Phosphorus Fertilization Affects Morphological, Physiological and Agronomic Characteristics of Faba Bean Cultivars
Abstract
1. Introduction
2. Materials and Methods
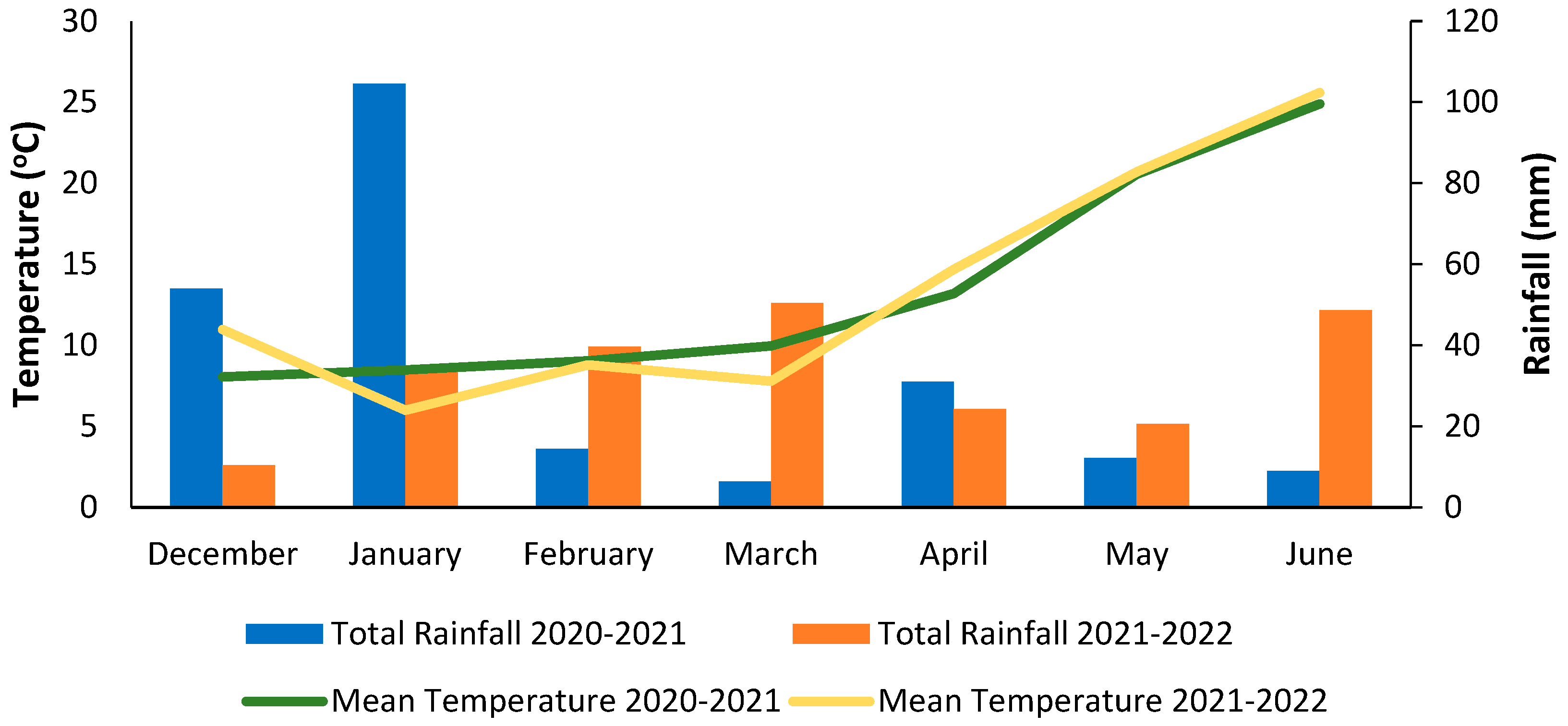
2.1. Experimental Protocol
2.2. Crop Species and Plant Cultivars Used in the Study
2.3. Experimental Design and Crop Management
2.4. Morphological Characteristics
2.4.1. Plant Height
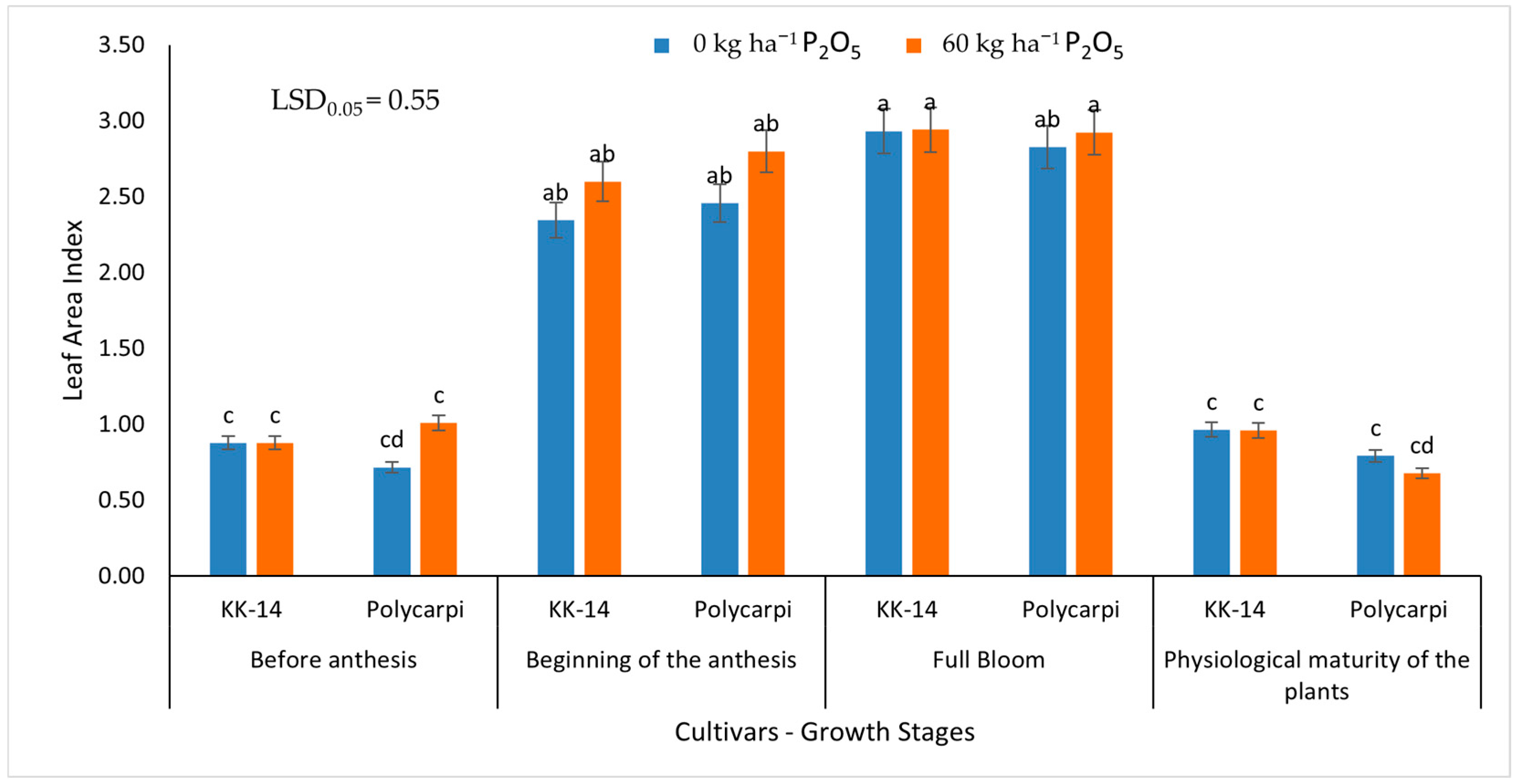
2.4.2. Leaf Area Index
2.5. Physiological Characteristics
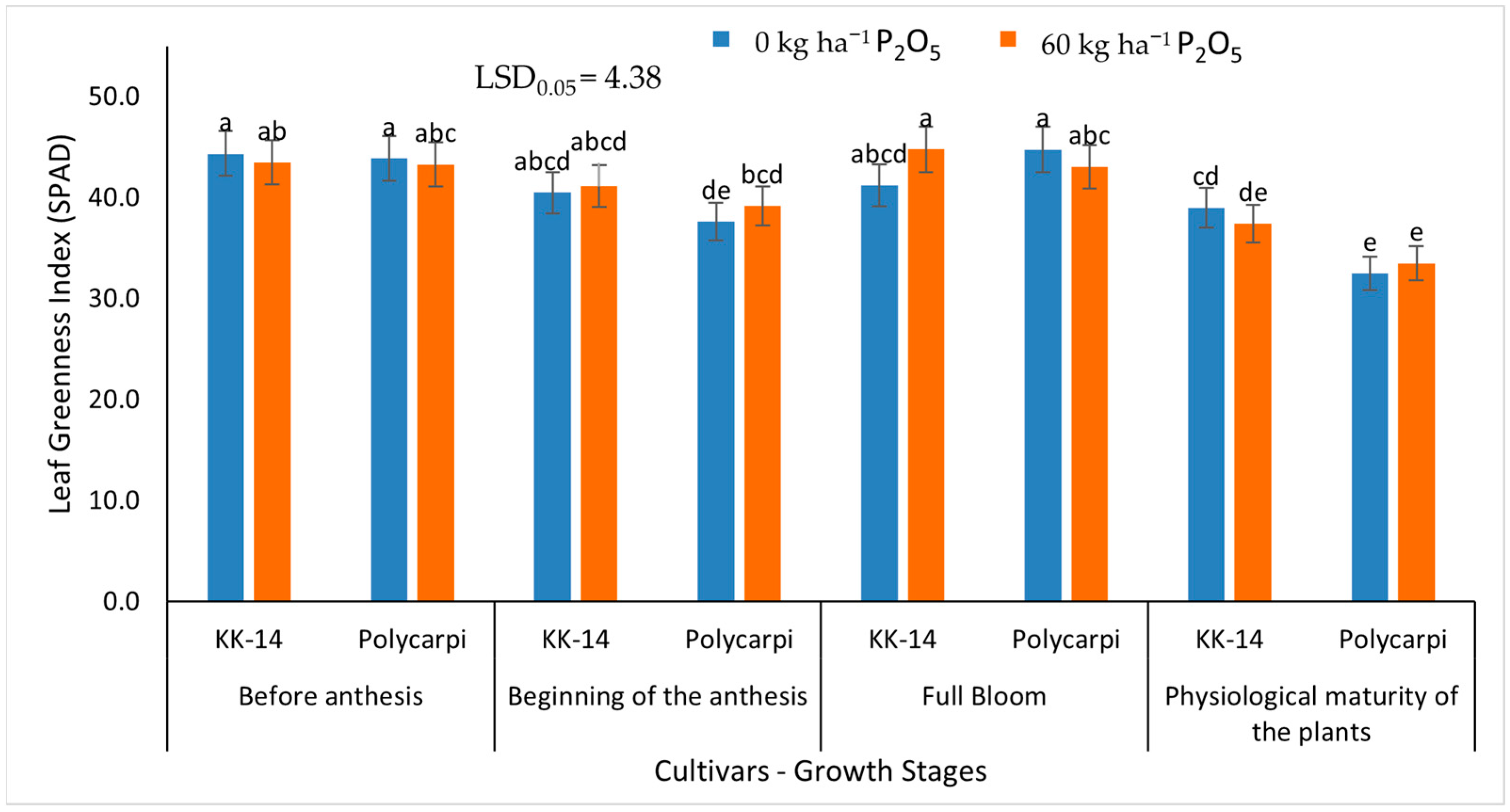
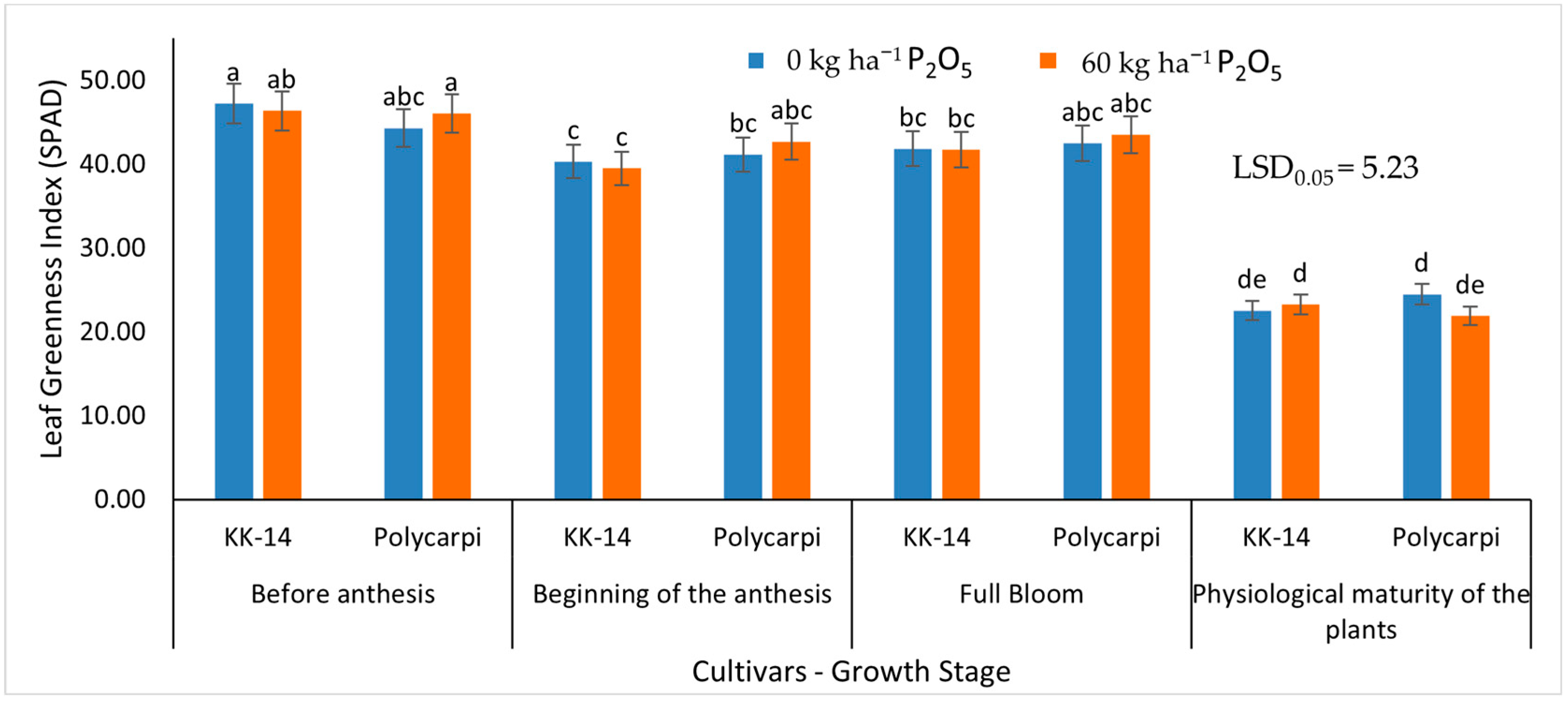
2.5.1. Leaf Greenness Index (SPAD)
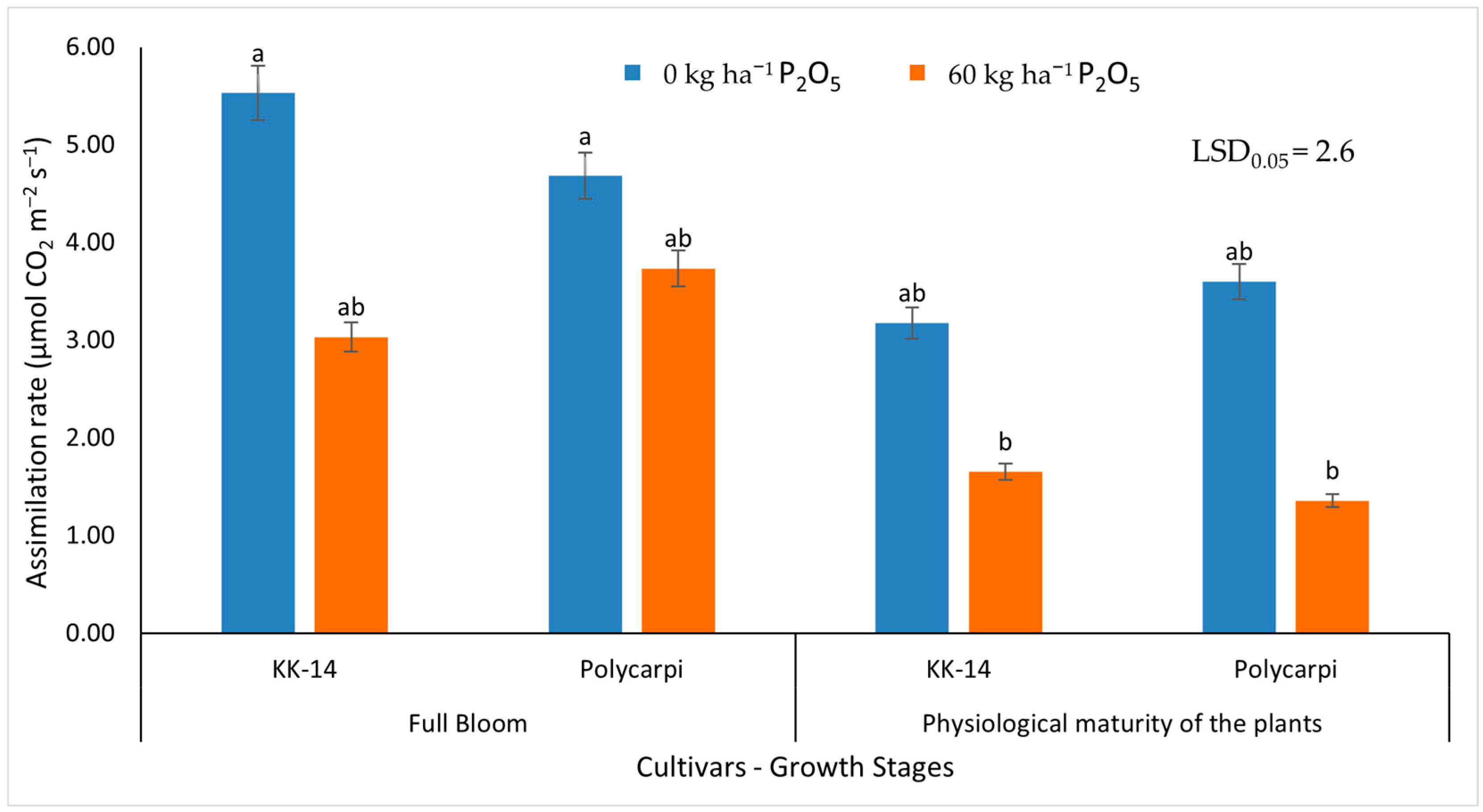
2.5.2. Gas Exchange Measurements
2.6. Seed Yield and Yield Components Determination
2.7. Water Use Efficiency
2.8. Phosphorus Use Efficiency
2.9. Statistical Analysis
3. Results
3.1. Morphological Characteristics
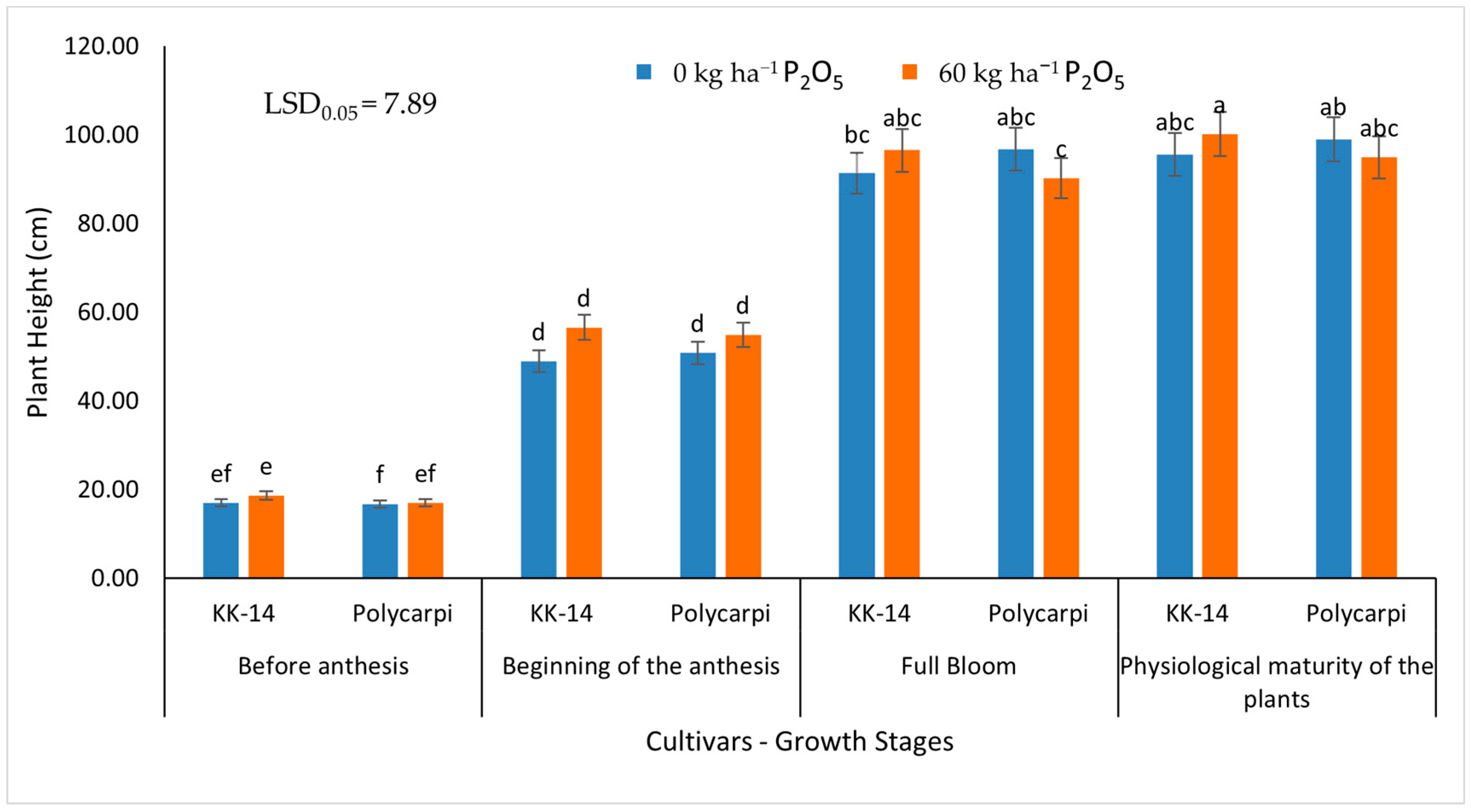
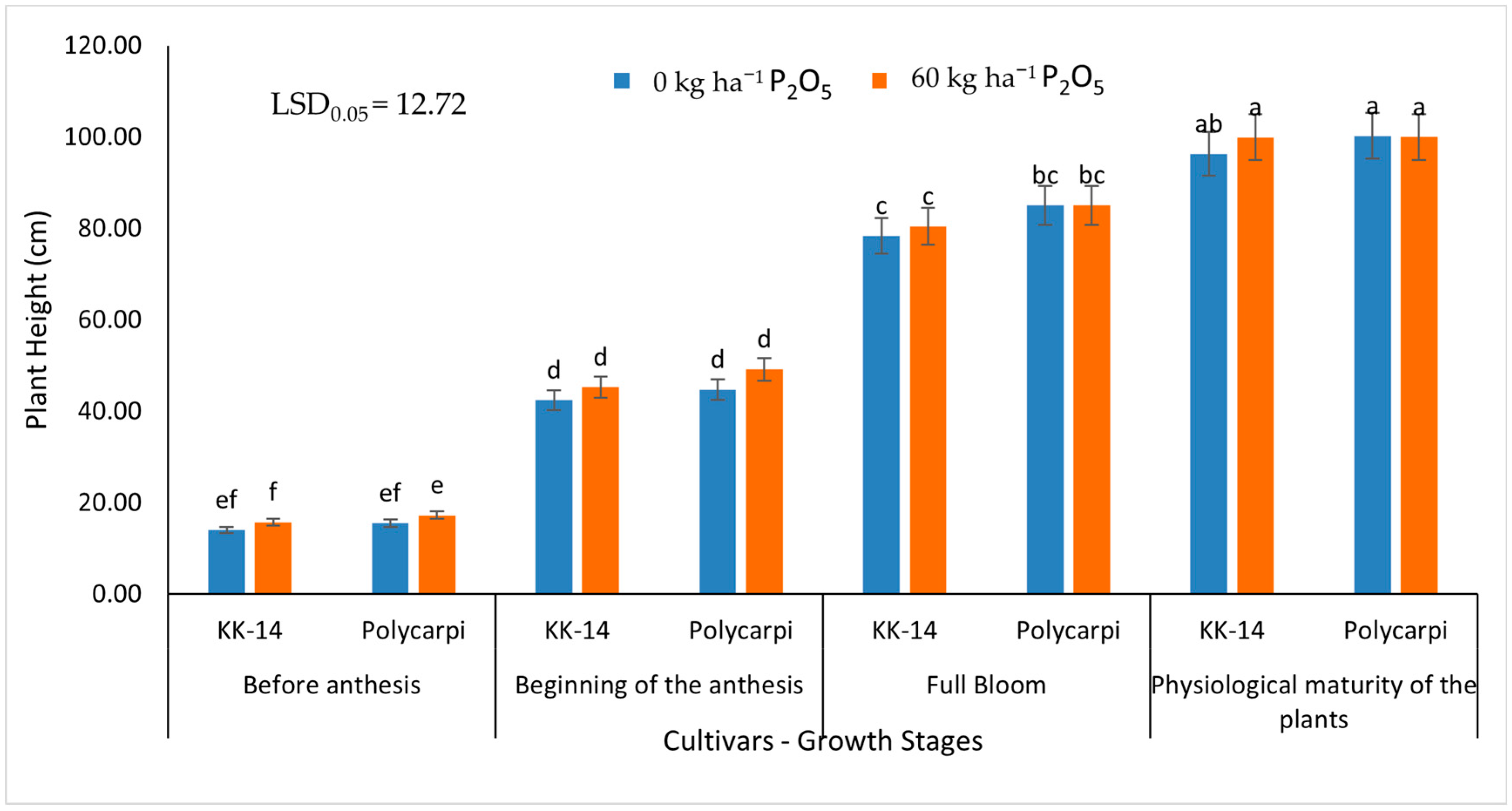
3.1.1. Plant Height
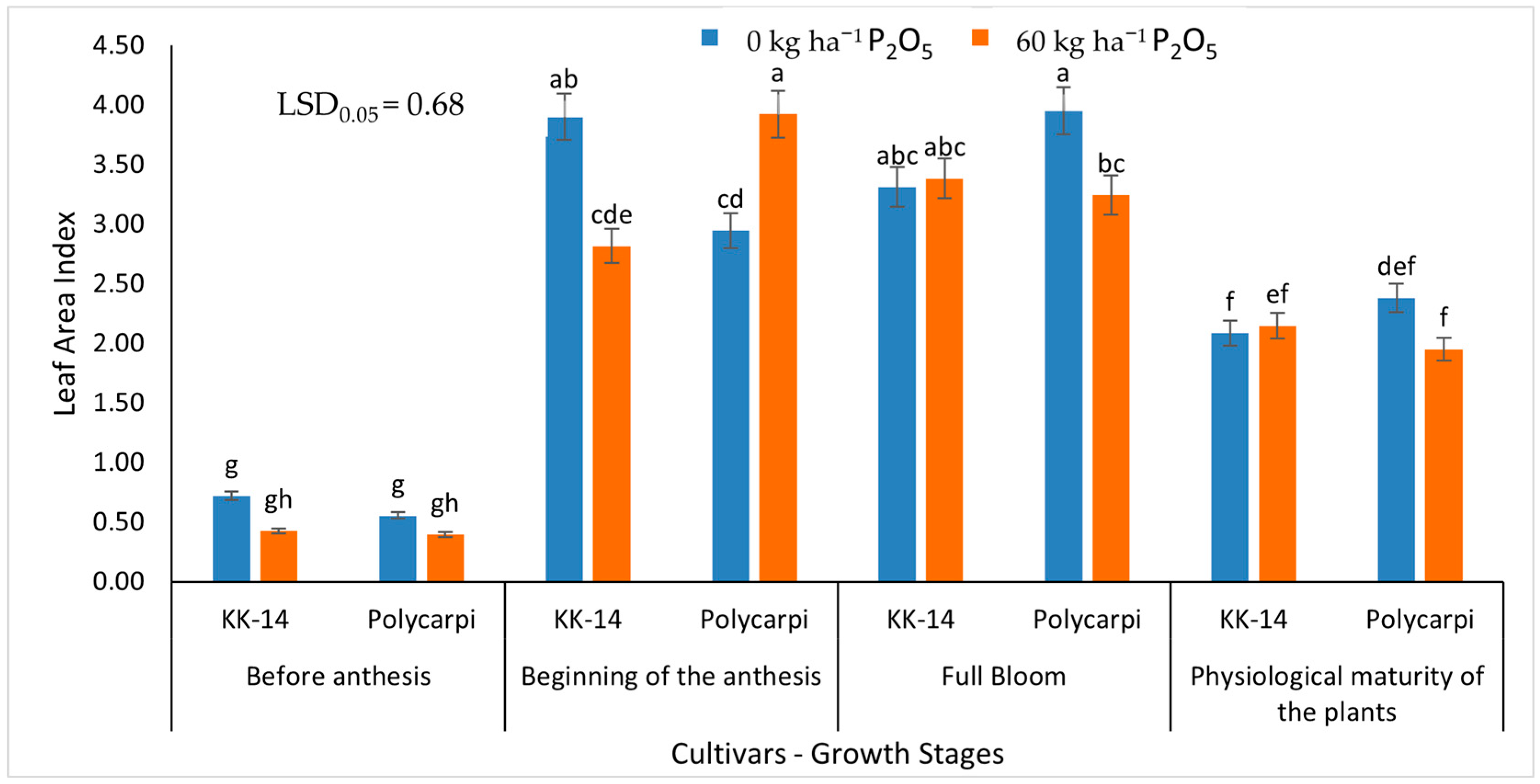
3.1.2. Leaf Area Index
3.2. Physiological Characteristics
3.2.1. Leaf Greenness Index (SPAD)
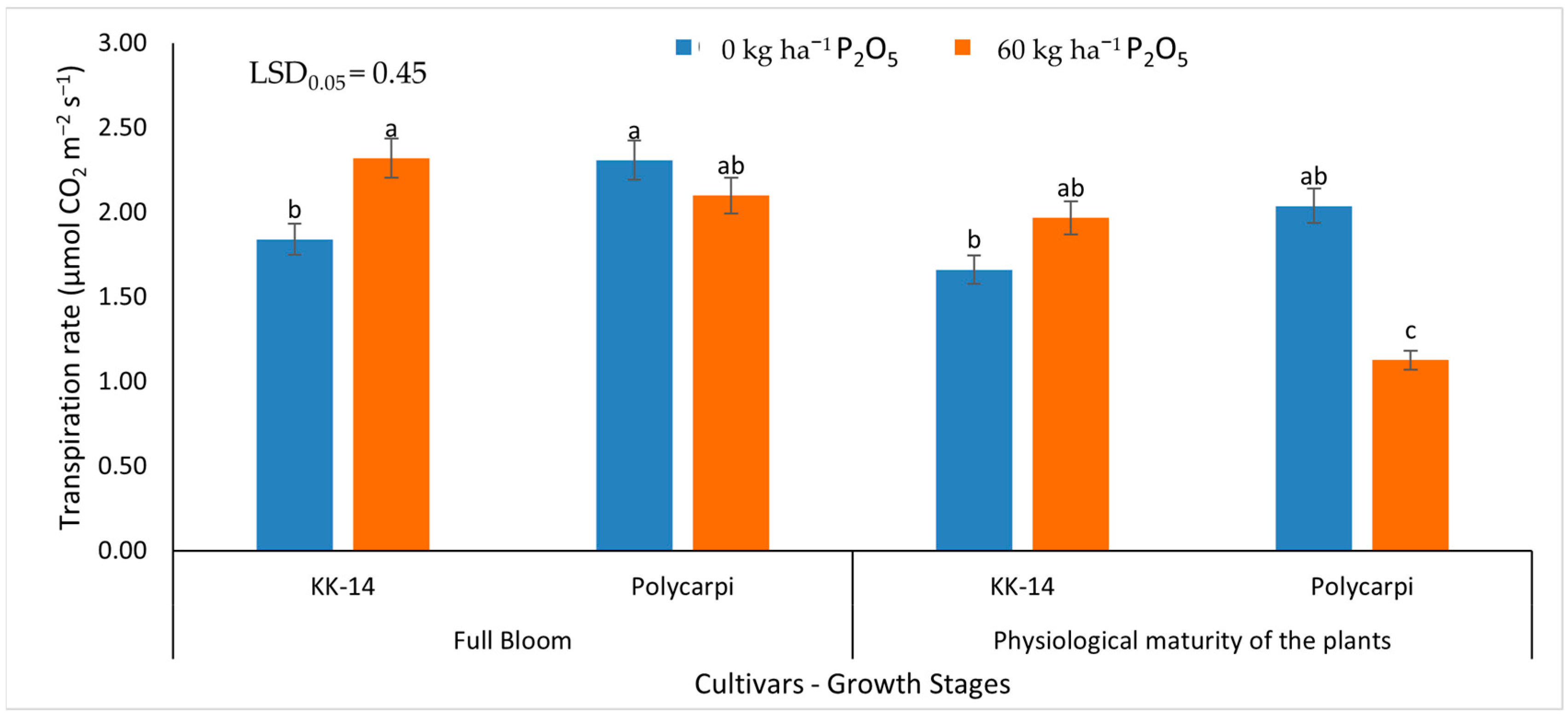
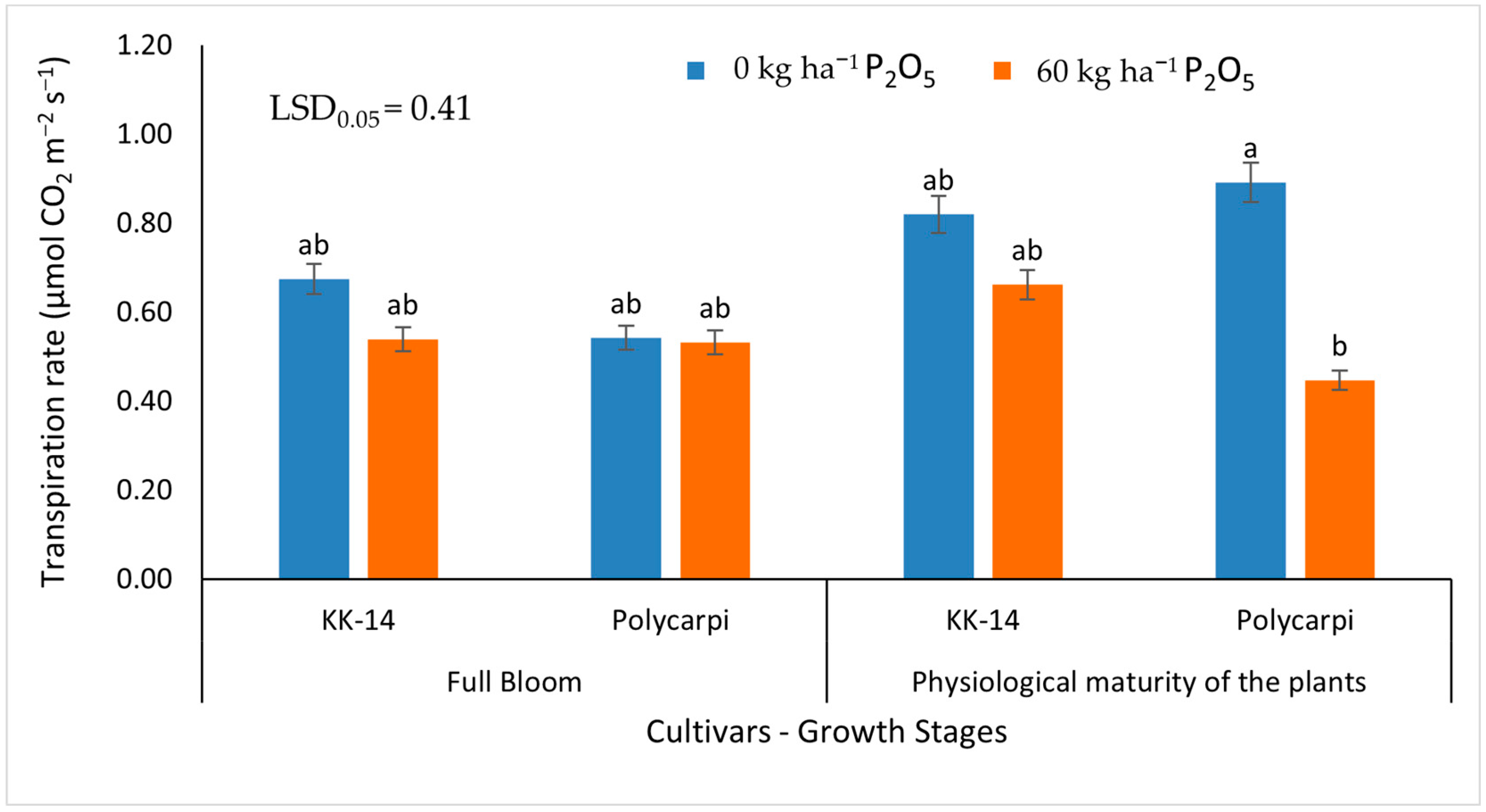
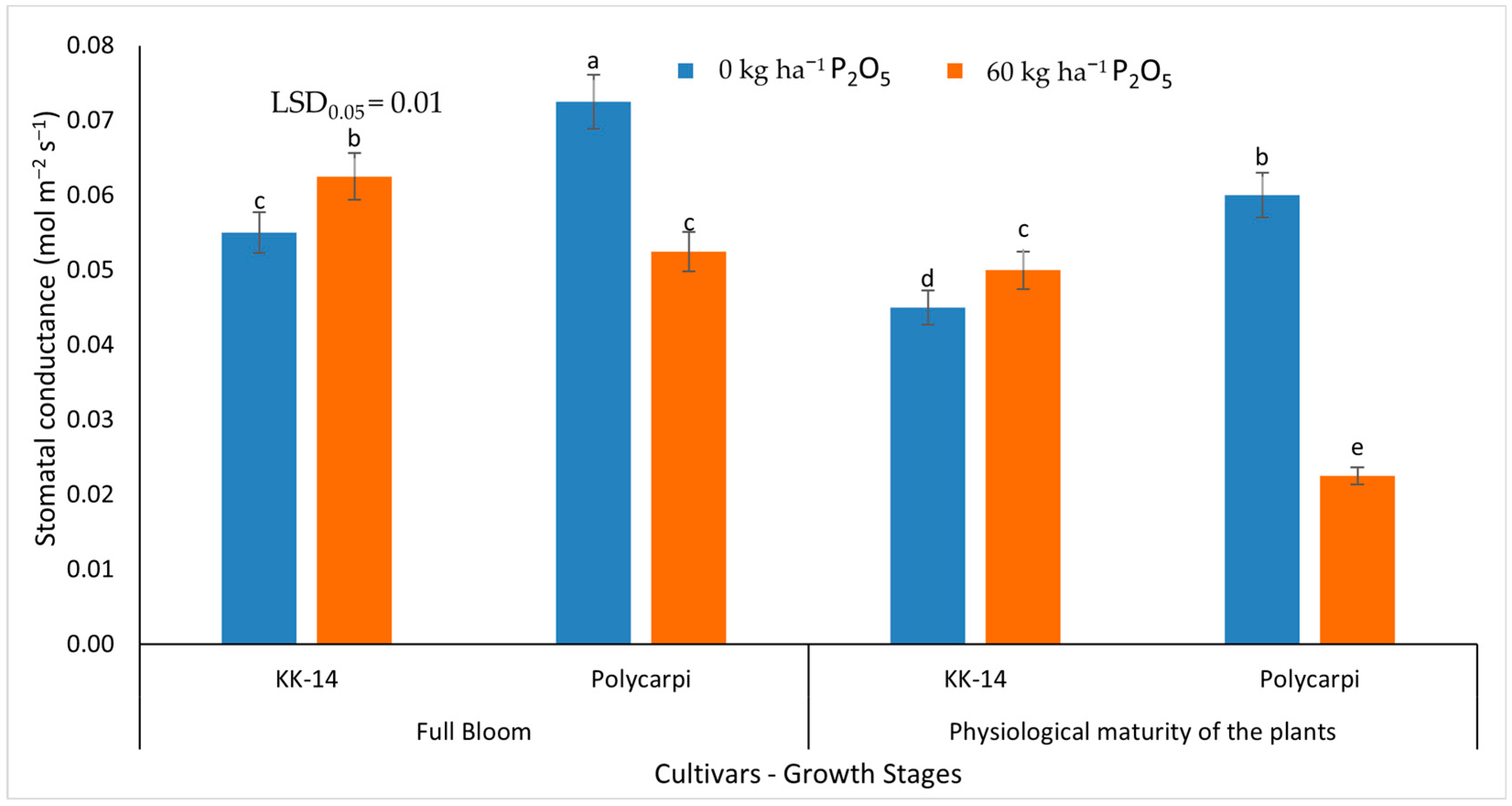
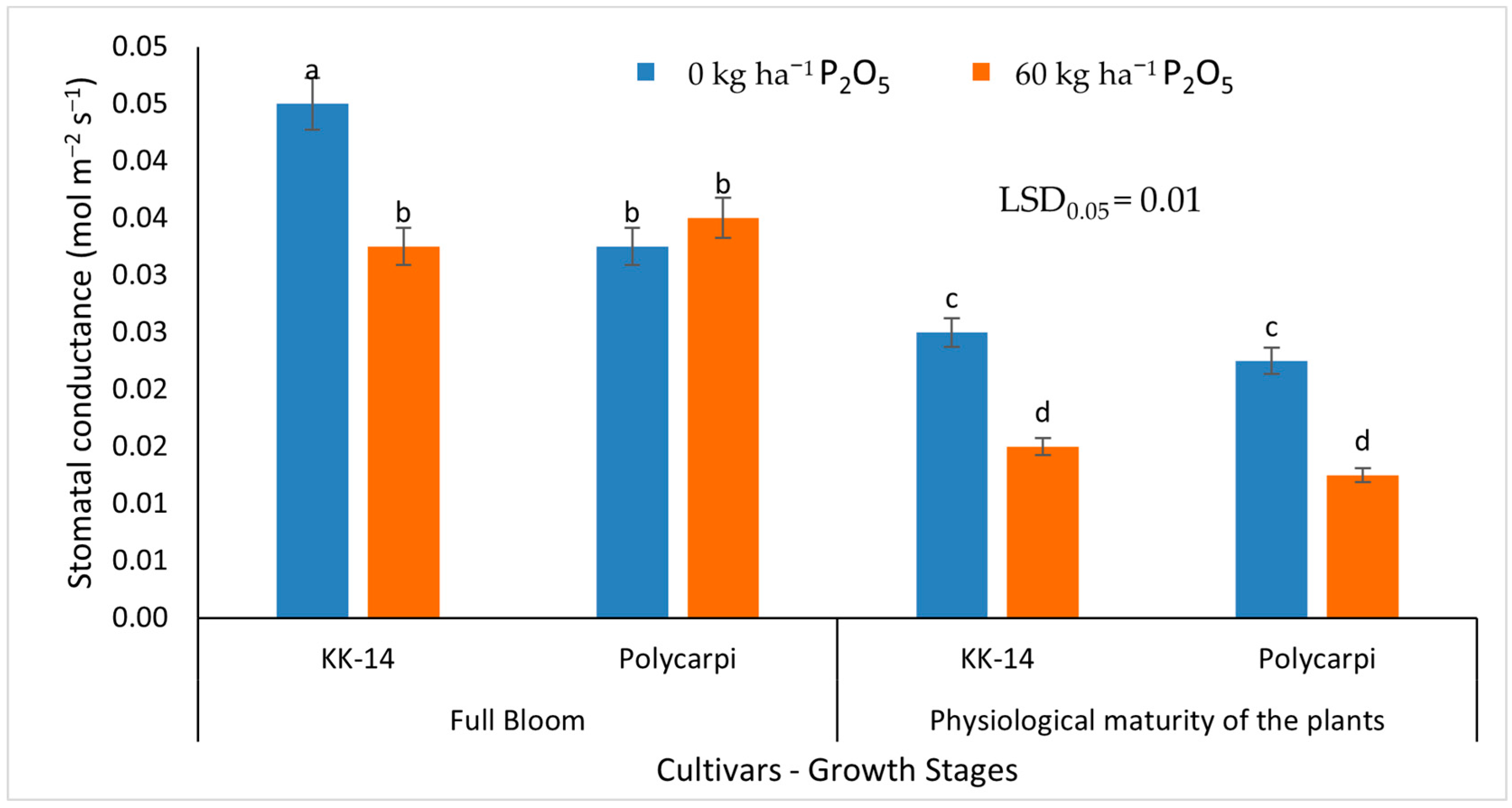
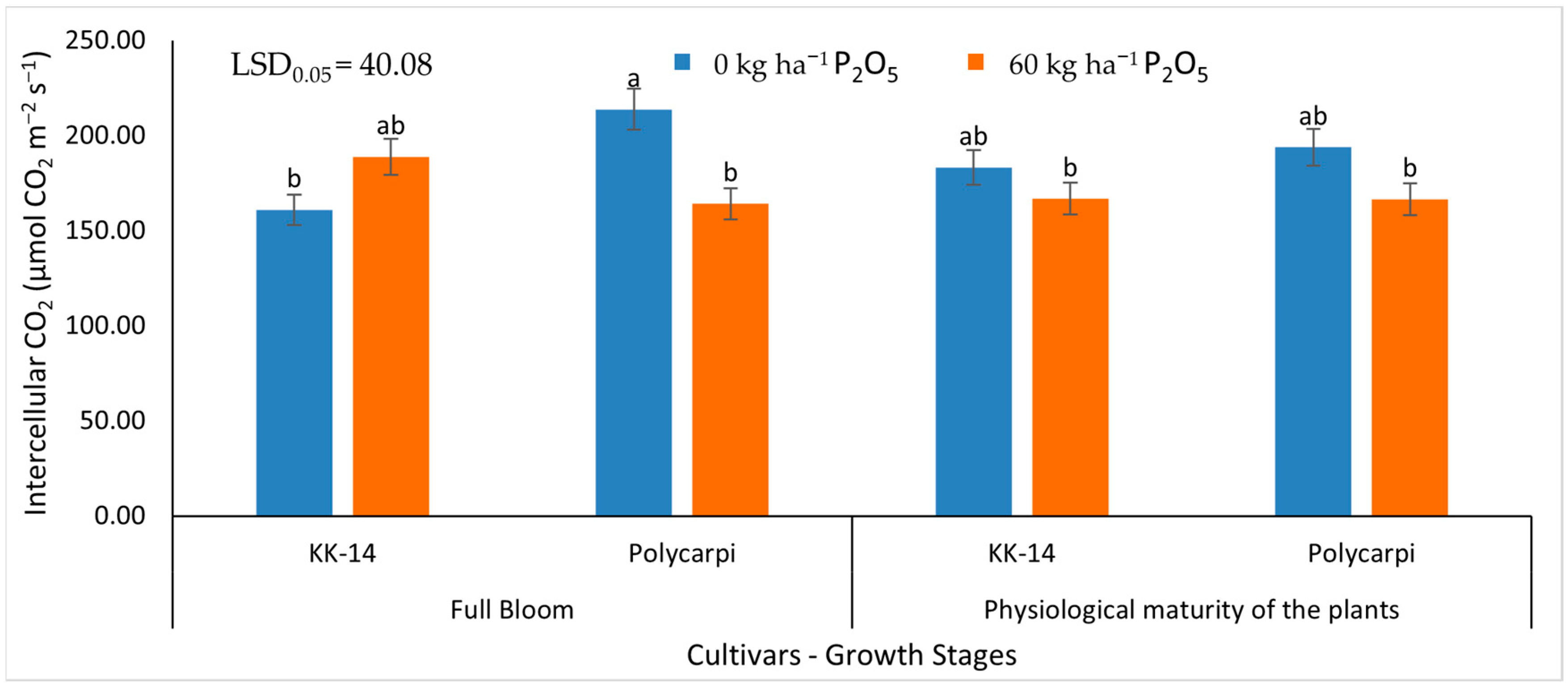
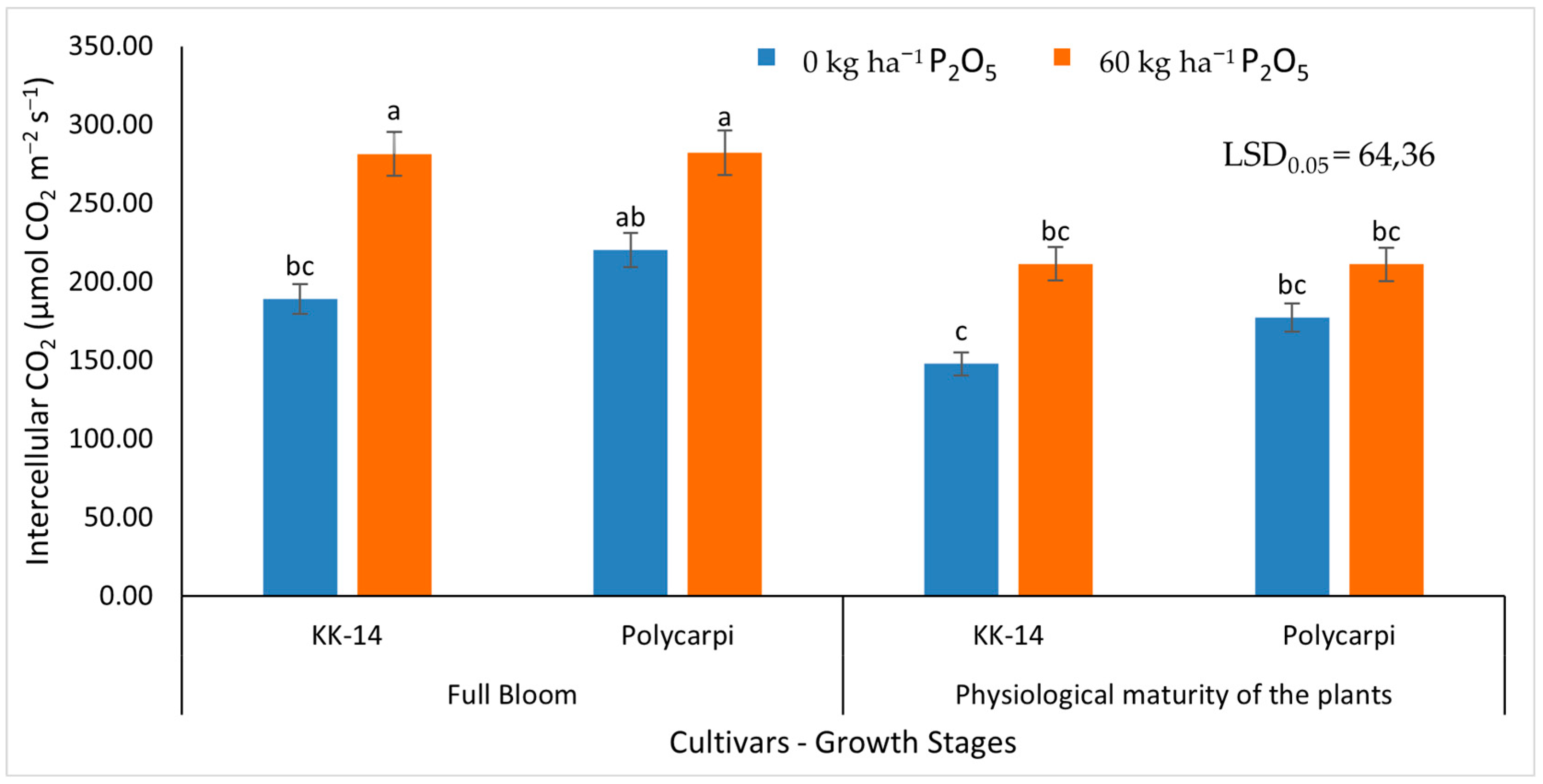
3.2.2. Gas Exchange Measurements
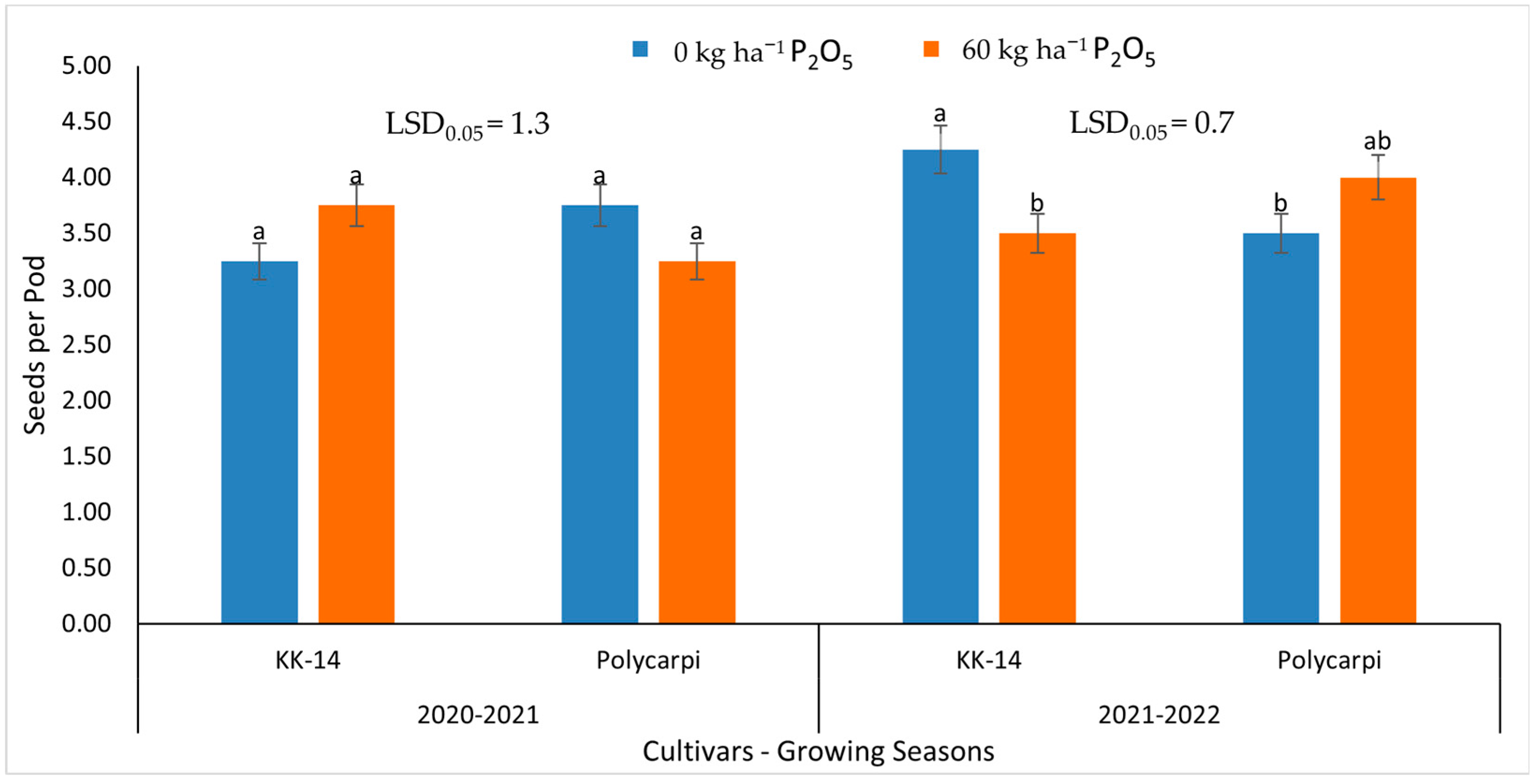
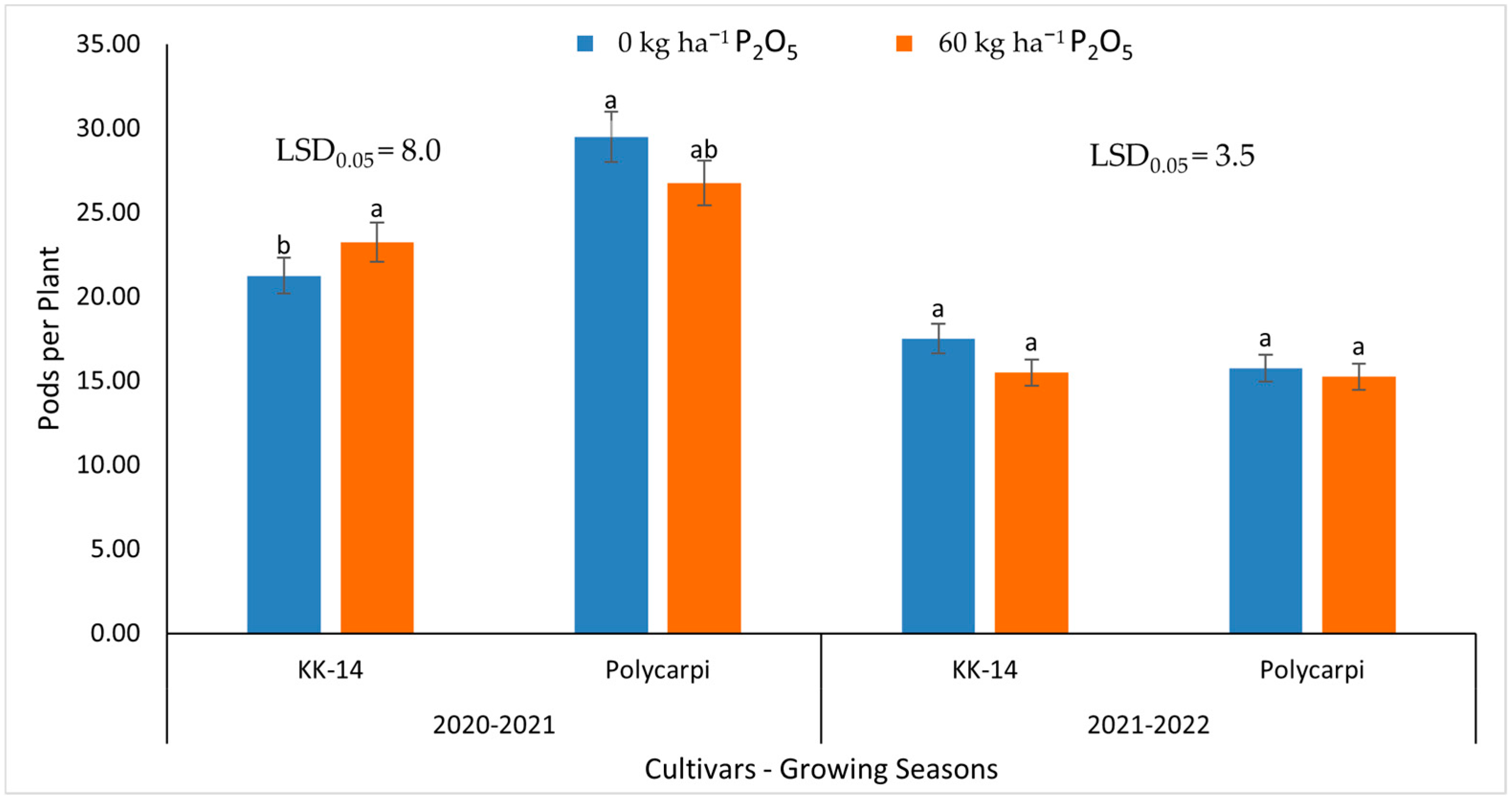
3.3. Seed Yield and Yield Components
3.4. Phosphorus Use Efficiency
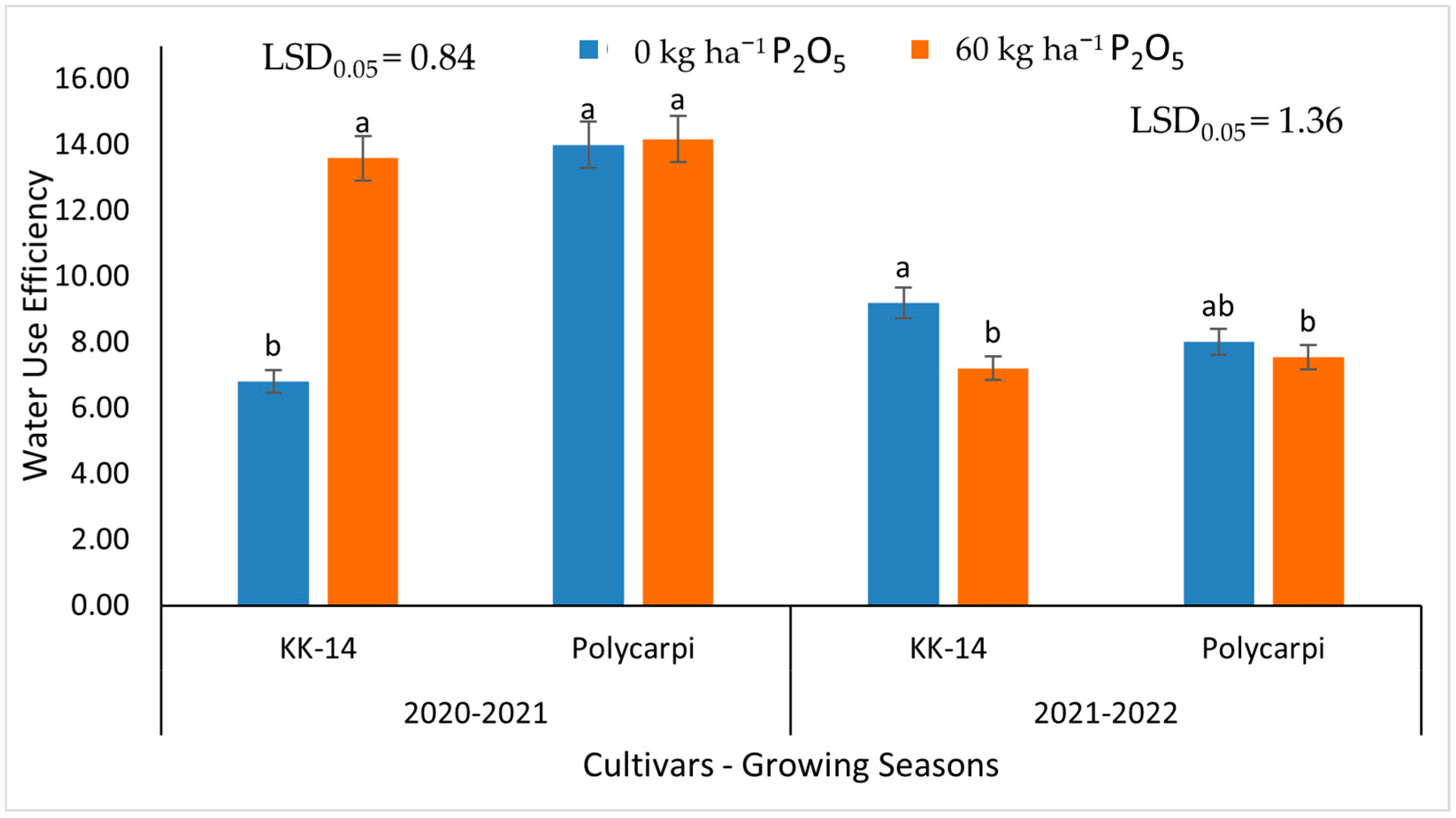
3.5. Water Use Efficiency
4. Discussion
4.1. Plant Height
4.2. Leaf Area Index
4.3. Leaf Greenness Index (SPAD)
4.4. Gas Exchange Measurements
4.5. Seed Yield and Yield Components Determination
4.6. Phosphorus Use Efficiency
4.7. Water Use Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araujo, S.S.; Beebe, S.; Crespi, M.; Delbreli, B.; Gonzaliz, E.M.; Gruber, V. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule performance within a changing environmental context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Boddey, R.M.; Gresshoff, P.M.; Hauggaard-Nielsen, H.; Alves, B., Jr.; Morrison, M.J. Legumes for mitigation of climate change and the provision of feedstock for biofuels and biorefineries. A review. Agron. Sustain. Dev. 2012, 32, 329–364. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Tomás, D.; Silva, M.; Lopes, S.; Viegas, W.; Veloso, M.M. Genetic Diversity and Population Structure in Vicia faba L. Landraces and Wild Related Species Assessed by Nuclear SSRs. PLoS ONE 2016, 11, e0154801. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.A. Response of some Faba Bean Cultivars (Vicia Faba L.) to Phosphorus Fertilization Under El-Tur and New Valley Conditions. Alex. Sci. Exch. J. 2023, 44, 93–108. [Google Scholar]
- Derese, T. Evaluation of Faba bean (Vicia faba L.) varieties for yield and yield contributing traits in the southern parts of Ethiopia. Acad. J. 2021, 8, 18–22. [Google Scholar]
- Abou-Khater, L.; Maalouf, F.; Jighly, A.; Rubiales, D.; Kumar, S. Adaptability and stability of faba bean (Vicia faba L.) accessions under diverse environments and herbicide treatments. Plants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Vogelsang-O’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; O’Mahony, J.A.; Arendt, E.K.; et al. Comparison of faba bean protein ingredients produced using dry fractionation and isoelectric precipitation: Techno-functional, nutritional and environmental performance. Foods 2020, 9, 322. [Google Scholar] [CrossRef]
- Robinson, G.H.J.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef]
- Koivisto, J.M.; Benjamin, L.R.; Lane, G.P.F.; Davies, W.P. Forage potential of semi-leafless grain peas. Grass Forage Sci. 2003, 58, 220–223. [Google Scholar] [CrossRef]
- Considine, M.J.; Siddique, K.H.; Foyer, C.H. Nature’s pulse power: Legumes, food security and climate change. J. Exp. Bot. 2017, 68, 1815–1818. [Google Scholar] [CrossRef]
- Valliyodan, B.; Ye, H.; Song, L.; Murphy, M.; Shannon, J.G.; Nguyen, H.T. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J. Exp. Bot. 2017, 68, 1835–1849. [Google Scholar] [CrossRef]
- Thuynsma, R.; Valentine, A.; Kleinert, A. Phosphorus deficiency affects the allocation of below-ground resources to combined cluster roots and nodules in Lupinus albus. J. Plant Physiol. 2014, 171, 285–291. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Sulieman, S.; Ha, C.V.; Schulze, J.; Tran, L.S.P. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, M.; Liu, J.; Allan, D.L.; Vance, C.P. Genomic and genetic control of phosphate stress in legumes. Plant Physiol. 2007, 144, 594–603. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges, and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Biostability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, M.M.; Hoekstra, A.Y. Global anthropogenic phosphorus loads to freshwater and associated grey water footprints and water pollution levels: A high-resolution global study. Water Resour. Res. 2018, 54, 345–358. [Google Scholar] [CrossRef]
- Fixen, P.E.; Johnston, A.M. World fertilizer nutrient reserves: A view to the future. J. Sci. Food Agric. 2012, 92, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.E.; Poulton, P.R.; Fixen, P.E.; Curtin, D. Phosphorus: Its efficient use in agriculture. Adv. Agron. 2014, 123, 177–228. [Google Scholar]
- Singh, A.K.; Bharati, R.C.; Manibhushan, N.C.; Pedpati, A. An assessment of faba bean (Vicia faba L.) current status and future prospect. Afr. J. Agric. Res. 2013, 8, 6634–6641. [Google Scholar]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [PubMed]
- Jones, D.L.; Oburger, E. Solubilization of Phosphorus by Soil Microorganisms. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus saturation and pH differentially regulate the efficiency of organic acid anion-mediated P solubilization mechanisms in soil. Plant Soil 2011, 341, 363–382. [Google Scholar]
- Wang, Q.; Wang, J.; Yang, Y.; Du, W.; Zhang, D.; Yu, D.; Cheng, H. A genome-wide expression profile analysis reveals active genes and pathways coping with phosphate starvation in soybean. BMC Genom. 2016, 17, 192. [Google Scholar]
- Henry, J.L.; Slinkard, A.E.; Hogg, T.J. The effect of phosphorus fertilizer on establishment, yield and quality of pea, lentil and faba bean. Can. J. Plant Sci. 2011, 75, 395–398. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of soil phosphorus on legume production. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 487–510. [Google Scholar]
- Adjei-Nsiah, S.; Alabi, B.U.; Ahiakpa, J.K.; Kanampiu, F. Response of Grain Legumes to Phosphorus Application in the Guinea Savanna Agro-Ecological Zones of Ghana. Agron. J. 2018, 110, 1089–1096. [Google Scholar]
- Papastylianou, P.; Vlachostergios, D.N.; Dordas, C.; Tigka, E.; Papakaloudis, P.; Kargiotidou, A.; Pratsinakis, E.; Koskosidis, A.; Pankou, C.; Kousta, A.; et al. Genotype X environment interaction analysis of faba bean (Vicia faba L.) for biomass and seed yield across different environments. Sustainability 2021, 13, 2586. [Google Scholar]
- Kalamartzis, I.; Dordas, C.; Georgiou, P.; Menexes, G. The use of appropriate cultivar of basil (Ocimum basilicum) can increase water use efficiency under water stress. Agronomy 2020, 10, 70. [Google Scholar]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar]
- Link, W.; Abdelmula, A.A.; von Kittlitz, E.; Bruns, S.; Riemer, H.; Stelling, D. Genotypic variation for drought tolerance in Vicia faba. Plant Breed. 1999, 118, 477–483. [Google Scholar]
- Bodner, G.; Kronberga, A.; Lepse, L.; Olle, M.; Vågen, I.M.; Rabante, L.; Fernandez, J.A.; Ntatsi, G.; Balliu, A.; Rewald, B. Trait identification of faba bean ideotypes for Northern European environments. Eur. J. Agron. 2018, 96, 1–12. [Google Scholar]
- Ulukan, H.; Culer, M.; Keskin, S. A path coefficient analysis of some yield and yield components in faba bean (Vicia faba L.) genotypes. Pak. J. Biol. Sci. 2003, 6, 1951–1955. [Google Scholar]
- Olle, M.; Williams, I.H.; Rosa, E. Selecting appropriate faba bean var. minor varieties for production under Northern European environmental conditions. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 432–438. [Google Scholar]
- Adak, M.S.; Kibritci, M. Effect of nitrogen and phosphorus levels on nodulation and yield components in faba bean (Vicia faba L.). Legum. Res. 2016, 39, 991–994. [Google Scholar]
- Ghizaw, A.; Mamo, T.; Yilma, Z.; Molla, A.; Ashagre, Y. Nitrogen and phosphorus effects on faba bean yield and some yield components. J. Agron. Crop. Sci. 1999, 182, 167–174. [Google Scholar]
- Oukaltouma, K.; El Moukhtari, A.; Lahrizi, Y.; Mouradi, M.; Farissi, M.; Willems, A.; Qaddoury, A.; Bekkaoui, F.; Ghoulam, C. Phosphorus deficiency enhances water deficit impact on some morphological and physiological traits in four faba bean (Vicia faba L.) varieties. Ital. J. Agron. 2021, 16, 1–13. [Google Scholar]
- Hashemabadi, D. Phosphorus fertilizers effect on the yield and yield components of faba bean (Vicia faba L.). Ann. Biol. Res. 2013, 4, 181–184. [Google Scholar]
- Nkaa, F.; Nwokeocha, O.W.; Ihuoma, O. Effect of phosphorus fertilizer on growth and yield of cowpea (Vigna unguiculata). J. Pharm. Biol. Sci. 2014, 9, 74–82. [Google Scholar]
- Yemane, A.; Skjelvåg, A.O. Effects of fertilizer phosphorus on yield traits of dekoko (Pisum sativum var. abyssinicum) under field conditions. J. Agron. Crop. Sci. 2003, 189, 14–20. [Google Scholar]
- Jia, Y.; Gray, V.M. Influence of phosphorus and nitrogen on photosynthetic parameters and growth in Vicia faba L. Photosynthetica 2004, 42, 535–542. [Google Scholar]
- Carpici, E.B. Changes in leaf area index, light interception, quality and dry matter yield of an abandoned rangeland as affected by the different levels of nitrogen and phosphorus fertilization. Turkish J. Field Crop. 2011, 16, 117–120. [Google Scholar]
- Dordas, C.A. Chlorophyll meter readings, N leaf concentration and their relationship with N use efficiency in oregano. J. Plant Nutr. 2017, 40, 391–403. [Google Scholar]
- Filiz, O.; Takil, E.; Kayan, N. The role of plant growth promoting rhizobacteria (PGPR) and phosphorus fertilization in improving phenology and physiology of bean (Phaseolus vulgaris L.). Appl. Ecol. Environ. Res. 2021, 19, 2507–2517. [Google Scholar]
- Lahmoud, A.M.; Laftta, W.M. Effect of planting distances and phosphate fertilization on two cultivars of broad bean (Vicia faba L). Int. J. Aquatic Sci. 2022, 13, 379–393. [Google Scholar]
- Saudy, H.; Noureldin, N.; Mubarak, M.; Fares, W.; Elsayed, M. Cultivar selection as a tool for managing soil phosphorus and faba bean yield sustainability. Arch. Agron. Soil Sci. 2020, 66, 414–425. [Google Scholar] [CrossRef]
- Fouda, K.F. Effect of phosphorus level and some growth regulators on productivity of faba bean (Vicia faba L.). Egypt. J. Soil Sci. 2017, 57, 73–87. [Google Scholar]
- Baccari, B.; Krouma, A. Rhizosphere Acidification Determines Phosphorus Availability in Calcareous Soil and Influences Faba Bean (Vicia faba) tolerance to P Deficiency. Sustainability 2023, 15, 6203. [Google Scholar] [CrossRef]
- Dordas, C.A.; Sioulas, C. Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind. Crops Prod. 2008, 27, 75–85. [Google Scholar] [CrossRef]
- Jia, Y.S.; Gray, V.M. The influence N and P supply on the short-term responses to elevated CO2 in faba bean (Vicia faba L.). S. Afr. J. Bot. 2007, 73, 466–470. [Google Scholar] [CrossRef][Green Version]
- Jin, J.; Tang, C.; Armstrong, R.; Sale, P. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient vertisol. Plant Soil 2012, 358, 91–104. [Google Scholar] [CrossRef]
- Oukaltouma, K.; El Moukhtari, A.; Lahrizi, Y.; Makoudi, B.; Mouradi, M.; Farissi, M.; Willems, A.; Qaddoury, A.; Bekkaoui, F.; Ghoulam, C. Physiological, biochemical and morphological tolerance mechanisms of faba bean (Vicia faba L.) to the combined stress of water deficit and phosphorus limitation. J. Soil Sci. Plant Nutr. 2022, 22, 1632–1646. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Cheto, S.; Oukaltouma, K.; Chamkhi, I.; Ibn Yassar, A.; Benmrid, B.; Qaddoury, A.; Kouisni, L.; Geistlinger, J.; Zeroual, Y.; Bargaz, A.; et al. Inoculation with rhizobacterial consortia alleviates combined water and phosphorus deficit stress in intercropped faba bean and wheat. Front. Sustain. Food Syst. 2023, 7, 203. [Google Scholar] [CrossRef]
- Khazaei, H.; Wach, D.; Pecio, A.; Vandenberg, A.; Stoddard, F.L. Genetic analysis of photosynthesis-related traits in faba bean (Vicia faba) for crop improvement. Plant Breed. 2019, 138, 761–769. [Google Scholar] [CrossRef]
- Jillani, G.; Sulieman, S.; Mühling, K.H. Carbohydrate utilization by belowground organs defines the capacity of faba bean to exploit organic phosphorus sources. J. Soil Sci. Plant Nutr. 2022, 185, 567–577. [Google Scholar] [CrossRef]
- Li, B.; Liu, J.; Shi, X.; Han, X.; Chen, X.; Wei, Y.; Xiong, F. Effects of belowground interactions on crop yields and nutrient uptake in maize-faba bean relay intercropping systems. Arch. Agron. Soil Sci. 2021, 69, 314–325. [Google Scholar] [CrossRef]
- Stoddard, F.L.; Balko, C.; Erskine, W.; Khan, H.R.; Link, W.; Sarker, A. Screening techniques and sources of resistance to abiotic stresses in cool season food legumes. Euphytica 2006, 147, 167–186. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.; Hauggaard, N.H. Review: Faba beans in cropping systems. Field Crop. Res. 2010, 115, 203–216. [Google Scholar] [CrossRef]
- Reckling, M.; Döring, T.F.; Bergkvist, G.; Chemielewski, F.-M.; Stoddard, F.L.; Watson, C.A.; Sedding, S.; Bachinger, J. Grain legume yield instability has increased over 60 years in long-term experiments as measured by a scale-adjusted coefficient of variation. Asp. Appl. Biol. 2018, 138, 15–20. [Google Scholar]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Effect of water stress during floral initiation, flowering and podding on the growth and yield of faba bean (Vicia faba L.). Eur. J. Agron. 1999, 11, 1–11. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, A.; Zaman, A. Effect of irrigation and phosphorus levels on broad bean (Vicia faba L.) for improving growth, yield and water extraction pattern. Legum. Res. 2017, 40, 257–263. [Google Scholar] [CrossRef][Green Version]
- Daoui, K.; Karrou, M.; Mrabet, R.; Fatemi, Z.; Draye, X.; Ledent, J.F. Genotypic variation of phosphorus use efficiency among Moroccan faba bean varieties (Vicia faba major) under rainfed conditions. J. Plant Nutr. 2012, 35, 34–48. [Google Scholar] [CrossRef]
- Di Paolo, E.; Garofalo, P.; Rinaldi, M. Irrigation and nitrogen fertilization treatments on productive and qualitative traits of broad bean (Vicia faba var. minor L.) in a Mediterranean environment. Legum. Res. 2015, 38, 209–218. [Google Scholar]
- Youseif, S.H.; Abd El-Megeed, F.H.; Saleh, S.A. Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low-fertility sandy soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef]
- Brahimi, S.; Toumatia, O.; Drevon, J.J.; Lazali, M.; Zitouni, A. Genotypic variability for tolerance to low soil phosphorus availability in faba bean (Vicia faba L.). J. Plant Nutr. 2022, 46, 167–183. [Google Scholar] [CrossRef]
- Bolland, M.D.A.; Siddique, K.H.M.; Brennan, R.F. Grain yield responses of faba bean (Vicia faba L.) to applications of fertiliser phosphorus and zinc. Austr. J. Exper. Agric. 2000, 40, 849–857. [Google Scholar] [CrossRef]
- Nebiyu, A.; Diels, J.; Boeckx, P. Phosphorus use efficiency of improved faba bean (Vicia faba) varieties in low-input agro-ecosystems. J. Soil Sci. Plant Nutr. 2016, 179, 347–354. [Google Scholar] [CrossRef]
- Amanuel, A.; Ku¨hne, R.F.; Tanner, D.G.; Vlek, P.L.G. Biological nitrogen fixation in faba bean (Vicia faba L.) in the Ethiopian highlands as affected by P fertilization and inoculation. Biol. Fert. Soils 2000, 32, 353–359. [Google Scholar] [CrossRef]
- Klippenstein, S.R.; Khazaei, H.; Vandenberg, A.; Schoenau, J. Nitrogen and phosphorus uptake and nitrogen fixation estimation of faba bean in western Canada. Agron. J. 2022, 114, 811–824. [Google Scholar] [CrossRef]
- Burman, U.; Garg, B.K.; Kathju, S. Effect of phosphorus application on cluster bean under different intensities of water stress. J. Plant Nutr. 2009, 32, 668–680. [Google Scholar] [CrossRef]
- Bishop, J.; Potts, S.G.; Jones, H.E. Susceptibility of faba bean (Vicia faba L.) to heat stress during floral development and anthesis. J. Agron. Crop. Sci. 2016, 202, 508–517. [Google Scholar] [CrossRef]
- Saxena, M.C. Some agronomic and physiological aspects of the important food legume crops in West Asia [Middle East]. In Food Legume Improvement and Development: Proceedings of the a Workshop Held at the University of Aleppo, Aleppo, Syria, 2–7 May 1978; IDRC: Ottawa, ON, Canada, 1979. [Google Scholar]
- Murinda, M.V.; Saxena, M.C. Agronomy of faba beans, lentils, and chickpeas. In Faba BEANS, Kabuli Chickpeas, and Lentils in the 1980s; Saxena, M.C., Verma, S., Eds.; ICARDA: Aleppo, Syria, 1985; pp. 229–244. [Google Scholar]
- Bond, D.A.; Lawes, D.A.; Hawtin, G.C.; Saxena, M.C.; Stephens, J.S. Faba Bean (Vicia faba L.). In Grain Legume Crops; Summer field, R.J., Roberts, E.H., Eds.; Mackeys of Chatham: Kent, UK, 1985; pp. 199–265. [Google Scholar]
- Papendick, R.I.; Chowdhury, S.L.; Johansen, C. Managing systems for increasing productivity of pulses in dryland agriculture. In World Crops: Cool Season Food Legumes: A Global Perspective of the Problems and Prospects for Crop Improvement in Pea, Lentil, Faba Bean and Chickpea; Springer: Dordrecht, The Netherlands, 1988; pp. 237–255. [Google Scholar]
- Tsigie, A.; Woldeab, A. Fertilizer response trials on highland food legumes [Pisum sativum, Lens culinaris, Cicer arietinum, Vicia faba]. In Proceedings of the First National Cool-Season Food Legumes Review Conference, Addis Abeba, Ethiopia, 16–20 December 1993; ICARDA: Beirut, Lebanon, 1995. [Google Scholar]
- Hebblethwaite, P.D.; Hawtin, G.C.; Latman, P.J.W. The husbandry of establishment and maintenance. Faba Bean 1983, 271–312. [Google Scholar]
- Saxena, M.C.; Wassimi, N. Crop-weed competition studies in lentils. Lens 1980, 7, 55–57. [Google Scholar]
- Algraishi, A.H.A.; Alogaidi, F.F.M. Evaluation of the performance of some cultivars of Faba Bean Vicia faba L. under different levels of phosphorus. J. Kerbala Agric. Sci. 2022, 9, 227–238. [Google Scholar] [CrossRef]
- Houassine, D.; Latati, M.; Rebouh, N.Y.; Gérard, F. Phosphorus acquisition processes in the field: Study of faba bean cultivated on calcareous soils in Algeria. Arch. Agron. Soil Sci. 2020, 66, 168–181. [Google Scholar] [CrossRef]
- Belouchrani, A.S.; Drouiche, N.; Ziche, Z.I.; Lounici, H. Study of the Effect of Phosphorus on Mineral Nutrition of Faba Bean «Vicia fabae L.». J. Plant Growth Regul. 2022, 42, 1750–1761. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; Al-Shameri, A.M.; Migdadi, H.M.; Ammar, M.H.; El-Harty, E.H.; Khan, M.A.; Farooq, M. Physiological and molecular characterization of faba bean (Vicia faba L.) genotypes for adaptation to drought stress. J. Agron. Crop. Sci. 2015, 201, 401–409. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Belal, E.E.; Rady, M.O.; Abd El-Mageed, S.A.; Mansour, E.; Awad, M.F.; Semida, W.M. Acidified biochar as a soil amendment to drought stressed (Vicia faba L.) plants: Influences on growth and productivity, nutrient status, and water use efficiency. Agronomy 2021, 11, 1290. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakaloudis, P.; Dordas, C. Phosphorus Fertilization Affects Morphological, Physiological and Agronomic Characteristics of Faba Bean Cultivars. Sustainability 2023, 15, 13172. https://doi.org/10.3390/su151713172
Papakaloudis P, Dordas C. Phosphorus Fertilization Affects Morphological, Physiological and Agronomic Characteristics of Faba Bean Cultivars. Sustainability. 2023; 15(17):13172. https://doi.org/10.3390/su151713172
Chicago/Turabian StylePapakaloudis, Paschalis, and Christos Dordas. 2023. "Phosphorus Fertilization Affects Morphological, Physiological and Agronomic Characteristics of Faba Bean Cultivars" Sustainability 15, no. 17: 13172. https://doi.org/10.3390/su151713172
APA StylePapakaloudis, P., & Dordas, C. (2023). Phosphorus Fertilization Affects Morphological, Physiological and Agronomic Characteristics of Faba Bean Cultivars. Sustainability, 15(17), 13172. https://doi.org/10.3390/su151713172





