1. Introduction
Dyes among others are the major components of the wastewater produced by various products such as varnishes, paints, leather, cosmetics, paper and pulp, and many others [
1]. These effluents are very dangerous to the environment as most of the dyes are very toxic to animals and plants. Crystal violet (CV) is one possessing negative effects on human and animals. Inhalation of the dye can lead to a breathing problems, profuse sweating, nausea, hypermotility, diarrhoea, and abdominal pain [
2]. It is used in biological staining, veterinary medicine, and as an additive in poultry food for bacterial growth necessary for animal digestion systems. Additionally CV has a negative impact on the Gram-positive bacteria, especially
Staphyloccocus aureus and yeast
Candida albicans [
3].
Different processes including, photodegradation [
4], flocculation [
5], chemical oxidation [
6,
7], biological processes [
8], and biocomposites [
9] are used for the removal of the dye from wastewaters [
10,
11,
12].
In order to reduce the costs of the dye remediation, adsorption is one of the most effective methods. The effectiveness of the adsorption is strongly connected with the used material as an adsorbent. Among the materials used as sorbents, one of the most examined materials is activated carbon. Unfortunately, due to the high cost, its use is limited and researchers are searching for cheaper solutions.
Various waste materials of organic origin such as peat, coconut husk and fibres, sawdust, cellulose waste, palm fibres, and orange peel are successfully used as adsorbent materials in processes of bioremediation of water intoxicated with dangerous dyes, and these materials can lower the costs of the process since they are easily obtainable [
13]. Beet pulp shreds material was previously studied as an adsorbent for the removal of heavy metals like copper, lead, zinc, and cadmium with high sorption capacity thus showing the utility of the material [
14,
15]
The present study shows the application of unmodified waste beet pulp shreds (BPS) as sorbent for the removal of crystal violet from model aqueous solutions and the antimicrobial activity of the obtained product. BPS is a byproduct produced in high amounts in sugar plants. It is composed of cellulose, lignin, sucrose, and functional groups like OH, COO, and CO. Due to the wide applicability of crystal violet in industry and its hydrophilic characteristic it can be seen as a violet/purple threat in effluents. The effect of various process parameters on the removal of the dye and the antimicrobial properties of the obtained product has been checked in this study. The obtained results have been examined for three isotherm models. Antimicrobial tests were performed with the use of various bacteria and yeast strains.
2. Materials and Methods
BPS was obtained from the local sugar company as the byproduct obtained in the process of sugar production. It is a natural, biodegradable, organic matter mostly used as an animal food additive. BPS was milled and sieved (0.5 mm ϕ) to obtain same particle size substance. In the next step, BPS was rinsed with deionized water 3 times to remove any impurities present in the material. The next step concerned drying the obtained material at 105 °C for 24 h. Reagents used in the tests were of analytical purity bought in the Sigma-Aldrich company (Berlin, Germany).
2.1. Methods
To acquire information about the specific surface area of the prepared sorbent, BET (Brunauer-Emmet-Teller method) analysis was performed using a Macrometric ASAP 2010 analyzer with a degassing station. A SEM-EDS microscope was used to obtain information about the surface of the adsorbent (Hitachi TM-3000 Scanning Electron Microscope with EDX X-ray microanalyzer, Hitachi, Kyoto, Japan) and EDX has been used to characterize the presence of elements on the BPS surface prior to and after the crystal violet removal.
Fourier-transform infrared (FT-IR) transmission spectra were registered in transmission mode using a Vertex 70v (Bruker Optics, Berlin, Germany) vacuum spectrometer. The pellets contain approximately 1 mg of sample added to 200 mg of KBr. The spectrometer was equipped with a DLaTGS Wide Range detector and a ceramic excitation source. The spectral resolution was 4 cm−1 and the number of scans were 256. Interpretation of the FT-IR results was possible by the use of software products OPUS (7.5 version) and GRAMS/AI 9.3 software packages. FT-IR spectroscopy made it possible to obtain information about the various bonds present in BPS and characterize the nature of the sorption.
The concentration of the crystal violet present in the solution after the process was measured using a UV-Vis spectrometer (Rayleigh UV-1800, Chelmsford, UK).
2.2. Sorption Studies
In the first part of the work, the sorption process for various concentrations of crystal violet onto ground sugar beet shreds was investigated. The BPS was previously rinsed with deionized water and dried (105 °C, 24 h). An amount of 0.5 g of organic material was weighed into six containers and 40 cm3 of crystal violet solution of appropriate concentration was added. The concentrations of the solutions were, respectively, 100 ppm, 200 ppm, 300 ppm, 400 ppm, and 500 ppm. After pouring the solution into the containers, all six of them were placed on a multi-station stirrer. The sorption times were 1, 3, 5, 10, 15, 30, 60 and 90 min, respectively. After a specified time, probes were filtered. At the end of the process (90 min) it was determined that the equilibrium state was reached. The sediment was obtained (BPSCV), and after filtering it, it was dried and used for further microbiological research. The filtrate was analyzed with a UV-Vis spectrophotometer to obtain information about the sorption capacity of BPS.
2.2.1. Equilibrium Studies
In order to evaluate the Langmuir, Freundlich and Temkin isotherms, the sorption capacity at any time (q
t mg/g), equilibrium (q
e mg/g), and the percentage removal (R
e %) were calculated.
Table 1 provides the linearized version of the used equations.
2.2.2. Sorption Kinetics
The CV sorption onto the BPS material and additional kinetic studies were carried out. The adsorption mechanism is connected with the physical, chemical properties of the adsorbent and the mass transport process [
19]. To analyze the experimental data of the CV adsorption onto BPS at different initial concentrations (100–500 ppm), constant mass (0.5 g), volume (40 cm
3), and natural CV solution pH, three kinetic models were chosen: the pseudo-first, the pseudo-second and the Weber-Morris model. The most suited model was selected based on the highest value of the correlation coefficient factor (R
2). The linear equations are presented in
Table 2.
2.3. Antimicrobial Characteristics of the Obtained Product
In the present publication, minimal inhibiting concentration (MIC) was determined. The antimicrobial effect of the BPSCV was carried out using a plate-diffusion test. The experiments were conducted against Gram-positive (Staphylococcus aureus ATCC 23235), Gram-negative bacteria (Escherichia coli ATCC BAA-2471, Pseudomonas aeruginosa ATCC BAA-1744) and yeasts (Candida albicans NCYC 1363).
2.3.1. Preparation of the Tested Material
Appropriate amounts of 0.2 g of obtained BPSCV obtained during the adsorption process with various concentrations of crystal violet (100–500 ppm) were chosen for microbial tests.
2.3.2. Preparation of Bacterial and Fungal Inoculum
A 24 h bacterial and fungal culture in the logarithmic phase of growth was taken and diluted in a YCU medium to obtain the inoculum of density equal to 1.5 × 108 cfu/mL measured using the Fuchs-Rosenthal chamber.
2.3.3. Determination of MIC
An amount of 0.1 mL of previously prepared bacterial inoculum was introduced to the prepared glass tubes with the tested materials (0.5 g) and optimal growth medium. As the control, medium without the addition of BPSCV was used. The results were obtained after 24 h at the temperature of 37 °C. The MIC concentration was the concentration that inhibits the growth of microorganisms with a 100% efficiency. The results were obtained by measuring the optical density of the medium after incubation with the use of a UV-Vis spectrometer at the 540 nm wavelength. To check the MIC parameter, the cell cultures without the addition of BPSCV was used as a negative control. Prepared samples were incubated for 24 h at 37 °C.
All of the above carried out experiments were conducted in triplicates. Standard deviation error was lower than 5%; thus, statistic data has not been provided.
3. Results and Discussion
3.1. Characterization of Materials
To analyze the specific surface area of BPS, the Macrometrics camera measured the pores. The samples prior to characterization were dried in the atmosphere of helium at 110 °C for 8 h. The obtained results were equal to 4.1244 m2/g.
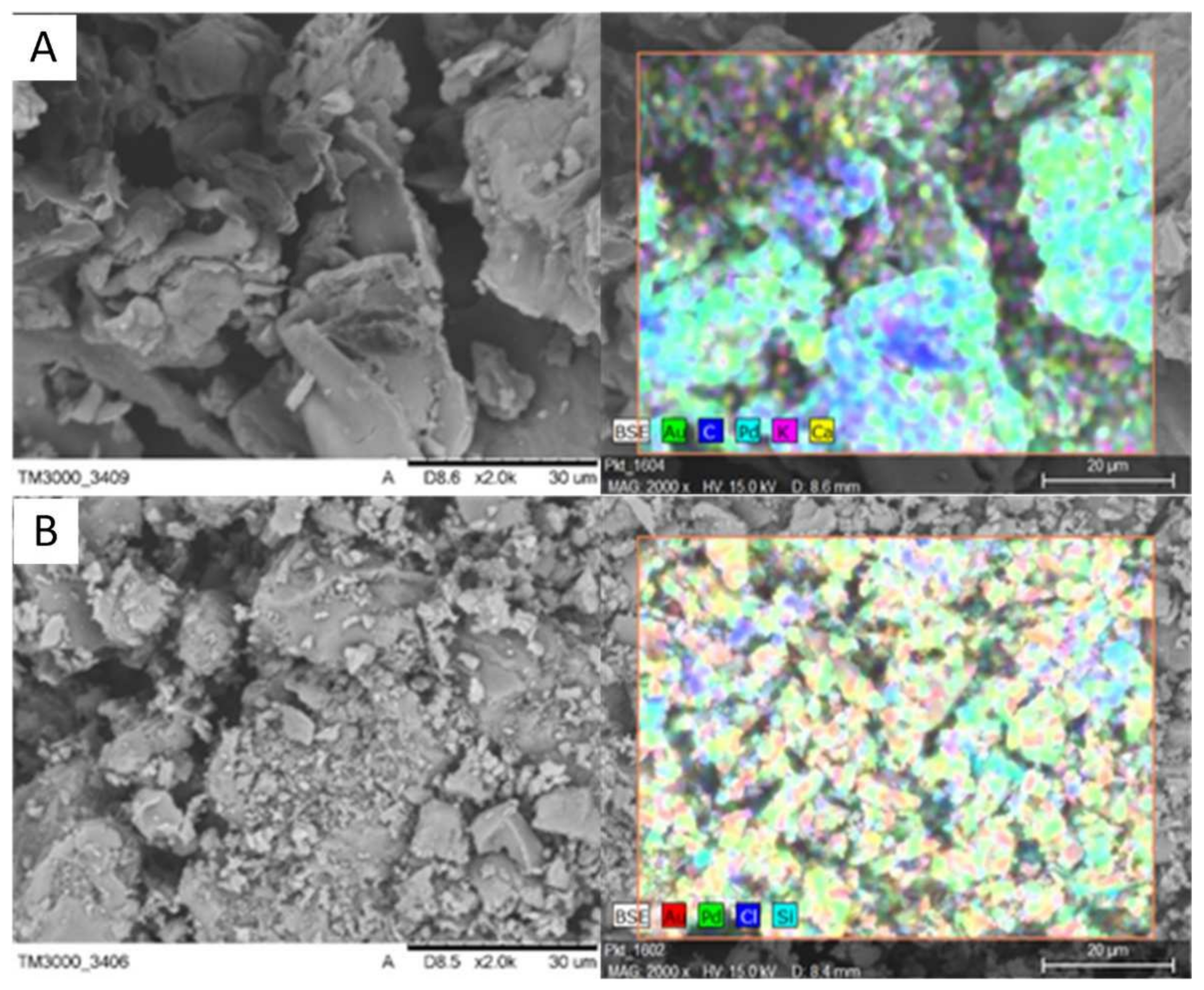
SEM microphotographs of the beet pulp shreds before and after crystal violet sorption are shown in
Figure 1. It can be seen that the organic material has a high number of pores and its structure is heterogenous, which is common for organic materials of plant origin. The main porosity is caused by the unique crystal building. The other can be connected with the sizes and other associated components.
Figure 1 shows the microphotographs of BPS and BPSCV made with a scanning electron microscope with an EDS analyzer.
On the microphotographs, one can see that after the adsorption the Cl ions coming from crystal violet, dye appeared on the surface, suggesting that the strong adhesion between the dye and material occurred.
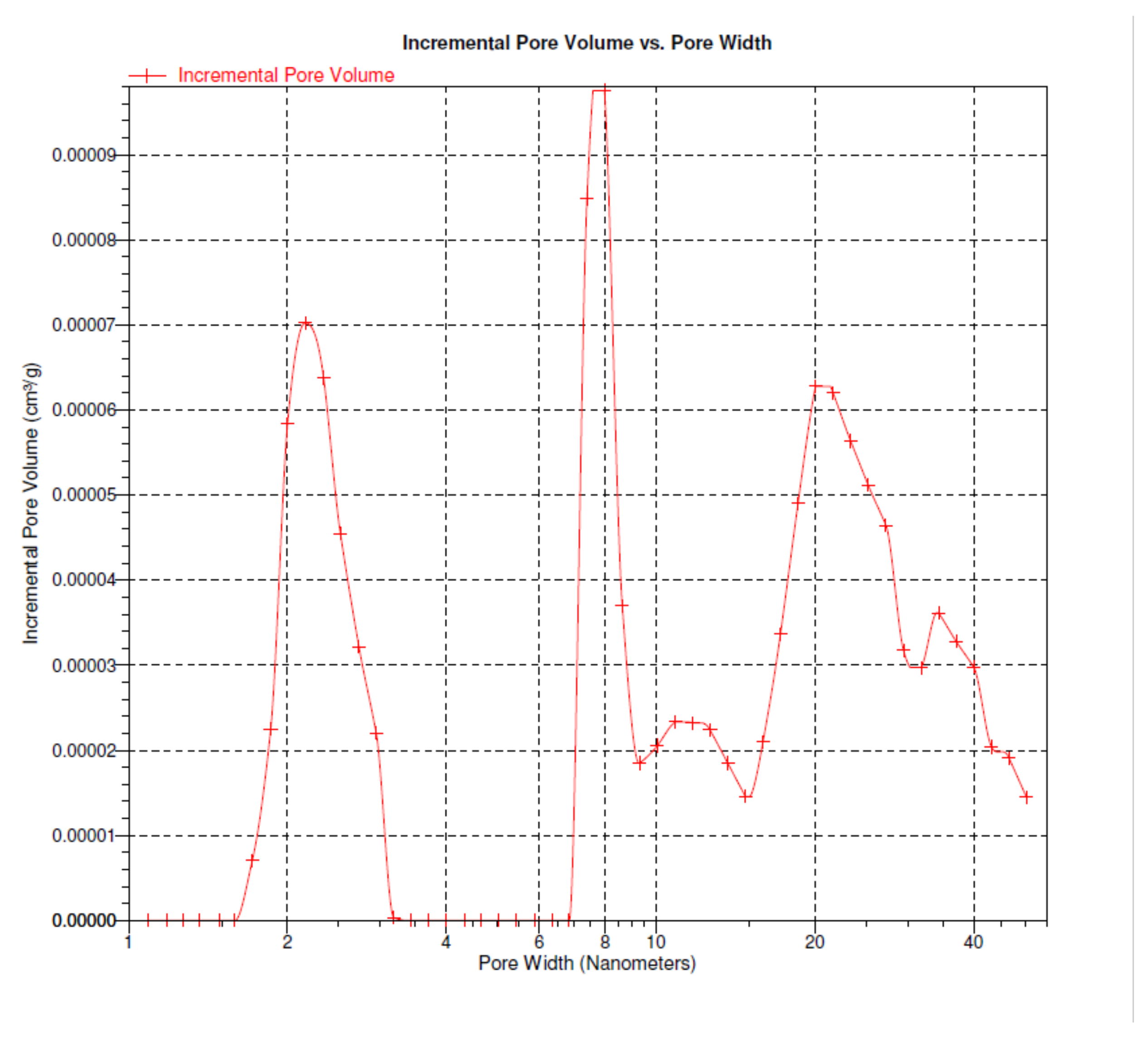
Figure 2 presents the porosity graphs showing the ratio of pore volumes to pore width. The pore volume of the probe is counted from the volume of condensed vapour. Analyzing pore volume plots as a function of its radius, the pore volume in the BPS can be defined.
3.2. FT-IR Study
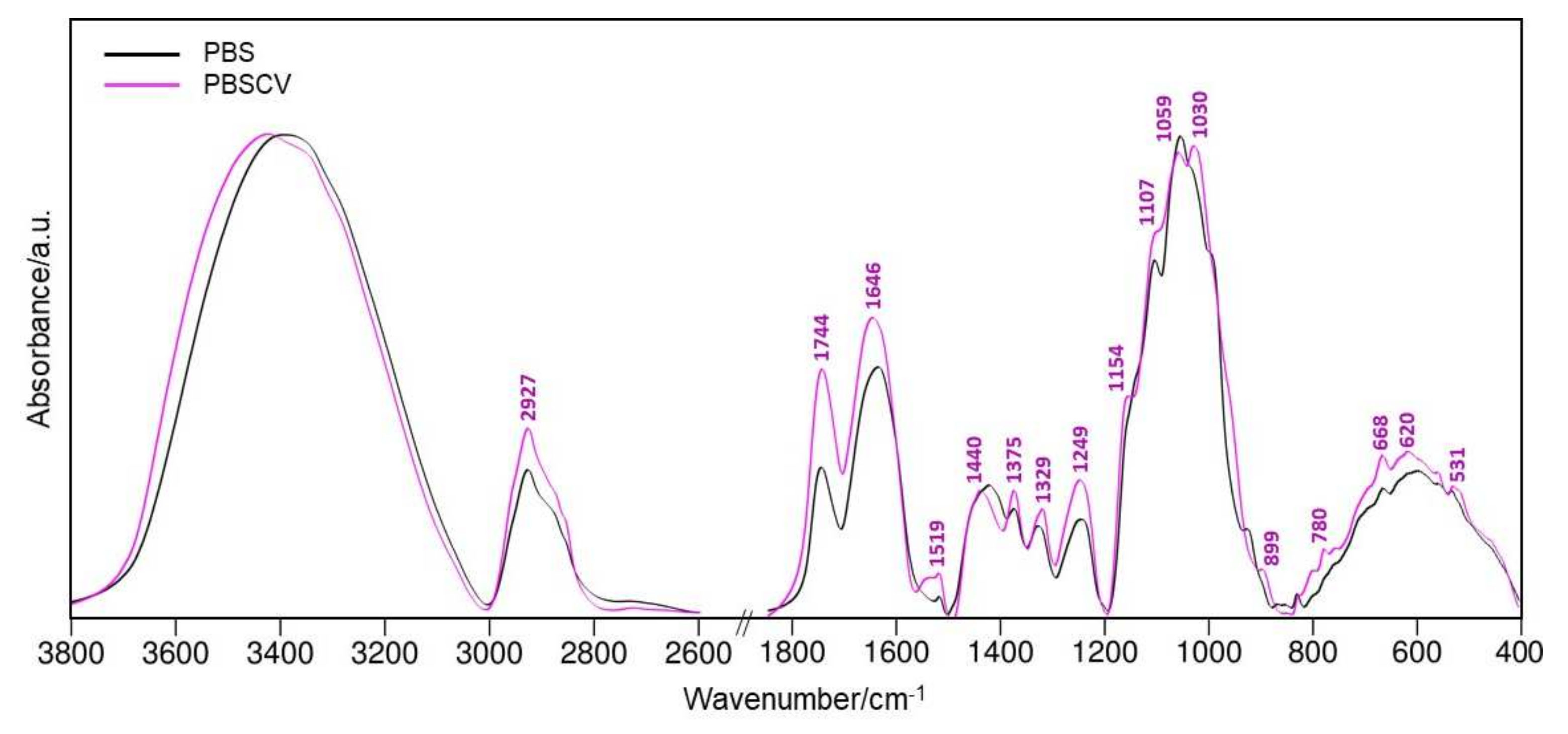
The FT-IR spectra before and after the sorption of CV onto the BPS are shown in
Figure 3. The performed analysis allowed us to determine the changes resulting from the sorption of crystal violet on BPS, and, as a result, to characterize the way of sorption. This demonstrates the significant influence of adsorbed CV on the chemical structure, the nature of the bonds in BPS, and the interaction of these two materials. As can be seen from the spectra, there are distinct changes in both the relative intensities of the bands and their frequencies (see
Table S1, Supporting Information for detailed band positions and assignments).
The most intense wide band at ~3408 cm
−1 corresponds to O-H stretching vibrations from cellulose, lignin, or pectin. Another strongly pronounced FT-IR signal appearing at the high-frequency range is the 2927 cm
−1 peak attributed to the C-H stretching oscillations, which probably originated from cellulose, hemicellulose, and lignin [
23,
24,
25,
26,
27]. Additional peaks assigned to the vibrations of the methylene and methyl groups appear at ~2964 cm
−1, 2879 cm
−1, and 2855 cm
−1 [
28,
29]. It is noteworthy that the relative intensity of the FT-IR peak arising at 1744 cm
−1 due to ν(C=O) from carboxylic acids, pectin esters or unconjugated ketones [
25,
30,
31] shows significant enhancement upon the adsorption of CV on BPS surface. At the same time, the share of the 1646 cm
−1 band in the BPSCV spectrum significantly increases. There is also a significant blue shift of the aforementioned spectral signal (Δ
= 11 cm
−1) in relation to the spectrum collected before the adsorption.
Some changes are also visible in the spectral range between 1200 cm
−1 and 1500 cm
−1, especially in the case of the 1249 cm
−1 band corresponding to the stretching vibrations of the C-C-O fragment in the esters (
Table S1) [
32].
Furthermore, the intensity of the 1030 cm
−1 band clearly increased after the removal process. This band can be attributed to C-O stretching vibrations due to glycosidic bonding, alcohol OH groups of sugars, or C-O-C stretching vibrations in lignin or hemicellulose [
26,
28]. At the same time, a decrease in the intensity of the 1059 cm
−1 band coming from the C=C stretching vibrations of pectins, is observed.
Based on the FT-IR results, it can be summarized that the observed spectral changes after the adsorption of CV on the BPS surface result from possible alterations in the chemical structure of BPS rather than from the spectral signals from the CV. The described spectral differences suggest the involvement of the aforementioned functional groups in the binding of CV to the BPS surface. These findings indicate the great potential of BPS as an effective biosorbent for reduction of CV as a hazardous dye present in wastewater.
3.3. Sorption Studies
To measure the sorption capacity of BPS, the equilibrium data for crystal violet dye sorption was analyzed. In
Figure 4, it can be seen that sorption capacity changes over time with dependence of the initial dye concentration.
Studying
Figure 4 it can be seen that the sorption equilibrium is reached after about the first 10 min from the beginning of the process. The highest adsorption capacity occurs in the first 8 min of the process. The reason for this phenomena can be probably related to the amount of the increased collisions between crystal violet dye and the surface of the adsorbent. Increasing the amount of the dye increases the sorption capacity (q
e) of the BPS from 3.94 to 20.91 mg/g. According to the obtained data, the removal of the dye from an aqueous solution can be seen at each used concentration ranging from 97% to 98% thus suggesting that the adsorbent can remove higher concentrations of crystal violet dye than used in this study. These results show that BPS has a large number of active sites with a high affinity to the crystal violet dye.
Equilibrium Studies
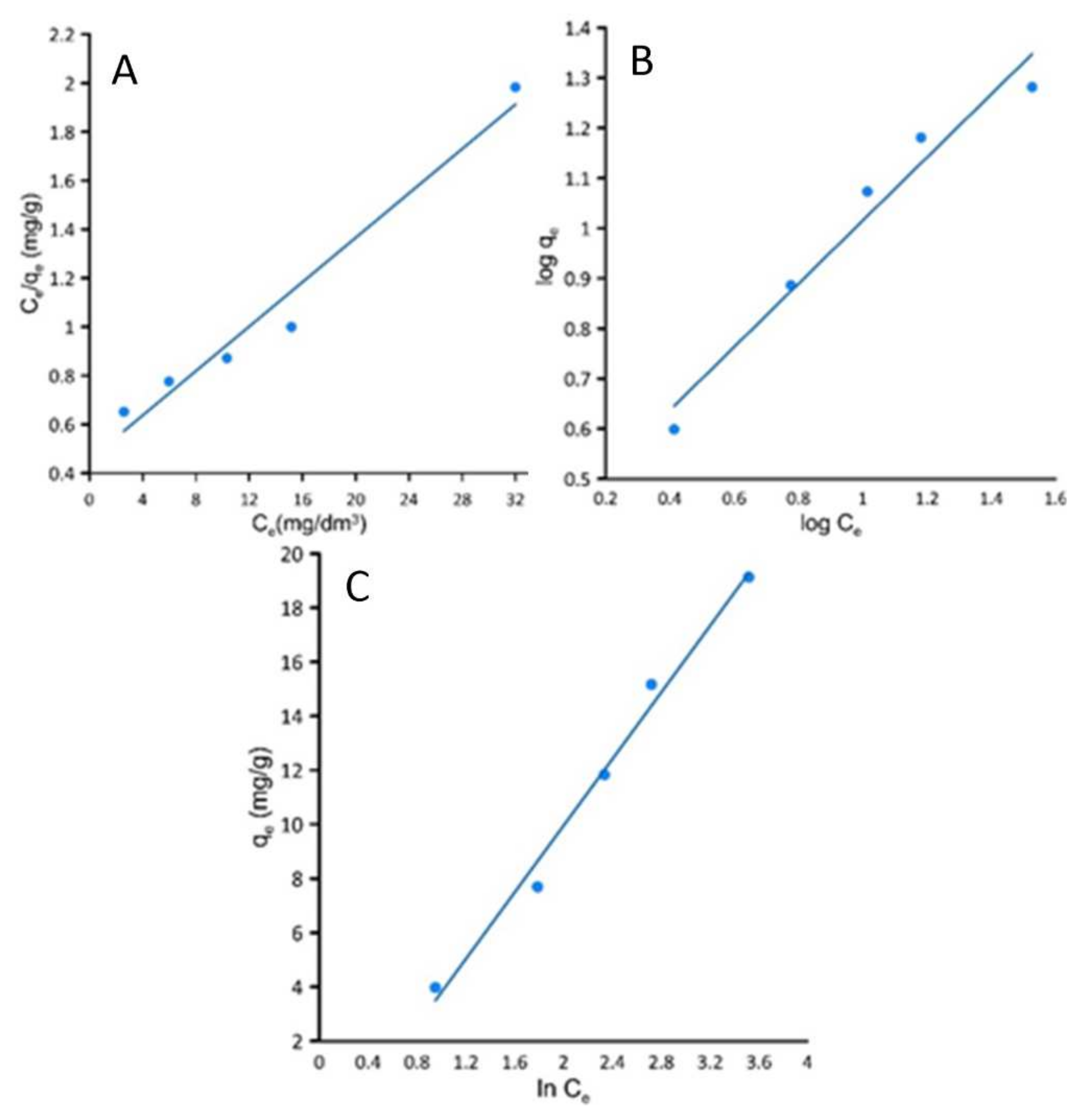
Three isotherm models were chosen for the presented study to acquire information about the sorption process in the equilibrium state. In
Figure 5 the Langmuir, Freundlich and Temkin models in linearized version are presented.
The parameters obtained through the calculations based on the isotherms at a constant temperature equal to 20 °C are presented in
Table 3.
Studying
Table 3, one can see that the highest coefficient factor R
2 is for the Langmuir isotherm model. Langmuir is connected with the thesis that the adsorbate covers the adsorbent with a monolayer and that interactions between the dye’s molecules are negligible. A relatively high value of Langmuir constant K
L represents a high affinity of active sites for crystal violet and a measure of the strength of connection between molecules and the adsorbent surface. The obtained R
L parameter from the Langmuir constant is in the range 0–1 suggesting that the process conditions are favourable.
Studying the parameters of Freundlich isotherm, it can be stated that the sorption process has a chemical nature according to the 1/n parameter which is in the range between 0 < 1 < n. The correlation coefficient corresponding to the Temkin isotherm model is quite low, suggesting that the model isn’t appropriate to describe the sorption process.
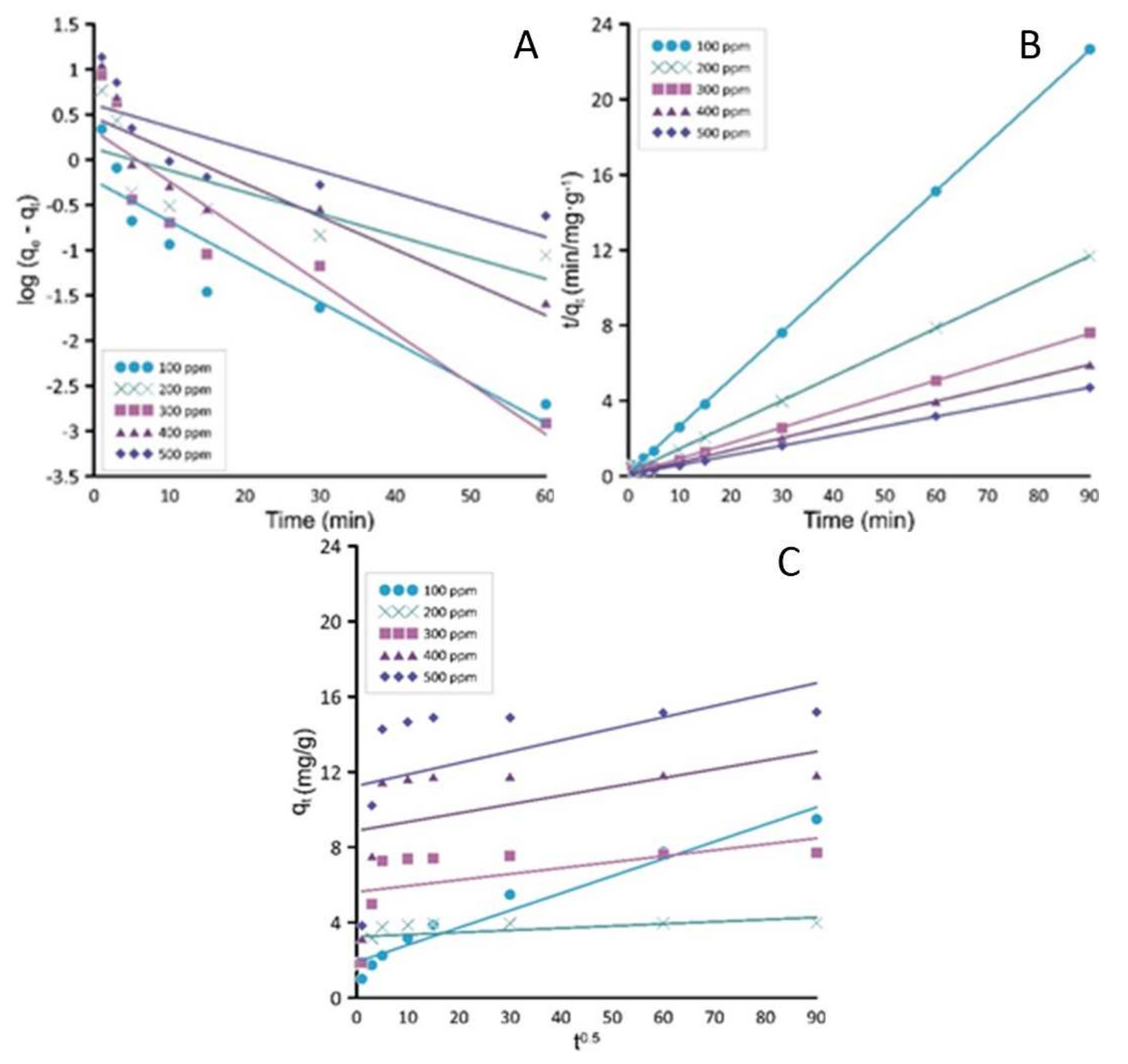
3.4. Kinetic Studies
The pseudo-first model is usually a better fit and chosen for low solute concentrations. The obtained data presented in
Table 4 and
Figure 6 are not in agreement with the calculated sorption capacity in equilibrium q
e and the value of coefficient factor R
2 is low for the adsorption data thus suggesting that the sorption process is not according to the pseudo-first-order kinetic model.
Using Weber Moris kinetic model by plotting qt vs. t, the parameter k can be calculated. The plot of qt vs. t0.5 is a line when the internal diffusion is the controlling process. If different, the phenomena is controlled by various processes. Researching obtained data, it can be seen that this model is not appropriate for CV sorption onto the BPS material.
The rate of the pseudo-second-order model is mostly connected with the quantity of solute adsorbed on the tested material and the quantity adsorbed at equilibrium [
33]. The obtained plots show alignment between the test values of maximum sorption capacity in equilibrium q
e (mg/g). In the case of all concentrations, the correlation coefficient R
2 is higher than 0.999. Comparing all three models’ prediction of the best fitting time relationship, it is suggested that pseudo-second-order describes the adsorption process of crystal violet dye onto the surface of waste beet pulp shreds. According to the best-fitted model, the limiting step is not connected with the boundary layer resistance. The external model will not provide information that the adsorption process mechanism and the rate-controlling step was chemical adsorption [
19].
3.5. Antimicrobial Properties
The antimicrobial properties of BPSCV material were carried out against three strains of bacteria:
Escherichia coli, Pseudomonas aeruginosa (Gram-negative bacteria), and
Staphylococcus aureus, (Gram-positive bacteria) and the yeast strain
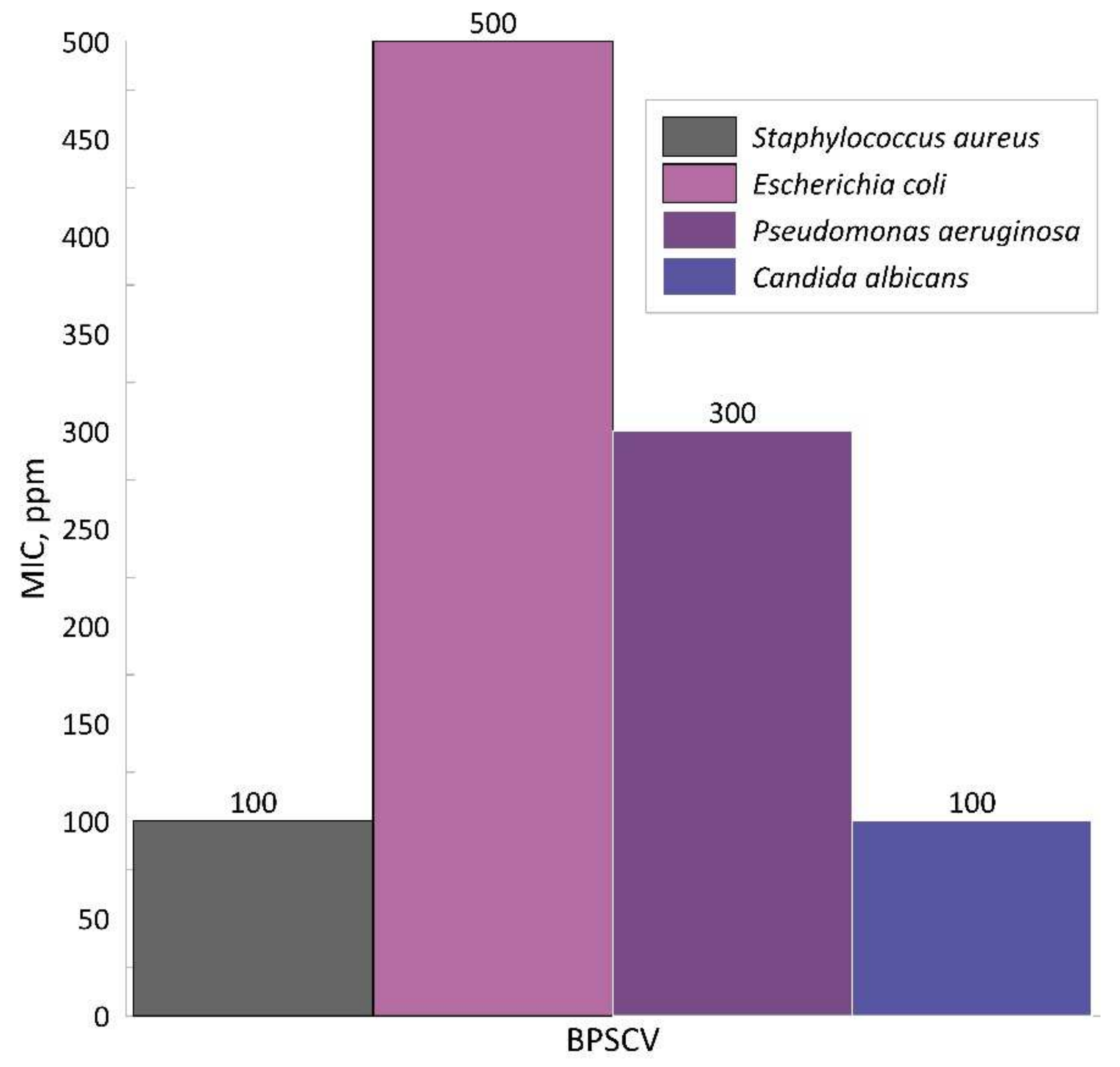
Candida albicans. In
Figure 7, the MIC results obtained for BPSCV are shown. The concentration of the dye is expressed in the
ppm units for better interpretation of the results. The unmodified BPS did not influenced the growth of the used microorganisms in the way that was expected because of the high number of saccharides present in the material which were a potential source of carbon. On the other hand, BPS modified with crystal violet dye even though it also contained saccharides showed high antimicrobial properties. Analysis of the growth of the microorganisms shows that the best inhibitory characteristics were provided by BPSCV in concentration: 100 ppm for
S. aureus and
C. albicans, 300 ppm for
P. aeruginosa and 500 ppm for
E. coli. The Gram-negative bacteria used in the study such as
Escherichia coli have less acidic components compared to the Gram-positive
Staphylococci group, so it is harder to combine with the dye for them. The possible explanation of the antibacterial properties of the product is connected with the formation of the complex, cell-dye. Bacteria
E. coli has high isoelectric points suggesting that it should be more resistant than the other species used in the present study [
34]. As is well known, the gram-negative bacterial cell wall is built differently in the comparison with the gram-positive bacterial cell wall. The first group’s cell wall is 30 nm thick while the second group’s cell wall is smaller, around 3–4 nm. Gram-negative bacteria contain a peptidoglycan cytoplasmic membrane in addition to an external membrane containing lipopolisaccharides. The Gram-positive bacteria have a cell membrane which is further away from the outer membrane and present peptidoglycan is more loosely packed which suggests more easy penetration of the dye into the cell [
34].
4. Conclusions
The presented study showed that BPS have a high affinity for crystal violet dye from aqueous solutions. The bioremediation process of CV was connected with different chemical and physical parameters. Studies carried out in equilibrium showed that the Langmuir isotherm was best fitted (correlation factor R2 was above 0.99). The highest sorption capacity ranged from 3.94 to 20.91 mg/g depending on the concentration used. The RL parameters for every study were between 0–1 suggesting favourable conditions of the dye removal. From the kinetic modelling it can be summarized that the adsorption is best fitted to the pseudo-second rate order model according to the correlation coefficient (>0.99). Summarizing it can be suggested that process is probably chemical what is following the FTIR analysis. The inhibiting properties of BPSCV in the growth of both G+ and G- bacteria and yeast have been confirmed. E. coli, S. aureus, P. aeruginosa, and C. albicans strains have been used to check the antimicrobial properties of the obtained BPSCV product. The properties and characteristics of the cell wall is one of the most important factors in the assessment of antimicrobial properties. The obtained material can be used as a remediating product to a microbiologically contaminated surface for its disinfection.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/su15010067/s1, Table S1: Wavenumber and suggested band assignments for FT-IR spectra before (BPS) and after the sorption of crystal violet onto the BPS (BPSCV).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by J.C. and P.S. Data curation was conducted by J.C., E.P. and C.P. The first draft of the manuscript was written by J.C. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and materials are available directly from the corresponding author.
Acknowledgments
This study was partially performed using equipment purchased in the frame of the project co-funded by the Małopolska Regional Operational Program Measure 5.1 Krakow Metropolitan Area as an important hub of the European Research Area for 2007–2013, project No. MRPO.05.01.00-12-013/15.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of Crystal Violet Dye from Wastewater by Surfactant-Modified Alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Fabryanty, R.; Valencia, C.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Removal of Crystal Violet Dye by Adsorption Using Bentonite—Alginate Composite. J. Environ. Chem. Eng. 2017, 5, 5677–5687. [Google Scholar] [CrossRef]
- Sehmi, S.K.; Noimark, S.; Bear, J.C.; Peveler, W.J.; Bovis, M.; Allan, E.; MacRobert, A.J.; Parkin, I.P. Lethal Photosensitisation of Staphylococcus Aureus and Escherichia Coli Using Crystal Violet and Zinc Oxide-Encapsulated Polyurethane. J. Mater. Chem. B 2015, 3, 6490–6500. [Google Scholar] [CrossRef]
- Pawar, K.K.; Chaudhary, L.S.; Mali, S.S.; Bhat, T.S.; Sheikh, A.D.; Hong, C.K.; Patil, P.S. In2O3 Nanocapsules for Rapid Photodegradation of Crystal Violet Dye under Sunlight. J. Colloid Interface Sci. 2020, 561, 287–297. [Google Scholar] [CrossRef]
- Wang, Y.; Geng, Q.; Yang, J.; Liu, Y.; Liu, C. Hybrid System of Flocculation-Photocatalysis for the Decolorization of Crystal Violet, Reactive Red X-3B, and Acid Orange II Dye. ACS Omega 2020, 5, 31137–31145. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan–Clay Composite as Highly Effective and Low-Cost Adsorbent for Batch and Fixed-Bed Adsorption of Methylene Blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly Effective Adsorption of Crystal Violet Dye from Contaminated Water Using Graphene Oxide Intercalated Montmorillonite Nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Roy, D.C.; Biswas, S.K.; Saha, A.K.; Sikdar, B.; Rahman, M.; Roy, A.K.; Prodhan, Z.H.; Tang, S.S. Biodegradation of Crystal Violet Dye by Bacteria Isolated from Textile Industry Effluents. PeerJ 2018, 2018, e5015. [Google Scholar] [CrossRef]
- Hamza, M.F.; Fouda, A.; Wei, Y.; El Aassy, I.E.; Alotaibi, S.H.; Guibal, E.; Mashaal, N.M. Functionalized Biobased Composite for Metal Decontamination—Insight on Uranium and Application to Water Samples Collected from Wells in Mining Areas (Sinai, Egypt). Chem. Eng. J. 2022, 431, 133967. [Google Scholar] [CrossRef]
- Kulkarni, M.R.; Revanth, T.; Acharya, A.; Bhat, P. Removal of Crystal Violet Dye from Aqueous Solution Using Water Hyacinth: Equilibrium, Kinetics and Thermodynamics Study. Resour. Technol. 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; ElHafeez, E.A.; Goda, E.S.; Lee, S.; Yoon, K.R. Smart Bactericidal Filter Containing Biodegradable Polymers for Crystal Violet Dye Adsorption. Cellulose 2019, 26, 9179–9206. [Google Scholar] [CrossRef]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A.; Dela Cruz, E.; Huyop, F. Biological Treatment of Real Textile Effluent Using Aspergillus Flavus and Fusarium Oxysporium and Their Consortium along with the Evaluation of Their Phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption Characteristics for the Removal of a Toxic Dye, Tartrazine from Aqueous Solutions by a Low Cost Agricultural by-Product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Joseph, O.; Rouez, M.; Métivier-Pignon, H.; Bayard, R.; Emmanuel, E.; Gourdon, R. Adsorption of Heavy Metals on to Sugar Cane Bagasse: Improvement of Adsorption Capacities Due to Anaerobic Degradation of the Biosorbent. Environ. Technol. 2009, 30, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Sciban, M.; Vulic, T.; Kukic, D.; Prodanovic, J.; Klasnja, M. Characterization of Raw and Treated Sugar Beet Shreds for Copper Ions Adsorption. Desalin. Water Treat. 2016, 57, 14590–14597. [Google Scholar] [CrossRef]
- Chwastowski, J.; Bradło, D.; Żukowski, W. Adsorption of Cadmium, Manganese and Lead Ions from Aqueous Solutions Using Spent Coffee Grounds and Biochar Produced by Its Pyrolysis in the Fluidized Bed Reactor. Materials 2020, 13, 2782. [Google Scholar] [CrossRef]
- Staroń, P.; Sorys, P.; Chwastowski, J. Equilibrium and Kinetic Study of Ammonium Sorption by Raphia Farinifera. Water. Air. Soil Pollut. 2019, 230, 243. [Google Scholar] [CrossRef]
- Staroń, P.; Chwastowski, J.; Banach, M. Sorption Behavior of Methylene Blue from Aqueous Solution by Raphia Fibers. Int. J. Environ. Sci. Technol. 2019, 2019, 4469. [Google Scholar] [CrossRef]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Abu Bakar, N.F.; Nik Him, N.R.; Lau, W.J. Adsorption Kinetics of Methylene Blue Dyes onto Magnetic Graphene Oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Alsaadi, M.A.; Hayyan, M.; Akib, S.; Ibrahim, R.K.; Hashim, M.A. Lead Removal from Water by Choline Chloride Based Deep Eutectic Solvents Functionalized Carbon Nanotubes. J. Mol. Liq. 2016, 222, 883–894. [Google Scholar] [CrossRef]
- Rahman, N.; Haseen, U. Equilibrium Modeling, Kinetic, and Thermodynamic Studies on Adsorption of Pb(II) by a Hybrid Inorganic−Organic Material: Polyacrylamide Zirconium(IV) Iodate. Ind. Eng. Chem. Res. 2014, 53, 8198–8207. [Google Scholar] [CrossRef]
- Markandeya, S.P.S.; Kisku, G.C. Linear and Non-Linear Kinetic Modeling for Adsorption of Disperse Dye in Batch Process. Res. J. Environ. Toxicol. 2015, 9, 320–331. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agricultural Residues-Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Singh, M. Isolation and Characterization of Cellulose Nanofibrils from Wheat Straw Using Steam Explosion Coupled with High Shear Homogenization. Carbohydr. Res. 2011, 346, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ibragić, S.; Smječanin, N.; Milušić, R.; Nuhanović, M. Pomelo Peel and Sugar Beet Pulp as Novel Biosorbents in Purification of Biodiesel. Biofuels 2021, 13, 755–762. [Google Scholar] [CrossRef]
- Sidi-Yacoub, B.; Oudghiri, F.; Belkadi, M.; Rodríguez-Barroso, R. Characterization of Lignocellulosic Components in Exhausted Sugar Beet Pulp Waste by TG/FTIR Analysis. J. Therm. Anal. Calorim. 2019, 138, 1801–1809. [Google Scholar] [CrossRef]
- Cherian, B.M.; Pothan, L.A.; Nguyen-Chung, T.; Mennig, G.; Kottaisamy, M.; Thomas, S. A Novel Method for the Synthesis of Cellulose Nanofibril Whiskers from Banana Fibers and Characterization. J. Agric. Food Chem. 2008, 56, 5617–5627. [Google Scholar] [CrossRef]
- Abou-Elseoud, W.S.; Hassan, E.A.; Hassan, M.L. Extraction of Pectin from Sugar Beet Pulp by Enzymatic and Ultrasound-Assisted Treatments. Carbohydr. Polym. Technol. Appl. 2021, 2, 100042. [Google Scholar] [CrossRef]
- Yue, Z.B.; Li, Q.; Li, C.C.; Chen, T.H.; Wang, J. Component Analysis and Heavy Metal Adsorption Ability of Extracellular Polymeric Substances (EPS) from Sulfate Reducing Bacteria. Bioresour. Technol. 2015, 194, 399–402. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Liu, Y.; Li, D.; Wang, L.J.; Adhikari, B.; Chen, X.D. Microwave-Driven Sugar Beet Pulp Liquefaction in Polyhydric Alcohols. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Proniewicz, L.M.; Paluszkiewicz, C.; Wesełucha-Birczyńska, A.; Majcherczyk, H.; Barański, A.; Konieczna, A. FT-IR and FT-Raman Study of Hydrothermally Degradated Cellulose. J. Mol. Struct. 2001, 596, 163–169. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.J.; Li, D.; Cheng, Y.L.; Adhikari, B. Preparation and Characterization of Cellulose Nanofibers from De-Pectinated Sugar Beet Pulp. Carbohydr. Polym. 2014, 102, 136–143. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Staroń, A.; Staroń, P.; Chmielowiec-Korzeniowska, A.; Drabik, A.; Tymczyna, L.; Banach, M. Preparation and of PVA-Based Compositions with Embedded Silver, Copper and Zinc Oxide Nanoparticles and Assessment of Their Antibacterial Properties. J. Nanobiotechnol. 2020, 18, 148. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).