Green Synthesis of Nickel and Copper Nanoparticles Doped with Silver from Hammada scoparia Leaf Extract and Evaluation of Their Potential to Inhibit Microorganisms and to Remove Dyes from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Synthesis of NiO/CuO and Ag-Doped NiO/CuO NPs
2.2. Characterizations
2.3. Photocatalytic Degradation
2.4. Adsorption Studies of Ag/CuO
2.5. Antimicrobial Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of NPs
3.1.1. FTIR
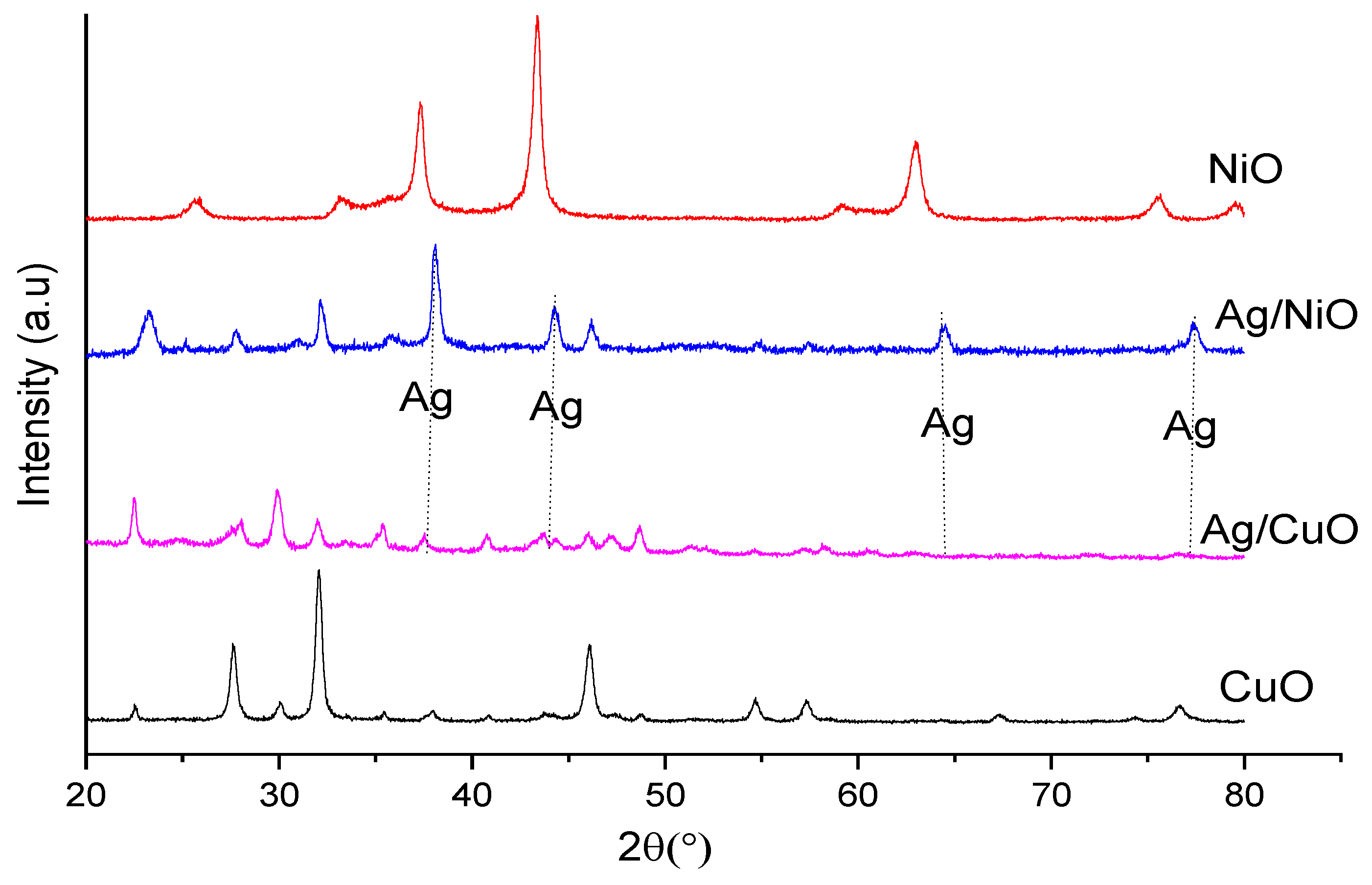
3.1.2. XRD
3.2. Adsorption Study
3.2.1. Effect of pH and Mass of Adsorbent
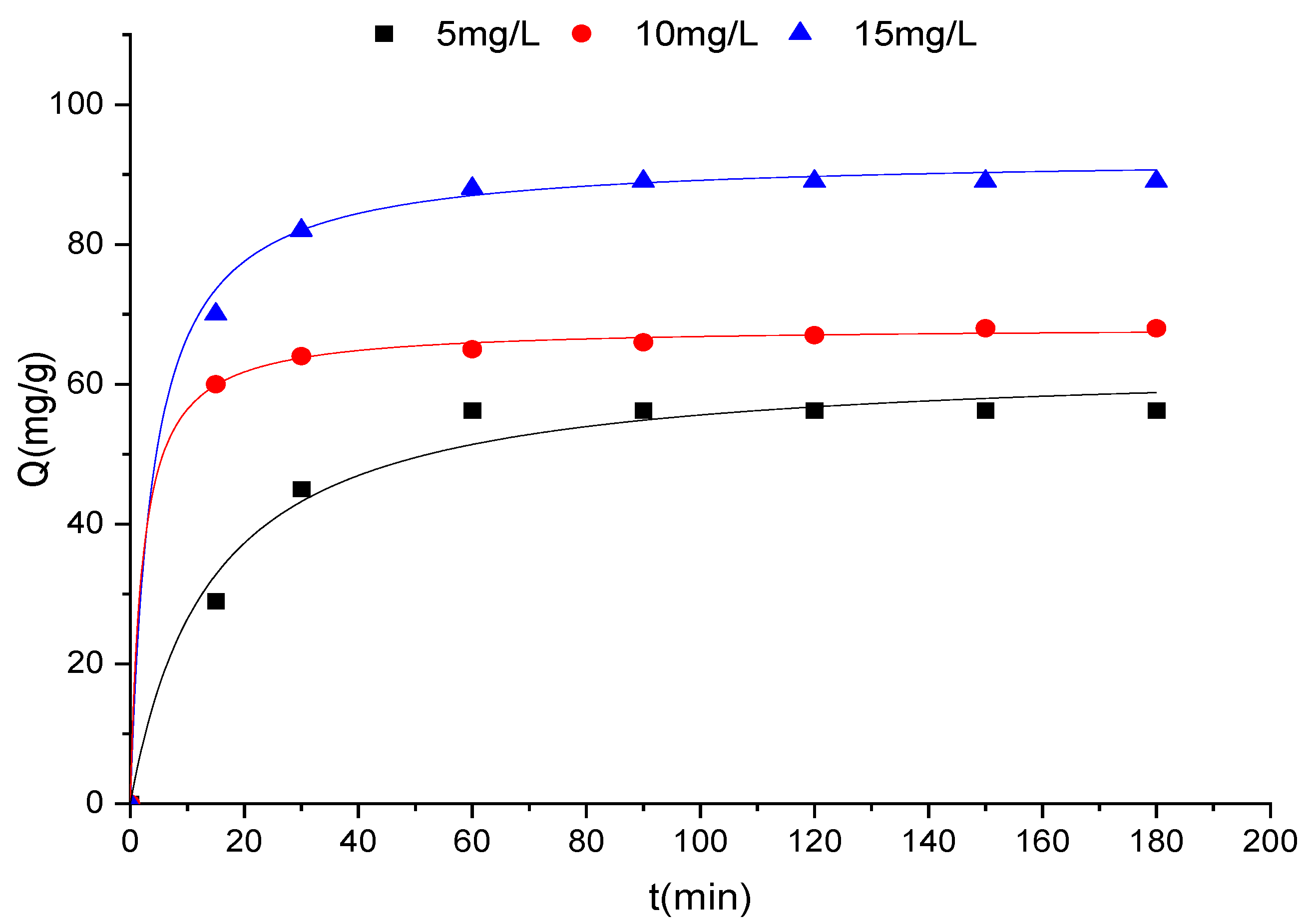
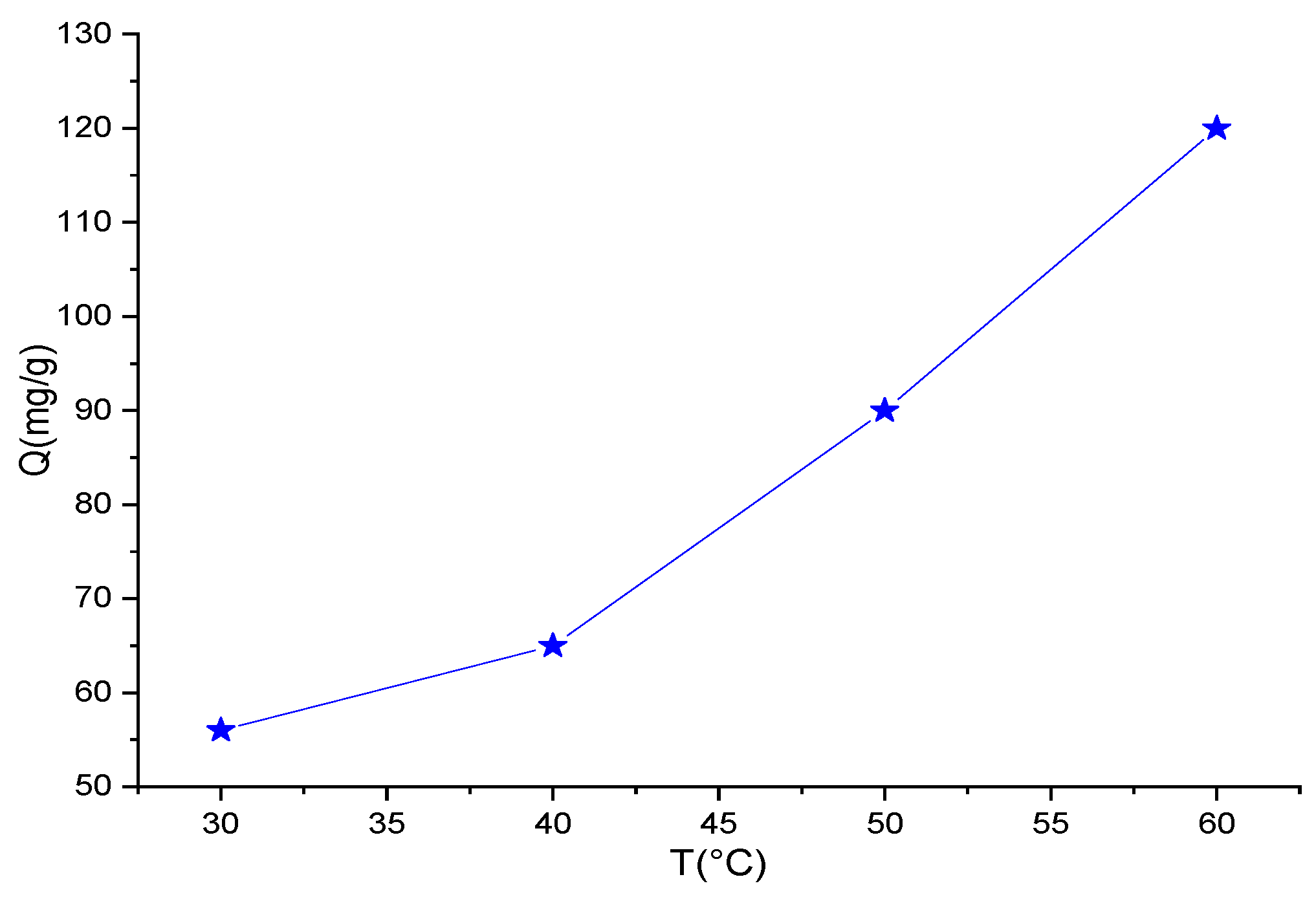
3.2.2. Adsorption Kinetics
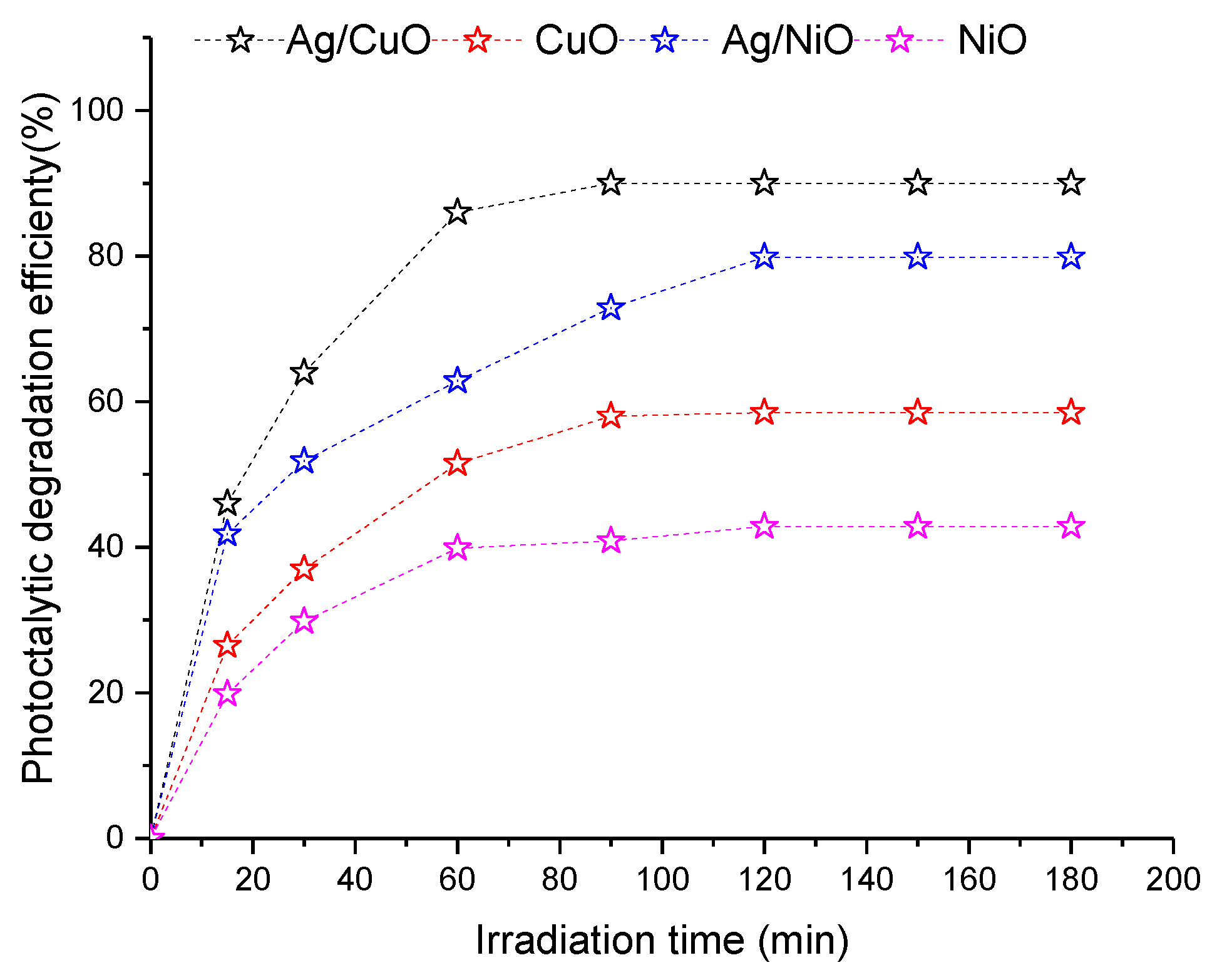
3.3. Photodegradation
3.4. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita Pepo l. J. Nanomater. 2019, 2019, 3476437. [Google Scholar] [CrossRef]
- Fuku, X.; Modibedi, M.; Mathe, M. Green Synthesis of Cu/Cu2O/CuO Nanostructures and the Analysis of Their Electrochemical Properties. SN Appl. Sci. 2020, 2, 902. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P. Prospects of Nanobiomaterials for Biosensing. Int. J. Electrochem. 2011, 2011, 125487. [Google Scholar] [CrossRef]
- Babu, A.T.; Antony, R. Green Synthesis of Silver Doped Nano Metal Oxides of Zinc & Copper for Antibacterial Properties, Adsorption, Catalytic Hydrogenation & Photodegradation of Aromatics. J. Environ. Chem. Eng. 2019, 7, 102840. [Google Scholar] [CrossRef]
- Mandal, G.; Ganguly, T. Applications of Nanomaterials in the Different Fields of Photosciences. Indian J. Phys. 2011, 85, 1229–1245. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of Gold Nanotriangles and Silver Nanoparticles Using Aloevera Plant Extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef]
- Jadhav, K.; Dhamecha, D.; Bhattacharya, D.; Patil, M. Green and Ecofriendly Synthesis of Silver Nanoparticles: Characterization, Biocompatibility Studies and Gel Formulation for Treatment of Infections in Burns. J. Photochem. Photobiol. B 2016, 155, 109–115. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Małek, K.; Celichowski, G.; Orlowski, P.; Krzyzowska, M.; Grobelny, J. The Synthesis of Monodisperse Silver Nanoparticles with Plant Extracts. Colloids Surf. B Biointerfaces 2019, 177, 19–24. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Taïr, K.; Kharoubi, O.; Taïr, O.A.; Hellal, N.; Benyettou, I.; Aoues, A. Aluminium-Induced Acute Neurotoxicity in Rats: Treatment with Aqueous Extract of Arthrophytum (Hammada scoparia). J. Acute Dis. 2016, 5, 470–482. [Google Scholar] [CrossRef]
- Boulanouar, B.; Abdelaziz, G.; Aazza, S.; Gago, C.; Miguel, M.G. Antioxidant Activities of Eight Algerian Plant Extracts and Two Essential Oils. Ind. Crops Prod. 2013, 46, 85–96. [Google Scholar] [CrossRef]
- Kharchoufa, L.; Bouhrim, M.; Bencheikh, N.; el Assri, S.; Amirou, A.; Yamani, A.; Choukri, M.; Mekhfi, H.; Elachouri, M. Acute and Subacute Toxicity Studies of the Aqueous Extract from Haloxylon Scoparium Pomel (Hammada scoparia (Pomel)) by Oral Administration in Rodents. Biomed. Res. Int. 2020, 2020, 4020647. [Google Scholar] [CrossRef]
- Haida, S.; Kribii, A. Chemical Composition, Phenolic Content and Antioxidant Capacity of Haloxylon Scoparium Extracts. S. Afr. J. Bot. 2020, 131, 151–160. [Google Scholar] [CrossRef]
- Bourogaa, E.; Bertrand, J.; Despeaux, M.; Jarraya, R.; Fabre, N.; Payrastre, L.; Demur, C.; Fournié, J.J.; Damak, M.; Feki, A.; et al. Hammada Scoparia Flavonoids and Rutin Kill Adherent and Chemoresistant Leukemic Cells. Leuk. Res. 2011, 35, 1093–1101. [Google Scholar] [CrossRef]
- Bouaziz, A.; Mhalla, D.; Zouari, I.; Jlaiel, L.; Tounsi, S.; Jarraya, R.; Trigui, M. Antibacterial and Antioxidant Activities of Hammada Scoparia Extracts and Its Major Purified Alkaloids. S. Afr. J. Bot. 2016, 105, 89–96. [Google Scholar] [CrossRef]
- Lamchouri, F.; Benali, T.; Bennani, B.; Toufik, H.; Ibn, L.; Hassani, M.; Bouachrine, M.; Lyoussi, B.; Ben, M.; Maroc, A. Preliminary Phytochemical and Antimicrobial Investigations of Extracts of Haloxylon Scoparium. J. Mater. Environ. Sci 2012, 3, 754–759. [Google Scholar]
- El-Shazly, A.; Wink, M. Tetrahydroisoquinoline and β-Carboline Alkaloids from Haloxylon Articulatum (Cav.) Bunge (Chenopodiaceae). Z. Fur Nat.—Sect. C J. Biosci. 2003, 58, 477–480. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Nalbone, L.; Filali, F.R.; Trabelsi, N.; el Majdoub, Y.O.; Bouchrif, B.; Giarratana, F.; Giuffrida, A. Comprehensive Evaluation on the Use of Thymus Vulgaris Essential Oil as Natural Additive against Different Serotypes of Salmonella Enterica. Sustainability 2021, 13, 4594. [Google Scholar] [CrossRef]
- Zhu, X.; Pathakoti, K.; Hwang, H.-M. Green Synthesis of Titanium Dioxide and Zinc Oxide Nanoparticles and Their Usage for Antimicrobial Applications and Environmental Remediation. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 223–263. [Google Scholar]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, H.; Singh, S.C. Green Synthesis of Nanoparticles and Its Potential Application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive Removal and Photocatalytic Degradation of Organic Pollutants Using Metal Oxides and Their Composites: A Comprehensive Review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Reddy, I.N.; Ravindranadh, K.; Reddy, K.R.; Shetti, N.P.; Kim, D.; Shim, J.; Aminabhavi, T.M. Copper-Doped ZrO2 Nanoparticles as High-Performance Catalysts for Efficient Removal of Toxic Organic Pollutants and Stable Solar Water Oxidation. J. Environ. Manag. 2020, 260, 110088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Kong, L.; Liu, L.; Luo, J.; Liu, B.; Zhou, Q.; He, F.; Xu, D.; Wu, Z. Preparation and Preferential Photocatalytic Degradation of Acephate by Using the Composite Photocatalyst Sr/TiO2-PCFM. Chem. Eng. J. 2019, 374, 852–862. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Khayi, S.; Oulghazi, S.; Bouchrif, B.; el Allaoui, A.; Ouhmidou, B.; Moumni, M. Antimicrobial Resistance, Virulence Genes, and Genetic Diversity of Salmonella Enterica Isolated from Sausages. Eur. J. Microbiol. Immunol. 2019, 9, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Filali, F.R.; Bouymajane, A.; Benhallam, F.; el Allaoui, A.; Chaiba, A.; Giarratana, F. Antibiotic Susceptibility Profile of Staphylococcus Aureus Isolated from Sausages in Meknes, Morocco. Vet. World 2018, 11, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Ed-Dra, A.; Filali, F.R.; lo Presti, V.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical Composition, Antioxidant Capacity and Antibacterial Action of Five Moroccan Essential Oils against Listeria Monocytogenes and Different Serotypes of Salmonella Enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef] [PubMed]
- Khaleed, A.A.; Bello, A.; Dangbegnon, J.K.; Momodu, D.Y.; Madito, M.J.; Ugbo, F.U.; Akande, A.A.; Dhonge, B.P.; Barzegar, F.; Olaniyan, O.; et al. Effect of Activated Carbon on the Enhancement of CO Sensing Performance of NiO. J. Alloys Compd. 2017, 694, 155–162. [Google Scholar] [CrossRef]
- Thakur, N.; Anu; Kumar, K. Effect of (Ag, Co) Co-Doping on the Structural and Antibacterial Efficiency of CuO Nanoparticles: A Rapid Microwave Assisted Method. J. Environ. Chem. Eng. 2020, 8, 104011. [Google Scholar] [CrossRef]
- Dehmani, Y.; Abouarnadasse, S. Study of the Adsorbent Properties of Nickel Oxide for Phenol Depollution. Arab. J. Chem. 2020, 13, 5312–5325. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Bimeghdar, S. Synthesis of Mesoporous NiO Nanoparticles and Their Application in the Adsorption of Cr(VI). Chem. Eng. J. 2014, 239, 105–113. [Google Scholar] [CrossRef]
- Ramzan, M.; Obodo, R.M.; Mukhtar, S.; Ilyas, S.Z.; Aziz, F.; Thovhogi, N. Green Synthesis of Copper Oxide Nanoparticles Using Cedrus Deodara Aqueous Extract for Antibacterial Activity. Mater. Today Proc. 2021, 36, 576–581. [Google Scholar] [CrossRef]
- Dehbi, A.; Dehmani, Y.; Omari, H.; Lammini, A.; Elazhari, K.; Abouarnadasse, S.; Abdallaoui, A. Comparative Study of Malachite Green and Phenol Adsorption on Synthetic Hematite Iron Oxide Nanoparticles (α-Fe2O3). Surf. Interfaces 2020, 21, 100637. [Google Scholar] [CrossRef]
- Dehmani, Y.; Alrashdi, A.A.; Lgaz, H.; Lamhasni, T.; Abouarnadasse, S.; Chung, I.M. Removal of Phenol from Aqueous Solution by Adsorption onto Hematite (α-Fe2O3): Mechanism Exploration from Both Experimental and Theoretical Studies. Arab. J. Chem. 2020, 13, 5474–5486. [Google Scholar] [CrossRef]
- Kundu, A.; Mondal, A. Kinetics, Isotherm, and Thermodynamic Studies of Methylene Blue Selective Adsorption and Photocatalysis of Malachite Green from Aqueous Solution Using Layered Na-Intercalated Cu-Doped Titania. Appl. Clay. Sci. 2019, 183, 105323. [Google Scholar] [CrossRef]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of Malachite Green and Methylene Blue by Fe0.01Ni0.01Zn0.98O/Polyacrylamide Nanocomposite Using Coupled Adsorption and Photocatalysis. Appl. Catal. B 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Luo, X.P.; Fu, S.Y.; Du, Y.M.; Guo, J.Z.; Li, B. Adsorption of Methylene Blue and Malachite Green from Aqueous Solution by Sulfonic Acid Group Modified MIL-101. Microporous Mesoporous Mater. 2017, 237, 268–274. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; Rehman, A. ur Combining Structurally Ordered Intermetallic Nodes: Kinetic and Isothermal Studies for Removal of Malachite Green and Methyl Orange with Mechanistic Aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated Biochar Derived from Opuntia Ficus-Indica for the Efficient Adsorption of Malachite Green Dye, Cu+2 and Ni+2 from Water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Rezaee, R.; Xie, Q.; You, L. Characterization of the Combined e Ff Ect of High Temperature and Moisture on Methane Adsorption in Shale Gas Reservoirs. J. Pet. Sci. Eng. 2019, 182, 106353. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Liu, S.; Li, W.; Tang, X. Temperature Effect on Gas Adsorption Capacity in Different Sized Pores of Coal: Experiment and Numerical Modeling. J. Pet. Sci. Eng. 2018, 165, 821–830. [Google Scholar] [CrossRef]
- Zhang, W.; Camarena, D.C. Development of ZnFe2O4 Nanoparticle Functionalized Baker’s Yeast Composite for Effective Removal of Methylene Blue via Adsorption and Photodegradation. J. Water Process Eng. 2020, 37, 101234. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Hydrothermal Synthesis of Hexagonal ZnO Nanoplates Used for Photodegradation of Methylene Blue. Optik 2021, 226, 165949. [Google Scholar] [CrossRef]
- de Oliveira Alves, F.H.; Araújo, O.A.; de Oliveira, A.C.; Garg, V.K. Preparation and Characterization of PAni(CA)/Magnetic Iron Oxide Hybrids and Evaluation in Adsorption/Photodegradation of Blue Methylene Dye. Surf. Interfaces 2021, 23, 100954. [Google Scholar] [CrossRef]
- Din, M.I.; Nabi, A.G.; Rani, A.; Aihetasham, A.; Mukhtar, M. Single Step Green Synthesis of Stable Nickel and Nickel Oxide Nanoparticles from Calotropis Gigantea: Catalytic and Antimicrobial Potentials. Environ. Nanotechnol. Monit. Manag. 2018, 9, 29–36. [Google Scholar] [CrossRef]
- Logeswari, P.; Silambarasan, S.; Abraham, J. Synthesis of Silver Nanoparticles Using Plants Extract and Analysis of Their Antimicrobial Property. J. Saudi Chem. Soc. 2015, 19, 311–317. [Google Scholar] [CrossRef]
- Sharma, P.; Pant, S.; Dave, V.; Tak, K.; Sadhu, V.; Reddy, K.R. Green Synthesis and Characterization of Copper Nanoparticles by Tinospora Cardifolia to Produce Nature-Friendly Copper Nano-Coated Fabric and Their Antimicrobial Evaluation. J. Microbiol. Methods 2019, 160, 107–116. [Google Scholar] [CrossRef]
- Geoprincy, G.; Saravanan, P.; Nagendra Gandhi, N.; Renganathan, S. A Novel Approach for Studying the Combined Antimicrobial Effects of Silver Nanoparticles and Antibiotics through Agar over Layer Method and Disk Diffusion Method. Dig. J. Nanomater. Biostruct. 2011, 6, 1557–1565. [Google Scholar]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic Antimicrobial Potential of Essential Oils in Combination with Nanoparticles: Emerging Trends and Future Perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzekri, O.; El Gamouz, S.; Ed-Dra, A.; Moussout, H.; Dehmani, Y.; Ziyat, H.; El Idrissi, M.; Choukrad, M.; Abouarnadasse, S. Green Synthesis of Nickel and Copper Nanoparticles Doped with Silver from Hammada scoparia Leaf Extract and Evaluation of Their Potential to Inhibit Microorganisms and to Remove Dyes from Aqueous Solutions. Sustainability 2023, 15, 1541. https://doi.org/10.3390/su15021541
Bouzekri O, El Gamouz S, Ed-Dra A, Moussout H, Dehmani Y, Ziyat H, El Idrissi M, Choukrad M, Abouarnadasse S. Green Synthesis of Nickel and Copper Nanoparticles Doped with Silver from Hammada scoparia Leaf Extract and Evaluation of Their Potential to Inhibit Microorganisms and to Remove Dyes from Aqueous Solutions. Sustainability. 2023; 15(2):1541. https://doi.org/10.3390/su15021541
Chicago/Turabian StyleBouzekri, Omayma, Sabah El Gamouz, Abdelaziz Ed-Dra, Hamou Moussout, Younes Dehmani, Hamid Ziyat, Mostafa El Idrissi, M’barek Choukrad, and Sadik Abouarnadasse. 2023. "Green Synthesis of Nickel and Copper Nanoparticles Doped with Silver from Hammada scoparia Leaf Extract and Evaluation of Their Potential to Inhibit Microorganisms and to Remove Dyes from Aqueous Solutions" Sustainability 15, no. 2: 1541. https://doi.org/10.3390/su15021541
APA StyleBouzekri, O., El Gamouz, S., Ed-Dra, A., Moussout, H., Dehmani, Y., Ziyat, H., El Idrissi, M., Choukrad, M., & Abouarnadasse, S. (2023). Green Synthesis of Nickel and Copper Nanoparticles Doped with Silver from Hammada scoparia Leaf Extract and Evaluation of Their Potential to Inhibit Microorganisms and to Remove Dyes from Aqueous Solutions. Sustainability, 15(2), 1541. https://doi.org/10.3390/su15021541






