’ adherence efficiency to barley roots for more than 4 weeks. Investigations addressing MF removal using different real matrices and investigations using different root plants are other gaps in the knowledge worth exploring. Finally, further investigations are needed to understand the effect of different types and shapes of MPs on the health of plants’ roots.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15042851/s1. Figure S1—Preliminary stage of barley cultivation for phytoremediation experiments (a) germination stage and (b) grown barley to be used in the study; Figure S2—Apparatus of vertical hydroponics system. System NP: no plants (control) and System P: with plants (barley); Figure S3—Variation of pH (n = 3) in the water reservoir of System NP and System P. Trendlines were added; Figure S4—Variation of cation concentration (n = 2) in the water reservoir of (a) System NP and (b) System P; Figure S5—(a) MFs captured among roots from the top row (b) MF are trapped in the healthy and neat root instead of dying, brown roots (c) intensive root network (d) existence of worm (e) existence of fungi (f) black spots on roots; Figure S6—Variation of EC (n = 3) in the water reservoir of System NP and System P. Trendlines were added; Table S1—Barley root growth during 4 weeks of vertical hydroponics; Figure S7—Variation of TOC (n = 3) in the water reservoir of System NP and System P. Trendlines were added; and Figure S8—Results of ANOVA analysis (n = 3) of MF abundance in the bottom, tap and pump of the water reservoir, where “a” means significantly different while “b” means insignificantly different. S1 means System 1 (System no plants—NP) and S2 means System 2 (System with plants—P).
Author Contributions
Conceptualization, D.C.-S. and L.C.C.; Formal analysis, N.d.S., D.C.-S., Y.Q., R.B. and F.G.; Investigation, Y.Q.; Methodology, D.C.-S., R.B. and F.G.; Project administration, L.C.C.; Resources, L.C.C.; Supervision, D.C.-S. and L.C.C.; Visualization, D.C.-S., N.d.S. and Y.Q.; Writing—original draft, N.d.S.; Writing—review and editing, N.d.S., D.C.-S., L.C.C., R.B. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Department of Civil, Environmental and Geomatic Engineering of University College London.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors acknowledge Utku Solpuker, Judith Zhou, and Melisa Canales for helping with the analyses and experimental setups.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency—ECHA Microplastics. Available online: https://echa.europa.eu/hot-opics/microplastics (accessed on 10 November 2022).
- Rillig, M.C.; Lehmann, A. Microplastic in Terrestrial Ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.M.; Hansen, B.H.; Frenzel, M.; Johnsen, H.; Altin, D. Uptake and Toxicity of Methylmethacrylate-Based Nanoplastic Particles in Aquatic Organisms. Environ. Toxicol. Chem. 2016, 35, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.-J. Effects of Micro- and Nanoplastics on Aquatic Ecosystems: Current Research Trends and Perspectives. Mar. Pollut. Bull. 2017, 124, 624–632. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Tallec, K.; Gonzalez-Fernandez, C.; Lambert, C.; Vincent, D.; Mazurais, D.; Zambonino-Infante, J.-L.; Brotons, G.; Lagarde, F.; Fabioux, C.; et al. Constraints and Priorities for Conducting Experimental Exposures of Marine Organisms to Microplastics. Front. Mar. Sci. 2018, 5, 252. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.R.B.O.; Lundebye, A.-K.; Guilhermino, L. Marine Microplastic Debris: An Emerging Issue for Food Security, Food Safety and Human Health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic Pollution, a Threat to Marine Ecosystem and Human Health: A Short Review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Lambert, S.; Scherer, C.; Wagner, M. Ecotoxicity Testing of Microplastics: Considering the Heterogeneity of Physicochemical Properties. Integr. Environ. Assess. Manag. 2017, 13, 470–475. [Google Scholar] [CrossRef]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in Aquatic Environments: Implications for Canadian Ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef]
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics Physicochemical Properties, Specific Adsorption Modeling and Their Interaction with Pharmaceuticals and Other Emerging Contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef] [PubMed]
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, Classification and Removal Efficiency of Microplastics in Wastewater Treatment Plants. Environ. Pollut. 2019, 255, 113326. [Google Scholar] [CrossRef] [PubMed]
- Polymer DataBase Polymer DataBase. Available online: http://polymerdatabase.com/more.html (accessed on 5 February 2022).
- Colin Hindle British Plastics Federation. Available online: https://www.bpf.co.uk/plastipedia/polymers/PP.aspx (accessed on 5 February 2022).
- de Dos Santos, N.O.; Busquets, R.; Campos, L.C. Insights into the Removal of Microplastics and Microfibres by Advanced Oxidation Processes. Sci. Total Environ. 2023, 861, 160665. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A. Sources and Dispersive Modes of Micro-Fibers in the Environment. Integr. Environ. Assess. Manag. 2017, 13, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are We Underestimating the Sources of Microplastic Pollution in Terrestrial Environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Talvitie, J.; Mikola, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How Well Is Microlitter Purified from Wastewater?—A Detailed Study on the Stepwise Removal of Microlitter in a Tertiary Level Wastewater Treatment Plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, F.; Lei, K.; Li, H.; Kang, Y.; Cui, S.; An, L. Microfiber Release from Different Fabrics during Washing. Environ. Pollut. 2019, 249, 136–143. [Google Scholar] [CrossRef]
- Lofty, J.; Muhawenimana, V.; Wilson, C.A.M.E.; Ouro, P. Microplastics Removal from a Primary Settler Tank in a Wastewater Treatment Plant and Estimations of Contamination onto European Agricultural Land via Sewage Sludge Recycling. Environ. Pollut. 2022, 304, 119198. [Google Scholar] [CrossRef]
- Kapp, K.J.; Miller, R.Z. Electric Clothes Dryers: An Underestimated Source of Microfiber Pollution. PLoS ONE 2020, 15, e0239165. [Google Scholar] [CrossRef]
- Lant, N.J.; Hayward, A.S.; Peththawadu, M.M.D.; Sheridan, K.J.; Dean, J.R. Microfiber Release from Real Soiled Consumer Laundry and the Impact of Fabric Care Products and Washing Conditions. PLoS ONE 2020, 15, e0233332. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Cai, Y. Focus Topics on Microplastics in Soil: Analytical Methods, Occurrence, Transport, and Ecological Risks. Environ. Pollut. 2020, 257, 113570. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially Charged Nanoplastics Demonstrate Distinct Accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Kim, L.; Kim, D.; An, S.; An, Y.-J. Microplastics from Shoe Sole Fragments Cause Oxidative Stress in a Plant (Vigna radiata) and Impair Soil Environment. J. Hazard. Mater. 2022, 429, 128306. [Google Scholar] [CrossRef]
- Li, J.; Yu, S.; Yu, Y.; Xu, M. Effects of Microplastics on Higher Plants: A Review. Bull. Environ. Contam. Toxicol. 2022, 109, 241–265. [Google Scholar] [CrossRef]
- Liao, Y.C.; Jahitbek, N.; Li, M.; Wang, X.L.; Jiang, L.J. Effects of Microplastics on the Growth, Physiology, and Biochemical Characteristics of Wheat (Triticum aestivum). Huanjing Kexue/Environmental. Sci. 2019, 40, 4661–4667. [Google Scholar] [CrossRef]
- Krahnstöver, T.; Santos, N.; Georges, K.; Campos, L.; Antizar-Ladislao, B. Low-Carbon Technologies to Remove Organic Micropollutants from Wastewater: A Focus on Pharmaceuticals. Sustainability 2022, 14, 11686. [Google Scholar] [CrossRef]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Removal of Pharmaceuticals and Personal Care Products in Aquatic Plant-Based Systems: A Review. Environ. Pollut. 2014, 184, 620–639. [Google Scholar] [CrossRef]
- Ndulini, S.F.; Sithole, G.M.; Mthembu, M.S. Investigation of Nutrients and Faecal Coliforms Removal in Wastewater Using a Hydroponic System. Phys. Chem. Earth Parts A/B/C 2018, 106, 68–72. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic Technology as Decentralised System for Domestic Wastewater Treatment and Vegetable Production in Urban Agriculture: A Review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A Review on Removing Pharmaceutical Contaminants from Wastewater by Constructed Wetlands: Design, Performance and Mechanism. Sci. Total Environ. 2014, 468–469, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Glennie, E.B.; Littlejohn, C.; Gendebien, A.; Hayes, A.; Palfrey, R.; Sivil, D.; Wright, K. Phosphates and Alternative Detergent Builders—Final Report; EU Environment Directorate: Swindon, Wiltshire, UK, 2002. [Google Scholar]
- Cardinale, M.; Ratering, S.; Suarez, C.; Zapata Montoya, A.M.; Geissler-Plaum, R.; Schnell, S. Paradox of Plant Growth Promotion Potential of Rhizobacteria and Their Actual Promotion Effect on Growth of Barley (Hordeum vulgare L.) under Salt Stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef]
- Dos Santos, N.O.; Teixeira, L.A.C.; Spadotto, J.C.; Campos, L.C. A Simple ZVI-Fenton Pre-Oxidation Using Steel-Nails for NOM Degradation in Water Treatment. J. Water Process Eng. 2021, 43, 102230. [Google Scholar] [CrossRef]
- Hay, J.; Khan, W.; Mead, A.J.C.; Seal, D.V.; Sugden, J.K. Membrane Filtration Method for Bacteriological Testing of Water: Enhanced Colony Visualization and Stability on Purification of Phenol Red Indicator. Lett. Appl. Microbiol. 1994, 18, 117–119. [Google Scholar] [CrossRef]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus Acquisition Efficiency Related to Root Traits: Is Mycorrhizal Symbiosis a Key Factor to Wheat and Barley Cropping? Front. Plant Sci. 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Porter, R.; Fitzsimons, D.W. The Use of Phosphate. In Phosphorus in the Environment: Its Chemistry and Biochemistry; Porter, R., Fitzsimons, D.W., Eds.; Novartis Foundation Symposia; John Wiley & Sons, Ltd.: Chichester, UK, 1978; Volume 57, pp. 253–268. ISBN 9780470720387. [Google Scholar]
- Ward, M.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Villanueva, C.; van Breda, S. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive Effects of Na+ and Cl− Ions on Barley Growth under Salinity Stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Sigma-Aldrich Detergent Properties and Applications. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biofiles/detergent-properties.html (accessed on 20 December 2022).
- Braga, J.K.; Varesche, M.B.A. Commercial Laundry Water Characterisation. Am. J. Anal. Chem. 2014, 05, 8–16. [Google Scholar] [CrossRef]
- Vardar, G.; Altıkatoğlu, M.; Ortaç, D.; Cemek, M.; Işıldak, İ. Measuring Calcium, Potassium, and Nitrate in Plant Nutrient Solutions Using Ion-Selective Electrodes in Hydroponic Greenhouse of Some Vegetables. Biotechnol. Appl. Biochem. 2015, 62, 663–668. [Google Scholar] [CrossRef]
- Johansen, C.; Edwards, D.G.; Loneragan, J.F. Interaction Between Potassium and Calcium in Their Absorption by Intact Barley Plants. I. Effects of Potassium on Calcium Absorption. Plant Physiol. 1968, 43, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, F.; Arzani, A.; Rahimmalek, M.; Sun, D.; Peng, J. Salinity Tolerance of Wild Barley Hordeum vulgare Ssp. Spontaneum. Plant Breed. 2020, 139, 304–316. [Google Scholar] [CrossRef]
- Zhou, G.; Johnson, P.; Ryan, P.R.; Delhaize, E.; Zhou, M. Quantitative Trait Loci for Salinity Tolerance in Barley (Hordeum vulgare L.). Mol. Breed. 2012, 29, 427–436. [Google Scholar] [CrossRef]
- Austin, Å.N.; Hansen, J.P.; Donadi, S.; Eklöf, J.S. Relationships between Aquatic Vegetation and Water Turbidity: A Field Survey across Seasons and Spatial Scales. PLoS ONE 2017, 12, e0181419. [Google Scholar] [CrossRef] [PubMed]
- Nahar, K.; Hoque, S. Phytoremediation to Improve Eutrophic Ecosystem by the Floating Aquatic Macrophyte, Water Lettuce (Pistia stratiotes L.) at Lab Scale. Egypt. J. Aquat. Res. 2021, 47, 231–237. [Google Scholar] [CrossRef]
- Abdul Aziz, N.I.H.; Mohd Hanafiah, M.; Halim, N.H.; Fidri, P.A.S. Phytoremediation of TSS, NH3-N and COD from Sewage Wastewater by Lemna Minor L., Salvinia Minima, Ipomea Aquatica and Centella Asiatica. Appl. Sci. 2020, 10, 5397. [Google Scholar] [CrossRef]
- Li, P.; Wang, C.; Liu, G.; Luo, X.; Rauan, A.; Zhang, C.; Li, T.; Yu, H.; Dong, S.; Gao, Q. A Hydroponic Plants and Biofilm Combined Treatment System Efficiently Purified Wastewater from Cold Flowing Water Aquaculture. Sci. Total Environ. 2022, 821, 153534. [Google Scholar] [CrossRef]
- García, J.; García-Galán, M.J.; Day, J.W.; Boopathy, R.; White, J.R.; Wallace, S.; Hunter, R.G. A Review of Emerging Organic Contaminants (EOCs), Antibiotic Resistant Bacteria (ARB), and Antibiotic Resistance Genes (ARGs) in the Environment: Increasing Removal with Wetlands and Reducing Environmental Impacts. Bioresour. Technol. 2020, 307, 123228. [Google Scholar] [CrossRef]
- Parnian, A.; Furze, J.N. Vertical Phytoremediation of Wastewater Using Vetiveria zizanioides L. Environ. Sci. Pollut. Res. 2021, 28, 64150–64155. [Google Scholar] [CrossRef]
- Kelvin, K.; Tole, M. The Efficacy of a Tropical Constructed Wetland for Treating Wastewater During the Dry Season: The Kenyan Experience. Water, Air, Soil Pollut. 2011, 215, 137–143. [Google Scholar] [CrossRef]
- Kato, T.; Kuroda, H.; Nakasone, H. Runoff Characteristics of Nutrients from an Agricultural Watershed with Intensive Livestock Production. J. Hydrol. 2009, 368, 79–87. [Google Scholar] [CrossRef]
- Goren, A.Y.; Yucel, A.; Sofuoglu, S.C.; Sofuoglu, A. Phytoremediation of Olive Mill Wastewater with Vetiveria zizanioides (L.) Nash and Cyperus alternifolius L. Environ. Technol. Innov. 2021, 24, 102071. [Google Scholar] [CrossRef]
- Wang, S.; Fan, W.; Zhang, H.; Zhao, X.; Bo, Y. Origin and Removal of Nitrite in Water. Hans J. Food Nutr. Sci. 2017, 06, 37–42. [Google Scholar] [CrossRef]
- Zou, J.; Wang, C.; Li, J.; Wei, J.; Liu, Y.; Hu, L.; Liu, H.; Bian, H.; Sun, D. Effect of Polyethylene (LDPE) Microplastic on Remediation of Cadmium Contaminated Soil by Solanum Nigrum L. J. Geosci. Environ. Prot. 2022, 10, 49–64. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Zain, M.; Yousef, N.; Yinghai, Z.; Azeem, K.; Zhou, P.; White, J.C.; et al. Microplastic and Nanoplastic Interactions with Plant Species: Trends, Meta-Analysis, and Perspectives. Environ. Sci. Technol. Lett. 2022, 9, 482–492. [Google Scholar] [CrossRef]
- Rozman, U.; Jemec Kokalj, A.; Dolar, A.; Drobne, D.; Kalčíková, G. Long-Term Interactions between Microplastics and Floating Macrophyte Lemna Minor: The Potential for Phytoremediation of Microplastics in the Aquatic Environment. Sci. Total Environ. 2022, 831, 154866. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; van Pelt, F.N.A.M.; O’Halloran, J.; Jansen, M.A.K. Adsorption, Uptake and Toxicity of Micro- and Nanoplastics: Effects on Terrestrial Plants and Aquatic Macrophytes. Environ. Pollut. 2021, 284, 117183. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, J.-J.; Gong, Y.-Y.; Li, Z.-J.; Zeng, E.Y. Strong Influence of Surfactants on Virgin Hydrophobic Microplastics Adsorbing Ionic Organic Pollutants. Environ. Pollut. 2020, 265, 115061. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics Accumulate on Pores in Seed Capsule and Delay Germination and Root Growth of the Terrestrial Vascular Plant Lepidium Sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the Commercial Seaweed Nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhang, G.; Liu, S.; Zou, H.; Wang, Z.; Zhu, W.; Geng, J. Interactive Effects of Microplastics and Selected Pharmaceuticals on Red Tilapia: Role of Microplastic Aging. Sci. Total Environ. 2021, 752, 142256. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wen, X.; Huang, D.; Zeng, G.; Deng, R.; Liu, R.; Zhou, Z.; Tao, J.; Xiao, R.; Pan, H. Microplastics Retention by Reeds in Freshwater Environment. Sci. Total Environ. 2021, 790, 148200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and Translocation of Nano/Microplastics by Rice Seedlings: Evidence from a Hydroponic Experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef] [PubMed]
- Urbina, M.A.; Correa, F.; Aburto, F.; Ferrio, J.P. Adsorption of Polyethylene Microbeads and Physiological Effects on Hydroponic Maize. Sci. Total Environ. 2020, 741, 140216. [Google Scholar] [CrossRef]
- Xu, D.; Yin, X.; Zhou, S.; Jiang, Y.; Xi, X.; Sun, H.; Wang, J. A Review on the Remediation of Microplastics Using Constructed Wetlands: Bibliometric, Co-Occurrence, Current Trends, and Future Directions. Chemosphere 2022, 303, 134990. [Google Scholar] [CrossRef]
- Dalmau-Soler, J.; Ballesteros-Cano, R.; Boleda, M.R.; Paraira, M.; Ferrer, N.; Lacorte, S. Microplastics from Headwaters to Tap Water: Occurrence and Removal in a Drinking Water Treatment Plant in Barcelona Metropolitan Area (Catalonia, NE Spain). Environ. Sci. Pollut. Res. 2021, 28, 59462–59472. [Google Scholar] [CrossRef]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and Fate of Microplastics at Two Different Drinking Water Treatment Plants within a River Catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and Removal of Microplastics in an Advanced Drinking Water Treatment Plant (ADWTP). Sci. Total Environ. 2020, 700, 134520. [Google Scholar] [CrossRef]
- Singh, N.; Mondal, A.; Bagri, A.; Tiwari, E.; Khandelwal, N.; Monikh, F.A.; Darbha, G.K. Characteristics and Spatial Distribution of Microplastics in the Lower Ganga River Water and Sediment. Mar. Pollut. Bull. 2021, 163, 111960. [Google Scholar] [CrossRef]
- Jiang, N.; Luo, W.; Zhao, P.; Ga, B.; Jia, J.; Giesy, J.P. Distribution of Microplastics in Benthic Sediments of Qinghai Lake on the Tibetan Plateau, China. Sci. Total Environ. 2022, 835, 155434. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Sahoo, B.K.; Das, A.; Nag, S.K.; Manna, R.K.; Behera, B.K.; Samanta, S. Occurrence, Fate and Removal of Microplastics as Heavy Metal Vector in Natural Wastewater Treatment Wetland System. Water Res. 2021, 192, 116853. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Cárdenas, A.; O’Halloran, J.; van Pelt, F.N.A.M.; Jansen, M.A.K. Rapid Fragmentation of Microplastics by the Freshwater Amphipod Gammarus duebeni (Lillj.). Sci. Rep. 2020, 10, 12799. [Google Scholar] [CrossRef] [PubMed]
- van Weert, S.; Redondo-Hasselerharm, P.E.; Diepens, N.J.; Koelmans, A.A. Effects of Nanoplastics and Microplastics on the Growth of Sediment-Rooted Macrophytes. Sci. Total Environ. 2019, 654, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).