Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent
Abstract
:1. Introduction
2. Methods and Materials
2.1. Microalgae and Brewery Wastewater
2.2. Microalgae Cultivation in Brewery Effluent
2.3. Growth and Microalgal Biomass Production
2.4. Nutrient Removal Efficiency and Removal Rate
2.5. Removal Rate Constant, and Biomass Yield Coefficient
2.6. Lipid Extraction
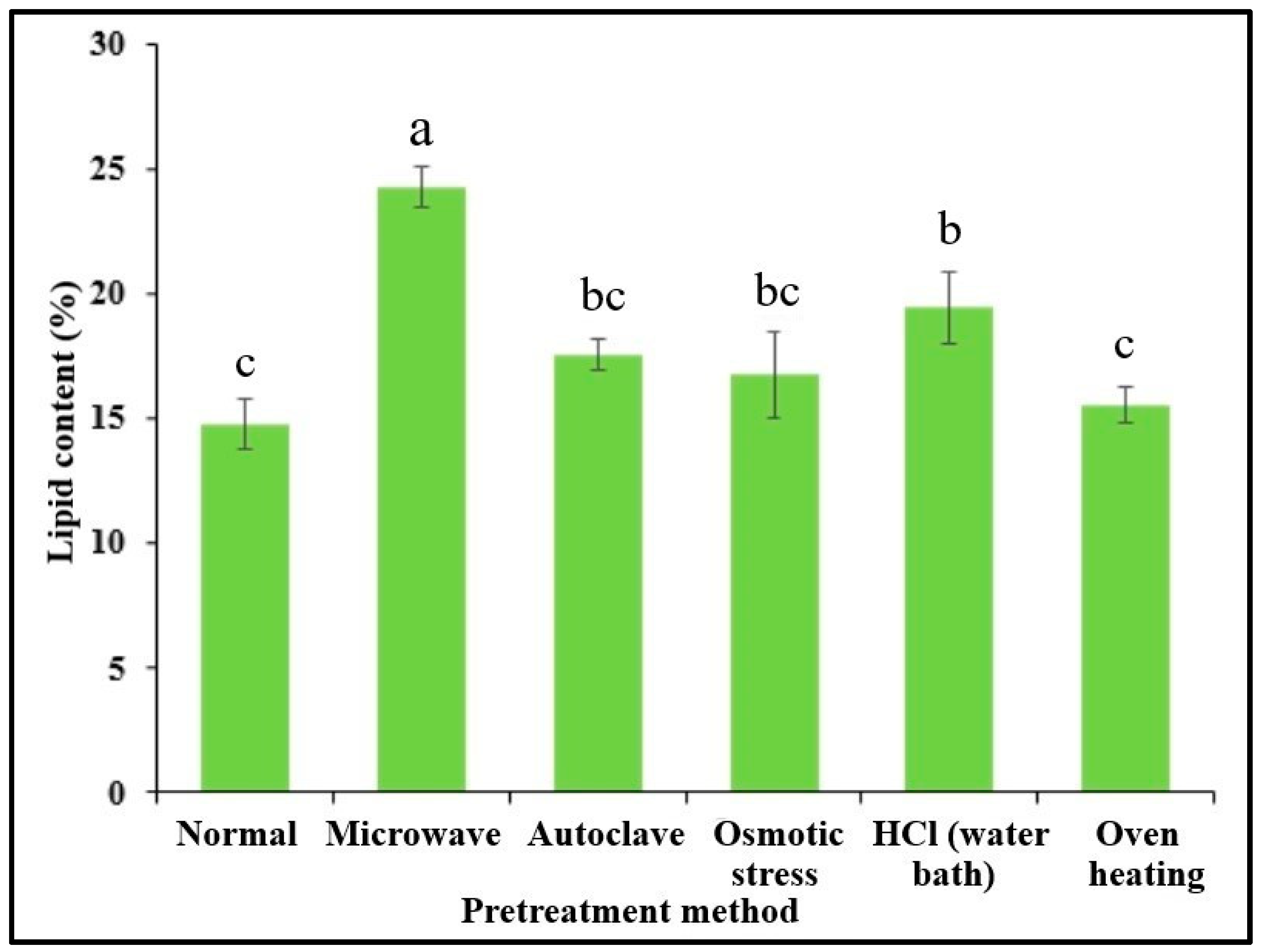
2.7. Pretreatment for Lipid Extraction
- ▪
- Autoclave pretreatment: A dry microalgal biomass of 0.5 g was mixed with 15 mL of distilled water and subjected to autoclaving at 121 °C for 15 min;
- ▪
- Microwave pretreatment: The same biomass was mixed with 15 mL of water and subjected to microwave radiation at 1000 W and 100 °C for 10 min;
- ▪
- Osmotic stress pretreatment: Another portion of the biomass (0.5 g) was blended with 10 mL of a 10% NaCl solution and incubated for 48 h to induce osmotic stress;
- ▪
- Oven heating: The biomass (0.5 g) was mixed with 15 mL of water and heated in an oven at 120 °C for 15 min;
- ▪
- Acid digestion: The biomass (0.5 g) was mixed with 15 mL of 3 N HCl solution and digested at 80 °C for 1 h in a water bath.
2.8. Statistical Analysis
3. Results and Discussion
3.1. Brewery Effluent Characterization
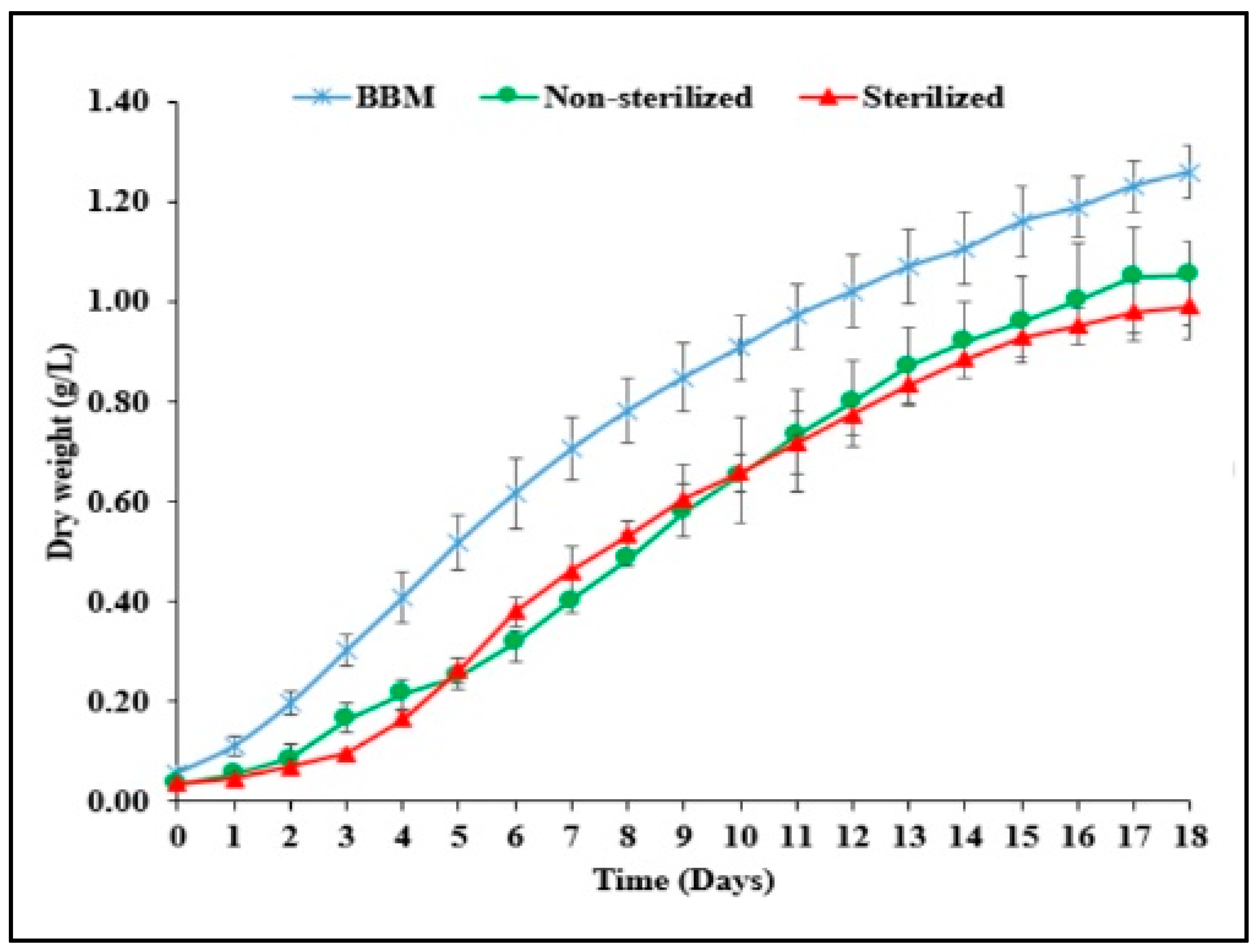
3.2. Growth and Biomass Production in Brewery Effluent
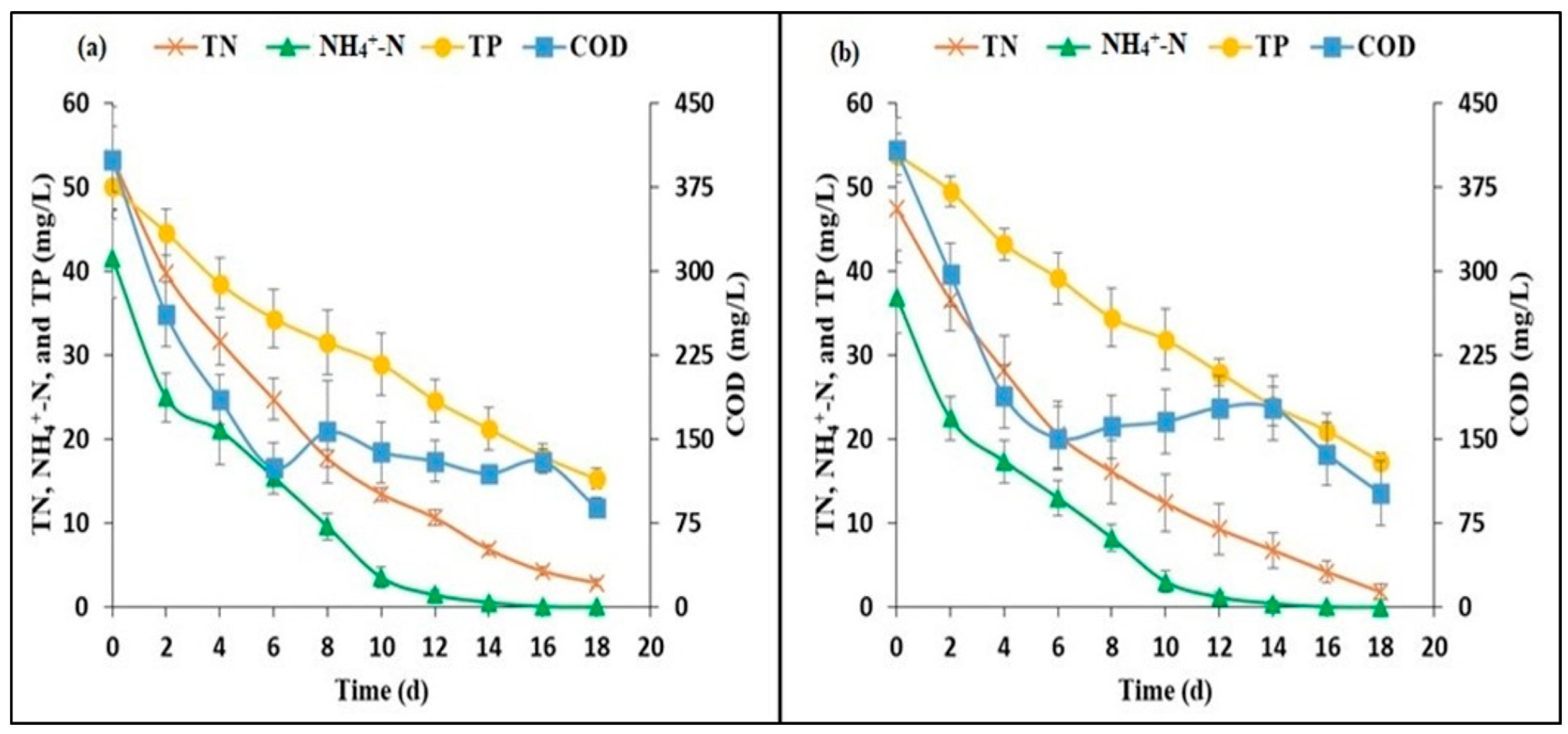
3.3. Nutrient and COD Removal Efficiency
3.4. Nutrient and COD Removal Rate
3.5. Nutrient Removal Rate Constant and Biomass Yield Coefficient
3.6. Lipid Production
3.7. Pretreatment for Lipid Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganeshkumar, V.; Subashchandrabose, S.R.; Dharmarajan, R. Use of mixed wastewaters from piggery and winery for nutrient removal and lipid production by Chlorella sp. MM3. Bioresour. Technol. 2018, 256, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.; Karemore, A.; Sen, R. Sustainable valorization of flue gas CO2 and wastewater for the production of microalgal biomass as a biofuel feedstock in closed and open reactor systems. RSC Adv. 2016, 6, 91111–91120. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Utilization of microalgae feedstock for concomitant production of bioethanol and biodiesel. Fuel 2018, 217, 458–466. [Google Scholar] [CrossRef]
- Barros, R.; Raposo, S.; Morais, E.G.; Rodrigues, B.; Afonso, V.; Gonçalves, P.; Marques, J.; Cerqueira, P.R.; Varela, J.; Teixeira, M.R.; et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of. Energies 2022, 15, 5713. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Process Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Baldev, E.; Ali, D.M.; Pugazhendhi, A.; Thajuddin, N. Wastewater as an economical and ecofriendly green medium for microalgal biofuel production. Fuel 2021, 294, 120484. [Google Scholar] [CrossRef]
- Tripathi, R.; Gupta, A.; Thakur, I.S. An integrated approach for phycoremediation of wastewater and sustainable biodiesel production by green microalgae, Scenedesmus sp. Renew. Energy 2019, 135, 617–625. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Yun, Y.-M.; Shin, H.-S.; Kim, H.-S.; Han, J.-I. Scenedesmus-based treatment of nitrogen and phosphorus from effluent of anaerobic digester and bio-oil production. Bioresour. Technol. 2015, 196, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Lee, S.-M. Enhanced nutrient removal from municipal wastewater assisted by mixotrophic microalgal cultivation using glycerol. Environ. Sci. Pollut. Res. 2016, 23, 10114–10123. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Eraky, M.; Alhajeri, N.S.; Osman, A.I.; Rooney, D.W. Cultivation of microalgae on liquid anaerobic digestate for depollution, biofuels and cosmetics: A review. Environ. Chem. Lett. 2022, 20, 3631–3656. [Google Scholar] [CrossRef]
- Tan, X.-B.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Lipids production and nutrients recycling by microalgae mixotrophic culture in anaerobic digestate of sludge using wasted organics as carbon source. Bioresour. Technol. 2020, 297, 122379. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Khalekuzzaman; Hossain, N.; Alamgir, M. Anaerobic digested effluent phycoremediation by microalgae co-culture and harvesting by Moringa oleifera as natural coagulant. J. Clean. Prod. 2021, 292, 126042. [Google Scholar] [CrossRef]
- Khalekuzzaman, M.; Bin Kabir, S.; Islam, B.; Datta, P.; Alam, A.; Xu, J. Enhancing microalgal productivity and quality by different colored photobioreactors for biodiesel production using anaerobic reactor effluent. Biomass Convers. Biorefin. 2021, 11, 767–779. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Pei, H.; Pan, J.; Jiang, L.; Hou, Q.; Han, F. Cultivation of microalgae using anaerobically digested effluent from kitchen waste as a nutrient source for biodiesel production. Renew. Energy 2018, 115, 276–287. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Sacchi, R.; Masi, P. Bioresource Technology Reports Techno-economic assessment of DHA-rich Aurantiochytrium sp. production using food industry by-products and waste streams as alternative growth media. Bioresour. Technol. Rep. 2022, 18, 100997. [Google Scholar] [CrossRef]
- Mercado, I.; Xavier, Á.; Verduga, M.; Cruz, A. Scenedesmus sp. Cultivated in the Wastewater of the Dairy Industry. Processes 2020, 8, 1458. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Galal, H.R.; Mousa, A.S.H.; Farghl, A.A.M. Coupling wastewater treatment, biomass, lipids, and biodiesel production of some green microalgae. Environ. Sci. Pollut. Res. 2023, 30, 35492–35504. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Post treatment of anaerobically treated brewery effluent using pilot scale horizontal subsurface flow constructed wetland system. Bioresour. Bioprocess. 2021, 8, 8. [Google Scholar] [CrossRef]
- Daba, C.; Atamo, A.; Dagne, M.; Gizeyatu, A.; Adane, M.; Embrandiri, A.; Gebrehiwot, M. Performance evaluation of a brewery wastewater treatment plant in Ethiopia: Implications for wetland ecosystem management. Lakes Reserv. 2022, 27, e12412. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Andersen, R.A.; Kawachi, M. Traditional Microalgae Isolation Techniques. In Algal Culturing Techniques; Andersen, R.A., Ed.; Elsevier/Academic Press: London, UK, 2005; pp. 83–100. [Google Scholar]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification and Use as Bioindicators; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 187–189. [Google Scholar]
- Shubert, E.; Gärtner, G. Nonmotile Coccoid and Colonial Green Algae. In Freshwater Algae of North America; Wehr, J.D., Sheath, R.G., Kociolek, J.P., Eds.; Elsevier Inc.: London, UK, 2015; pp. 315–373. [Google Scholar]
- Oliveira, O.; Gianesella, S.; Silva, V.; Mata, T.; Caetano, N. Lipid and carbohydrate profile of microalga isolated wastewater. Energy Procedia 2017, 136, 468–473. [Google Scholar] [CrossRef]
- Ansari, A.A.; Khoja, A.H.; Nawar, A.; Qayyum, M.; Ali, E. Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl. Water Sci. 2017, 7, 4151–4158. [Google Scholar] [CrossRef]
- Li, Y.-R.; Tsai, W.-T.; Hsu, Y.-C.; Xie, M.-Z.; Chen, J.-J. Comparison of autotrophic and mixotrophic cultivation of green microalgal for biodiesel production. Energy Procedia 2014, 52, 371–376. [Google Scholar] [CrossRef]
- Lee, Y.; Chen, W.; Shen, H.; Han, D.; Li, Y.; Jones, H.D.T.; Timlin, J.A.; Hu, Q. Basic Culturing and Analytical Measurement Techniques. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2013; pp. 37–68. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; Clesceri, L.S., Greenberg, A.E., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Tan, Y.H.; Chai, M.K.; Na, J.Y.; Wong, L.S. Microalgal Growth and Nutrient Removal Efficiency in Non-Sterilised Primary Domestic Wastewater. Sustainability 2023, 15, 6601. [Google Scholar] [CrossRef]
- Yang, I.-S.; Salama, E.-S.; Kim, J.-O.; Govindwar, S.P.; Kurade, M.B.; Lee, M.; Roh, H.-S.; Jeon, B.-H. Cultivation and harvesting of microalgae in photobioreactor for biodiesel production and simultaneous nutrient removal. Energy Conersion Manag. 2016, 117, 54–62. [Google Scholar] [CrossRef]
- Hach. Model DR/2400 Spectrophotometer; Hach Company: Loveland, CO, USA, 2002. [Google Scholar]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M. Enhanced Microalgae Growth for Biodiesel Production and Nutrients Removal in Raw Swine Wastewater by Carbon Sources Supplementation. Waste Biomass Valorization 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Silva, N.F.P.; Gonçalves, A.L.; Moreira, F.C.; Silva, T.F.C.V.; Martins, F.G.; Alvim-ferraz, M.C.M.; Boaventura, R.; Vilar, V.; Pires, J. Towards sustainable microalgal biomass production by phycoremediation of a synthetic wastewater: A kinetic study. Algal Res. 2015, 11, 350–358. [Google Scholar] [CrossRef]
- Liu, X.; Ying, K.; Chen, G.; Zhou, C.; Zhang, W.; Zhang, X.; Cai, Z.; Holmes, T.; Tao, Y. Growth of Chlorella vulgaris and nutrient removal in the wastewater in response to intermittent carbon dioxide. Chemosphere 2017, 186, 977–985. [Google Scholar] [CrossRef]
- Pandey, P.; Shi, J. Assessing Nutrient Removal Kinetics in Flushed Manure Using Chlorella vulgaris Biomass Production. Front. Bioeng. Biotechnol. 2017, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Srivastava, S.; Kumar, S. Sequential optimization of essential nutrients addition in simulated dairy effluent for improved Scenedesmus sp. ASK22 growth, lipid production and nutrients removal. Biomass Bioenergy 2019, 128, 105319. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- EEPA (Ethiopian Environmental protection Authority). Environmental Standards for Industrial Pollution Control in Ethiopia; Environmnetal Protection Authority: Addis Ababa, Ethiopia, 2003; p. 12. [Google Scholar]
- Alayu, E.; Yirgu, Z. Advanced technologies for the treatment of wastewaters from agro-processing industries and cogeneration of by-products: A case of slaughterhouse, dairy and beverage industries. Int. J. Environ. Sci. Technol. 2018, 15, 1581–1596. [Google Scholar] [CrossRef]
- Farooq, W.; Lee, Y.-C.; Ryu, B.-G.; Kim, B.-H.; Kim, H.-S.; Choi, Y.-E.; Yang, J.-W. Two-stage cultivation of two Chlorella sp. strains by simultaneous treatment of brewery wastewater and maximizing lipid productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef]
- Darpito, C.; Shin, W.-S.; Jeon, S.; Lee, H.; Nam, K.; Kwon, J.-H.; Yang, J.-W. Cultivation of Chlorella protothecoides in anaerobically treated brewery wastewater for cost-effective biodiesel production. Bioprocess. Biosyst. Eng. 2014, 38, 523–530. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Li, D.; Amoah, P.K.; Chen, B.; Xue, C.; Hu, X.; Gao, K.; Deng, X. Feasibility of Growing Chlorella sorokiniana on Cooking Cocoon Wastewater for Biomass Production and Nutrient Removal. Appl. Biochem. Biotechnol. 2019, 188, 663–676. [Google Scholar] [CrossRef]
- Liu, X.; Hong, Y. Microalgae-Based Wastewater Treatment and Recovery with Biomass and Value-Added Products: A Brief Review. Curr. Pollut. Rep. 2021, 7, 227–245. [Google Scholar] [CrossRef]
- Udaiyappana, A.F.M.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Mohd Yasin, N.; Nazashida Mohd Hakimi, N.I. Microalgae-bacteria interaction in palm oil mill effluent treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Marques, P.A.; Ferreira, A.F.; Dias, A.P.; Pinheiro, H.M.; Reis, A.; Gouveia, L. Scenedesmus obliquus mediated brewery wastewater remediation and CO2 biofixation for green energy purposes. J. Clean. Prod. 2017, 165, 1316–1327. [Google Scholar] [CrossRef]
- Kumar, P.K.; Krishna, S.V.; Naidu, S.S.; Verma, K.; Bhagawan, D.; Himabindu, V. Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions: Comparative study. Carbon Resour. Convers. 2019, 2, 126–133. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Yun, Y.-M.; Shin, H.-S.; Han, J.-I. Cultivation of four microalgae species in the effluent of anaerobic digester for biodiesel production. Bioresour. Technol. 2017, 224, 738–742. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2014, 38, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef]
- Whitton, R.; Le Mével, A.; Pidou, M.; Ometto, F.; Villa, R.; Jefferson, B. Influence of microalgal N and P composition on wastewater nutrient remediation. Water Res. 2016, 91, 371–378. [Google Scholar] [CrossRef]
- Beuckels, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Choi, W.J.; Chae, A.N.; Guen, K.; Joonhong, S.; Byung, P.; Lee, C. Effect of trophic conditions on microalga growth, nutrient removal, algal organic matter, and energy storage products in Scenedesmus (Acutodesmus) obliquus KGE-17 cultivation. Bioprocess Biosyst. Eng. 2019, 42, 1225–1234. [Google Scholar] [CrossRef]
- Babiak, W.; Krzeminska, I. Extracellular Polymeric Substances (EPS) as Microalgal Bioproducts: A Review of Factors Affecting EPS Synthesis and Application in Flocculation Processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Choi, H. Parametric study of brewery wastewater effluent treatment using Chlorella vulgaris microalgae. Environ. Eng. Res. 2016, 21, 401–408. [Google Scholar] [CrossRef]
- Nayak, M.; Karemore, A.; Sen, R. Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 bio fi xation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar] [CrossRef]
- Mennaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater treatment by seven species of microalgae and an algal bloom: Biomass production, N and P removal kinetics and harvestability. Water Res. 2015, 83, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-Z.; Shao, Y.; Luo, S.; Zeng, F.-J.; Tian, G.-M. Nutrient removal from piggery wastewater by Desmodesmus sp. CHX1 and its cultivation conditions optimization. Environ. Technol. 2019, 40, 2739–2746. [Google Scholar] [CrossRef]
- Aslan, S.; Kapdan, I.K. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol. Eng. 2006, 28, 64–70. [Google Scholar] [CrossRef]
- Lv, J.; Guo, B.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Integration of wastewater treatment and flocculation for harvesting biomass for lipid production by a newly isolated self-flocculating microalga Scenedesmus rubescens SX. J. Clean. Prod. 2019, 240, 118211. [Google Scholar] [CrossRef]
- Medrano-Barboza, J.; Herrera-Rengifo, K.; Aguirre-Bravo, A.; Ramírez-Iglesias, J.R.; Rodríguez, R.; Morales, V. Pig Slaughterhouse Wastewater: Medium Culture for Microalgae Biomass Generation as Raw Material in Biofuel Industries. Water 2022, 14, 3016. [Google Scholar] [CrossRef]
- Diniz, G.S.; Silva, A.F.; Araújo, O.Q.F.; Chaloub, R.M. The potential of microalgal biomass production for biotechnological purposes using wastewater resources. J. Appl. Phycol. 2017, 29, 821–832. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Kamaraj, B.; Cornejo, P. Scenedesmus sp. strain SD07 cultivation in municipal wastewater for pollutant removal and production of lipid and exopolysaccharides. Environ. Res. 2023, 218, 115051. [Google Scholar] [CrossRef]
- Ma, C.; Wen, H.; Xing, D.; Pei, X.; Zhu, J.; Ren, N.; Liu, B. Molasses wastewater treatment and lipid production at low temperature conditions by a microalgal mutant Scenedesmus. Biotechnol. Biofuels 2017, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Thangam, K.R.; Santhiya, A.; Sri, S.A.; MubarakAli, D.; Karthikumar, S.; Kumar, R.S.; Thajuddin, N.; Soosai, M.R.; Varalakshmi, P.; Moorthy, I.G.; et al. Biorefinery approaches based concomitant microalgal biofuel production and wastewater treatment. Sci. Total Environ. 2021, 785, 147267. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Yasin, N.H.M. Microalgae acclimatization in industrial wastewater and its effect on growth and primary metabolite composition. Algal Res. 2021, 53, 102163. [Google Scholar] [CrossRef]
- de Souza Silva, A.P.F.; Costa, M.C.; Lopes, A.C.; Neto, E.F.A.; Leitão, R.C.; Mota, C.R.; dos Santos, A.B. Comparison of pretreatment methods for total lipids extraction from mixed microalgae. Renew. Energy 2014, 63, 762–766. [Google Scholar] [CrossRef]
- Yu, X.; Dong, T.; Zheng, Y.; Miao, C.; Chen, S. Investigations on cell disruption of oleaginous microorganisms: Hydrochloric acid digestion is an effective method for lipid extraction. Eur. J. Lipid Sci. Technol. 2015, 117, 730–737. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2018, 31, 61–88. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
| Parameters | Concentration (mg/L, Except for pH) | |
|---|---|---|
| Non-Sterilized Effluent | Sterilized Effluent | |
| pH | 7.47 ± 0.18 | 9.00 ± 0.11 |
| COD | 399 ±29.14 | 408 ± 33.79 |
| NH4+-N | 41.52 ±4.73 | 36.86 ± 4.18 |
| TN | 53.42 ± 6.19 | 47.50 ± 5.06 |
| PO43−-P | 37.78 ± 2.64 | 39.28 ± 3.00 |
| TP | 50.01 ± 2.44 | 53.88 ± 2.44 |
| Brewery Effluent | |||
|---|---|---|---|
| Parameters | BBM | Non-Sterilized | Sterilized |
| Biomass concentration (g/L) | 1.26 ± 0 a | 1.05 ± 0.10 b | 0.992 ± 0.06 b |
| Specific growth rate (d−1) | 0.34 ± 0.01 a | 0.31 ± 0.02 ac | 0.28 ± 0.02 c |
| Doubling time (d) | 2.46 ± 0.97 a | 2.43 ± 0.15 b | 2.63 ± 0 c |
| Biomass productivity (mg/L/d) | 93.30 ± 9.23 a | 64.33 ± 6.26 b | 63.27 ± 3.13 b |
| Pseudo-First-Order Constant (knutrient, 1/d) | Biomass Yield Coefficient (Y, mg Biomass/mg Nutrient) | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Sterilized Effluent | Sterilized Effluent | Non-Sterilized Effluent | Sterilized Effluent | |||||
| Parameter | k | R2 | k | R2 | Y | R2 | Y | R2 |
| COD | 0.06 | 0.76 | 0.05 | 0.69 | 0.006 | 0.64 | 0.005 | 0.54 |
| TN | 0.16 | 0.99 | 0.16 | 0.96 | 0.026 | 0.96 | 0.027 | 0.98 |
| NH4+-N | 0.37 | 0.94 | 0.38 | 0.94 | 0.035 | 0.93 | 0.039 | 0.96 |
| TP | 0.06 | 0.99 | 0.06 | 0.99 | 0.035 | 0.97 | 0.030 | 0.99 |
| Type of Wastewater | Culture Volume (Liter) | Culture Period (day) | Biomass Concentration (g/L) | Biomass Productivity (mg/L/d) | Lipid Content (%) | Lipid Productivity (mg/L/d) | Reference |
|---|---|---|---|---|---|---|---|
| Institution wastewater | 0. 25 * | 12 | 0.445 | 58.70 | 13.00 | 7.63 + | [27] |
| Molasses wastewater | 0. 25 * | 7 | 2.5 | 357.14 + | 28.9 | 94.4 | [68] |
| Municipal wastewater | 3.00 * | 4 | 0.217 | 54.20 | 12.50 | 6.77 + | [66] |
| Aquaculture wastewater | 1.00 * | 14 | 1.25 | 89.61 | 30.85 | 27.65 | [10] |
| Dairy wastewater | 0. 15 ** | 11 | - | 1750 | 51.00 | 892.5 + | [18] |
| Domestic wastewater | 1.00 * | 22 | 0.836 | - | 20.47 | 8.56 | [7] |
| Domestic wastewater | 20.0 ** | 14 | 0.95 | - | 50.50 | 19.00 | [69] |
| Pig slaughterhouse wastewater | 40.0 ** | 11 | 0.2 | 18 | 16.25 | 0.274 | [65] |
| Municipal wastewater | 1.00 * | 14 | 1.54 | 128.61 | 33.00 | 42.44 + | [67] |
| Non-sterilized brewery effluent | 2.00 * | 18 | 1.05 | 63.27 | 13.67 | 8.72 | This study |
| Sterilized brewery effluent | 2.00 * | 18 | 0.992 | 64.33 | 14.79 | 9.58 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yirgu, Z.; Asfaw, S.L.; Dekebo, A.H.; Khan, M.M.; Aragaw, T. Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent. Sustainability 2023, 15, 15403. https://doi.org/10.3390/su152115403
Yirgu Z, Asfaw SL, Dekebo AH, Khan MM, Aragaw T. Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent. Sustainability. 2023; 15(21):15403. https://doi.org/10.3390/su152115403
Chicago/Turabian StyleYirgu, Zenebe, Seyoum Leta Asfaw, Ahmed Hussen Dekebo, Mohammed Mazharuddin Khan, and Temesgen Aragaw. 2023. "Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent" Sustainability 15, no. 21: 15403. https://doi.org/10.3390/su152115403
APA StyleYirgu, Z., Asfaw, S. L., Dekebo, A. H., Khan, M. M., & Aragaw, T. (2023). Simultaneous Phycoremediation and Lipid Production by Microalgae Grown in Non-Sterilized and Sterilized Anaerobically Digested Brewery Effluent. Sustainability, 15(21), 15403. https://doi.org/10.3390/su152115403






