Abstract
The aim of the study is to determine the effectiveness of nano-silicon dioxide (NaSiO2) application on the tolerance of strawberry plants exposed to drought stress under in vitro conditions. Drought stress was induced with polyethylene glycol (PEG-6000). In the experiment, the effects of PEG 6000 concentrations (0, 4, 8%) and NaSiO2 concentrations (0, 50, 100 mg L−1) on in vitro strawberry plants were determined. Plants treated with PEG 6000 showed reduced vegetative growth parameters, but this decrease was reduced with NaSiO2 application. The addition of NaSiO2 enhanced shoot and leaf growth, SPAD index, and the leaf relative water content (LRWC) of in vitro strawberry plants. NaSiO2 at 50 mg L−1 induced the maximum shoot and root fresh weight (1.20 g, 1.24 g, respectively) and length (40.09 mm, 34.26 mm, respectively), leaves number (16.67 pieces/plant) and SPAD index 53.57 among 4% and 8% PEG applications. When the superoxide dismutase (SOD) and catalase (CAT) activities were examined, the results showed that the application of NaSiO2 enhanced drought stress tolerance by promoting certain antioxidant responses by increasing SOD and CAT activities under drought stress. Our results suggest that the application of NaSiO2 can help maintain the devastating impact of drought stress and markedly enhance all the examined parameters in the Albion strawberry cultivar under in vitro conditions.
1. Introduction
The climate crisis has a significant impact on agricultural production and reported that its effects will increase significantly in the next 20 years [1]. Changing climatic conditions such as floods, droughts, and extreme temperatures lead to a decrease in the productivity of arable agricultural lands and agricultural products [2,3]. In order to ensure sustainability in crop production, it is necessary to take measures against the rapidly increasing arid conditions. The most influential factor on agricultural production is drought, which affects plant growth, causes serious decreases in yield, and negatively affects sustainable productivity [2,3,4]. Considering that approximately 80% of water resources are currently consumed by irrigated agriculture [5,6], it is seen that agricultural production is under serious threat due to increasingly arid conditions. In order to minimize the effects of agricultural drought and ensure sustainability, it is necessary to take measures and develop appropriate strategies as soon as possible.
Drought reduces the leaf’s relative water content, membrane stability index, net CO2 assimilation rate, stomatal conductivity, transpiration, and chlorophyll content [7,8,9] and therefore decreases the root and leaf growth [10,11,12,13], fresh and dry masses, leaf area and number [14] in plants.
Strawberries (Fragaria x ananassa Duch.), which are defined as a functional food source beneficial to human health due to its rich content of antioxidants, polyphenols, fiber, vitamins, and many nutritional elements [15,16,17], are the most consumed berry fruit in the world [18]. This herbaceous plant is affected by environmental factors and frequently exposed to abiotic stress [8,19]. In arid and semi-arid areas, problems such as low precipitation, high evaporation, and temperature, negatively affect strawberry cultivation [20,21]. It has been reported in various studies that the growth and development of strawberry plants are affected by drought [8,9,10,11,12,22]. Today, biotechnological methods are widely used in agricultural production, and in vitro propagation techniques have been widely implemented for efficient strawberry production [23]. In vitro systems are rapid screening experiments that focus on quantitative features [24]. In addition, PEG can be used as an osmotic regulator in in vitro systems to simulate drought stress, as it reduces water potentiation [25,26].
The measures that can be taken to mitigate the negative effects of drought are limited. One of the methods that can facilitate crop production under drought-stress conditions is to develop drought-tolerant plant varieties through breeding methods. However, this method is not advantageous in terms of time and cost. In recent years, various exogenous applications of organic solutions or some NPs that allow the increase of plant tolerance to abiotic stress conditions have attracted attention [3,8,10,11,27,28,29]. Some nanoparticle applications, which have a less harmful environmental impact compared to many conventional products, are known to have the potential to increase tolerance to drought [10,27,30,31]. External applications of nanoparticles (NPs) contribute to strong root tissue formation, regulation of antioxidant enzyme activity, and cellular water balance [7,27,30,32,33]. One of the precursors of these nanoparticles is SiO2, which has recently been shown to play an effective role in regulating various mechanisms involved in abiotic stress in plants [34,35,36,37,38]. Silicon (Si) is recognized as one of the most valuable elements for plant life [39]. NaSi supplementation affects the activation of photosynthetic enzymes, the activation of antioxidant defense systems, and increases water use efficiency, root growth, and hydraulic conductivity, thus it can be effective in eliminating the negative effects of abiotic stress conditions and contribute to vegetative growth [7,10,40,41]. Avestan et al. investigated the effects of NaSiO2 on strawberry plants under salt stress conditions and reported that nanosilicon application suppressed the negative effect of salinity on anatomical and physiological changes in plants [42]. Zahedi et al. elucidated the effect of SiO2 and NasiO2 on modulating the adverse effects of drought stress on strawberry plants grown in a greenhouse [43]. They announced that foliar application of SiO2 and NaSiO2 increased osmolytes and improved chlorophylls in strawberry plants under drought stress. The researchers also noted that strawberry plants exposed to drought stress treated with Si showed an improvement in antioxidant activity and phenolic compounds as non-enzymatic antioxidants. Seyed Hajizadeh et al. studied the effect of NaSiO2 under drought conditions simulated with PEG in Damask rose genotypes (Maragheh and Kashan) under in vitro culture [44]. In the study, it was reported that drought stress caused a significant decrease in chlorophyll, fresh/dry weight, and biomass, but NaSiO2 application improved the leaf area index (LAI), chlorophyll, and biomass under severe water deficiency. Sharf-Eldin et al. investigated the response of corn seedlings to NaSiO2 application under drought stress and reported that 0.25 g L−1 foliar application of NaSiO2 provided a significant improvement in the growth index of corn plants exposed to drought stress [45]. Researchers also declared that these particles caused a significant increase in chlorophyll, proline, cell membrane integrity, leaf water content, SOD, CAT, and guaiacol peroxidase (G-POX) levels. Sohby et al. evaluated the effect of nano-silicone and nano-chitosan on two citrus rootstocks grown under salt stress and determined that these two nanoparticles significantly improved full vegetative growth parameters and antioxidant defense enzymes. In this study, it was aimed to determine the effectiveness of NaSiO2 on the tolerance of ‘Albion’ strawberry cultivar exposed to drought stress in vitro conditions [46].
2. Materials and Methods
2.1. Plant Material
‘Albion’ strawberry cultivar was used as the donor plant in the experiment. The plants used in the experiment were grown in the glass greenhouse located in the Akdeniz University Research and Application Area. About 2–3 cm long runner tips were collected from donor plants and excised for aseptic sterilization (Figure 1a). Explants were washed with tap water for 2 h, then surface sterilized with 70% ethanol solution for 30 s. and washed with sterilized distilled water twice. After that, samples were soaked in 10% sodium hypochlorite solution for 10 min then vigorously rinsed with sterilized double-distilled water. The leaves, scales, and hairs on the disinfected explants were cleaned under aseptic conditions under a binocular microscope and meristems were obtained [47]. Before starting the research, preliminary experiments were carried out and it was decided to use the combination with the best results. Meristems were transferred to a full-strength MS starting medium containing 1.5 mg/l IBA and 1 mg/l IAA and were cultured in a climate chamber (25 ± 2 °C, 16/8 photoperiod, 40 μmol m−2 s−1 irradiance intensity) for 35 days [47] (Figure 1b). These plantlets were subcultured for three cycles on a 4-weekly interval before the drought and NaSiO2 treatments (Figure 1c). The media pH was adjusted to 5.7 before autoclaving at 121 °C for 20 min.
Figure 1.
Different steps during in vitro propagation of strawberry cultivar ‘Albion’ (a) explants used for meristem isolation; (b) plantlet shooting from an isolated meristem; (c) in vitro plants subcultured for three cycles on a 4-weekly interval.
2.2. Nanoparticles
The experiment was conducted in the tissue culture laboratory of Akdeniz University in 2019–2020. The NaSiO2 (Nanografi Chemical Company, Ankara, Turkey) average diameter was between 15–35 nm and had a purity of 99.5% in the study. The morphological study of nanoparticles was done by scanning electron microscope (SEM) (ZEİSS-LEO 1430, ZEİSS, Oberkochen, Germany) (Figure 2). Drought stress was imposed using polyethylene glycol (PEG 6000, Sigma Aldrich, St. Louis, MO, USA).

Figure 2.
Imaging of the NaSiO2 Scanning electron microscopy (SEM).
2.3. Preparation of Treatments
Within the scope of the study, different PEG 6000 (0, 4, 8%) and NaSiO2 concentrations (0, 50, 100 mg L−1) were used to determine the effects of NaSiO2 application on the tolerance of strawberry plants exposed to drought stress in vitro. Combinations of NaSiO2 and PEG concentrations are given in Table 1.

Table 1.
Combinations of NaSiO2 and PEG concentrations used in this study.
Deionized water was used to prepare suspensions at different concentrations (50 and 100 mg L−1) of NaSiO2. These mixtures were homogenized with a sonicator (JL-360, Shanghai, USA at 40 kHz) for 30 min. The obtained homogenized mixture was used in the preparation of a MS medium containing PEG at different concentrations without waiting. Similarly trained plantlets were sub-cultured into a new MS medium which contain different doses of NaSiO2 and PEG-6000 and were cultured for 35 days (25 ± 2 °C, 16/8 photoperiod, 40 μmol m−2 s−1 irradiance intensity).
The experimental design was 2 factorial, arranged in a completely randomized design with 3 replications. Fifteen jars were used as each replicate and one plantlet was transferred in each jar. All measurements were performed 35 after transferring the plants to the described media.
2.4. Growth Measurement
At the end of the experimental period, growth measurements were recorded on five randomly selected plants per treatment. Healthy plantlets were removed from glass jars and gently washed with tap water to remove the culture medium attached to the roots. Root and shoot length and shoot diameter were measured with a digital caliper. The fresh weight of the shoot and roots were measured using a digital balance (Kern ABJ 320-4NM, KERN & SOHN GmbH, Balingen, Germany) with a sensitivity of 0.0001 g. Dry weights were determined by drying the samples at 65 °C for 48 h in an oven.
2.5. Physiological Parameters
The SPAD index was measured with a non-destructive field chlorophyll meter (SPAD–502, Konica Minolta Sensing, Inc., Tokyo, Japan) by an average of twenty readings per sample. Measurements were taken between 10:00 a.m.–12:00 p.m., on the three most light-exposed leaves.
The leaf relative water contents (LRWC) were determined on three leaves of the same age and following the method of Sanchez et al. [48]. The LRWC was calculated as follows:
LRWC = (FW − DW)/(TW − DW) × 100
FW: Fresh weight, DW: Dry weight, TW: Turgid weight.
2.6. Enzyme Activities Assay
The procedure of enzyme extraction was done under 4 °C. Enzymes were extracted from 1 g leaf samples kept at −80 °C with 5 mL of extraction buffer, using a mortar and pestle, containing a 0.1 M phosphate buffer (pH 7.8). The homogenized suspension was obtained by centrifuging at 10,000 rpm for 10 min at 4 °C. The supernatant was used for assaying superoxide dismutase (SOD) and catalase (CAT). Spectrophotometric analysis was conducted on a Shimadzu 2401 UV/visible light spectrophotometer.
Catalase activity (CAT) was determined by the disappearance of H2O2 by measuring the decrease in absorbance at 240 nm for 1 min due to the H2O2 [49]. The reaction mixture contained 25 mM phosphate buffer (pH 7.0), 10 mM H2O2, and an enzyme.
SOD activity was defined as the photochemical reduction of nitro blue tetrazolium (NBT), according to Bayer and Fridovich [50]. One-unit SOD activity was defined as the amount of SOD required for 50% inhibition of NBT (p-nitro blue tetrazolium chloride) reduction measured at 560 nm of the reaction mixture containing 50 mM phosphate buffer (pH 7.8), 33 μM NBT, 10 mM l-methionine, 0.66 mM EDTA and 3.3 μM riboflavin.
2.7. Statistical Analysis
Analysis of variance (ANOVA) was applied to the data using the SPSS/PC+ Statistics software package (SPSS 23.0, SPSS, Inc., Chicago, IL, USA). Differences among treatments were analyzed taking p ≤ 0.05 as significance according to Duncan’s multiple range test. Pearsion correlation was done using SPSS 23.0 to demonstrate the correlation between the various measurements and their relationship with the different treatments.
3. Results
3.1. Growth Measurement
Mean squares due to different concentrations of NaSiO2, PEG, and their interaction were significant for root and shoot growth of in vitro strawberries (Table 2). The highest responses of shoot and root fresh weight, shoot and root length, were obtained on the medium supplemented with 50 and 100 mg L−1 NaSiO2 among all treatments (Table 2). Plants had water stress induced by PEG application, the increased PEG in the medium exerted a negative influence on the vegetative growth of shoots, whereas, NaSiO2 treatment supplemented in the growth medium, stimulated a positive response in growth and development parameters. NaSiO2 at 50 mg L−1 induced the maximum shoot fresh weight (1.20 g) among 4% and 8% PEG applications (Table 2). There was no statistical difference between the mean SD values among the vegetative growth parameters evaluated. As seen in Table 2, the average highest root weight was similarly measured in T2 (1.57 g) and T3 (1.53 g) applications. The lowest average value was determined in the T7 (0.47 g). The effect of Si had a similar effect on almost all vegetative parameters, and it was also found to be effective on root length in tolerating the adverse conditions created by drought. The highest mean root lengths among all treatments were obtained in the control group (T1, T2, T3) and T5 group. An average root length of 34.26 mm was obtained from 50 mg L−1 NaSiO2 treatments, among 4% PEG applications in which the drought effect was created. In the 4% PEG application, in which NaSiO2 was not applied, an average root length of 16.69 mm was obtained, which is even less than half of this value.

Table 2.
Influence PEG and NaSiO2 treatment on vegetative growth of strawberry plants.
Considering all the data obtained, it has been proven that the addition of NaSiO2 is effective in in vitro strawberry cultivation. On the other hand, it was determined that the addition of 50 mg L−1 NaSiO2 to the medium was effective in improving shoot and root growth parameters at 4% PEG concentrations while adding 100 mg NaSiO2 gave better results when the PEG concentration increased to 8%.
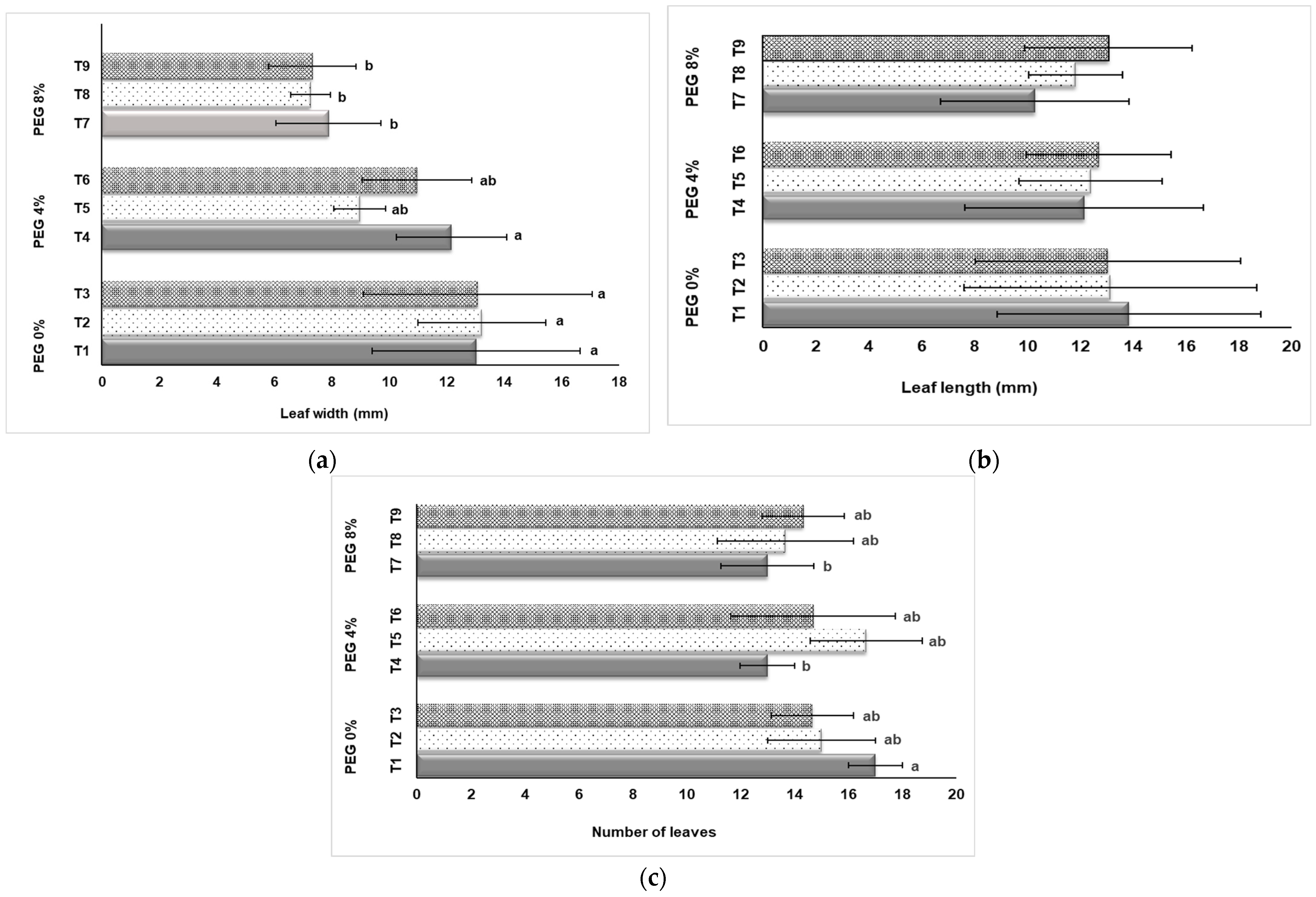
We documented the growth of plantlets in terms of leaf growth (Figure 3). Responses to drought conditions differed according to the doses of PEG application and NaSiO2 treatment. It was determined that the effects of PEG and NaSiO2 applications on leaf width were significant (Figure 3a). The highest average leaf width values among the different treatments were obtained in plants without PEG application (T1, T2, T3). In the groups in which 8% PEG was applied, drought stress was effective and thus leaf width values decreased. However, as seen in Figure 3a, the use of NaSiO2 had a positive effect in the control group (T1, T2, T3) on the increase in leaf width. According to the results obtained, leaf length was not affected statistically (p ≤ 0.05) by the PEG and NaSiO2 applications. When Figure 3b is examined, it is seen that; while the highest value was seen in the 0% PEG + 0 mg L−1 NaSiO2 with 13.85 mm, the lowest value was observed with 10.30 mm in the application of 8% PEG +0 mg L−1 NaSiO2. According to the evaluated results, the highest average leaves number was seen in the 0% PEG + 0 mg L−1 NaSiO2 application (17 pieces/plant), followed by 4% PEG + 50 mg L−1 NaSiO2 application (16.67 pieces/plant) (Figure 3c). The lowest value was obtained from plants (8% PEG + 0 mg L−1 NaSiO2; 13 pieces/plant) in which the highest PEG dose was applied and NaSiO2 was not applied. It was observed that 4% and 8% PEG application decreased the number of leaves, while NaSiO2 treatment was found to improve the number of leaves (Figure 3c).
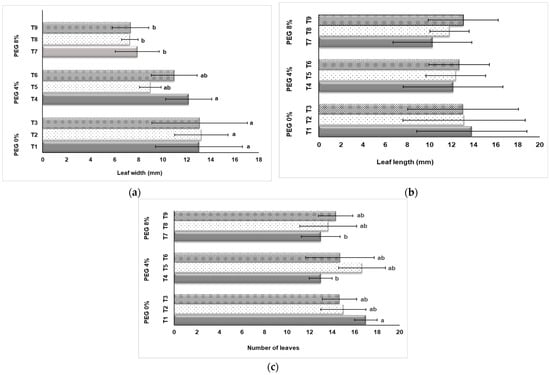
Figure 3.
Influence of PEG and NaSiO2 treatment in in vitro strawberry leaves: (a) Leaf width (mm); (b) Leaf length (mm); (c) Number of leaves. (T1) 0% PEG + 0 mg L−1 NaSiO2; (T2) 0% PEG + 50 mg L−1 NaSiO2; (T3) 0% PEG + 100 mg L−1 NaSiO2; (T4) 4% PEG + 0 mg L−1 NaSiO2; (T5) 4% PEG + 50 mg L−1 NaSiO2; (T6) 4% PEG + 100 mg L−1 NaSiO2; (T7) 8% PEG + 0 mg L−1 NaSiO2; (T8) 8% PEG + 50 mg L−1 NaSiO2; (T9) 8% PEG + 100 mg L−1 NaSiO2. Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
3.2. SPAD Index and Leaf Relative Water Content (LRWC)
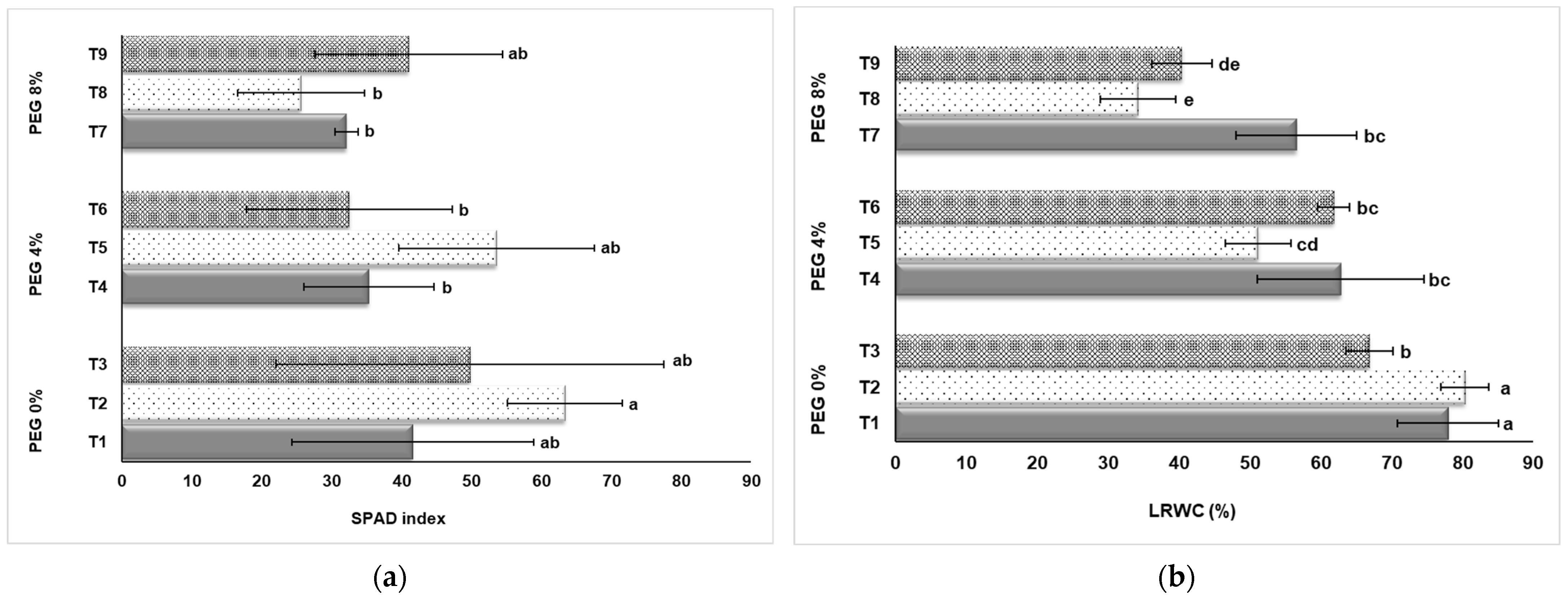
As seen in Figure 4a, four treatment groups showed a decreasing trend in the SPAD index due to drought stress caused by PEG application. However, the T2 group had the highest SPAD value (63.37) among the control groups without PEG application. When the control group (T1, T2, and T3) was examined; it was observed that the SPAD index increased significantly with NaSiO2 application. In this case, it can be said that silicon application has a significant effect on the SPAD index in strawberry cultivation in vitro. A significant effect can be observed when looking at the SPAD values of the plants in the groups T4, T5, and T6 in which drought stress is created by 4% PEG application. The average SPAD values of the T5 plants (4% PEG + 50 mg L−1 NaSiO2), were determined as 53.57. This value was determined as 35.34 in the T4 group without NaSiO2 application. In the T7, T8, and T9 groups in which 8% PEG was applied; higher SPAD index values were obtained from the T9 application compared to the T7 and T8 applications, and this group was statistically in the same group as the T1 group in which drought stress was not applied (Figure 4a).
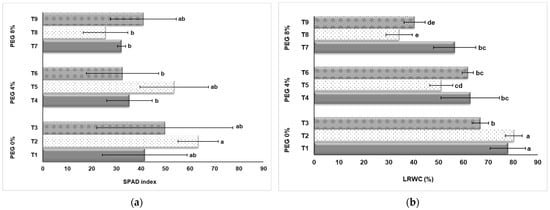
Figure 4.
Effect of PEG and NaSiO2 treatment on SPAD index (a) and LRWC (b) in strawberry leaves under in vitro conditions. (T1) 0% PEG + 0 mg L−1 NaSiO2; (T2) 0% PEG + 50 mg L−1 NaSiO2; (T3) 0% PEG + 100 mg L−1 NaSiO2; (T4) 4% PEG + 0 mg L−1 NaSiO2; (T5) 4% PEG + 50 mg L−1 NaSiO2; (T6) 4% PEG + 100 mg L−1 NaSiO2; (T7) 8% PEG + 0 mg L−1 NaSiO2; (T8) 8% PEG + 50 mg L−1 NaSiO2; (T9) 8% PEG + 100 mg L−1 NaSiO2. Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
Drought stress significantly reduced LRWC content in in vitro strawberries (Figure 4b). The LRWC of plants exposed to drought stress decreased by up to 57% (T1: 80.40%, T8: 34.21%). In the study, it is seen in Figure 4b that the effect of NaSiO2 on LRWC, which is aimed to determine its effectiveness in tolerating the effects of drought stress, is low. When the control group (T1, T2, and T3) in which drought stress was not applied was examined, there was no significant difference between T1 without NaSiO2 application and T2 application with 50 mg L−1 NaSiO2. The LRWC content of the T3 group, on which 100 mg L−1 NaSiO2 was applied, remained lower than T1 and T2. A similar situation was also reflected in the results obtained from plants in the T4, T5, T6, T7, T8, and T9 groups treated with 4% PEG and 8% PEG (Figure 4b).
3.3. SOD and CAT Activity
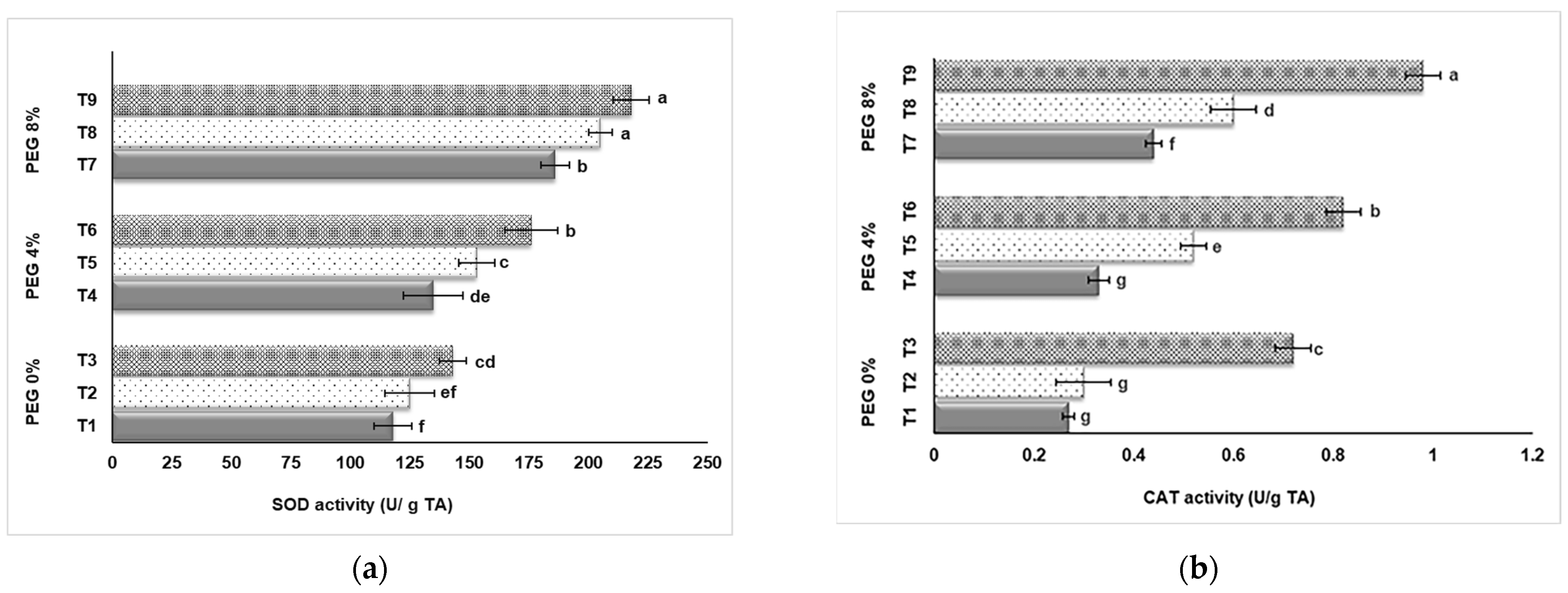
As a result of our research, the effects of PEG and NaSiO2 treatment on the SOD and CAT activities of strawberry leaves grown in in vitro conditions were found to be statistically significant (p < 0.05). Figure 5 shows that strawberry plants grown in in vitro conditions respond to drought stress and the SOD and CAT activity values increase in direct proportion to increasing PEG doses. Also, the addition of NaSiO2 caused an increase in enzyme activities in all applications. 8% PEG application (T7, T8, T9) had higher enzyme activity than 4% and control group (T1, T2, T3). Our data showed that the highest SOD value was determined in application T9 (8% PEG + 100 mg L−1 NaSiO2) with 218 U/g TA, followed by application T8 (8% PEG + 50 mg L−1 NaSiO2) with a value of 205 U/g TA (Figure 5a). The lowest value was found in the control group (T1) with 118 U/g TA. 8% PEG+ 100 mg L−1 NaSiO2 application up-regulated SOD activity. CAT activity values obtained from different applications are shown in Figure 5b. The highest value was observed with 0.98 U/g TA in the T9 (8% PEG + 100 mg L−1 NaSiO2) application, while the lowest value was found in the control group (T1) with 0.27 U/g TA.
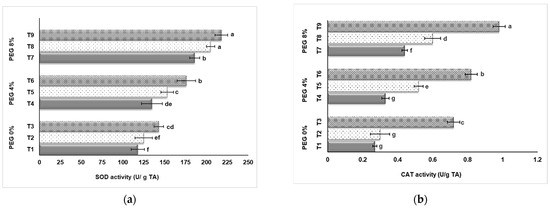
Figure 5.
Effect of PEG and NaSiO2 treatment on SOD (a) and CAT (b) activities in strawberry leaves under in vitro conditions. (T1) 0% PEG + 0 mg L−1 NaSiO2; (T2) 0% PEG + 50 mg L−1 NaSiO2; (T3) 0% PEG + 100 mg L−1 NaSiO2; (T4) 4% PEG + 0 mg L−1 NaSiO2; (T5) 4% PEG + 50 mg L−1 NaSiO2; (T6) 4% PEG + 100 mg L−1 NaSiO2; (T7) 8% PEG + 0 mg L−1 NaSiO2; (T8) 8% PEG + 50 mg L−1 NaSiO2; (T9) 8% PEG + 100 mg L−1 NaSiO2. Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
3.4. Correlations
The correlations between the growth parameters and physiological traits are shown in Table 3. A significant relationship was observed between shoot and root growth. Among the parameters that were measured, a positive correlation was found between RW and SL, RL, LW, LRWC, and the SPAD index. Also, SL exhibited a positive correlation with RL, LW, and LRWC. Enzymatic antioxidants (SOD and CAT) were negatively correlated with some growth parameters; RW, SL, LW and LRWC (Table 3).

Table 3.
Pearson’s correlation coefficients between the growth parameters and physiological traits.
4. Discussion
Strawberries are a major horticultural plant worldwide and the growth and development of this plant can be affected by different factors [51]. Responding to abiotic stresses has been one of the main subjects of research since it is very important in terms of plant productivity [52,53]. This research involved the NaSiO2 effect on in vitro strawberry growth under various levels of PEG-induced drought stress. It has been reported in various previous studies that the application of different nanoparticles to strawberry plants in in vitro and ex vitro conditions has significant effects on plant growth, crop quality, and yield, and also reduces the effect of abiotic stress factors [7,10,27,30,54,55]. One of the most important signs observed in plants exposed to abiotic stress is growth inhibition [20]. Our data showed that additions of 50 or 100 mg L−1 NaSiO2 increased the shoot and root growth of strawberry plants in stress or in non-stressed conditions. The significant (p < 0.05) decrease in growth parameters of in vitro plants (Table 2) in PEG applications could be due to the reduction of water absorption and consequently to decreased cell division and elongation [56]. NaSiO2 at 50 mg L−1 induced the maximum shoot (1.20 g) and root (1.24 g) fresh weight as well as shoot (40.09 mm) and root (34.26 mm) length, among 4% and 8% PEG applications. These results are congruent with the results of Mozafari et al. [27] which reported the significant effect of iron nanoparticles (FeNPs) and salicylic acid (SA) on in vitro strawberry plants under various concentrations of drought stress. Similar results were obtained in two different studies investigating the effects of FeNPs on strawberry plants grown in vitro under salt [54] and drought stress [30]. In both studies, it is reported that the application of FeNPs under stress conditions can reduce the negative effects of stress. Zahedi et al. reported that spraying solutions containing SiO2, Se, and Se/SiO2 (50 and 100 mg L−1) nanoparticles improved the growth parameters of strawberry plants grown under normal and drought stress conditions [10].
According to the findings of this study, leaf width and leaf number of in vitro strawberries were significantly affected by the combined application of NaSiO2 and PEG. NaSiO2 increases the leaf width of in vitro strawberry plants in non-stress conditions. In contrast, in drought stress conditions (4% and 8%PEG) NaSiO2 had negative effects on this parameter (Figure 3). Using NaSiO2 under drought stress or without stress, on the other hand, improved leaves number, which can be attributed to the important role of NaSiO2 in drought conditions. Zahedi et al., states that in a similar study, strawberry plants treated with SiO2 showed an increase in the number of plant leaves by 29% and 42%, respectively, in moderate and severe drought conditions compared to strawberry plants not treated with Si. The researchers also reported that the SiO2-nanoparticles formed had a better effect on the number of leaves than SiO2 [43]. Studies that fully demonstrate the effectiveness of silica nanoparticles are still limited. According to Sun et al., the application of 2000 mg L−1 mesoporous silica nanoparticles (Si NP) had no adverse effects in terms of oxidative status or cell membrane integrity in both wheat and lupine [57], while the same Si NP concentration decreased root and stem biomass and plant height in cotton (Gossypium hirsutum L.) [58]. The effect of Si NP application on plant growth and development is affected by the properties of the material such as size and shape, as well as the application stage, and biomechanical and physical factors [59]. One of the important effects of drought on plants is the production of fewer leaves or the production of leaves with a smaller surface area [60]. In previous studies, silicon application improved the biomass accumulation of the strawberry plant [7]; this may be correlated with higher leaves number during stress conditions. Under drought stress conditions, the application of NaSiO2 significantly increased the leaves number of apples (Malus domestica Borkh.) [61], feverfew (Tanacetum parthenium L.) [62] and wheat [63].
50 mg L−1 NaSiO2 application significantly increased the SPAD index in the control group without drought stress (T1, T2, T3) and in the group with 4% PEG application (T4, T5, T6) (Figure 4a). However, in the group where the drought level increased and 8% PEG was applied; it is seen that the highest mean value was determined in the T9 group, which was administered 100 mg L−1 of NaSiO2. The decrease in the SPAD index, which is expected to be positively correlated with photosynthesis, leads to the limitation of plant biomass and yield [63,64]. Various studies have reported that the adverse effects of drought on the SPAD index were alleviated by silicon application [65,66,67]. Our study shows that LRWC decreases as the PEG concentrations increase. The RWC (Relative Water Content) decreases in response to drought stress [68]. As a result of the decrease in turgor pressure in drought conditions, cell division, and cell elongation are restricted, and as a result of this situation, the development of plants and leaves decreases [63,69]. With the restriction of access to water, the loss of leaf water content occurs, followed by impaired cell growth and metabolism, which eventually leads to the development of secondary damage to the leaf due to drought [70,71,72]. The addition of 50 mg L−1 of NaSiO2 increased the LRWC under unstressed conditions. Under drought conditions where 4% PEG was applied, the LRWC was not significantly changed in its presence compared to the absence of additional NaSiO2. In the stress conditions created by 8% PEG application, NaSiO2 application had a negative effect on the LRWC. Some studies, in contrast to our findings, reported that silicon supplementation reduces the osmotic potential of strawberry leaves and increases turgor pressure under drought stress. Therefore, it has been suggested that silicon supplementation can increase the water content of plants [8,10]. On the other hand, in line with our study findings; Mali and Aery also reported that the RWC content decreased with silicon application [73]. Researchers speculated that this reduction in RWC may be due to a lack of water in the soil or root systems, which cannot compensate for water lost by transpiration through reduced absorbent surface [74]. In addition to all these, it can be thought that the effectiveness of silicone application varies in all studies, and this change may vary according to growing conditions and application dose.
Our results indicated that the application of NaSiO2 elevated the CAT and SOD activity in stressed plants. 100 mg L−1 NaSiO2 had the highest effect between all treatments. Silicon, which regulates endogenous plant hormones, resulted in improved tolerance to drought stress [75]. A number of ecological conditions can cause the production of reactive oxygen species (ROS), which is formed as a by-product of plant metabolic processes [76,77]. Plants need to balance/maintain ROS levels within the cell through enzymatic and non-enzymatic activities to cope with oxidative damage under conditions of abiotic stress [78,79,80,81,82]. Antioxidant enzyme activities can be improved by CAT activation, which scavenges ROS, participates in the defense mechanism against H2O2 increase, and controls H2O2 levels in cells [76,83,84]. Also, if the activation of SOD is combined with other ROS-scavenging enzymes, defensive strategies can be provided to attenuate the oxidative burst in plants under arid conditions [85,86]. It has been reported that nano-silicone has a protective effect on plants through the regulation of antioxidant systems, and phytohormone regulation induced by Si addition results in improved tolerance to drought stress [87,88]. In a recent study, SiNPs were shown to increase the tolerance of roses (Rosa damascena Mill.) to PEG-induced drought stress by decreasing the H2O2 concentration and increasing the activity of antioxidant enzymes [89]. The results obtained from this study show that the addition of NaSiO2 alleviates the effects of drought stress simulated with PEG in vitro and agrees with the studies in the literature. Results from in vitro systems cannot always be expected to show the same effect in vivo and applications may need to be tested under field conditions. In addition, although nanotechnology is a rapidly growing and developing sector today, the product range can still be expensive for many countries—especially developing countries—and is not economically accessible everywhere. However, it is also known that there is a high amount of product and yield loss due to drought all over the world every year [90]. Precautions against drought are thought to be important for strawberries, which are sensitive to water stress. Therefore, from this point of view, silicon supplementation is thought to be responsible for increased tolerability in drought-stressed plants.
5. Conclusions
The current study investigated the effects of NaSiO2 application on drought tolerance under in vitro culture induced by PEG and its effects on strawberry growth and development. Drought stress leads to adverse effects in plants, which is associated with the reduction of growth parameters. According to the results, the addition of NaSiO2 improved water stress tolerance in strawberry plants in vitro by increasing shoot weight and length, root weight and length, number of leaves, SPAD index, SOD, and CAT activity. Hence NaSiO2 application could alleviate the oxidative damage of in vitro strawberry plants under drought conditions, which could contribute to the improvement of drought tolerance.
Author Contributions
Conceptualization, S.Ş. and C.N.D.; methodology, S.Ş., H.S. and C.N.D.; data curation, S.Ş., H.S. and C.N.D.; writing—original draft preparation, S.Ş. and C.N.D.; writing—review and editing, S.Ş. and H.S.; supervision, S.Ş.; project administration, S.Ş.; funding acquisition, S.Ş. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Akdeniz University, Scientific Research Projects Coordination Unit, Antalya, Turkey, grant number FYL-2020-5229 (only for research funding).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within this article.
Acknowledgments
The authors extend their appreciation to Akdeniz University, Scientific Research Projects Coordination Unit, Antalya, Turkey, grant number FYL-2020-5229 (only for research funding).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ali, S.; Liu, Y.; Ishaq, M.; Shah, T.; Abdullah; Ilyas, A.; Din, I.U. Climate change and its impact on the yield of major food crops: Evidence from Pakistan. Foods 2017, 6, 39. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2020, 302, 110733. [Google Scholar] [CrossRef]
- Şener, S.; Türemiş, N.F.; Tanır, F. Agrochemical Usage for Sustainable Fruit Production and Human Health. In Agrochemicals Detection, Treatment and Remediation, 1st ed.; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 291–305. [Google Scholar]
- Abdelaal, K.; Alkahtani, M.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The role of plant growth-promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Kummu, M.; Guillaume, J.H.A.; de Moel, H.; Eisner, S.; Flörke, M.; Porkka, M.; Siebert, S.; Veldkamp, T.I.E.; Ward, P.J. The world’s road to water scarcity: Shortage and stress in the 20th century and pathways towards sustainability. Sci. Rep. 2016, 6, 38495. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. National Water Footprint Accounts: The Green, Blue and Gray Water Footprint of Production and Consumption; Value of Water Research Report Series No. 50; UNESCO-IHE: Delft, The Netherlands, 2011; Volume 2. [Google Scholar]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef]
- Moradtalab, N.; Hajiboland, R.; Aliasgharzad, N.; Hartmann, T.E.; Neumann, G. Silicon and the Association with an Arbuscular-Mycorrhizal Fungus (Rhizophagus clarus) Mitigate the Adverse Effects of Drought Stress on Strawberry. Agronomy 2019, 9, 41. [Google Scholar] [CrossRef]
- Ghaderi, N.; Siosemardeh, A. Response to drought stress of two strawberry cultivars (cv. Kurdistan and Selva). Hortic. Environ. Biotechnol. 2011, 52, 6–12. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and Silica Nanostructure-Based Recovery of Strawberry Plants Subjected to Drought Stress. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Grant, O.M.; Johnson, A.W.; Davies, M.J.; James, C.M.; Simpson, D.W. Physiological and morphological diversity of cultivated strawberry (Fragaria × ananassa) in response to water deficit. Environ. Exp. Bot. 2010, 68, 264–272. [Google Scholar] [CrossRef]
- Blanke, M.M.; Cooke, D.T. Effects of flooding and drought on stomatal activity, transpiration, photosynthesis, water potential and water channel activity in strawberry stolons and leaves. Plant Growth Regul. 2004, 42, 153–160. [Google Scholar] [CrossRef]
- Razavi, F.; Pollet, B.; Steppe, K.; Van Labeke, M.C.; Labeke, M.C. Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 2008, 46, 631–633. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Jalde, S.S.; Patel, R.V. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22002. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO Statistical Database (FAOSTAT). 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 13 April 2023).
- Scherr, K.D.; Jamieson, M.A. Abiotic and biotic drivers of strawberry productivity across a rural-urban gradient. Basic Appl. Ecol. 2021, 57, 65–77. [Google Scholar] [CrossRef]
- Şener, S. ; Abiotic Stress Factors and Strawberry Cultivation. Impact of Climate Change on Agriculture, 1st ed.; Akçal, A., Çamoğlu, G., Tan, S., Eds.; Holistence Publications: Çanakkale, Turkey, 2021; pp. 111–125. [Google Scholar]
- De Bossoreille de Ribou, S.; Douam, F.; Hamant, O.; Frohlich, M.W.; Negrutiu, I. Plant science and agricultural productivity: Why are we hitting the yield ceiling? Plant Sci. 2013, 210, 159–176. [Google Scholar] [CrossRef]
- Kaman, H.; Gübbük, H.; Tezcan, A.; Can, M.; Özbek, Ö. Water-yield relationship of greenhouse-grown strawberry under limited irrigation. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 13235. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, S.H.; Chung, M.Y.; Park, S.K.; Kim, C.K. In vitro propagation method for production of morphologically and genetically stable plants of different strawberry cultivars. Plant Methods 2019, 15, 36. [Google Scholar] [CrossRef]
- Munns, R.; Husain, S.; Rivelli, A.R.; James, R.A.; Condon, A.; Lindsay, M.P.; Lagudah, E.S.; Schachtman, D.P.; Hare, R.A. Avenues for Increasing Salt Tolerance of Crops, and the Role of Physiologically Based Selection Traits. In Progress in Plant Nutrition: Plenary Lectures of the XIV International Plant Nutrition Colloquium; Springer Nature: Hannover, The Netherlands, 2002; pp. 93–105. [Google Scholar]
- Mehmandar, M.N.; Rasouli, F.; Giglou, M.T.; Zahedi, S.M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Tajaragh, R.P.; Ryant, P.; Mlcek, J. Polyethylene Glycol and Sorbitol-Mediated In Vitro Screening for Drought Stress as an Efficient and Rapid Tool to Reach the Tolerant Cucumis melo L. Genotypes. Plants 2023, 12, 870. [Google Scholar] [CrossRef]
- Gopal, J.; Iwama, K. In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep. 2007, 26, 693–700. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tissue Organ Cult. 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Zahid, G.; Iftikhar, S.; Shimira, F.; Ahmad, H.M.; Kacar, Y.A. An overview and recent progress of plant growth regulators (PGRs) in the mitigation of abiotic stresses in fruits: A review. Sci. Hortic. 2023, 309, 111621. [Google Scholar] [CrossRef]
- Yosefi, A.; Mozafari, A.A.; Javadi, T. In vitro assessment of strawberry (Fragaria × ananassa Duch.) plant responses to water shortage stress under nano-iron application. Vitro Cell. Dev. Biol. Plant 2022, 58, 499–510. [Google Scholar] [CrossRef]
- Snehal, S.; Lohani, P. Silica nanoparticles: Its green synthesis and importance in agriculture. J. Pharm. Phytochem. 2018, 7, 3383–3393. [Google Scholar]
- Rahman, M.H.; Hasan, M.N.; Khan, M.Z.H. Study on different nano fertilizers influencing the growth, proximate composition and antioxidant properties of strawberry fruits. J. Agric. Res. 2021, 6, 100246. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A.; Hallaj, R. Influence of nano-ZnO on microbial growth, bioactive content and postharvest quality of strawberries during storage. Innov. Food Sci. Emerg. Technol. 2016, 35, 168–176. [Google Scholar] [CrossRef]
- Caubet, M.; Cornu, S.; Saby, N.P.A.; Meunier, J.D. Agriculture increases the bioavailability of silicon, a beneficial element for crop, in temperate soils. Sci. Rep. 2020, 10, 19999. [Google Scholar] [CrossRef]
- Behboudi, F.; Sarvestani, T.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A. Improving growth and yield of wheat under drought stress via application of SiO2 nanoparticles. J. Agric. Sci. Technol. 2018, 20, 1479–1492. [Google Scholar]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A Review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestic, M.; Xie, W. Silicon: An essential element for plant nutrition and phytohormones signalling mechanism under stressful conditions. Plant Growth. Regul. 2022, 100, 301–319. [Google Scholar] [CrossRef]
- Wadas, W.; Kondraciuk, T. Effect of silicon on micronutrient content in new potato tubers. Int. J. Mol. Sci. 2023, 24, 10578. [Google Scholar] [CrossRef] [PubMed]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Soleymanzadeh, R.; Iranbakhsh, A.; Habibi, G.; Ardebili, Z.O. Soil supplementation with silicon nanoparticles to alleviate toxicity signs of salinity in strawberry. Iran. J. Plant Physiol. 2022, 12, 4099–4109. [Google Scholar]
- Moradi, P.; Vafaee, Y.; Mozafari, A.A.; Tahir, N.A.-R. Silicon nanoparticles and methyl jasmonate improve physiological response and increase expression of stress-related genes in strawberry cv. Paros under salinity stress. Silicon 2022, 14, 10559–10569. [Google Scholar] [CrossRef]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Barker, A.V. Effects of nanosilicon dioxide on leaf anatomy, chlorophyll fluorescence, and mineral element composition of strawberry under salinity stress. J. Plant Nutr. 2021, 44, 3005–3019. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculík, M. Comparative morphological, physiological and molecular analyses of drought-stressed strawberry plants affected by SiO2 and SiO2-NPs foliar spray. Sci. Hortic. 2023, 309, 111686. [Google Scholar] [CrossRef]
- Seyed Hajizadeh, H.; Azizi, S.; Rasouli, F.; Kaya, O. Evaluation of nano-silicon efficiency on compatible solutes and nutrient status of Damask rose affected by in vitro simulated drought stress. Chem. Biol. Technol. Agric. 2023, 10, 22. [Google Scholar] [CrossRef]
- Sharf-Eldin, A.A.; Alwutayd, K.M.; El-Yazied, A.A.; El-Beltagi, H.S.; Alharbi, B.M.; Eisa, M.A.M.; Alqurashi, M.; Sharaf, M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Response of Maize Seedlings to Silicon Dioxide Nanoparticles (SiO2NPs) under Drought Stress. Plants 2023, 12, 2592. [Google Scholar] [CrossRef]
- Sohby, M.K.; Khalil, H.A.; Eissa, A.M.; Fekry, W.M. Influence of nano-silicon and nano-chitosan on growth, ion content, and antioxidant defense enzyme of two citrus rootstocks under salinity conditions. Mesop. J. Agric. 2023, 51, 147–166. [Google Scholar] [CrossRef]
- Duran, C.N. Determination of the Effects of Nano Silicone Dioxide Application on the Tolerance of Strawberry Plants Exposed to Drought Stress in vitro Conditions. Master’s Thesis, Akdeniz University, Antalya, Türkiye, 2020. [Google Scholar]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crop. Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Cakmak, I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium-and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot. 1994, 45, 1259–1266. [Google Scholar] [CrossRef]
- Bayer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Şener, S.; Türemiş, N.F. Effects of several cultivars’, mulch and fertilizer applications on plant growth and development criteria and plant’s nutrition elements uptake in organic strawberry plantation in Nevşehir city. Asian J. Agric. Rural. Dev. 2016, 6, 221–228. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Nasırcılar, A.G.; Ulukapı, K.; Sener, S. Exogenous salicylic acid applications as an example of molecules effective in abiotic stress tolerance in plants. Turk. J. Agric. Food Sci. Technol. 2019, 7, 5–10. [Google Scholar]
- Dedejani, S.; Mozafari, A.A.; Ghaderi, N. Salicylic acid and iron nanoparticles application to mitigate the adverse effects of salinity stress under in vitro culture of strawberry plants. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 821–831. [Google Scholar] [CrossRef]
- Mozafari, A.A.; Ghaderi, N.; Havas, F.; Dedejani, S. Comparative investigation of structural relationships among morphophysiological and biochemical properties of strawberry (Fragaria × ananassa Duch.) under drought and salinity stresses: A study based on in vitro culture. Sci. Hortic. 2019, 256, 108601. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014, 5, 12–50. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Ghaderi, N.; Normohammadi, S.; Javadi, T. Morpho-physiological responses of strawberry (Fragaria x ananassa) to Exogenous Salicylic Acid Application under Drought Stress. J. Agric. Sci. Technol. 2015, 17, 167–178. [Google Scholar]
- Avestan, S.; Naseri, L.; Barker, A.V. Evaluation of nanosilicon dioxide and chitosan on tissue culture of apple under agar-induced osmotic stress. J. Plant Nutr. 2017, 40, 2797–2807. [Google Scholar] [CrossRef]
- Esmaili, S.; Tavallali, V.; Amiri, B. Nano-Silicon Complexes Enhance Growth, Yield, Water Relations and Mineral Composition in Tanacetum parthenium under Water Deficit Stress. Silicon 2020, 13, 2493–2508. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K.; Chen, G.; Wang, S.; Zhang, C. Effects of silicon on growth of wheat under drought. J. Plant Nutr. 2003, 26, 1055–1063. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Ahmed, M.; Asif, M.; Hassan, F.-U. Augmenting drought tolerance in sorghum by silicon nutrition. Acta Physiol. Plant. 2014, 36, 473–483. [Google Scholar] [CrossRef]
- Ahmed, M.; Hassen, F.U.; Qadeer, U.; Aslam, M.A. Silicon application and drought tolerance mechanism of sorghum. Afr. J. Agric. Res. 2011, 6, 594–607. [Google Scholar]
- Bolat, I.; Bakır, A.G.; Korkmaz, K.; Gutiérrez-Gamboa, G.; Kaya, O. Silicon and Nitric Oxide Applications Allow Mitigation of Water Stress in Myrobalan 29C Rootstocks (Prunus cerasifera Ehrh.). Life 2022, 12, 1273. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Pessarakli, M. Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J. Plant Nutr. 2016, 39, 1001–1015. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Zhao, C.X. Water-deficit stress-induced anatomical changes in higher plants. Comptes Rendus Biol. 2008, 331, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Yadollahi, A.; Arzani, K.; Imani, A.; Aghaalikhani, M. Gas-exchange response of almond genotypes to water stress. Photosynthetica 2015, 53, 29–34. [Google Scholar] [CrossRef]
- Karimi, S.; Karami, H.; Mokhtassi-Bidgoli, A.; Tavallali, V.; Vahdati, K. Inducing drought tolerance in greenhouse grown Juglans regia by imposing controlled salt stress: The role of osmotic adjustment. Sci. Hortic. 2018, 239, 181–192. [Google Scholar] [CrossRef]
- Pei, Z.; Ming, D.; Liu, D.; Wan, G.; Geng, X.; Gong, H.; Zhou, W. Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat (Triticum aestivum L.) seedlings. J. Plant Growth Regul. 2010, 29, 106–115. [Google Scholar] [CrossRef]
- Mali, M.; Aery, N.C. Influence of silicon on growth, relative water contents and uptake of silicon, calcium and potassium in wheat grown in nutrient solution. J. Plant Nutr. 2008, 31, 1867–1876. [Google Scholar] [CrossRef]
- Gadallah, M.A.A.; Ramadan, T. Effects of zinc and salinity on growth and anatomical structure of Carthamus tinctorius L. Biol. Plant. 1997, 39, 411–418. [Google Scholar] [CrossRef]
- Yin, L.; Wang, S.; Tanaka, K.; Fujihara, S.; Itai, A.; Den, X.; Zhang, S. Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. Plant Cell Environ. 2016, 39, 245–258. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Ma, X.; Qalekhani, F. Effects of engineered aluminium and nickel oxide nanoparticles on the growth and antioxidant defense systems of Nigella arvensis L. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unraveling salt stress signalling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Zaid, A.; Wani, S.H. Reactive Oxygen Species Generation, Scavenging and Signaling in Plant Defence Responses. In Bioactive Molecules in Plant Defense; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 111–132. [Google Scholar]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll Fluorescence Imaging for Early Detection of Drought and Heat Stress in Strawberry Plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Song, G.; Hou, W.; Gao, Y.; Wang, Y.; Lin, L.; Zhang, Z.; Niu, Q.; Ma, R.; Mu, L.; Wang, H. Effects of CuO nanoparticles on Lemna minor. Bot. Stud. 2016, 57, 1–8. [Google Scholar] [CrossRef]
- Verma, K.K.; Song, X.P.; Joshi, A.; Tian, D.D.; Rajput, V.D.; Singh, M.; Arora, J.; Minkina, T.; Li, Y.R. Recent Trends in Nano-Fertilizers for Sustainable Agriculture under Climate Change for Global Food Security. Nanomaterials 2022, 12, 173. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Mohammadi, H.; Kariman, K. Nanosilicon-based recovery of barley (Hordeum vulgare) plants subjected to drought stress. Environ. Sci. Nano 2020, 7, 443–461. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Corpas, F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021, 412, 125254. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, H.S.; Azizi, S.; Rasouli, F.; Okatan, V. Modulation of physiological and biochemical traits of two genotypes of Rosa damascena Mill. by SiO2-NPs under in vitro drought stress. BMC Plant Biol. 2022, 22, 538. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.; Noce, S.; Antonelli, M.; Caporaso, L. Complex drought patterns robustly explain global yield loss for major crops. Sci. Rep. 2022, 12, 5792. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).