Effect of P-Dipping on Growth of NERICA 4 Rice in Different Soil Types at Initial Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physiochemical Characteristics of the Experimental Soils
2.2. Experimental Design and the Environmental Condition
2.3. Data Collection and Measurements
2.4. Statistical Analyses
3. Results
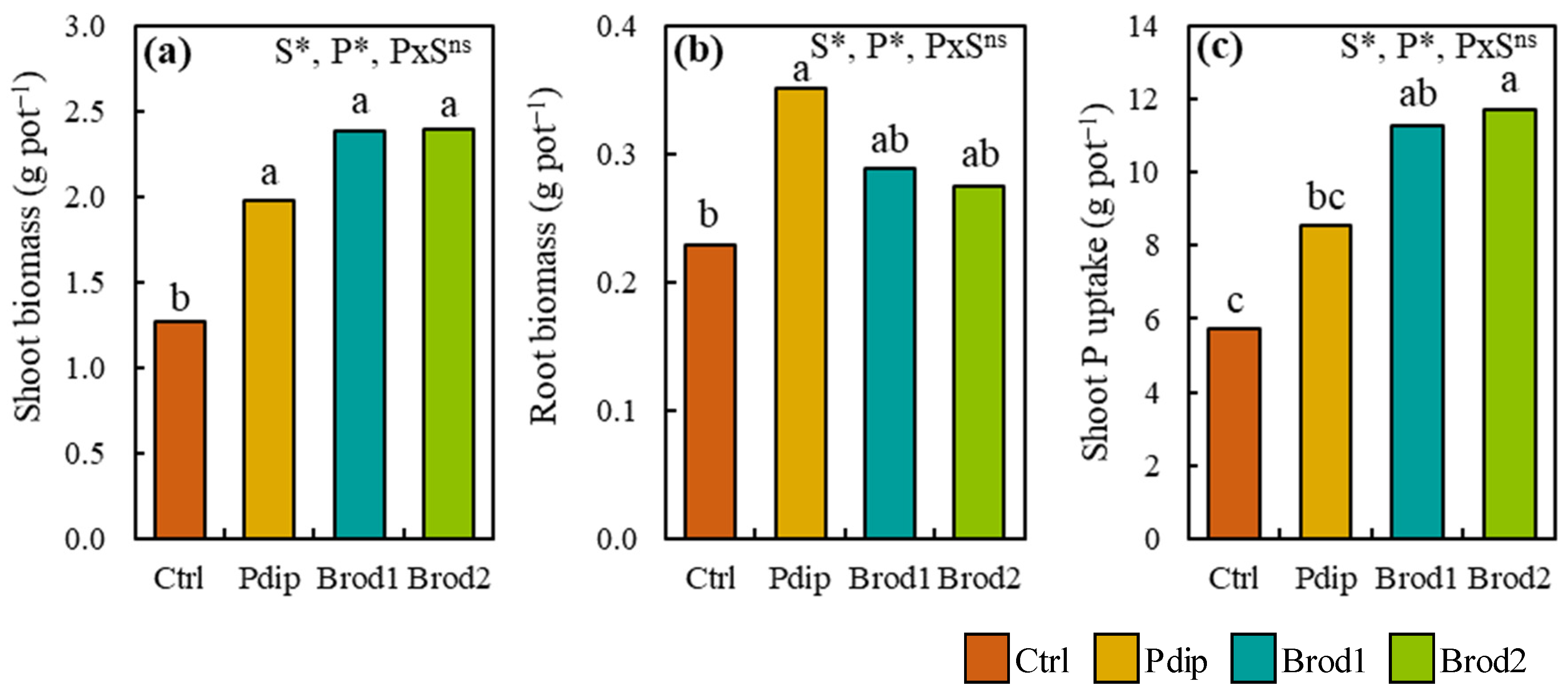
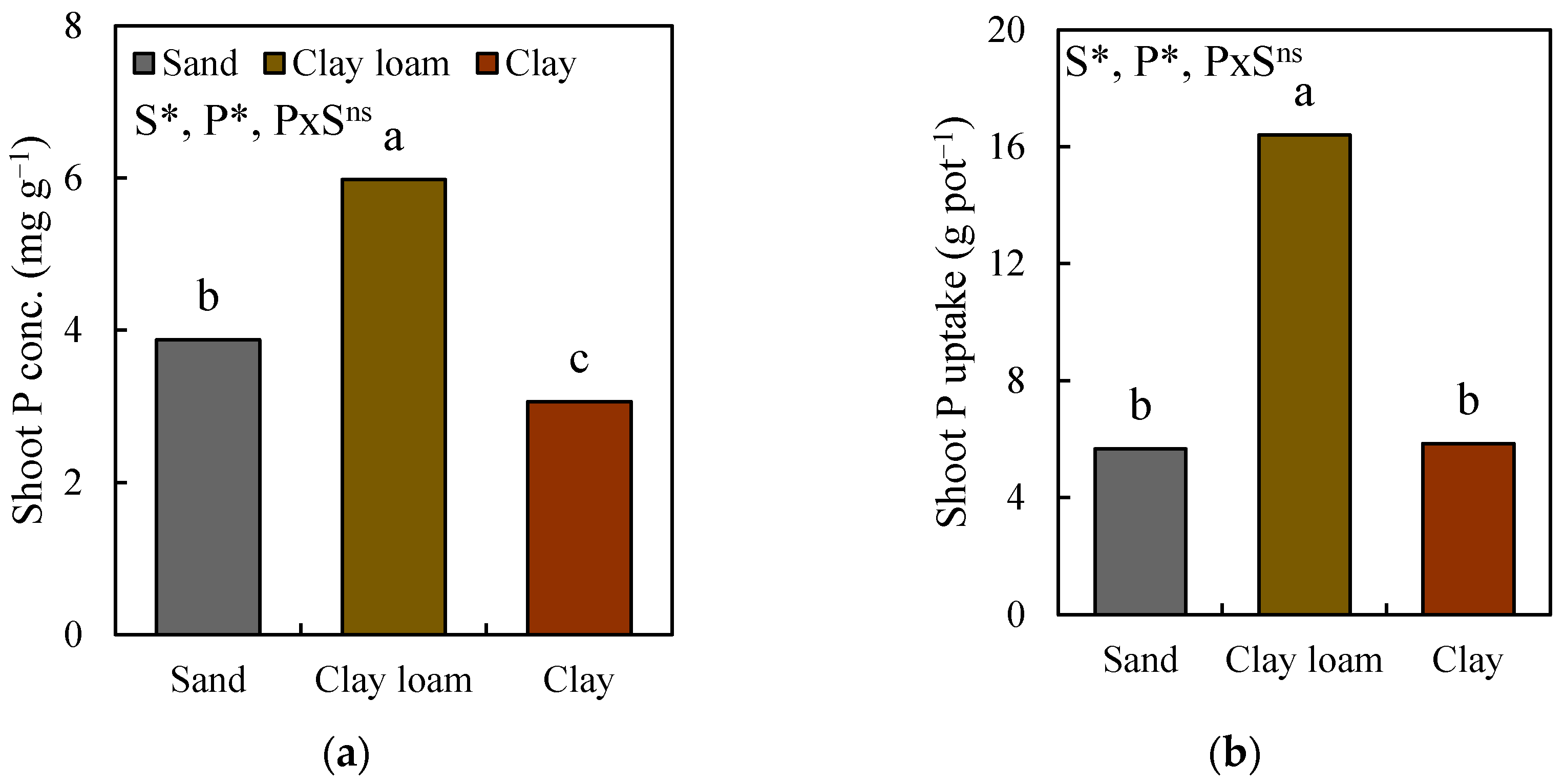
3.1. Changes in Shoot Biomass, Root Biomass, and Shoot P Uptake
3.2. Changes in Shoot Physiology and Morphology
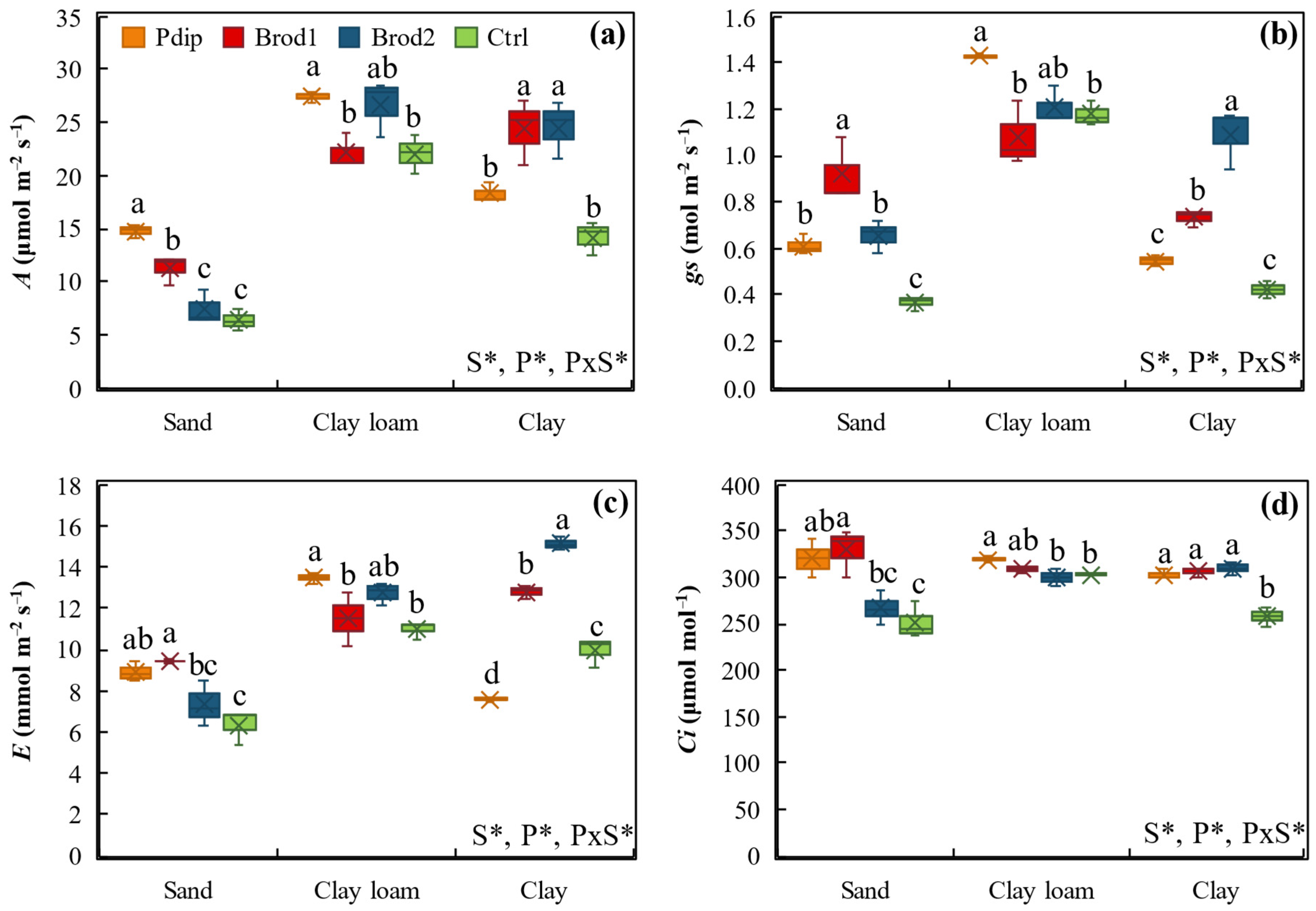
3.3. Gas Exchange Parameters
3.4. Changes in Root Morphology and Shoot P Uptake
4. Discussion
4.1. Soil Texture and P-Dipping Effects on Rice Shoot Morphology
4.2. Changes in Photosynthetic Rate under Different Soil Textures
4.3. Changes in Root Morphology and the Effect on Shoot P Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diagne, A.; Alia, D.Y.; Amovin-Assagba, E.; Wopereis, M.C.; Saito, K.; Nakelse, T. Farmer perceptions of the biophysical constraints to rice production in sub-Saharan Africa, and potential impact of research. In Realizing Africa’s Rice Promise; CABI: Wallingford, UK, 2013; pp. 46–68. [Google Scholar] [CrossRef]
- Saito, K.; Nelson, A.; Zwart, S.; Niang, A.; Sow, A.; Yoshida, H.; Wopereis, M. Towards a Better Understanding of Biophysical Determinants of Yield Gaps and the Potential for Expansion of the Rice Area in Africa; CABI: Wallingford, UK, 2013; pp. 188–203. [Google Scholar] [CrossRef]
- Tanaka, A.; Johnson, J.-M.; Senthilkumar, K.; Akakpo, C.; Segda, Z.; Yameogo, L.P.; Bassoro, I.; Lamare, D.M.; Allarangaye, M.D.; Gbakatchetche, H.; et al. On-farm rice yield and its association with biophysical factors in sub-Saharan Africa. Eur. J. Agron. 2017, 85, 1–11. [Google Scholar] [CrossRef]
- Bationo, A.; Hartemink, A.; Lungo, O.; Naimi, M.; Okoth, P.; Smaling, E.; Thiombiano, L. African Soils: Their Productivity and Profitability of Fertilizer Use: Background Paper for the African Fertilizer Summit 9–13th June 2006, Abuja, Nigeria; IFDC: Muscle Shoals, AL, USA, 2006. [Google Scholar]
- Sileshi, G.W.; Kihara, J.; Tamene, L.; Vanlauwe, B.; Phiri, E.; Jama, B. Unravelling causes of poor crop response to applied N and P fertilizers on African soils. Exp. Agric. 2022, 58, e7. [Google Scholar] [CrossRef]
- Stocking, M.A. Tropical soils and food security: The next 50 years. Science 2003, 302, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Batjes, N.H. ISRIC-WISE Derived Soil Properties on a 5 by 5 Arc-Minutes Global Grid (Ver. 1.2); ISRIC-World Soil Information: Wageningen, The Netherlands, 2012. [Google Scholar]
- Shang, C.; Zelazny, L.W. Selective dissolution techniques for mineral analysis of soils and sediments. In Methods of Soil Analysis Part 5—Mineralogical Methods; Soil Science Society of America, Inc.: Madison, WI, USA, 2008; Volume 5, pp. 33–80. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Sie, M.; Hijmans, R.; Otsuka, K. Increasing rice production in sub-Saharan Africa: Challenges and opportunities. Adv. Agron. 2007, 94, 55–133. [Google Scholar] [CrossRef]
- Vandamme, E.; Ahouanton, K.; Mwakasege, L.; Mujuni, S.; Mujawamariya, G.; Kamanda, J.; Senthilkumar, K.; Saito, K. Phosphorus micro-dosing as an entry point to sustainable intensification of rice systems in sub-Saharan Africa. Field Crops Res. 2018, 222, 39–49. [Google Scholar] [CrossRef]
- Nakamura, S.; Fukuda, M.; Nagumo, F.; Tobita, S. Potential utilization of local phosphate rocks to enhance rice production in sub-Saharan Africa. Jpn. Agric. Res. Q. JARQ 2013, 47, 353–363. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Gill, M.A.; Qureshi, R.H. Genotypic variations of phosphorus utilization efficiency of crops. J. Plant Nutr. 2001, 24, 1149–1171. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cakmak, I. Genotypic variation in phosphorus efficiency between wheat cultivars grown under greenhouse and field conditions. Soil Sci. Plant Nutr. 2006, 52, 470–478. [Google Scholar] [CrossRef]
- Wissuwa, M.; Ae, N. Genotypic variation for tolerance to phosphorus deficiency in rice and the potential for its exploitation in rice improvement. Plant Breed. 2001, 120, 43–48. [Google Scholar] [CrossRef]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009, 59, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Pratt, S.; Batstone, D.J. Phosphorus recovery from wastewater through microbial processes. Curr. Opin. Biotechnol. 2012, 23, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Rakotoson, T.; Amery, F.; Rabeharisoa, L.; Smolders, E. Soil flooding and rice straw addition can increase isotopic exchangeable phosphorus in P-deficient tropical soils. Soil Use Manag. 2014, 30, 189–197. [Google Scholar] [CrossRef]
- Shenker, M.; Seitelbach, S.; Brand, S.; Haim, A.; Litaor, M. Redox reactions and phosphorus release in re-flooded soils of an altered wetland. Eur. J. Soil Sci. 2005, 56, 515–525. [Google Scholar] [CrossRef]
- De Bauw, P.; Smolders, E.; Verbeeck, M.; Senthilkumar, K.; Houben, E.; Vandamme, E. Micro-dose placement of phosphorus induces deep rooting of upland rice. Plant Soil 2021, 463, 187–204. [Google Scholar] [CrossRef]
- McKenzie, R.; Roberts, T. Soil and fertilizers phosphorus update. In Proceedings of the Alberta Soil Science Workshop Proceedings, Edmonton, AB, Canada, 20–22 February 1990; pp. 20–22. [Google Scholar] [CrossRef]
- Smith, S.E.; Dickson, S.; Smith, F.A. Nutrient transfer in arbuscular mycorrhizas: How are fungal and plant processes integrated? Funct. Plant Biol. 2001, 28, 685–696. [Google Scholar] [CrossRef]
- Tabo, R.; Bationo, A.; Amadou, B.; Marchal, D.; Lompo, F.; Gandah, M.; Hassane, O.; Diallo, M.K.; Ndjeunga, J.; Fatondji, D. Fertilizer microdosing and “warrantage” or inventory credit system to improve food security and farmers’ income in West Africa. In Innovations as Key to the Green Revolution in Africa: Exploring the Scientific Facts; Springer: Berlin/Heidelberg, Germany, 2011; pp. 113–121. [Google Scholar]
- Odama, E.; Tsujimoto, Y.; Yabuta, S.; Akagi, I.; Sakagami, J.-I. P-dipping improved NERICA 4 rice seedling resilience to water and nutrient stresses under rainfed-like conditions. Rhizosphere 2023, 26, 100688. [Google Scholar] [CrossRef]
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M.; Andrianary, B.H. Localized phosphorus application via P-dipping doubles applied P use efficiency and avoids weather-induced stresses for rice production on P-deficient lowlands. Eur. J. Agron. 2023, 149, 126901. [Google Scholar] [CrossRef]
- Rakotoarisoa, N.M.; Tsujimoto, Y.; Oo, A.Z. Dipping rice seedlings in P-enriched slurry increases grain yield and shortens days to heading on P-deficient lowlands in the central highlands of Madagascar. Field Crops Res. 2020, 254, 107806. [Google Scholar] [CrossRef]
- Alhaj Hamoud, Y.; Wang, Z.; Guo, X.; Shaghaleh, H.; Sheteiwy, M.; Chen, S.; Qiu, R.; Elbashier, M.M. Effect of irrigation regimes and soil texture on the potassium utilization efficiency of rice. Agronomy 2019, 9, 100. [Google Scholar] [CrossRef]
- Dou, F.; Soriano, J.; Tabien, R.E.; Chen, K. Soil texture and cultivar effects on rice (Oryza sativa L.) grain yield, yield components and water productivity in three water regimes. PLoS ONE 2016, 11, e0150549. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.D.L.; Soratto, R.P.; Fernandes, A.; Dias, P.H. Phosphorus fertilization and soil texture affect potato yield. Rev. Caatinga 2018, 31, 541–550. [Google Scholar] [CrossRef]
- Mojid, M.A.; Mousumi, K.A.; Ahmed, T. Performance of wheat in five soils of different textures under freshwater and wastewater irrigation. Agric. Sci. 2020, 2, 89. [Google Scholar] [CrossRef]
- Azam, M.G.; Sarker, U.; Uddin, M.S. Screening maize (Zea mays L.) genotypes for phosphorus deficiency at the seedling stage. Turk. J. Agric. For. 2022, 46, 802–821. [Google Scholar] [CrossRef]
- Jabborova, D. The effects of Pseudomonas koreensis IGPEB 17 and arbuscular mycorrhizal fungi on growth and physiological properties of ginger. Turk. J. Agric. For. 2022, 46, 488–495. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: Shifted hot spots and nutrient imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.; Holland, E.; Johnes, P.; Katzenberger, J.; Martinelli, L.A.; Matson, P. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, L.C.; Searle, P.L.; Daly, B.K. Methods for chemical analysis of soils. NZ Soil Bur. Sci. Rep. 1987, 80, 71–76. [Google Scholar]
- Piper, C. Soil and Plant Analysis; Hassel Press: Adelaide, Australia, 1950; 368p. [Google Scholar]
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M. Optimizing the phosphorus concentration and duration of seedling dipping in soil slurry for accelerating the initial growth of transplanted rice. Agronomy 2020, 10, 240. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Nziguheba, G.; Merckx, R.; Palm, C.A. Soil phosphorus dynamics and maize response to different rates of phosphorus fertilizer applied to an Acrisol in western Kenya. Plant Soil 2002, 243, 1–10. [Google Scholar] [CrossRef]
- Miller, S.S.; Liu, J.; Allan, D.L.; Menzhuber, C.J.; Fedorova, M.; Vance, C.P. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiol. 2001, 127, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Matar, A.; Torrent, J.; Ryan, J. Soil and fertilizer phosphorus and crop responses in the dryland Mediterranean zone. Adv. Soil Sci. 1992, 18, 81–146. [Google Scholar] [CrossRef]
- Takahashi, Y.; Katoh, M. Root response and phosphorus uptake with enhancement in available phosphorus level in soil in the presence of water-soluble organic matter deriving from organic material. J. Environ. Manag. 2022, 322, 116038. [Google Scholar] [CrossRef] [PubMed]
- De Bauw, P.; Vandamme, E.; Senthilkumar, K.; Lupembe, A.; Smolders, E.; Merckx, R. Combining phosphorus placement and water saving technologies enhances rice production in phosphorus-deficient lowlands. Field Crops Res. 2019, 236, 177–189. [Google Scholar] [CrossRef]
- Oo, A.Z.; Tsujimoto, Y.; Rakotoarisoa, N.M.; Kawamura, K.; Nishigaki, T. P-dipping of rice seedlings increases applied P use efficiency in high P-fixing soils. Sci. Rep. 2020, 10, 11919. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, O.; Higuchi, K.; Miwa, E.; Tadano, T. Growth injury induced by high pH in rice and tomato. Soil Sci. Plant Nutr. 2010, 56, 407–411. [Google Scholar] [CrossRef]
- Turner, A.J.; Arzola, C.I.; Nunez, G.H. High pH stress affects root morphology and nutritional status of hydroponically grown Rhododendron (Rhododendron spp.). Plants 2020, 9, 1019. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Q.; Sheng, W.-p.; Li, S.-g.; Tian, J. Determination of leaf carbon isotope discrimination in C4 plants under variable N and water supply. Sci. Rep. 2017, 7, 351. [Google Scholar] [CrossRef]
- Lloret, P.G.; Casero, P.J. Lateral root initiation. In Plant Roots: The Hidden Half; CRC Press: Boca Raton, FL, USA, 2002; Volume 3. [Google Scholar]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef]
- Raven, J.A.; Edwards, D. Roots: Evolutionary origins and biogeochemical significance. J. Exp. Bot. 2001, 52, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mo, Q.; Han, X.; Hui, D.; Shen, W. Fine root dynamics responses to nitrogen addition depend on root order, soil layer, and experimental duration in a subtropical forest. Biol. Fertil. Soils 2019, 55, 723–736. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Spilles, A.; Bonkowski, M. C:N:P stoichiometry and nutrient limitation of the soil microbial biomass in a grazed grassland site under experimental P limitation or excess. Ecol. Process. 2012, 1, 6. [Google Scholar] [CrossRef]
- He, Y.; Liao, H.; Yan, X. Localized supply of phosphorus induces root morphological and architectural changes of rice in split and stratified soil cultures. Plant Soil 2003, 248, 247–256. [Google Scholar] [CrossRef]
- Lynch, P.; Bates, R. Root hairs confer a competitive advantage under low P availability. Plant Soil 2001, 236, 243–250. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.; Brown, K.; Lynch, J. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Ho, M.D.; Rosas, J.C.; Brown, K.M.; Lynch, J.P. Root architectural tradeoffs for water and phosphorus acquisition. Funct. Plant Biol. 2005, 32, 737–748. [Google Scholar] [CrossRef]
- Prescott, C.E.; Grayston, S.J.; Helmisaari, H.-S.; Kaštovská, E.; Körner, C.; Lambers, H.; Meier, I.C.; Millard, P.; Ostonen, I. Surplus carbon drives allocation and plant–soil interactions. Trends Ecol. Evol. 2020, 35, 1110–1118. [Google Scholar] [CrossRef]
- Wang, R.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J. Ecol. 2021, 109, 3699–3709. [Google Scholar] [CrossRef]
- Aziz, T.; Ahmed, I.; Farooq, M.; Maqsood, M.A.; Sabir, M. Variation in phosphorus efficiency among Brassica cultivars I: Internal utilization and phosphorus remobilization. J. Plant Nutr. 2011, 34, 2006–2017. [Google Scholar] [CrossRef]
- de Souza Campos, P.M.; Meier, S.; Morales, A.; Borie, F.; Cornejo, P.; Ruiz, A.; Seguel, A. Root traits distinguish phosphorus acquisition of two wheat cultivars growing in phosphorus-deficient acid soil. Rhizosphere 2022, 22, 100549. [Google Scholar] [CrossRef]
- De Bauw, P.; Vandamme, E.; Lupembe, A.; Mwakasege, L.; Senthilkumar, K.; Merckx, R. Architectural root responses of rice to reduced water availability can overcome phosphorus stress. Agronomy 2018, 9, 11. [Google Scholar] [CrossRef]
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef]
- Irfan, M.; Abbas, M.; Shah, J.A.; Akram, M.A.; Depar, N.; Memon, M.Y. Biomass and phosphorus accumulation, partitioning and remobilization during grain development in wheat under phosphorus deficiency. Intr. J. Agric. Biol. 2019, 21, 351–358. [Google Scholar] [CrossRef]
- Wikström, F. A theoretical explanation of the Piper-Steenbjerg effect. Plant Cell Environ. 1994, 17, 1053–1060. [Google Scholar] [CrossRef]
| Property | Kagoshima 1 | Tanegashima 1 | Tokunoshima 1 |
|---|---|---|---|
| WRB classification | Arenosols | Andosols | Acrisols |
| pH (1:2.5 H2O) | 8.8 | 4.9 | 5.8 |
| EC (mS m−1) | 44.0 | 16.3 | 28.9 |
| Total N (%) | 0.02 | 0.19 | 0.10 |
| Total organic C (%) | 0.05 | 1.62 | 0.52 |
| C:N ratio | 0.9 | 8.5 | 5.3 |
| Organic matter content (%) | 0.09 | 2.79 | 0.90 |
| Available P (mg kg−1) | 24.5 | 186.5 | 18.3 |
| Al oxalate (mg g−1) | 9.1 | 25.2 | 13.2 |
| Fe oxalate (mg g−1) | 1.7 | 6.9 | 1.8 |
| Sand (%) | 95.6 | 30.8 | 12.6 |
| Clay (%) | 2.7 | 43.4 | 79.7 |
| Silt (%) | 1.7 | 25.8 | 7.7 |
| Textural name | Sand | Clay loam | Clay |
| Treatments | Application Rate (mg P2O5 pot−1) | Application Method | Timing |
|---|---|---|---|
| P application | |||
| Pdip | 68.7 | P-dipping 2 | At transplanting |
| Brod1 | 43.1 | Broadcasting | At transplanting |
| Brod2 | 85.9 | Broadcasting | At transplanting |
| Ctrl | 0 | - | - |
| Soil texture | Quantity (kg soil pot−1) | Field condition volumetric moisture content (% w/w) | |
| Sand | 1.5 | 32 | |
| Clay loam | 1.5 | 42 | |
| Clay | 1.5 | 48 |
| Soil Texture (S) | Phosphorus Treatment (P) | Plant Height | Leaf Age | Leaf Area | SPAD |
|---|---|---|---|---|---|
| (cm) | (cm2 pot−1) | Value | |||
| Sand | Ctrl | 55.6 ± 1.8 c | 5.0 ± 0.01 b | 197.3 ± 10.9 b | 14.3 ± 2.4 b |
| Pdip | 63.1 ± 2.5 b | 5.0 ± 0.01 b | 238.4 ± 17.4 b | 21.7 ± 1.1 a | |
| Brod1 | 66.2 ± 2.7 b | 7.3 ± 0.58 a | 312.0 ± 17.4 a | 24.0 ± 1.3 a | |
| Brod2 | 72.6 ± 2.1 a | 7.2 ± 1.44 a | 305.7 ± 27.5 a | 17.6 ± 0.1 b | |
| Clay loam | Ctrl | 81.7 ± 2.8 b | 9.5 ± 0.87 a | 447.2 ± 10.7 a | 43.5 ± 0.8 b |
| Pdip | 87.3 ± 3.2 ab | 9.3 ± 0.58 a | 501.3 ± 16.7 a | 47.1 ± 0.3 a | |
| Brod1 | 89.6 ± 1.7 a | 9.2 ± 0.58 a | 499.5 ± 35.6 a | 45.6 ± 0.6 a | |
| Brod2 | 86.9 ± 0.9 ab | 8.8 ± 0.29 a | 500.7 ± 12.0 a | 46.1 ± 0.6 a | |
| Clay | Ctrl | 65.0 ± 5.9 b | 4.7 ± 0.58 b | 180.1 ± 30.9 c | 39.7 ± 1.4 c |
| Pdip | 81.0 ± 5.5 a | 5.0 ± 0.01 b | 265.2 ± 28.3 b | 43.5 ± 0.6 b | |
| Brod1 | 84.8 ± 5.9 a | 6.8 ± 0.29 a | 368.2 ± 10.3 a | 45.8 ± 0.4 a | |
| Brod2 | 89.2 ± 1.0 a | 7.0 ± 0.50 a | 418.4 ± 10.1 a | 47.9 ± 0.6 a | |
| Two-way ANOVA | S | * | * | * | * |
| P | * | * | * | * | |
| S × P | * | * | * | * |
| Soil Texture (S) | Phosphorus Treatment (P) | Total Root Length | Root Surface Area | Root Volume | Root Length Ratio | Root Mass Ratio | Root to Shoot Ratio |
|---|---|---|---|---|---|---|---|
| (m pot−1) | (cm2 pot−1) | (cm3 pot−1) | (m g−1) | (g g−1) | |||
| Sand | Ctrl | 33.9 ± 1.3 c | 519.7 ± 6.8 c | 4.5 ± 0.1 a | 29.0 ± 1.1 ab | 0.13 ± 0.02 a | 0.15 ± 0.03 a |
| Pdip | 52.0 ± 0.8 a | 557.6 ± 8.7 b | 4.8 ± 0.3 a | 38.2 ± 2.6 a | 0.12 ± 0.01 ab | 0.13 ± 0.01 a | |
| Brod1 | 52.5 ± 0.9 a | 588.6 ± 9.9 a | 4.9 ± 0.4 a | 27.8 ± 5.8 ab | 0.11 ± 0.01 ab | 0.12 ± 0.02 ab | |
| Brod2 | 36.9 ± 0.9 b | 361.6 ± 6.4 d | 2.8 ± 0.1 b | 20.3 ± 5.2 c | 0.09 ± 0.02 b | 0.10 ± 0.02 ab | |
| Clay loam | Ctrl | 27.2 ± 6.0 b | 404.2 ± 5.9 d | 4.1 ± 0.1 c | 13.6 ± 1.2 ab | 0.25 ± 0.04 a | 0.13 ± 0.06 a |
| Pdip | 31.9 ± 6.4 b | 481.6 ± 9.4 c | 4.8 ± 0.1 c | 9.8 ± 2.9 b | 0.24 ± 0.04 a | 0.12 ± 0.04 a | |
| Brod1 | 43.0 ± 5.9 b | 670.9 ± 29.4 b | 6.7 ± 0.4 b | 12.6 ± 2.0 b | 0.14 ± 0.01 b | 0.08 ± 0.01 a | |
| Brod2 | 63.7 ± 6.2 a | 852.4 ± 29.7 a | 8.1 ± 0.9 a | 20.2 ± 4.0 a | 0.13 ± 0.02 b | 0.09 ± 0.02 a | |
| Clay | Ctrl | 60.2 ± 0.2 c | 717.2 ± 7.2 c | 6.7 ± 0.2 b | 47.1 ± 8.4 a | 0.11 ± 0.05 a | 0.33 ± 0.06 a |
| Pdip | 115.9 ± 3.0 a | 1286.3 ± 23.9 a | 11.8 ± 0.5 a | 53.2 ± 3.9 a | 0.10 ± 0.03 a | 0.33 ± 0.08 a | |
| Brod1 | 66.1 ± 3.7 c | 781.8 ± 25.5 b | 7.3 ± 0.6 b | 25.2 ± 1.0 b | 0.08 ± 0.01 a | 0.17 ± 0.01 b | |
| Brod2 | 75.2 ± 3.5 b | 692.5 ± 23.0 c | 6.4 ± 0.5 b | 27.4 ± 6.3 b | 0.08 ± 0.02 a | 0.15 ± 0.03 b | |
| Two-way ANOVA | S | * | * | * | * | * | * |
| P | * | * | * | * | * | * | |
| S × P | * | * | * | * | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odama, E.; Tsujimoto, Y.; Yabuta, S.; Akagi, I.; Chepkoech, R.; Soe, I.; Sakagami, J.-I. Effect of P-Dipping on Growth of NERICA 4 Rice in Different Soil Types at Initial Growth Stages. Sustainability 2023, 15, 15402. https://doi.org/10.3390/su152115402
Odama E, Tsujimoto Y, Yabuta S, Akagi I, Chepkoech R, Soe I, Sakagami J-I. Effect of P-Dipping on Growth of NERICA 4 Rice in Different Soil Types at Initial Growth Stages. Sustainability. 2023; 15(21):15402. https://doi.org/10.3390/su152115402
Chicago/Turabian StyleOdama, Emmanuel, Yasuhiro Tsujimoto, Shin Yabuta, Isao Akagi, Rael Chepkoech, Ibrahim Soe, and Jun-Ichi Sakagami. 2023. "Effect of P-Dipping on Growth of NERICA 4 Rice in Different Soil Types at Initial Growth Stages" Sustainability 15, no. 21: 15402. https://doi.org/10.3390/su152115402
APA StyleOdama, E., Tsujimoto, Y., Yabuta, S., Akagi, I., Chepkoech, R., Soe, I., & Sakagami, J.-I. (2023). Effect of P-Dipping on Growth of NERICA 4 Rice in Different Soil Types at Initial Growth Stages. Sustainability, 15(21), 15402. https://doi.org/10.3390/su152115402







