Abstract
Magnesium nutrition in plants has remained largely unexplored compared to other essential elements. Although the impact of magnesium deficiency on plants has been reported from numerous studies, the responses of plants to excess magnesium salt levels have received less attention. Using five different magnesium levels (0, 500, 1000, 1500, and 2000 ppm) and two magnesium sources (MgSO4 and MgCl2), this study evaluated the effect of excess magnesium salts on rice production and associated physiological processes on a hybrid rice cultivar ‘XP 753’. Rice morphological and physiological parameters, including plant growth, biomass, root morphological features, tissue and grain mineral concentrations, membrane injury (MI), chlorophyll, malondialdehyde (MDA) concentrations, proline concentrations, as well as gas exchange parameters, were evaluated. A dose-dependent reduction in above- and below-ground shoot and root morphological features was observed under the application of magnesium salts on the soil substrate. Analysis of physiological parameters demonstrated that an inhibition in plant growth, biomass, and yield was due to the decrease in total chlorophyll content, net photosynthesis rate, and membrane stability in rice. Furthermore, this study showed that the application of magnesium salts to soil interfered with the uptake and translocation of minerals and significantly increased reactive oxygen species (ROS), malondialdehyde (MDA), and proline levels, indicating the toxic effects of excess magnesium salts on rice plants.
1. Introduction
Magnesium (Mg) is an essential nutrient for plant growth and development, and it plays a crucial role in many physiological processes and enzyme functions. It has a central role in chlorophyll formation, metabolism of carbohydrates, and cell membrane stabilization. Magnesium is also pivotal for the function of numerous cellular enzymes, including adenosine triphosphatases (ATPases), RNA polymerases, phosphatases, protein kinases, glutathione synthase, and carboxylases [1,2,3]. More importantly, maintaining Mg homeostasis is essential in plants as the activities of a number of chloroplast enzymes are also strongly impacted by slight changes in the levels of Mg2+ ions in the cytosol and the chloroplast [4,5]. Although Mg is one of the most abundant available minerals on earth, it is highly prone to leaching because Mg2+ ions, the only available form of Mg for plant absorption, have the largest hydrated radius compared to other divalent cations [1,6]. Moreover, Mg2+ absorption is also impacted by the addition of excessive K+ and NH4+ fertilizers in soil, causing Mg deficiencies in plants [7,8]. Although the responses of the plants to Mg deficiency have been well reported, the opposite, excess soil Mg levels, has not been well studied.
It is obvious that the status of Mg content in the plant cell affects the cellular metabolism, impacting various phenotypic and physiological parameters. Excess cellular Mg2+ leads to the substitution of Ca2+ and K+, causing an impairment in cell wall stability and cell membrane permeability [9,10]. The surplus presence of Mg salt in the media had a toxic effect on the radicles of Kalidium capsicum, and the unhealthy radicle symptoms from excess Mg were different from the osmotic effect caused by the supply of polyethylene glycol (PEG) in the media [11]. In bean plants (Phaseolus vulgaris L.), the application of 120 mM MgCl2 and MgSO4 salt to the soil decreased shoot and root biomass by 4.3- and 6.0-fold, respectively, indicating the toxic effect of Mg salt to the plants [12]. Moreover, muskmelon shoot growth was severely reduced by MgCl2 and MgSO4 salts compared to the sodium (Na+) salt, suggesting crops might be more sensitive to magnesium salts than sodic salts [13]. Furthermore, oversupply of Mg drastically increased the transport of metabolites from sources to sink tissues, negatively impacting the abundance of metabolites in tomato fruits [14,15]. In rice, excess magnesium present in a culture solution not only limited the shoot growth to 54–67% of that in the control but also slightly reduced Ca and K contents in the tissue [16].
Plants responses to environmental stresses including excess magnesium salt depend upon the magnitude and the duration of stress. It is known that environmental stresses, such as salt, drought, UV, and nutrient deficiencies, induce the production of reactive oxygen species (ROS) [17,18]. Elevated production of ROS leads to damage in the cell at the molecular level, which is known as oxidative stress [19]. The excess ROS are subsequently converted to their most stable forms, including hydrogen peroxide (H2O2), via cellular machinery. Some of the potential downstream effects of the overaccumulation of ROS in plant cells include elevated malondialdehyde (MDA) production, reduced soluble sugar, and reduced leaf chlorophyll content [20,21]. Plants have developed an array of cellular responses to overcome the deleterious effects of ROS overaccumulation. One such response includes the production of free proline, which plays a major role in resistance to environmental stress. Proline not only participates in osmotic adjustment but also functions as enzyme protectants, free radical scavenger, and cell redox balancer [22,23].
Rice (Oryza sativa L.) is one of the most widely consumed cereal grains in the world and is the staple food of more than half of the global population. Soil analysis reports of rice fields in Texas, U.S.A., indicated elevated levels of Mg element (Supplementary Table S1). However, studies on the effect of excess magnesium supply on rice production and its associated biochemical and physiological processes have been rarely reported. We hypothesized that high Mg supply may impair nutrient uptake and carbon assimilation in rice plants by altering enzyme functions, ultimately reducing crop growth and grain yield. Therefore, the aim of this study is to characterize the impact of an excess supply of magnesium salt in rice production and the underlying physiological processes. To manifest this goal, a greenhouse trial was conducted using two magnesium sources, magnesium sulfate (MgSO4) and magnesium chloride (MgCl2), and a hybrid rice cultivar (‘XP 753’).
2. Materials and Methods
2.1. Trial 1: Biomass and Yield Analysis
A greenhouse trial was conducted from September 2020 to February 2021 at the Texas A & M AgriLife Research Center near Beaumont Texas. The soil for this study was collected from a rice field at the research center. The collected soil was a League clay soil (fine, montmorillonitic, Entic Pelludert) constituting 28% silt, 58% clay, 1.2% organic matter, and pH 5.5. The experiment was arranged in a completely randomized design with two Mg sources (MgSO4 vs. MgCl2) and five Mg concentrations (0, 500, 1000, 1500, and 2000 ppm). The trial was conducted using polyethylene pots (22 cm diameter, 22 cm deep) filled with 3.5 kg of air-dried soil with four replications per treatment. Before transplanting into soil, the seeds of a hybrid rice cultivar ‘XP 753’ were incubated at 25 °C for 48 h in petri dishes with moist filter paper. Six pre-germinated seedlings were transplanted to each pot and then pots were thinned to three rice plants 12 days after planting. Each pot was occasionally irrigated until a permanent flood was established 25 days after planting. A depth of 3 cm of standing water above the surface of the soil was maintained in each pot until drainage before harvest.
For biomass analysis, above- and below-ground tissues were harvested at the maximum tillering stage. Harvested tissues were carefully washed using tap water and were dried in an oven at 65 °C for 3 d, and their respective dry weights were recorded. For yield and yield component analysis, plants were manually harvested, the harvested rice bundles were dried, and above-ground dry biomass was recorded. Yield components, including grain yield per plant, number of grains per plant, and 1000-grain weight, were measured.
Gas exchange measurements were performed on the uppermost fully expanded leaves of the main tiller in a rice plant at the maximum tillering stage using an LI-6400 portable photosynthesis system (LI-COR Inc., Lincoln, NE, USA). The rate of net CO2 assimilation was assessed at 25 °C, 400 μmol CO2 mol−1 under 1500 μmol m−2 s−1 light between 10:00 a.m. and 12:00 p.m. local time. Following photosynthetic measurements, the leaf area was recorded and used for standardization.
The Soil Plant Analysis Development (SPAD) readings were also measured on the two uppermost fully expanded leaves of the main tiller in a rice plant at the maximum tillering stage using a SPAD 502 Chlorophyll Meter (Minolta, Osaka, Japan). Two SPAD readings were taken from each leaf, and two plants were randomly selected from each pot.
The harvested biomass and grain tissues were dried at 65 °C for 2 d. Tissue samples, equivalent to 1 g (gm) dry weight, were then ground with a mechanical grinder. Following grinding, the plant’s minerals (B, Ca, Fe, K, Mg, Mn, Na, P, S, and Zn) were determined via inductively coupled plasma (ICP) spectrometry (Spectro Analytical Instruments Inc., 1 Executive Dr Ste 101, Chelmsford, MA, USA) using nitric acid digestion method [24].
2.2. Trial 2: Root Morphology Anlaysis
Pre-germinated seedlings, three per pot, were placed in vertical soil pots containing different levels of Mg salts. The pots were thinned to one rice plant per pot at 10 days, and the plants were grown for an additional two weeks. At the end of the trial, rice roots were harvested, washed properly with tap water, and root morphology features were measured using WinRHIZO 2012a (Regent Instruments, Inc., Quebec, QC, Canada).
2.3. Trial 3: Germination and Hypocotyl Elongation
For the germination experiment, rice seeds were directly placed in soil pots containing different levels of magnesium salts and grown under greenhouse conditions for seven days. The number of germinated seedlings was counted at 4, 5, 6, and 7 days after sowing.
2.4. Trial 4: Stress Evaluation Experiemnt
Pre-germinated seedlings, three per pot, were placed in small soil pots containing different levels of Mg salts. The pots were thinned to one rice plant per pot two weeks after transplanting, and the plants were grown for an additional two weeks. The shoot tissues were harvested at the end of the trial. Tissues were then washed immediately using deionized water, frozen in liquid nitrogen, and stored at −80 °C. The frozen tissues were later ground in liquid nitrogen and were used for the following physiological assays.
For total H2O2 content, 100 mg frozen shoot tissue sample was mixed with 1.0 mL of 50 mM potassium phosphate (K-PO4) (pH 7) and incubated on ice for 10 min. Following centrifugation at 4 °C for 20 min at 4640× g, the supernatant was used for H2O2 content evaluation. An aliquot of the supernatant (200 µL) was mixed with 200 µL of 0.1% Titanium Chloride (TiCl4) in 20% H2O2 (v/v) and incubated at room temperature for 5 min. The solution was then centrifuged at 4640× g for 10 min at room temperature, and the absorbance was recorded at 410 nm using a microplate reader. The H2O2 content in the tissue was calculated using the extinction coefficient (e = 0.28 µ mol−1 cm−1) [25].
Total chlorophyll was extracted using 3 mL of 100% methanol from 50 mg of pulverized leaf tissues on ice. The solution was centrifuged at 4 °C for 20 min at 4640× g. Using a microplate reader, the absorbance of the supernatant was measured at 652.0 nm and 665.2 nm [26].
Lipid peroxidation was analyzed with the thiobarbituric acid (TBA) test, which measures malondialdehyde (MDA) as an end product of lipid peroxidation [27]. Frozen tissue (50 mg) was extracted in 1 mL of 80% (v/v; 13.7 M) ethanol on ice. The supernatant (0.5 mL) was centrifuged at 4640× g for 10 min at 4 °C and mixed with 0.5 mL of 20% (w/v; 1.22 M) trichloroacetic acid containing 0.65% (w/v; 0.05 M) thiobarbituric acid. The mixture was incubated at 95 °C for 30 min and then immediately cooled on ice. The mixture was later centrifuged at 4640× g for 10 min at 4 °C, and the absorbance of the supernatant was quantified at 532 nm, subtracting the value for non-specific absorption at 600 nm. The MDA concentration was calculated from the extinction coefficient of 155 mM−1 cm−1.
2.5. Statistical Analysis
Statistical analyses were performed in RStudio Version 1.4.1717. One-way ANOVA was conducted in R to evaluate the effect of magnesium salt sources on plant growth, biomass, yield, root parameters, and other associated physiological processes. The comparison between the mean values was carried out using Fisher LSD test using Agricolae package in R (https://cran.r-project.org/package=agricolae, accessed on 24 August 2023) [28].
3. Results
3.1. Germination and Hypocotyl Elongation
Germination and hypocotyl elongation experiments were conducted to determine the effect of excess Mg supply at the rice seed and seedling stages. Excess magnesium supply reduced seed germination, starting at a concentration of 500 ppm; however, the germination was severely reduced at higher Mg doses, such as 1500 ppm and 2000 ppm (Figure 1a,b). At lower concentrations (500 ppm), the germination was reduced by 56% and 12%, respectively, for MgCl2 and MgSO4 salts compared to non-stress conditions (Figure 1c). At the highest Mg dose (2000 ppm), seed germination was reduced by 84% for MgSO4 salt, and no seed germination was observed under MgCl2 salt (Figure 1c). Similar to the germination, the hypocotyl elongation was also significantly reduced with an additional supply of magnesium to the substrate (Figure 1d). The reduction in hypocotyl growth was minimal at a low Mg concentration (500 ppm); however, at a high concentration, 1500 ppm, the hypocotyl growth was reduced by 62% and 85% for MgSO4 and MgCl2 salts, respectively (Figure 1d). No elongation was observed at the highest Mg dose, 2000 ppm. Interestingly, the effect of MgCl2 on seed germination and hypocotyl elongation was more pronounced compared to the MgSO4 salt at any dose.
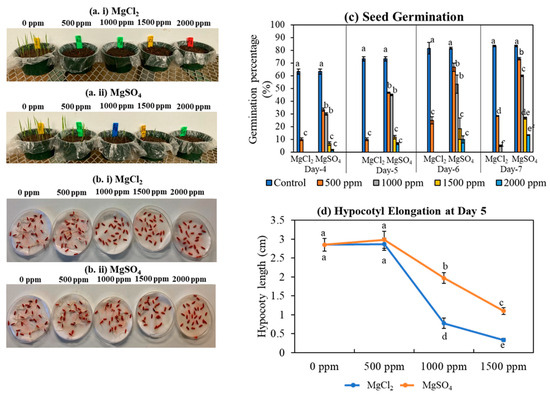
Figure 1.
Excess Mg reduced germination and hypocotyl elongation in rice. (a,b) Photos of germinated rice seeds exposed to different levels of magnesium salts. (a(i,ii)) Seeds were directly sown on soil treated with 0, 500, 1000, 1500, and 2000 ppm concentration of magnesium salts. Photographs were taken at 7 DAS. (b(i,ii)) Seeds were sown in petri dishes with wet filter papers containing 0, 500, 1000, 1500, and 2000 ppm of magnesium salts. Images were taken five days after incubation. (c) Germination percentage of rice seeds grown under different levels of magnesium salts. (d) Hypocotyl elongation of rice seeds incubated under different levels of magnesium salts. Data represents means ± SE. Different letters (a, b, c, d, e, and f) indicate significant differences between means (n = 20, Fisher LSD test at α = 0.05).
3.2. Early Vegetative Growth and Biomass
Both sources of magnesium salt caused a dose-dependent reduction in the number of tillers, shoot biomass, and root biomass (Figure 2b). The number of tillers per plant reduced from an average of 5.2 per plant under non-stress control conditions to approximately 3.0 and 1.4 tillers per plant at the highest Mg level (2000 ppm) for MgSO4 and MgCl2, respectively (Figure 2a). Similar to the tiller number per plant, shoot and root biomass were reduced from 16 gm and 12 gm per plant under non-stress conditions to 7.0 gm and 2.3 gm per plant, respectively, at the highest Mg level (2000 ppm) for both Mg salts (Figure 2c,d). Interestingly, under excess soil Mg supply, the shoot biomass was reduced by two-fold, whereas the root biomass was reduced by more than three-fold.
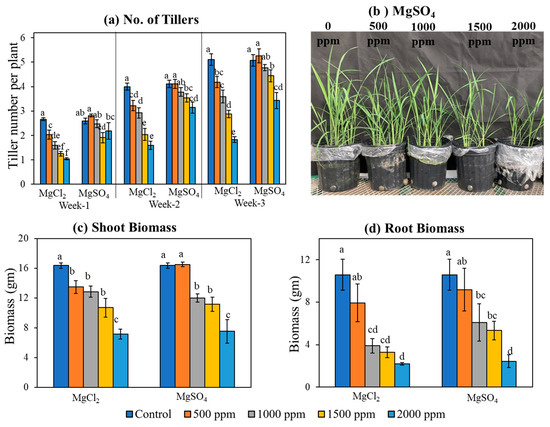
Figure 2.
Excess Mg reduced tiller number and crop biomass in rice. (a) Tiller no. of rice plants grown under different levels of magnesium salts. (b) Photos of rice plants exposed to different concentrations of magnesium sulfate salts. (c,d) Shoot and root biomass of rice plants grown under different levels of magnesium salts. Data represent means ± SE. Different letters (a, b, c, d, e, and f) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
3.3. Root Morphology
The impact of excess magnesium salt on root morphological parameters was analyzed on soil-grown rice plants using WinRhizo Pro 2012 (Figure 3a). Maximum root depth, average root diameter, total root surface area, and total root volume were reduced by excess magnesium supply (Figure 3). The root depth was reduced by more than 40% due to the addition of magnesium salts to the soil, from 28 cm under normal conditions to an average of 16 cm under the higher magnesium salt levels, 1500 and 2000 ppm (Figure 3b). Similarly, root surface area and root volume were reduced by an average of 85% and 86%, respectively, due to the addition of magnesium salts (Figure 3c,d). Interestingly, only MgCl2 salt had an impact on rice root diameter (Figure 3e). The root diameter was reduced by more than two-fold with the addition of MgCl2 to the soil; however, the effect was not observed with MgSO4 salt treatment (Figure 3e). Altogether, consistent with the previous observations, magnesium chloride salt had a more pronounced effect on rice root morphological features, such as root depth, root surface area, root volume, and root diameter, than magnesium sulfate salt.
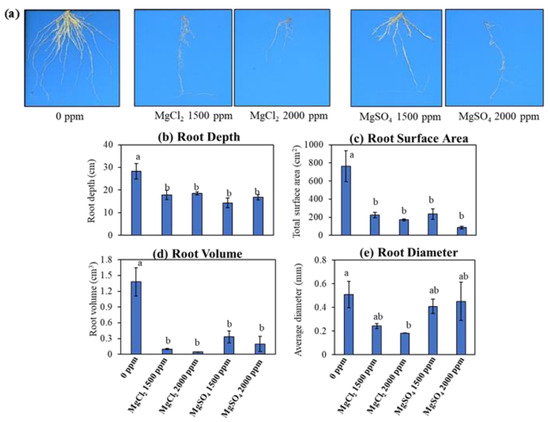
Figure 3.
Effect of magnesium salt input on rice root morphological features. (a) Scanned images of rice roots exposed to 0 ppm, 1500 ppm, and 2000 ppm of magnesium salts. (b–e) Root morphology of ‘XP 753’ rice grown under different concentrations of magnesium salts. Data represent means ± SE. Different letters (a and b) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
3.4. Tissue Mineral Contents
The addition of magnesium salts altered mineral contents in both roots and shoots. Magnesium salt application on soil caused a dose-dependent increase in root Mg concentrations (Figure 4). The root Mg concentration in roots was approximately 1100 ppm under non-treated conditions and was increased by more than three-fold at the highest Mg dose, 2000 ppm. Similarly, nitrogen percentage in roots increased from 1.1% from normal conditions to 1.57% under the highest 2000 ppm dose of MgCl2 salt; however, root N level was not significantly enhanced by the addition of MgSO4 salt. Moreover, our analysis showed that root calcium and copper levels were also significantly increased, whereas iron level was significantly decreased by the addition of magnesium salts on the soil (Figure 4). Surprisingly, no significant change in root potassium, sodium, zinc, or manganese content was observed under magnesium salt treatment.
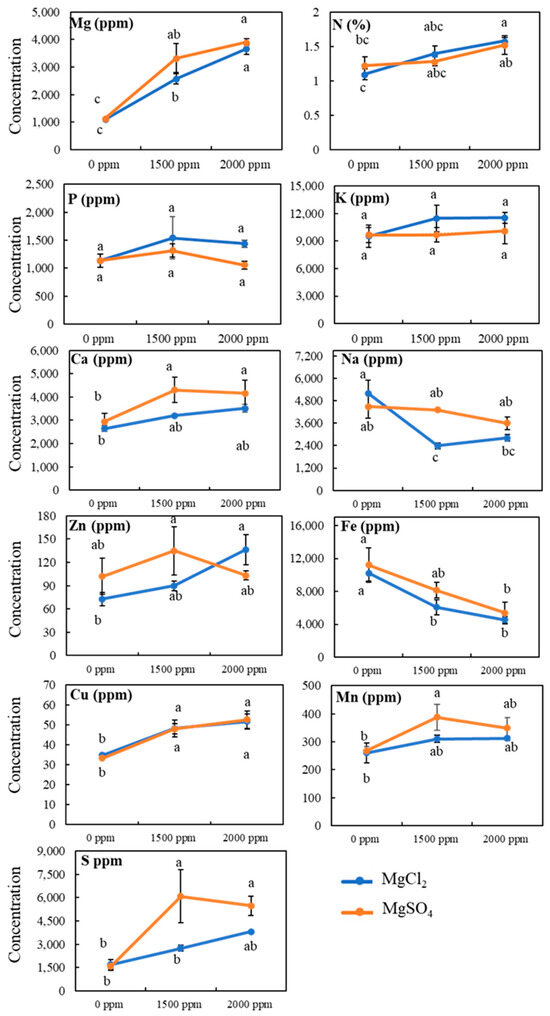
Figure 4.
Effect of magnesium salt treatment on root mineral contents. Data represent means ± SE. Different letters (a, b, and c) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
Similar to the root, the addition of magnesium salts to the soil induced changes in shoot mineral contents (Figure 5). The Mg concentration in shoots increased by more than 1.5-fold at the highest Mg dose (2000 ppm). Shoot Mg level increased from 2100 ppm under control conditions to 3100 ppm and 3600 ppm under the highest dose (2000 ppm) of MgCl2 and MgSO4, respectively. Similarly, shoot N level was also increased by the application of both magnesium salts, MgSO4 and MgCl2, to the soil. MgSO4 salt treatment increased shoot N level from 2.2% under control conditions to 2.58% and 3.1% for 1500 and 200 ppm, respectively. Furthermore, shoot N level also increased from 1.9% under control to 3.1% and 3.5%, respectively, for 1500 and 2000 ppm of MgCl2 salt. In contrast to root, the shoot potassium concentration increased from 32,000 ppm under control conditions to 39,000 and 40,000 ppm at the highest dose (2000 ppm) application of MgSO4 and MgCl2 salts, indicating the positive impact of magnesium salts on shoot potassium content. Moreover, our analysis showed that shoot copper level was also significantly increased, whereas sodium level was significantly decreased by the addition of magnesium salts on the soil. No significant change in shoot phosphorus, calcium, or iron content was observed under the application of either magnesium salt treatment.
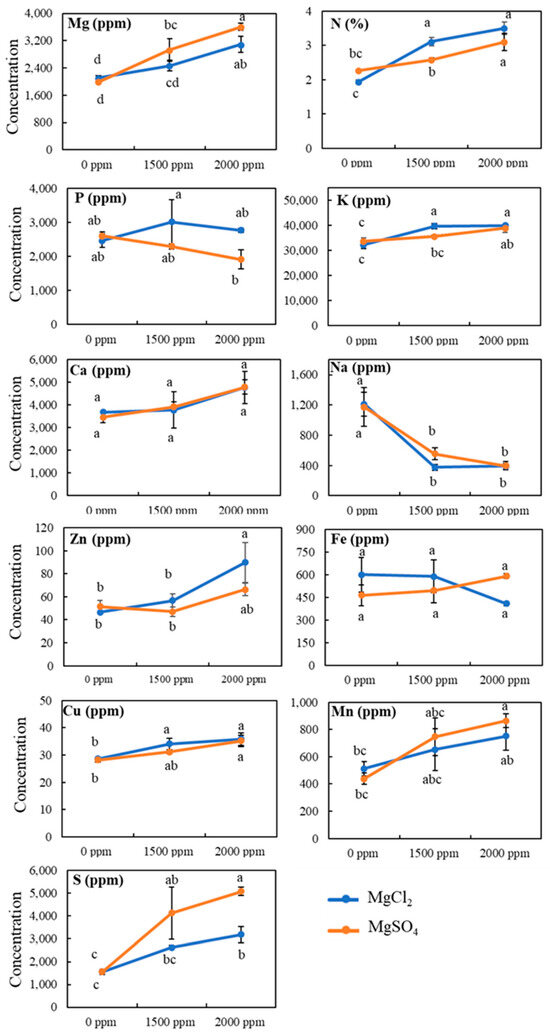
Figure 5.
Effect of magnesium salt treatment on shoot mineral contents. Data represent means ± SE. Different letters (a, b, c, and d) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
3.5. Yield and Biomass
Excess magnesium salt supply caused a significant decrease in total grain yield, above-ground biomass, and grain number per plant (Figure 6). The grain yield decreased from an average of 11 gm per plant under control conditions to 2.2 gm and 4.4 gm per plant under the 2000 ppm dose of MgCl2 and MgSO4, respectively (Figure 6a). Similar to yield, the addition of Mg salt on soil resulted in a dose-dependent reduction in above-ground biomass. The total above-ground biomass reduced from 23.0 gm per plant under control conditions to 16.5 gm and 11.3 gm per plant under 1500 ppm and 2000 ppm doses of MgCl2, respectively (Figure 6b). Similarly, with MgSO4 salt treatment, the biomass per plant was also reduced from 23.0 gm per plant under control conditions to 20.7 gm to 14.3 gm per plant under 1500 and 2000 ppm of MgSO4 salt, respectively (Figure 6b). Although no significant change was observed for 1000-grain weight under magnesium salt treatment (Figure 6c), the grain number per plant significantly decreased from 490 to 165 and 251 for MgCl2 and MgSO4, respectively, under the highest dose, 2000 ppm, of Mg salt (Figure 6d).
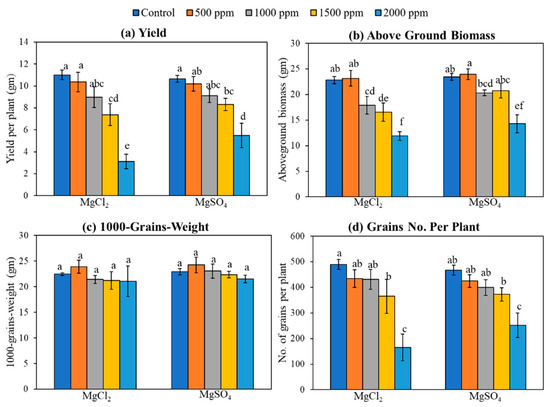
Figure 6.
Impact of excess magnesium salt on yield and yield parameters in a rice variety ‘XP 753’. Data represent means ± SE. Different letters (a, b, c, d, e, and f) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
We further evaluated the effect of magnesium salt treatment on grain mineral contents (Figure 7). Unlike tissue nitrogen content, grain nitrogen content decreased from 2.3% under normal conditions to 2.1% and 1.8% for MgCl2 and MgSO4, respectively, under the highest dose, 2000 ppm, of magnesium salt. Surprisingly, no significant change in grain magnesium content was observed for either source, MgCl2 and MgSO4, of magnesium salts. Similarly, soil magnesium application did not alter other grain mineral concentrations, including phosphorus, potassium, sodium, zinc, iron, and manganese. However, grain copper, sulfur, and calcium concentrations were significantly increased under magnesium salt treatment.
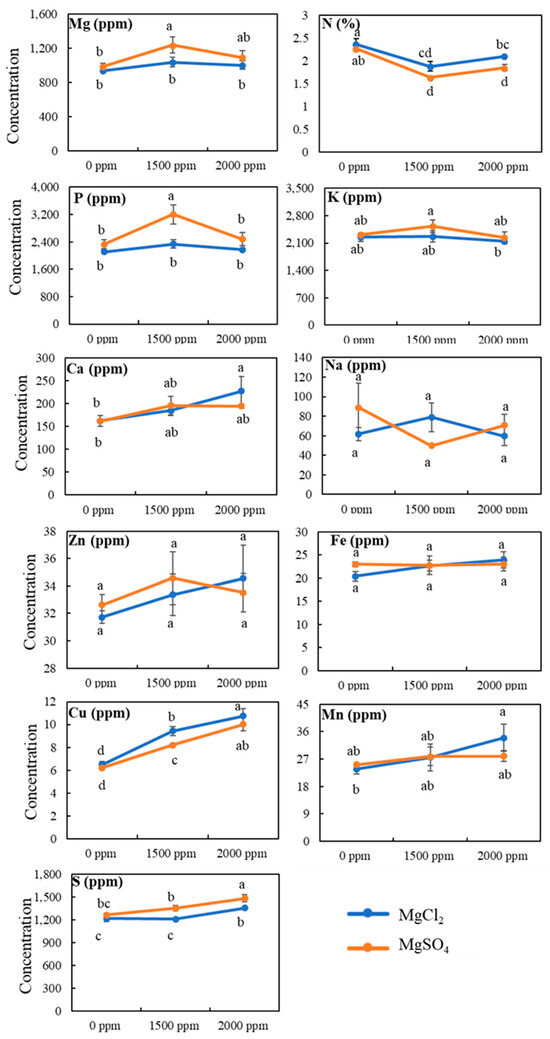
Figure 7.
Effect of magnesium salt treatment on grain minerals contents. Data represent means ± SE. Different letters (a, b, c, and d) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
3.6. Photosynthesis, SPAD, Total Chlorophyll Content, and Membrane Damage
Net photosynthesis rates in rice were significantly reduced by the excess supply of magnesium salts to the soil (Figure 8a). A nominal reduction in net photosynthesis was observed under lower doses of magnesium salts; however, at higher magnesium concentrations, net photosynthesis was significantly reduced by 16% and 13%, respectively, for 2000 ppm MgCl2 and MgSO4 (Figure 8a). Although no significant change in leaf SPAD readings was observed in this study (Figure 8b), total chlorophyll content decreased significantly with the addition of magnesium salt on soil (Figure 8c). The total chlorophyll content decreased by 12% and 14% at the highest salt dose, 2000 ppm, for MgSO4 and MgCl2, respectively. On the other hand, membrane relative injury was significantly increased by the addition of magnesium salt in the soil (Figure 8d). Leaf cell membrane damage was induced by 39% and 68%, respectively, for 1500 and 2000 ppm of MgCl2. Similarly, membrane damage was induced by 26% and 42% for 1500 and 2000 ppm of MgSO4 salt.
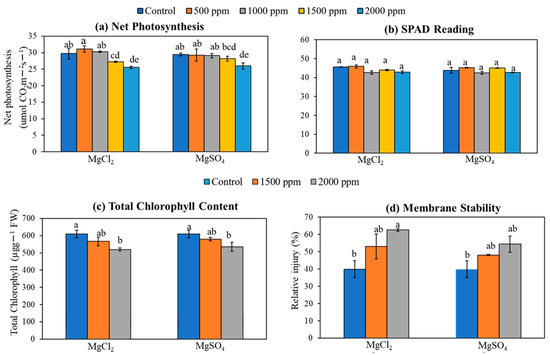
Figure 8.
Effect of magnesium salt doses on (a) net photosynthesis, (b) SPAD reading, (c) membrane stability, and (d) total chlorophyll content. Data represent means ± SE. Different letters (a, b, c, d, and e) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
3.7. ROS, Free Proline, and Membrane Lipid Peroxidation (MDA)
The application of magnesium salt stimulated a dose-dependent significant increase in the reactive oxygen species (ROS), as measured using the H2O2 assay (Figure 9a). Compared to the non-treated conditions, the addition of magnesium salts on soil enhanced tissue hydrogen peroxide by more than two-fold. Similarly, magnesium salt treatment to soil significantly induced lipid peroxidation, as measured using MDA (Figure 9b) and free proline levels (Figure 9c) in rice leaf tissues.
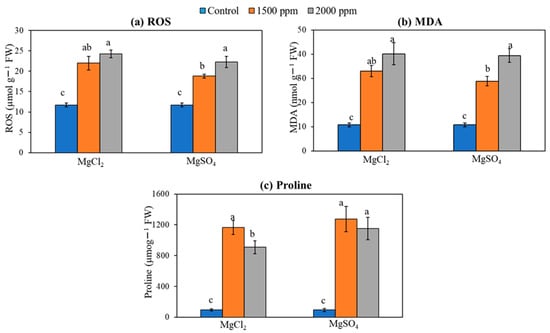
Figure 9.
Effect of magnesium salt doses on (a) ROS, (b) MDA, and (c) proline contents. Data represent means ± SE. Different letters (a, b, and c) indicate significant differences between means (n = 3, Fisher LSD test at α = 0.05).
4. Discussion
Soil salinity is a major abiotic stress restricting the use of land for agriculture because it limits the growth and development of most crop plants [15,29,30]. Improving productivity under these physiologically stressful conditions is a major scientific challenge because salinity has different effects at different developmental stages in different crops [31]. Using a commercial hybrid rice cultivar, ‘XP 753’, this study evaluated the impact of elevated magnesium soil salinity on rice production, physiology, and grain mineral contents.
Our results indicate that rice plants cultivated with excess levels of magnesium salts show a significant reduction in growth parameters in the ‘XP 753’ rice variety. Rice plants cultivated under magnesium salts demonstrated a significant dose-dependent reduction in seed germination, hypocotyl elongation, tiller number per plant, crop biomass, and final grain yield. Furthermore, the application of magnesium salt demonstrated a significant reduction in root morphological parameters, including root depth, root surface area, and root volume, implying the toxic effect of excess Mg on rice root growth. Despite a significant reduction in root features, the rice variety was able to maintain nutrient absorption from the soil, as indicated by the root and shoot mineral concentrations. This is consistent with the previous results demonstrated in rice and soybean plants under magnesium fertilization. Magnesium supply supported nitrogen uptake in soybean by differentially regulating the expression of the nitrate transporter gene (NRT2.1/2.2) [32]. Similarly, magnesium fertilization in soil augmented N fertilizer recovery in rice by inducing high fertilizer N uptake [33]. However, it is worth mentioning that these studies were conducted under magnesium-deficient conditions. Our study showed an increase in root nitrogen uptake in rice, even under high magnesium conditions, indicating that excess magnesium supply may not interfere with the uptake and translocation of nitrogen in plants. Furthermore, this study also showed that excess magnesium supply enhanced shoot potassium (K) concentrations. Previous studies have demonstrated a moderate synergistic to antagonistic interaction between potassium and magnesium uptake in plants. An increase in K concentration in plants significantly reduced Mg concentrations in both shoots and roots [7]. Nonetheless, an increase in Mg concentration in roots tends to improve root to shoot K translocation [7], indicating moderate synergistic effects between Mg and K. In contrast, Kobayashi et al. [16] demonstrated induced root K contents and reduced shoot K content with elevated MgCl2 levels, suggesting the inhibitory effect of magnesium on root to shoot K translocation. The synergistic effect between Mg supply and shoot K content observed in this study may indicate the regulatory roles of potassium in alleviating magnesium toxicity in this hybrid rice variety.
It has been widely recognized that the presence of salt, such as magnesium salt, on a substrate upregulates the production of reactive oxygen species (ROS) in plants in a number of ways [17,18,34]. An overaccumulation of ROS leads to unwanted oxidative damage in plants, consequently impacting chlorophyll biosynthesis, leaf membrane stability, and photosynthetic activities [19,35,36]. The results presented in this paper demonstrate that elevated Mg salt doses increased hydrogen peroxide (H2O2) contents, MDA concentrations, and relative membrane injury and decreased net photosynthesis and chlorophyll content, indicating Mg toxicity in rice plants. Proline accumulation is a well-known resistance response to salt stress in plants [23,37]. Nguyen et al. [38] found that an improvement in salinity resistance was attributed to the accumulation of proline in Nipponbare rice. Similarly, Zheng et al. [39] found that exogenous application of proline resulted in an improvement in salinity resistance in Eurya emarginata. These positive impacts are mainly driven by enhanced nutrient and water acquisition and by improving antioxidant activities and K+ accumulation by plants. The present data show a dose-dependent increase in free proline in the shoots of rice plants treated with excess Mg. Such a high proline accumulation could help the rice plants to avoid the potentially toxic impact of excess Mg; this beneficial effect of proline was manifested as no leaf burning or plant death and continuous plant growth until the maturity stage.
5. Conclusions
Recent soil analysis reports of rice fields in Texas showed very high levels of magnesium salts. Being a central atom in the chlorophyll molecule, an adequate supply of magnesium to the substrate is essential for smooth photosynthetic activities. Nevertheless, this study revealed that magnesium salinity caused dose-dependent biomass and growth restriction by depleting chlorophyll contents and limiting the net CO2 assimilation in rice plants. Moreover, the toxic impact of magnesium salt, even at concentrations such as 500 and 1000 ppm presented in this paper, suggests a revisit to the previous recommendation regarding magnesium fertilization for field crops including rice. Furthermore, future studies to identify tolerant rice genotypes and/or best management practices, including the application of calcium and potassium fertilizers, to overcome the inhibitory effect of excess magnesium salt are critical to address the concerns of Texas rice farmers.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su152215741/s1, Table S1: Soil magnesium levels from rice fields in Texas.
Author Contributions
All authors provided critical feedback and helped shape the research, analysis, and manuscript. Conceptualization, S.L. and F.D.; methodology, S.L. and F.D.; software, S.L.; validation, S.L., L.T. and F.D.; formal analysis, S.L., L.T. and F.D.; investigation, S.L.; resources, F.D.; data curation, S.L. and F.D.; writing—original draft preparation, S.L. and F.D.; writing—review and editing, all authors; visualization, S.L.; supervision, F.D.; project administration, F.D.; funding acquisition, F.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Texas Rice Research Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is provided through the Supplementary Table.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Cell Develop. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Cakmak, I.; Wang, S.; Zhang, F.; Guo, S. Synergistic and antagonistic interactions between potassium and magnesium in higher plants. Crop J. 2021, 9, 249–256. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Vyshpolsky, F.; Qadir, M.; Karimov, A.; Mukhamedjanov, K.; Bekbaev, U.; Paroda, R.; Karajeh, F. Enhancing the productivity of high-magnesium soil and water resources in Central Asia through the application of phosphogypsum. Land Degrad. Develop. 2008, 19, 45–56. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterization of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar] [CrossRef]
- Tobe, K.; Li, X.; Omasa, K. Effects of sodium, magnesium and calcium salts on seed germination and radicle survival of a halophyte, Kalidium caspicum (Chenopodiaceae). Aust. J. Bot. 2002, 50, 163–169. [Google Scholar] [CrossRef]
- Kant, C.; Aydin, A.; Turan, M. Ameliorative effect of hydro gel substrate on growth, inorganic ions, proline, and nitrate contents of bean under salinity stress. J. Plant Nutr. 2008, 31, 1420–1439. [Google Scholar] [CrossRef]
- Nukaya, A.; Masui, M.; Ishida, A. Salt tolerance of muskmelons as affected by various salinities in nutrient solution culture. J. Jpn. Soc. Hort. Sci. 1983, 52, 167–173. [Google Scholar] [CrossRef][Green Version]
- Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative metabolomics unravel the effect of magnesium oversupply on tomato fruit quality and associated plant metabolism. Metabolites 2019, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.X.; Kwon, M.C.; Lee, S.; Jung, E.S.; Lee, C.H.; Sung, J. Effects of nutrient and water supply during fruit development on metabolite composition in tomato fruits (Solanum lycopersicum L.) grown in magnesium excess soils. Front. Plant Sci. 2020, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of excess magnesium on the growth and mineral content of rice and Echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. ROS and oxidative stress: Origin and implication. In Reactive Oxygen Species in Plant Biology; Springer: New Delhi, India, 2019; pp. 1–31. [Google Scholar]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Mattioli, R.; Costantino, P.; Trovato, M. Proline accumulation in plants: Not only stress. Plant Signal. Behav. 2009, 4, 1016–1018. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L.; Soltanpour, P.N. A nitric acid and plant digest method for use with inductively coupled plasma spectrometry. Commun. Soil Sci. Plant. 1989, 14, 969–980. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae Tutorial; Version 1.3-5; Universidad Nacional Agraria: La Molina, Peru, 2021. [Google Scholar]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Alpuerto, J.B.; Han, A.; Fukao, T. The central negative regulator of flooding tolerance, the PROTEOLYSIS 6 branch of the N-degron pathway, adversely modulates salinity tolerance in Arabidopsis. Plants 2020, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Peng, W.T.; Qi, W.L.; Nie, M.M.; Xiao, Y.B.; Liao, H.; Chen, Z.C. Magnesium supports nitrogen uptake through regulating NRT2.1/2.2 in soybean. Plant Soil 2020, 457, 97–111. [Google Scholar] [CrossRef]
- Choudhury, T.M.A.; Khanif, Y.M. Evaluation of effects of nitrogen and magnesium fertilization on rice yield and fertilizer nitrogen efficiency using 15N tracer technique. J. Plant Nutr. 2001, 24, 855–871. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Liu, J.; Islam, M.T.; Sherif, S.M. Changes in reactive oxygen species, antioxidants and carbohydrate metabolism in relation to dormancy transition and bud break in apple (Malus × domestica Borkh) cultivars. Antioxidants 2021, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How does proline treatment promote salt stress tolerance during crop plant development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Das Bhowmik, S.; Long, H.; Cheng, Y.; Mundree, S.; Hoang, L.T.M. Rapid accumulation of proline enhances salinity tolerance in Australian wild rice Oryza australiensis Domin. Plants 2021, 10, 2044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Zhao, L.Y.; Wu, C.W.; Shen, B.; Zhu, A.Y. Exogenous proline reduces NaCl-induced damage by mediating ionic and osmotic adjustment and enhancing antioxidant defense in Eurya emarginata. Acta Physiol. Plant. 2015, 37, 181. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).