Evaluation of Soil Organic Carbon Stability in Different Land Uses in Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils and Study Sites
2.2. Soil Sampling and Analysis
2.3. Calculations and Statistics
3. Results
3.1. Physical and Chemical Soil Indicators in Different Land Use
3.2. Carbon and Nitrogen Concentrations in Soil Microbial Biomass and Microbial Respiration
3.3. Relationships between Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biermann, F.; Hickmann, T.; Sénit, C.A.; Beisheim, M.; Bernstein, S.; Chasek, P.; Grob, L.; Kim, R.E.; Kotzé, L.J.; Nilsson, M.; et al. Scientific evidence on the political impact of the Sustainable Development Goals. Nat. Sustain. 2022, 5, 795–800. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lützow, M.; Martin-Spiotta, E.; van Weswmael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Jensen, J.L.; Schjønning, P.; Watts, C.W.; Christensen, B.T.; Peltre, C.; Munkholm, L.J. Relating soil C and organic matter fractions to soil structural stability. Geoderma 2019, 337, 834–843. [Google Scholar] [CrossRef]
- Laganière, J.; Angers, D.A.; Paré, D. Carbon accumulation in agricultural soils after afforestation: A meta-analysis. Glob. Chang. Biol. 2020, 16, 439–453. [Google Scholar] [CrossRef]
- Guillaume, T.; Makowski, D.; Libohova, Z.; Bragazza, L.; Sallaku, F.; Sinaj, S. Soil organic carbon saturation in cropland-grassland systems: Storage potential and soil quality. Geoderma 2022, 406, 115529. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, B.; Dijkstra, F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Murphy, B.W. Impact of soil organic matter on soil properties—A review with emphasis on Australian soils. Soil Res. 2015, 53, 605–635. [Google Scholar] [CrossRef]
- Soucémarianadin, L.N.; Cécillon, L.; Guenet, B.; Chenu, C.; Baudin, F.; Nicolas, M.; Girardin, C.; Barré, P. Environmental factors controlling soil organic carbon stability in French forest soils. Plant Soil 2018, 426, 267–286. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, V.K. Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ. 2018, 634, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Garau, G.; Morillas, L.; Roales, J.; Castaldi, P.; Mangia, N.P.; Spano, D.; Mereu, S. Effect of monospecific and mixed Mediterranean tree plantations on soil microbial community and biochemical functioning. Appl. Soil Ecol. 2019, 140, 78–88. [Google Scholar] [CrossRef]
- Houfani, A.A.; Větrovsky, T.; Navarrete, O.U.; Štursová, M.; Tláskal, V.; Beiko, R.G.; Boucherba, N.; Baldrian, P.; Benallaoua, S.; Jorquera, M.A. Cellulase-Hemicellulase Activities and Bacterial Community Composition of Different Soils from Algerian Ecosystems. Microb. Ecol. 2019, 77, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Creamer, C.A.; Menezes, A.B.; Krull, E.S.; Sanderman, J.; Newton-Walters, R.; Farrell, M. Microbial community structure mediates response of soil C decomposition to litter addition and warming. Soil Biol. Biochem. 2015, 80, 175–188. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; McGuire, A.D.; Schädel, C.; Grosse, G.; Harden, J.W.; Hayes, D.J.; Hugelius, G.; Koven, C.D.; Kuhry, P.; Lawrence, D.M.; et al. Climate change and the permafrost carbon feedback. Nature 2015, 520, 171–179. [Google Scholar] [CrossRef]
- Högberg, M.N.; Högberg, P.; Myrold, D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 2007, 150, 590–601. [Google Scholar] [CrossRef]
- Drum, C.G.; Vogel, J.G.; Gezan, S.A.; Jokela, E.J. Belowground processes for two loblolly pine (Pinus taeda L.) families respond differently to the intensity of plantation management. For. Ecol. Manag. 2019, 441, 293–301. [Google Scholar] [CrossRef]
- Walkiewicz, A.; Bieganowski, A.; Rafalska, A.; Khalil, M.I.; Osborne, B. Contrasting effects of forest type and stand age on soil microbial activities: An analysis of local scale variability. Biology 2021, 10, 850. [Google Scholar] [CrossRef]
- IPCC. 2006 IPCC Guidelines for National Greenhouse Gas Inventories. In Prepared by the National Greenhouse Gas Inventories Programme; Eggleston, H.S., Buendia, L., Miwa, K., Ngara, T., Tanabe, K., Eds.; IGES: Kanagawa, Japan, 2006. [Google Scholar]
- WRB World Reference Base for Soil Resources 2014, Update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015; p. 192.
- Muraškienė, M. Evaluation of Soil Microbial Biomass Carbon and Nitrogen in Lithuania in Different Land Use. Ph.D. Dissertation, Lithuanian Research Centre for Agriculture and Forestry, Kaunas, Lithuania, 2023; p. 164, (In Lithuanian with English Summary). [Google Scholar]
- ISO 11272; Soil Quality—Determination of Dry Bulk Density. International Organization for Standardization: Geneva, Switzerland, 1998; pp. 1–10.
- ISO 11277-2020; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material–Method by Sieving and Sedimentation. International Organization for Standardization: Geneva, Switzerland, 2020; 38p.
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005; pp. 1–7.
- ISO 11261; Soil Quality—Determination of Total Nitrogen–Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995; pp. 1–4.
- ISO 10694; Soil Quality—Determination of Organic and Total Carbon after Dry Combustion (Elementary Analysis). International Organization for Standardization: Geneva, Switzerland, 1995; pp. 1–7.
- Faithfull, N.T. Methods in agricultural chemical analysis. In A Practical Handbook; University of Wales: Aberystwyth, UK, 2002; p. 289. [Google Scholar]
- ISO 8245; Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC). International Organization for Standardization: Geneva, Switzerland, 1999; pp. 1–11.
- ISO 14240–2; Soil Quality—Determination of Soil Microbial Biomass. Part 2: Fumigation–Extraction Method. International Organization for Standardization: Geneva, Switzerland, 1997; pp. 1–12.
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- ISO 16072; Soil Quality–Laboratory Methods for Determination of Microbial Soil Respiration. International Organization for Standardization: Geneva, Switzerland, 2002; pp. 1–19.
- Schinner, F.; Ohlinger, R.; Kandler, E.; Margesin, R. Bodenbiologische Arbeitsmethoden; Springer: Berlin, Germany, 1993; p. 640. (In German) [Google Scholar]
- Vesterdal, L.; Schmidt, I.K.; Callesen, I.; Nilson, L.O.; Gundersen, P. Carbon and nitrogen in forest floor and mineral soil under six common European tree species. For. Ecol. Manag. 2008, 255, 35–48. [Google Scholar] [CrossRef]
- Armolaitis, K.; Varnagirytė-Kabašinskienė, I.; Žemaitis, P.; Stakėnas, V.; Beniušis, R.; Kulbokas, G.; Urbaitis, G. Evaluation of organic carbon stocks in mineral and organic soils in Lithuania. Soil Use Manag. 2022, 38, 355–368. [Google Scholar] [CrossRef]
- Pranskevičius, M. Research and Assessment of Total Carbon Content and Carbon Dioxide Emissions from Soils of Different Land-Use Purpose. PhD Dissertation, Lithuanian Research Centre for Agriculture and Forestry, Kėdainiai, Lithuania, 2011; p. 140. (In Lithuanian). [Google Scholar]
- Jílková, V.; Straková, P.; Frouz, J. Foliage C:N ratio, stage of organic matter decomposition and interaction with soil affect microbial respiration and its response to C and N addition more than C:N changes during decomposition. Appl. Soil Ecol. 2020, 152, 103568. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Li, Y.; Liu, Y.; Xu, J.; Di, H. Effects of long-term mowing on the fractions and chemical composition of soil organic matter in a semiarid grassland. Biogeosciences 2017, 14, 2685–2696. [Google Scholar] [CrossRef]
- Mayer, M.; Prescott, C.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 1–25. [Google Scholar] [CrossRef]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Song, Y.; Liu, C.; Song, C.; Wang, X.; Ma, X.; Gao, J.; Gao, S.; Wang, L. Linking soil organic carbon mineralization with soil microbial and substrate properties under warming in permafrost peatlands of Northeastern China. Catena 2021, 203, 105348. [Google Scholar] [CrossRef]
- Anayeva, N.D.; Polyanskaya, L.M.; Susyan, E.A.; Vasenkina, I.V.; Wirth, S.; Zvyagintsev, D.G. Comparative assessment of soil microbial biomass determined by the methods of direct microscopy and substrate-induced respiration. Microbiology 2008, 77, 404–412. [Google Scholar]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017, 7, 44641. [Google Scholar] [CrossRef]
- Bradford, M.A.; McCulley, R.L.; Crowther, T.W.; Oldfield, E.E.; Wood, S.A.; Fierer, N. Cross-biome patterns in soil microbial respiration predictable from evolutionary theory on thermal adaptation. Nat. Ecol. Evol. 2019, 3, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ayres, E.; Stelzer, H.; Berg, S.; Wallenstein, M.D.; Simmons, B.L.; Wall, D.H. Tree species traits influence soil physical, chemical, and biological properties in high elevation forests. PLoS ONE 2009, 4, e5964. [Google Scholar] [CrossRef] [PubMed]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]
- Woloszczyk, P.; Fiencke, C.; Elsner, D.C.; Gordsen, E.; Pfeiffer, E.M. Spatial and temporal patterns in soil organic carbon, microbial biomass and activity under different land-use types in a long-term soil-monitoring network. Pedobiologia 2020, 80, 150642. [Google Scholar] [CrossRef]
- Mendoza, B.; Béjar, J.; Luna, D.; Osorio, M.; Jimenez, M.; Melendez, J.R. Differences in the ratio of soil microbial biomass carbon (MBC) and soil organic carbon (SOC) at various altitudes of Hyperalic alisol in the Amazon region of Ecuador. F1000Reserch 2020, 9, 443. [Google Scholar] [CrossRef]
- Lepcha, N.T.; Devi, N.B. Effect of land use, season, and soil depth on soil microbial biomass carbon of Eastern Himalayas. Ecol. Process. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Dyckmans, J.; Joergensen, R.G.; Pries, J.; Raubuch, M.; Sehy, U. Adenylates as an estimate of microbial biomass C in different soil groups. Soil Biol. Biochem. 2003, 35, 1485–1491. [Google Scholar] [CrossRef]
- Susyan, E.A.; Wirth, S.; Ananyeva, N.D.; Stolnikova, E.V. Forest succession on abandoned arable soils in European Russia—Impacts on microbial biomass, fungal-bacterial ratio, and basal CO2 respiration activity. Eur. J. Soil Biol. 2011, 47, 169–174. [Google Scholar] [CrossRef]
- Frieder, J.K.; Scheller, E. Composition of hydrolysable amino acids in soil organic matter and soil microbial biomass. Soil Biol. Biochem. 2002, 34, 315–325. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Soil microbial biomass: The eco-physiological approach. Soil Biol. Biochem. 2010, 42, 2039–2043. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; Brussaard, L. Soil quality A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Li, J.; Zhao, B.; Li, X.; Jiang, R.; So, H.B. Effects of long-term combined application of organic and mineral fertilizers on microbial biomass, soil enzyme activities and soil fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar] [CrossRef]
- Schweizer, S.A.; Mueller, C.W.; Höschen, C.; Ivanov, P.; Kögel-Knabner, I. The role of clay content and mineral surface area for soil organic carbon storage in an arable toposequence. Biogeochemistry 2021, 156, 401–420. [Google Scholar] [CrossRef]
- Poeplau, C.; Jacobs, A.; Don, A.; Vos, C.; Schneider, F.; Wittnebel, M.; Tiemeyer, M.; Heidkamp, A.; Prietz, R.; Flessa, H. Stocks of organic carbon in German agricultural soils—Key results of the first comprehensive inventory. J. Plant Nutr. Soil Sci. 2020, 183, 665–681. [Google Scholar] [CrossRef]
- Lavallee, J.; Soong, J.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Rocci, K.S.; Lavallee, J.M.; Steward, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 731, 148569. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Anderson, T.H.; Wolters, V. Carbon and nitrogen relationships in the microbial biomass of soils in beech (Fagus sylvatica L.) forests. Biol. Fertil. Soils 1995, 19, 141–147. [Google Scholar] [CrossRef]
- Wild, B.; Schnecker, J.; Knoltsch, A.; Takriti, M.; Mooshammer, M.; Gentsch, N.; Mikutta, R.; Alves, R.J.E.; Gittel, A.; Lashchinskiy, N.; et al. Microbial nitrogen dynamics in organic and mineral soil horizons along a latitudinal transect in western Siberia. Glob. Biogeochem. Cycles 2015, 29, 567–582. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, W.; Wang, J.; Wang, Z.; Zhou, Z.; Liu, S. No-tillage combined with appropriate amount of straw returning increased soil biochemical properties. Sustainability 2022, 14, 4875. [Google Scholar] [CrossRef]
- Partey, S.T.; Preziosi, R.F.; Robson, G.D. Improving maize residue use in soil fertility restoration by mixing with residues of low C-to-N ratio: Effects on C and N mineralization and soil microbial biomass. Soil Sci. Plant. Nutr. 2014, 14, 518–531. [Google Scholar] [CrossRef]
- Hannula, E.S.; Morriën, E. Will fungi solve the carbon dilemma? Geoderma 2022, 413, 115767. [Google Scholar] [CrossRef]
- Brust, G.E. Management Strategies for Organic Vegetable Fertility. In Safety and Practice for Organic Food; Biswas, D., Micallef, S.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 193–212. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Campiglia, E.; Grego, S. Chemical and biological indicators of soil quality in organic and conventional farming systems in Central Italy. Ecol. Indic. 2006, 6, 701–711. [Google Scholar] [CrossRef]
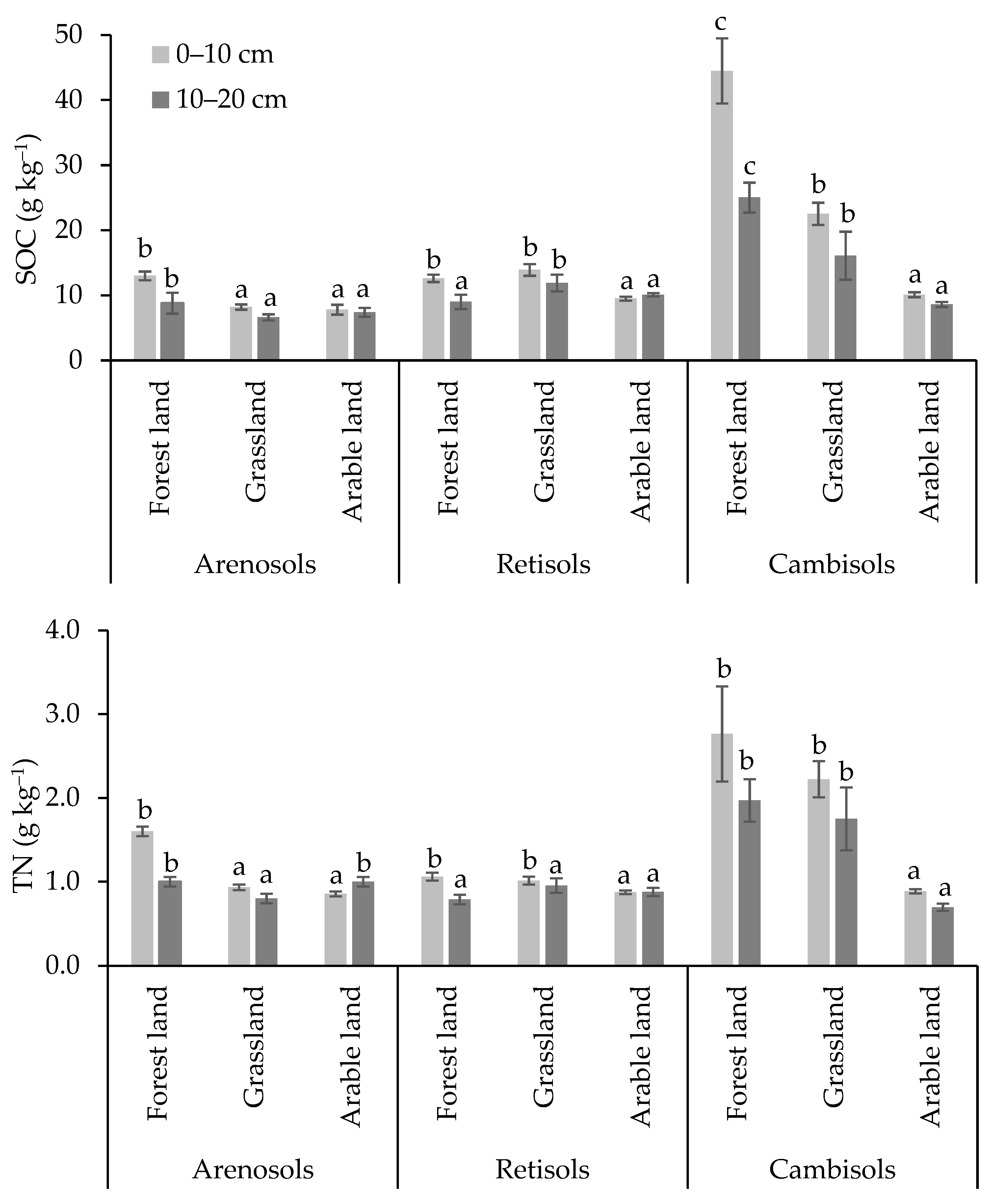
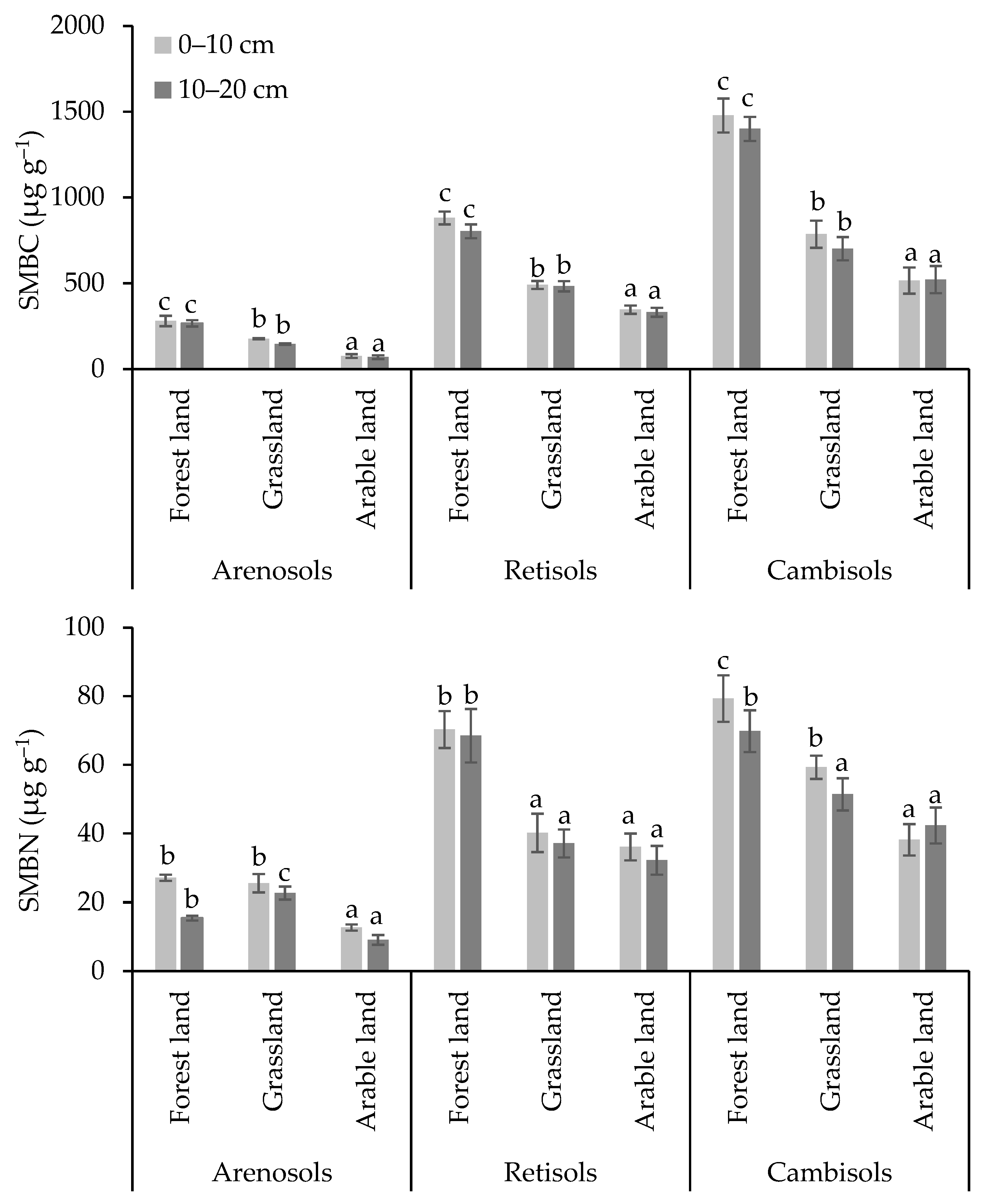

| Study Site | Soil [21] | Study Plot Description |
|---|---|---|
| Site 1, 54°10′ N, 24°25′ E | Haplic Arenosol | Forest land: 90% Betula pendula Roth; 10% Pinus sylvestris L. Stand age 50 years; mean tree diameter (DBH) 17 cm; mean tree height (H) 20.9 m; stand volume 135 m3 ha−1. |
| Perennial grassland: Hieracium pilosella L., Oenothera biennis L., Achillea millefolium L., Fragaria vesca L. | ||
| Arable land: Secale cereale L. | ||
| Site 2, 55°41′ N, 21°30′ E | Dystric Bathygleyic Glossic Retisol | Forest land: 90% Betula pendula; 10% Picea abies (L.) H. Karst.; naturally regenerated forest on abandoned agricultural land. Stand age 25 years; mean tree DBH 14 cm; mean tree H 15.3 m; stand volume 93 m3 ha−1. |
| Perennial grassland: Achillea millefolium L., Hieracium pilosella L., Campanula patula L., Holcus lanatus L. | ||
| Arable land: Secale cereale | ||
| Site 3, 55°41′ N, 21°30′ E | Endocalcari- Epihypogleyic Cambisol | Forest land: 70% Betula pendula; 20% Populus tremula L.; 10% Quercus robur L. Stand age 92 years; mean tree DBH 36 cm; mean tree H 30.1 m; mean stand volume 378 m3 ha−1. |
| Perennial grassland: Medicago sativa L., Taraxacum officinale L., Galega orientalis L., Lolium temulentum L., Trifolium repens L. | ||
| Arable land: Pisum sativum L. |
| Land Use | SOC Stocks (t ha–1) | TN Stocks (t ha–1) | ||
|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| Arenosols | ||||
| Forest land | 16.3 ± 1.8 b | 13.6 ± 2.6 b | 2.0 ± 0.1 b | 1.5 ± 0.1 b |
| Grassland | 11.2 ± 1.0 a | 8.5 ± 0.4 a | 1.3 ± 0.1 a | 1.0 ± 0.1 a |
| Arable land | 11.6 ± 0.4 a | 10.4 ± 0.7 a | 1.3 ± 0.0 a | 1.4 ± 0.1 a |
| Retisols | ||||
| Forest land | 18.5 ± 1.4 b | 14.3 ± 1.9 a | 1.5 ± 0.1 b | 1.3 ± 0.1 a |
| Grassland | 20.2 ± 1.4 b | 18.6 ± 1.6 b | 1.5 ± 0.1 b | 1.5 ± 0.1 a |
| Arable land | 13.5 ± 0.6 a | 16.3 ± 0.2 a | 1.2 ± 0.0 a | 1.4 ± 0.1 a |
| Cambisols | ||||
| Forest land | 46.5 ± 5.2 c | 27.3 ± 2.5 b | 2.7 ± 0.6 b | 2.1 ± 0.3 b |
| Grassland | 32.6 ± 6.6 b | 25.3 ± 5.6 b | 3.2 ± 0.2 b | 2.8 ± 0.6 b |
| Arable land | 15.9 ± 0.8 a | 14.5 ± 0.7 a | 1.4 ± 0.1 a | 1.2 ± 0.1 a |
| Land Use | Fulvic Acid (%) | Humic Acid (%) | Insoluble Residue (%) | WEOC (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| Arenosols | ||||||||
| Forest land | 0.74 ± 0.14 b | 0.73 ± 0.15 b | 0.22 ± 0.06 b | 0.18 ± 0.01 c | 99.04 ± 0.03 a | 99.09 ± 0.08 a | 527 ± 26 c | 367 ± 174 a |
| Grassland | 0.50 ± 0.13 a | 0.48 ± 0.06 a | 0.15 ± 0.01 a | 0.08 ± 0.00 a | 99.35 ± 0.10 b | 99.44 ± 0.04 b | 457 ± 50 b | 316 ± 47 a |
| Arable land | 0.39 ± 0.02 a | 0.40 ± 0.02 a | 0.15 ± 0.01 a | 0.14 ± 0.01 b | 99.46 ± 0.05 b | 99.46 ± 0.05 b | 353 ± 41 a | 346 ± 47 a |
| Retisols | ||||||||
| Forest land | 0.13 ± 0.01 b | 0.10 ± 0.00 b | 0.35 ± 0.07 b | 0.29 ± 0.02 b | 99.52 ± 0.07 a | 99.16 ± 0.03 a | 627 ± 20 b | 463 ± 22 a |
| Grassland | 0.11 ± 0.02 b | 0.10 ± 0.01 b | 0.32 ± 0.01 b | 0.29 ± 0.03 b | 99.58 ± 0.01 a | 99.61 ± 0.04 a | 657 ± 43 b | 587 ± 3 b |
| Arable land | 0.07 ± 0.00 a | 0.07 ± 0.00 a | 0.19 ± 0.03 a | 0.22 ± 0.02 a | 99.74 ± 0.03 b | 99.72 ± 0.03 b | 423 ± 43 a | 440 ± 40 a |
| Cambisols | ||||||||
| Forest land | 0.49 ± 0.02 c | 0.26 ± 0.06 c | 1.20 ± 0.15 c | 0.57 ± 0.07 c | 98.31 ± 0.17 a | 99.17 ± 0.08 a | 1727 ± 23 c | 1073 ± 201 c |
| Grassland | 0.16 ± 0.01 b | 0.12 ± 0.01 b | 0.48 ± 0.03 b | 0.24 ± 0.03 b | 99.36 ± 0.02 a | 99.64 ± 0.04 b | 1210 ± 35 b | 853 ± 28 b |
| Arable land | 0.05 ± 0.01 a | 0.06 ± 0.00 a | 0.17 ± 0.01 a | 0.14 ± 0.02 a | 99.81 ± 0.04 b | 99.82 ± 0.02 c | 546 ± 42 a | 407 ± 34 a |
| Land Use | SOC:TN | SMBC/SOC (%) | SMBN/TN (%) | SMBC:SMBN | ||||
|---|---|---|---|---|---|---|---|---|
| 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | 0–10 cm | 10–20 cm | |
| Arenosols | ||||||||
| Forest land | 8.2 ± 0.6 a | 8.9 ± 1.7 a | 2.2 ± 0.1 b | 3.1 ± 0.1 c | 1.0 ± 0.0 a | 1.6 ± 0.0 b | 17.6 ± 2.0 b | 17.4 ± 1.1 b |
| Grassland | 8.8 ± 0.2 a | 8.4 ± 0.9 a | 2.5 ± 0.1 b | 2.3 ± 0.1 b | 2.7 ± 0.2 c | 2.8 ± 0.3 c | 7.9 ± 0.9 a | 6.7 ± 1.0 a |
| Arable land | 9.1 ± 0.3 a | 7.4 ± 0.3 a | 1.0 ± 0.0 a | 0.9 ± 0.0 a | 1.5 ± 0.1 b | 0.9 ± 0.1 a | 6.0 ± 1.3 a | 7.6 ± 1.2 a |
| Retisols | ||||||||
| Forest land | 11.9 ± 0.2 a | 11.4 ± 0.6 a | 6.9 ± 0.5 b | 7.8 ± 0.5 c | 5.6 ± 0.4 b | 6.7 ± 0.5 c | 12.5 ± 1.7 b | 12.7 ± 1.6 b |
| Grassland | 13.7 ± 0.3 b | 12.5 ± 0.6 a | 3.5 ± 0.1 a | 4.1 ± 0.1 b | 4.1 ± 0.1 a | 3.9 ± 0.2 b | 12.2 ± 1.6 b | 13.0 ± 2.3 b |
| Arable land | 10.8 ± 0.3 a | 11.6 ± 0.4 a | 3.7 ± 0.1 a | 3.3 ± 0.3 a | 4.2 ± 0.5 a | 2.9 ± 0.5 a | 9.6 ± 1.9 a | 9.7 ± 1.5 a |
| Cambisols | ||||||||
| Forest land | 17.2 ± 2.4 b | 12.8 ± 0.7 b | 3.3 ± 0.2 a | 5.9 ± 0.4 b | 2.9 ± 0.0 a | 4.1 ± 0.0 b | 18.6 ± 2.5 b | 18.4 ± 2.6 b |
| Grassland | 9.9 ± 1.4 a | 9.3 ± 0.9 a | 3.5 ± 0.1 a | 4.4 ± 0.2 a | 2.7 ± 0.1 a | 2.9 ± 0.2 a | 13.3 ± 2.6 a | 13.6 ± 1.5 a |
| Arable land | 11.3 ± 0.2 a | 12.5 ± 0.7 b | 5.1 ± 0.3 b | 6.1 ± 0.4 b | 3.3 ± 0.1 b | 4.1 ± 0.4 b | 13.5 ± 2.1 a | 12.3 ± 1.2 a |
| Soil Quality Indicators | Variation Component (%) | Significance of Soil Layer | |
|---|---|---|---|
| Soil Type | Land Use | ||
| BD (g cm–3) | 0.0 | 71.7 | ** |
| Sand (%) | 87.8 | 9.3 | – |
| Silt (%) | 86.4 | 10.5 | – |
| Clay (%) | 74.7 | 14.7 | – |
| Soil pHCaCl2 | 66.6 | 15.6 | * |
| SOC (g kg−1) | 29.5 | 45.9 | ** |
| TN (g kg−1) | 29.5 | 45.9 | ** |
| SOC:TN | 36.2 | 32.2 | – |
| Humic acid (%) | 18.9 | 61.8 | *** |
| Fulvic acid (%) | 83.1 | 9.7 | ** |
| Insoluble residue (%) | 14.0 | 61.1 | *** |
| WEOC (mg kg−1) | 39.4 | 41.0 | ** |
| SMBC (µg g−1) | 70.5 | 19.1 | * |
| SMBN (µg g−1) | 87.6 | 10.4 | *** |
| Microbial CO2 (mg g−1 per day) | 71.5 | 25.8 | *** |
| Soil Quality Indicators | BD | SOC | TN | SOC:TN | pHCaCl2 | Sand | Silt | Clay | Humic Acid | Fulvic Acid | Insoluble Residue | WEOC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g cm−3) | (g kg−1) | (%) | (%) | (mg kg−1) | ||||||||
| All soil types | ||||||||||||
| SMBC * | −0.29 ** | 0.80 | 0.69 | 0.58 | 0.28 | −0.53 | 0.51 | 0.47 | 0.78 | −0.27 | −0.38 | 0.74 |
| SMBN | −0.12 | 0.67 | 0.56 | 0.58 | 0.35 | −0.68 | 0.65 | 0.61 | 0.69 | −0.39 | −0.23 | 0.67 |
| Microbial CO2 | −0.28 | 0.83 | 0.70 | 0.68 | 0.34 | −0.58 | 0.54 | 0.54 | 0.83 | −0.36 | −0.35 | 0.83 |
| Arenosols | ||||||||||||
| SMBC | 0.02 | 0.49 | 0.50 | −0.08 | −0.03 | −0.39 | 0.40 | 0.23 | 0.19 | 0.87 | −0.81 | 0.11 |
| SMBN | −0.02 | 0.43 | 0.51 | −0.21 | 0.12 | −0.24 | 0.25 | 0.10 | 0.16 | 0.82 | −0.76 | 0.14 |
| Microbial CO2 | −0.19 | 0.44 | 0.36 | 0.12 | −0.52 | −0.09 | 0.04 | 0.38 | 0.56 | −0.09 | −0.09 | 0.47 |
| Retisols | ||||||||||||
| SMBC | 0.01 | 0.14 | 0.18 | 0.02 | −0.61 | −0.71 | 0.70 | 0.57 | 0.61 | 0.71 | −0.69 | 0.32 |
| SMBN | 0.03 | −0.01 | 0.07 | −0.13 | −0.64 | −0.58 | 0.57 | 0.51 | 0.44 | 0.60 | −0.52 | 0.17 |
| Microbial CO2 | −0.21 | 0.75 | 0.62 | 0.67 | −0.04 | −0.81 | 0.80 | 0.64 | 0.60 | 0.78 | −0.65 | 0.67 |
| Cambisols | ||||||||||||
| SMBC | −0.87 | 0.81 | 0.74 | 0.38 | −0.74 | 0.06 | 0.31 | −0.43 | 0.75 | 0.82 | −0.78 | 0.71 |
| SMBN | −0.90 | 0.84 | 0.80 | 0.42 | −0.82 | 0.20 | 0.09 | −0.45 | 0.85 | 0.86 | −0.86 | 0.79 |
| Microbial CO2 | −0.92 | 0.90 | 0.84 | 0.48 | −0.81 | 0.11 | 0.20 | −0.42 | 0.91 | 0.93 | −0.92 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muraškienė, M.; Armolaitis, K.; Varnagirytė-Kabašinskienė, I.; Baliuckas, V.; Aleinikovienė, J. Evaluation of Soil Organic Carbon Stability in Different Land Uses in Lithuania. Sustainability 2023, 15, 16042. https://doi.org/10.3390/su152216042
Muraškienė M, Armolaitis K, Varnagirytė-Kabašinskienė I, Baliuckas V, Aleinikovienė J. Evaluation of Soil Organic Carbon Stability in Different Land Uses in Lithuania. Sustainability. 2023; 15(22):16042. https://doi.org/10.3390/su152216042
Chicago/Turabian StyleMuraškienė, Milda, Kęstutis Armolaitis, Iveta Varnagirytė-Kabašinskienė, Virgilijus Baliuckas, and Jūratė Aleinikovienė. 2023. "Evaluation of Soil Organic Carbon Stability in Different Land Uses in Lithuania" Sustainability 15, no. 22: 16042. https://doi.org/10.3390/su152216042
APA StyleMuraškienė, M., Armolaitis, K., Varnagirytė-Kabašinskienė, I., Baliuckas, V., & Aleinikovienė, J. (2023). Evaluation of Soil Organic Carbon Stability in Different Land Uses in Lithuania. Sustainability, 15(22), 16042. https://doi.org/10.3390/su152216042






