Abstract
The current study aimed to investigate the viability and characteristics of Scenedesmus sp. as an adsorbent system to remove lead (Pb) and cadmium (Cd) through an in vitro exposure to a metal solution. In batch sorption experiments, the effects of pH, contact time, initial concentration of metal ions, and sorbent dosage on the adsorption process were trialed. The ideal biosorption conditions for each of the two metals were recorded. The biosorption process was quick, and the equilibrium times for the above-mentioned metals were recorded as 90 and 60 min, with removal percentages of 85% and 83%, respectively. The point zero charge of algal biomass was 4.5, which indicates a negative charge on the surface of the biosorbent. The model-based assessment of the biosorption process was revealed to have followed pseudo-second-order kinetics. The adsorption isotherms for lead and cadmium achieved a best fit with the Langmuir model, with monolayer biosorption capacities of 102 and 128 mg g−1, respectively. The desorption of both metals achieved more than 70% by using HCl. The FT-IR revealed the presence of hydroxyl and amine groups on the surface of the adsorbent that are involved in the biosorption process, and morphological changes were assessed by SEM. Hence, Scenedesmus sp. from a Himalayan provenance showed considerable promise as an alternate sorbent for the treatment of heavy-metal-contaminated wastewater.
1. Introduction
For humanity, the greatest challenges are the remediation and management of heavy metals in the environment. Due to their toxicity and persistence in aquatic environments, heavy metal ions are of great interest. Heavy metals (HMs) are present in the natural ecosystem due to increased industrial and anthropogenic activities [1]. These contaminants cause ecological diversity loss as well as an increase in toxins in the grazing food chain [2]. The term “heavy metals” refers to substances that have a high atomic weight and a higher density than that of water [3,4,5]. These heavy metal ions are very mobile in water and are thought to be harmful even in small amounts. Consequently, it is necessary to eliminate these heavy metal ions from the aquatic system [6]. According to the WHO, the metals that are receiving the most attention are arsenic, chromium, cadmium, aluminum, copper, lead, cobalt, mercury, iron, zinc, and nickel. Lead and cadmium were chosen for biosorption investigations because of their extensive commercial use. Lead and cadmium, which are carcinogens, enter the ecosystems through industrial effluents. Numerous researchers have become interested in the removal and control of these metals from wastewater [7,8,9,10]. The maximum limits of lead and cadmium suggested by the WHO are 0.05 and 0.01 [11]. HMs are hazardous to plants, animals, and the overall health of the ecosystem [12]. Plants absorb HMs, notably through their roots, and they enter the food chain. High lead concentrations in soils can reduce production by causing plants to grow dark green leaves, less foliage, drooping and elderly leaves, and dwarf brown roots [13,14]. Excess chromium, cadmium, lead, and manganese intake can result in increased transpiration [15]. HMs increase the number of reactive oxygen species [16] in the environment, which can affect fish and other aquatic life [17]. Humans are usually exposed to HMs as a result of the consumption of foods cultivated in polluted soil, which causes serious problems [12], as shown in Figure 1.
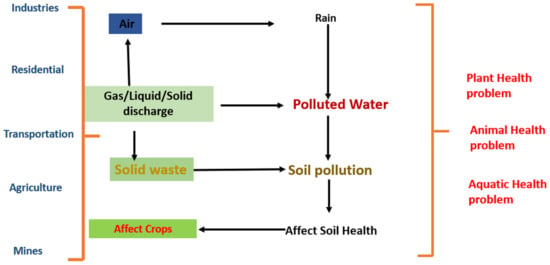
Figure 1.
Sources and effects of heavy metals.
Membrane filtration, adsorption, chemical precipitation, reverse osmosis, ion exchange, electro-dialysis, solvent extraction, chemical oxidation/reduction, and other traditional physicochemical procedures have been used to eliminate heavy metal pollutants from contaminated water. These methods have limitations, such as being less efficient at low heavy metal concentrations, producing toxic sludge, and having high capital investment and operating costs [18,19]. Recently, microalgae have emerged as a good choice among microorganisms for the removal of heavy metals from wastewater [20]. Microalgae are small algae that require light for their growth [21]. They are capable of capturing CO2 gas and converting it into high-value byproducts such as lipids, vitamins, and carbohydrates [22]. However, the production of microalgae is not economically possible due to photosynthesis and high growth and harvesting expenses [23]. When compared to agricultural crops and land plants, microalgae exhibit faster growth rates and higher biomass productivity [24]. Microalgae are known for their excellent capabilities regarding the biosorption process [25]. They have minerals and biochemical compositions, especially functional groups, which are suitable for the adsorption process [1]. Green algae (Scenedesmus spp.) have a wide distribution in all aquatic environments, and they are easily collected and cultured [26].
The use of algal biomass for the biosorption of heavy metals is interesting due to its low cost, ease of recovery and reuse, large contact area, and high efficiency in short contact durations [27]. According to the previous literature [28], biosorption is a passive process that allows for the removal of organic contaminants by microalgae. The process of biosorption employing microalgae is said to comprise adsorption, complexation, surface precipitation, ion exchange, and electrostatic interaction [29]. Therefore, the efficacy of adsorbing contaminants is substantially impacted by the binding moieties on the surface [30]. Algal biomass can be employed as an adsorbent to treat wastes due to its surface characteristics and retention capacities [29]. The biosorption of heavy metals from wastewater may be aided by carbohydrates, intercellular gaps, and sulfated polysaccharides present on the adsorbent cell wall [31]. Additionally, several binding sites, including COO−, SO42−, and NH2− functional groups, are universally present on the surface of algae [1]. Scenedesmus sp. and Synechocystis sp. were employed for the adsorption processes to eliminate diclofenac from water, and it was noted that the biomass of green algae (Scenedesmus sp.) can eliminate 28 mg/g of diclofenac, and the biomass of blue-green algae (Synechocystis) can eliminate 20 mg/g [32]. Additionally, E. intestinalis, C. glomerata, and M. amoena have been shown by [33] to have good adsorption capacity to eliminate chromium from an aqueous medium. C. glomerata exhibits maximal adsorption capacity and removes 66.6% of chromium by using amorphous algal cells [33].
There have been numerous prior studies that have used microalgal species such as Stigonema sp., Aphanothece halophytica, Spirulina, Chlorella Vulgaris, Chlamydomonas reinhardtii, Neocholoris oleoabundans, Chlorococcum sp., Chroococcus paris, Scenedesmus sp., and Phormidium sp. as biosorbents [34,35,36]. These species have maximum adsorption capacities (qmax) exceeding 50 mg g−1 against cadmium, lead, zinc, copper, and mercury. These findings show that microalgae are an effective alternative for removing these contaminants when compared to other biosorption materials [37,38].
No research study has been conducted so far on the biosorption of lead and cadmium on Scenedesmus sp. collected from Poonch Azad Kashmir. In this work, Scenedesmus sp. was first used for heavy metal removal as a biosorbent. sp. Genus Scenedesmus sp. H9 shows a promising characteristic, and it is not a hazardous microorganism for humans and the environment. The Genus of Scenedesmus sp. does not cause toxicity. The reason behind using only these metals as the criterion for search is that both elements have been mentioned in the literature owing to the high potential risk caused to public health and the environment. Hence, as a novelty, Scenedesmus sp. was used for the first time as a biosorbent for the removal of lead and cadmium from an aqueous medium.
The objective of this study was to investigate the usage of the amorphous biomass of Scenedesmus sp. as a sorbent for removing lead and cadmium from aqueous solutions. Furthermore, the impacts of several parameters such as solution pH, contact time, initial concentration of metal ions, and adsorbent dosage on the process of biosorption were investigated. The experimental data were also validated by using different kinetics and isotherms, and the desorption of a metal-loaded biosorbent was also studied. The FT-IR and SEM analyses were carried out to understand the mechanism of adsorption of heavy metals.
2. Materials and Methods
2.1. Preparation of Adsorbent
The microalgal sp. were collected from a stream in Hajira Poonch Azad Kashmir. Samples (H9) were brought into the Plant and Algal Genetic Laboratory of Quaid-a-Azam University, Islamabad. With a slight modification, the method of [39] was followed for the preparation of biosorbent. After being streaked, the samples were placed onto agar plates with Bold Basal medium and cultured for 15 days at 25 °C under continuous illumination from white LED lights with a light intensity of 1500 lux. Colonies were picked when they emerged and then re-inoculated in sterile agar plates to produce axenic cultures. The morphological and molecular analysis of isolated species revealed that it was a Scenedesmus sp. member. The axenic inoculum was cultured for three days at 25 °C under steady illumination of 1500 lux of white light in 200 mL of modified Bold Basal Medium (BBM).
During the exponential phase of growth, the algal strain (Scenedesmus sp.) biomass was harvested, centrifuged twice at 5000 rpm for 10 min to get cell pellet, and then washed with sterile deionized water, dried, powdered, sieved, and kept at 4 °C for later use [40].
2.2. Preparation of Adsorbate
Metal stock solutions were prepared by dissolving known amounts of metals of Pb (NO3)2 and CdCl2 and (1000 mg L−1) in distilled water. Stock solutions were diluted to make the needed concentrations for the experiments. The pH of both solutions was adjusted between 2.0 to 8.0 by using HCl and NaOH (827 Metrohm benchtop pH meter, New York, NY, USA) [26].
2.3. Point of Zero Charge
Point of zero charge (PZC) of algal biosorbent was determined by the salt addition method with slight modifications [41]. NaNO3 solutions (0.1 M) were collected in different flasks. HCl and NaOH (0.1 M) solutions were used to achieve different levels of pH (2–10). A total of 1 g of algal biosorbent was added into each flask and placed on a shaker at 150 rpm for 24 h. The contents were filtered, and the pH of each filtrate was recorded. The PZC value of algal biosorbent was calculated by plotting the graph of initial pH versus pH change.
2.4. Adsorption Experiment
Biosorption tests were conducted to study the effects of pH, contact time, initial concentrations of known amounts of metal ions, and biosorbent dosage in the synthetic solutions. The ideal pH was investigated using 100 mg L−1 lead and cadmium concentrations with a 60-min contact duration. The bioremediation of lead and cadmium of Scenedesmus sp. was performed with various pH ranges from 2 to 8 at metal concentration of 100 mg L−1 and contact time of 60 min. The effect of contact time was investigated by taking samples at predetermined intervals of time (5, 10, 15, 20, 30, 60, 90, and 120 min), 100 mg L−1 of metal concentration, pH 6 for lead, 7 for cadmium, and 1 g L−1 of adsorbent dosage. After adjusting the medium’s pH and contact time, tests were run with various initial concentrations of metal ions while maintaining optimized pH and contact time. This experiment was conducted at 23 °C, and 1 g L−1 of amorphous algal biosorbent was combined with a metal ion solution using the concentration of 20 to 120 mg L−1 at optimum pH for lead and cadmium. Another experiment was conducted at pH 6 and 7 for lead and cadmium and the optimized contact time at 23 °C to determine the effect of biosorbent dose that is in the range of 0.5–2 g L−1. The studies were investigated in 250 mL flasks that were filled with 100 mL of a metal solution (1000 mg L−1 of metal ions), and samples were mixed in a shaker at 150 rpm. Filtered metal solutions that had been combined with sorbent were then used to measure the concentration of metals in the solutions using atomic absorption spectroscopy (Agilent Technologies, MC187906, Malaysia) with a detection limit of 0.1–30 mg L−1 for lead and 0.02–3 mg L−1 for cadmium. The experiment was performed in triplicate. To determine the effectiveness of metal biosorption, the amount of sorbate adsorbed on the algal biomass (biosorbent) was estimated as follows (in mg g−1 and removal percentage) [11,26].
Here, qe in Equation (1) quantifies the adsorbed ions (sorbate) in milligrams per gram in the biosorbent, where the metal concentrations of Ci (initial) and Ce (final) were measured by atomic absorption spectrometry; V stands for the volume of the synthetic medium, while M stands for the biomass of the biosorbent (g).
2.5. Kinetic Study
Several empirical formulas were used to study the data. To analyze the dynamics of heavy metal removal biosorption processes and assess its efficiency in rate-controlling activities, pseudo-first-order [42], pseudo-second-order [43], and intra-particle diffusion models [44] were used. The studies were performed under experimental conditions of Ci = 100 mg L−1, pH 6 and 7 for lead and cadmium, sorbent dose = 1 g L−1, and various contact times (5, 10, 15, 20, 30, 60, 90, 120 min). Equations (3)–(5) are written as follows:
where k1 and K2 represent the pseudo-first- and second-order rate constants (mg g−1). The plot between log (qe − qt) and time revealed a straight line, and the slope’s value, − is used to derive the k1 value presented in Figure 7a. As demonstrated in Figure 7b, the graph of t/qt vs. time shows a linear trend. In Equation (3), ki denotes rate constant of the intra-particle diffusion as mg g−1 min−1. As illustrated in Figure 7c, the graph between (qt) against time (t0.5) must be linear and is deemed a rate-limiting step when the line crosses through the origin. The acquired constants and qe values from the graphs were considered and are shown in Table 1.

Table 1.
A comparison among biosorption rate constants of adsorption kinetics models.
2.6. Equilibrium Isotherms
Adsorption isotherms model is employed to analyze and distinguish the biosorptive behavior of various biosorbents. The equilibrium isotherms studies were carried out under experimental conditions of Ci = 100 mg L−1, pH 6 and 7 for lead and cadmium, biosorbent dose = 1 g L−1, contact times 60 min with different metal concentrations (20, 40, 60, 80, 100, 120 mg L−1). The biosorption data was investigated using the Langmuir, Freundlich, and Temkin adsorption isotherms. The Langmuir isotherm suggests adsorbate monolayer adsorption on adsorbent surfaces [45]. The Langmuir adsorption isotherm [46] is specified by Equation (6).
qe is the quantity of biosorbent adsorbed at equilibrium (mg g−1) onto the biomass of biosorbent), qmax is a constant that relates to the maximum amount of biosorbent that can be adsorbed by the biomass of algae forming a monolayer (mg g−1), b is the Langmuir constant (L/mg) for binding adsorbate on the sorbent sites, and Ce is equilibrium biosorbent concentration in medium following the biosorption process (mg L−1).
Freundlich isotherm is used to assess the biosorption processes that take place on heterogeneous surfaces. The surface heterogeneity, active sites, and their energies were defined using this isotherm model [47]. The Freundlich adsorption isotherm [46] can be presented as:
where qe is the quantity of sorbate adsorbed on the sorbent, KF denotes the Freundlich isotherm constant that measures the capacity of biosorption, concentration of sorbate (mg L−1) at equilibrium is Ce, and n is a constant that links the effectiveness and energy of adsorption. The biosorption capability of the biosorbent that is binding to the sorbate raises the value of KF. The value of 1/n is an important ratio for determining the suitability of adsorption.
Temkin isotherm states that increasing surface coverage reduces the adsorption heat for all molecules in the layer. This model is only applicable to moderate concentration ranges. The equation of this isotherm [48] is as follows:
2.7. Desorption and Regeneration Studies
The reusability of the adsorbent was assessed by the given methodology to efficiently desorb the metal ions and regenerate the biomass for reuse. The repetition of cycles (three cycles) was carried out utilizing the above preparations to carry out the successful experiment of adsorption and desorption. Hydrochloric acid was used to desorb metal ions (0.1 M). Desorption experiments were performed by mixing metal-loaded adsorbent with 50 mL of desorption medium (0.1 M HCl) and agitating at 150 rpm [49]. Each cycle includes primarily the sorption and then the desorption, followed by regeneration. The equations for the efficiency of desorption and regeneration of biosorbents are described as follows [50].
2.8. Characterization of Adsorbents
Treated and untreated biomass of Scenedesmus sp. were examined with a PerkinElmer Spectrum 65 FTIR within the wave number 4000–500 cm−1 under ambient conditions. The evaluation involved three samples (H9 control, H9 lead, H9 cadmium) of H9 species. This method was used to find the functional groups relevant to the biosorption of metal ions by the biosorbent. Scanning electron microscopy (JSM5910, JEOL Japan) was used to examine its morphology both before and after being exposed to lead and cadmium.
3. Results and Discussion
3.1. Effect of pH
In the biosorption process, pH is an essential factor. It affects the solubility of metals and the degree of dissociation of functional groups on the surface of sorbents [51]. The impact of pH on the adsorption of lead and cadmium onto dry biomass of Scenedesmus sp. was studied at pH 2–8, and the results are depicted in Figure 2.
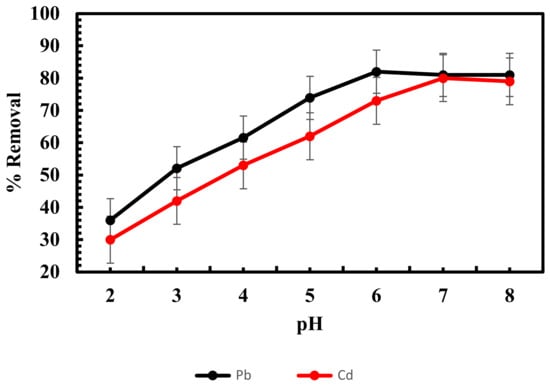
Figure 2.
Influence of pH on adsorption of lead and cadmium by Scenedesmus sp.
The biosorption capacities of lead and cadmium were low at the initial pH but significantly increased when the pH of the solution increased to 6 for lead and 7 for cadmium with removal percentages of 82 and 80. With further increases in pH, the biosorption capacity remained nearly unchanged. The following tests were run at an initial pH of 6 (lead) and 7 (cadmium). Metal ions and protons were clearly in competition for sorption sites at pH 2; this prevents lead and cadmium from readily attaching to the sites, so the biosorption of lead and cadmium is reduced at low pH [25,52,53]. The increased biosorption of lead and cadmium by the algal biomass above pH 5 could be explained by the fact that at a higher solution pH, more negative charges become accessible. As a result, the attraction of negatively charged cell walls to metal ions would increase [54]. The adsorption of heavy metal ions using various types of biomasses has been studied, and similar outcomes have been observed [55,56].
The charge on the sorbent surface is important in determining the interaction between adsorbent and adsorbate; therefore, there is a need to know the surface charge of the adsorbent while studying the sorption process. The positive charge on the surface of the sorbent is indicated by pH < pHPZC, whereas the negative surface is shown by pH > pHPZC [57]. In the current study, the pHPZC of algal biosorbent is 4.5 (Figure 3). Based on the pHPZC values of the sorbents, the optimum pH values of the adsorbent and adsorbate were 6.0 and 7.0 for lead and cadmium. When the solution pH is above pHPZC, the adsorbent will be negatively charged and bind more positive charges due to the electrostatic force of interaction [58].
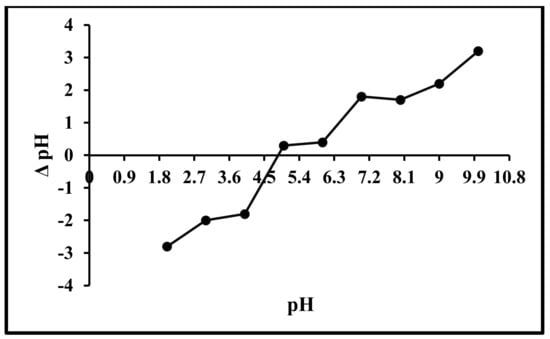
Figure 3.
Point of zero charge curve of Scenedesmus sp.
3.2. Effect of Contact Time
The biosorption process is greatly influenced by the effect of contact time. Figure 4 demonstrates that the effect of contact time affected the sorption of metal ions. These findings showed that the biosorption of both metals was quick in the first 20 min, then gradually increased until equilibrium was reached for lead and cadmium, respectively, at 90 and 60 min, then became practically constant. As a result, contact times of 90 and 60 min were chosen as the best times for lead and cadmium, respectively.
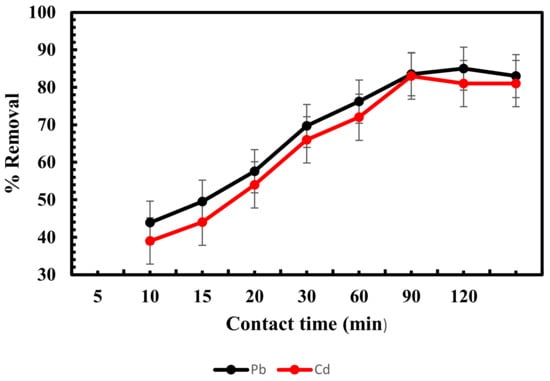
Figure 4.
Influence of contact time on adsorption of lead and cadmium by Scenedesmus sp.
Similar behavior for the biosorption of lead and cadmium on freshwater algal biomass was described by [40]. All the adsorbent surface sites were initially empty, and numerous functional groups found on the adsorbent’s surface could be used for the binding and biosorption of metal ions [59]. Since there was a higher concentration of lead and cadmium in the solution than those that were attached to the surface, there was a significant likelihood that the free metal ions would be absorbed by sorbents. The biosorption process got slower until it achieved equilibrium as the saturation of binding sites on the adsorbent increased and the lead and cadmium contents in the solution gradually declined [60,61]. After 90 and 60 min, the removal percentages of lead and cadmium achieved their maximum values, and these values showed that a contact time of 90 min for lead and 60 min for cadmium was sufficient for subsequent experiments.
3.3. Effect of Initial Metal Concentration
Figure 3 shows the adsorption process of lead and cadmium of Scenedesmus biomass at varying initial cadmium and lead concentrations. Batch experiments at pH 6 and 7 were used to study the biosorption of lead and cadmium ions at different initial metal concentrations. However, the effective adsorption of lead and cadmium by dry biomass of Scenedesmus sp. increases with increasing concentrations of lead and cadmium with adsorption capacities of 83.2 and 78 mg g−1 and then attains equilibrium, as seen in Figure 5.
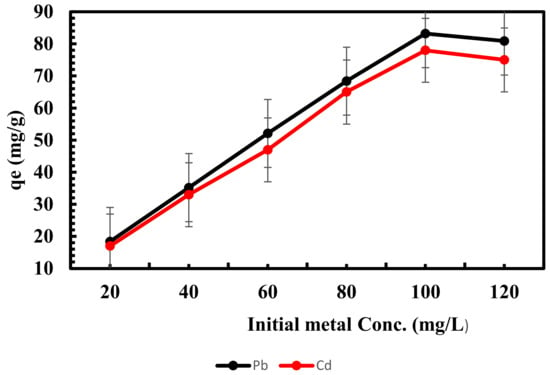
Figure 5.
Influence of initial metal conc. on adsorption of lead and cadmium by Scenedesmus sp.
The initial high concentrations of heavy metals developed a strong force that overcame all mass transfer resistances from the liquid to solid phases and enhanced the frequency of collisions between sorbate and the sorbent surface, which is the primary cause of this adsorption behavior [62]. The adsorption capacities of lead and cadmium increased as the initial concentration of metal ions increased (up to 100 mg/L) and then decreased slightly because the decrease in the number of effective adsorption sites on the surface of the adsorbent [63,64] found a similar outcome for the elimination of cadmium by biomass from Oscillatoria sp.
3.4. Effect of Biomass Concentration
The amount of sorbent used in the experiments is a crucial factor in determining its ability to remove lead and cadmium at specific metal concentrations. The findings, as shown in Figure 6, suggest that an increase in biomass increases the lead and cadmium adsorption capacity of Scenedesmus sp. Upon further increasing the adsorbent dosage, the biosorption capacity decreases for Scenedesmus biomass.
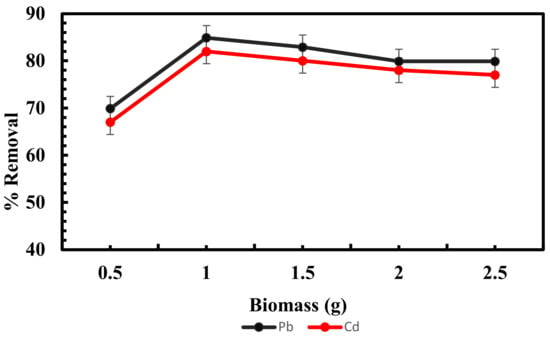
Figure 6.
Influence of biomass on adsorption of lead and cadmium by Scenedesmus sp.
With an increasing algal biomass concentration, the adsorption capacity may decrease due to the splitting effect of the concentration gradient between biosorbent and biosorbent. This results in a decrease in the number of metal ions adsorbed onto the unit weight of Scenedesmus biomass. The biosorption of dye by raw and activated date pits [65] showed a similar trend of decreasing adsorption ability with increasing biosorbent dosage.
The cause for this behavior was that as the sorbent concentration increased, the sorbent surface area also increased, enabling a greater number of binding sites for metal ions [66]. After reaching equilibrium, there was no increase in the adsorption capacity. The aggregation of the sorbent at high concentrations of algal biosorbent may be the cause of this phenomenon, which may have reduced the overall surface area and, consequently, the number of effective adsorption sites [67]. Similar findings have been reported by another study [11].
3.5. Biosorption Kinetics
The experimentally measured and computed values of qe, the correlation coefficient (R2), and the kinetic rate constants k1 and k2 are displayed in Table 1. Correlation coefficients of lead and cadmium at different contact times for different kinetics models have been calculated and compared. Table 1 shows that the pseudo-second-order has higher R2 values (0.998 lead and 0.9972 cadmium) than the pseudo-first-order (0.789 for lead and 0.742 for cadmium) and intra-particle models (0.797 for lead and 0.798 for cadmium).
This demonstrates that the pseudo-second-order accurately describes the kinetics of lead and cadmium biosorption by Scenedesmus biomass [62]. Additionally, qe(cal) using the pseudo-second-order is more similar to the qe(exp) obtained by experimentation. Similar findings regarding the removal of cadmium and lead by green algae were reported by [26]. The plots of different kinetics models are shown in Figure 7.
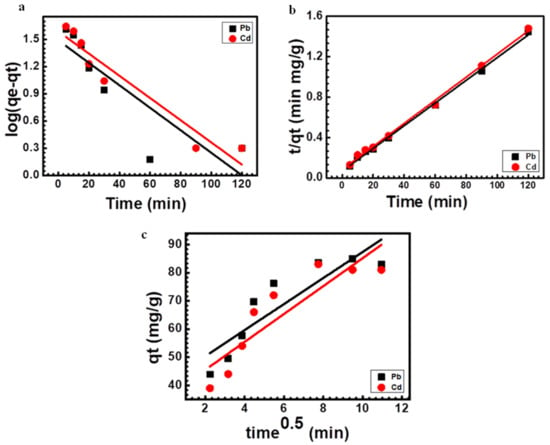
Figure 7.
Kinetics plots for lead and cadmium sorption onto Scenedesmus sp. (a) pseudo-first-order (b) pseudo-second-order (c) intra-particle diffusion.
3.6. Adsorption Isotherms
Table 2 contains the correlation coefficients (R2) and adsorption isotherm constants derived from the Langmuir, Freundlich, and Temkin isotherms. The results shown in Table 2 reveal that Langmuir isotherm was the best fit since it exhibited a greater regression coefficient (R2 0.990 for lead and 0.990 for cadmium) than Freundlich’s isotherm (R2 0.902 for lead and 0.917 for cadmium) and Temkin’s isotherm (R2 0.936 for lead and 0.911 for cadmium) (Khan et al., 2016). On the other hand, the highest predicted biosorption capacity (qmax) by the Langmuir model was calculated to be 102 mg g−1 for lead and 128 mg g−1 for cadmium, respectively (Table 2).

Table 2.
Parameters of isotherms for the adsorption of metals by Scenedesmus sp.
The monolayer adsorption of lead and cadmium onto Scenedesmus sp. biomass is confirmed by the best fit of data in Langmuir isotherm. Since the Langmuir equation assumes a uniform surface, the homogeneous distribution of active sites on the surface of Scenedesmus sp. may be the cause of the Langmuir isotherm’s fit to the experimental results [68,69]. The plots of various isotherms are presented in Figure 8.
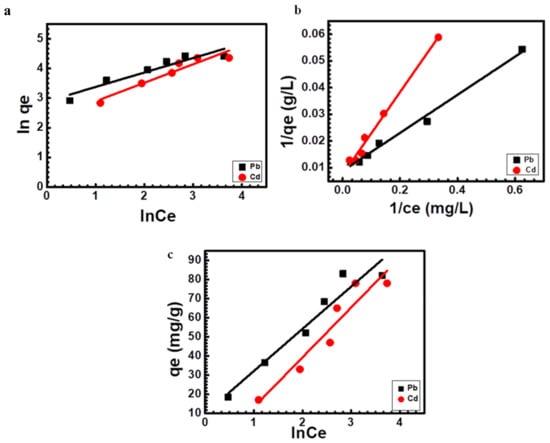
Figure 8.
Isotherms for lead and cadmium sorption onto Scenedesmus sp. (a) Freundlich (b) Langmuir (c) Temkin isotherms.
3.7. Comparison with Other Biosorbents
The adsorption potential of H9 sp. must be compared with other adsorbents, as shown in Table 3. It shows that Scenedesmus sp. H9 has good adsorption capacity when compared with other biosorbents due to differences in the properties of different sorbents.

Table 3.
A comparison of adsorption capacity between different biosorbents and Scenedesmus sp.
3.8. Desorption and Regeneration Studies
To reduce operating costs in the sorption process, the sorbent must be renewed after the sorption process. In this study, HCl is used to desorb lead and cadmium from metal-loaded green biomass. Figure 9 shows the collected results of the desorption experiment.
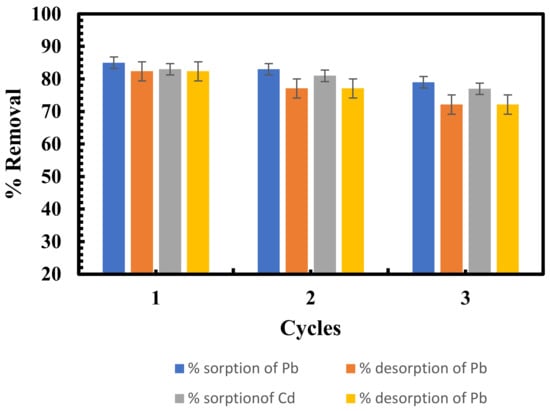
Figure 9.
Sorption-desorption efficiency of lead and cadmium by Scenedesmus sp.
The reuse of a sorbent was investigated through three cycles of studies. It was found that more than 70% of the sorbed lead and cadmium was desorbed from green biomass in the three cycles. The desorption % decreased after the first cycle as the number of cycles increased. This decrease in sorption effectiveness could be attributed to the loss of green biomass during sorption-desorption processes due to acid degradation of biomass [75]. The findings indicate that sorbent generated from Scenedesmus sp. can be effectively employed and reused for metal sorption. A strategy for the desorption and regeneration of algal biosorbents is presented in Figure 10.
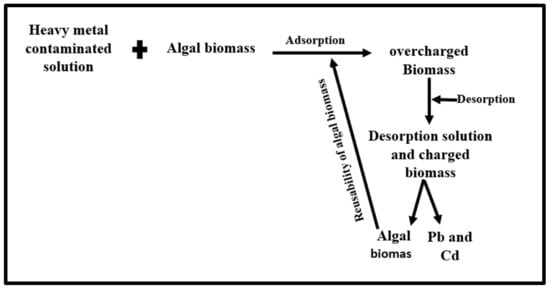
Figure 10.
Strategy for desorption and regeneration and reusability of algal sorbents.
3.9. Characterization
The biosorption process and metal ion interaction with Scenedesmus sp. were also studied. The FTIR technique was used to investigate raw and metal-loaded algal biomass in order to identify distinctive peaks associated with the complex matrix and functional groups involved in metal adsorption. The FTIR spectra of raw Scenedesmus sp. in Figure 11 revealed an absorbance peak (black) at 3184 cm−1, which corresponded to the N-H stretching vibration. The alcoholic O-H stretching vibration was attributed to the peak at 2935 cm−1 and the N-H binding vibration to the peak at 1625 cm−1. The FTIR spectra demonstrated that the surface of Scenedesmus sp. was covered with hydroxyl and amines [51].
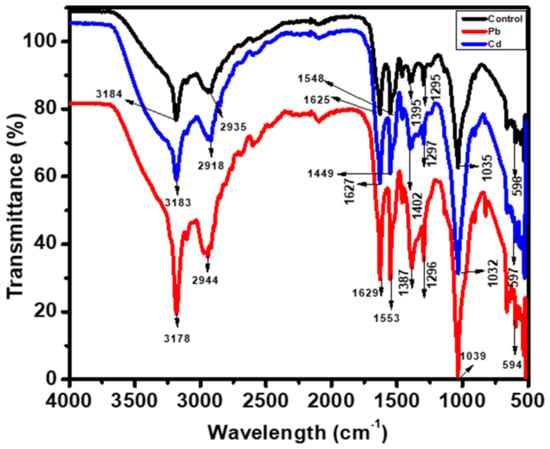
Figure 11.
FT-IR spectra of Scenedesmus sp.
The functional groups associated with metal sorption were determined by comparing the spectra of raw (black peak) and metal-loaded Scenedesmus sp. (red and blue peaks). In the metal-loaded spectrum (red and blue peaks) displayed in Figure 11, the relative intensity of the N-H bands decreased, indicating that lead and cadmium interacted with an amine. Furthermore, the O-H absorption band at 2935 cm−1 was discovered to shift at 2944 (lead) and 2918 cm−1 (cadmium). Similarly, the absorption band shifted from 1625 cm−1 to 1629 (lead) and 1627 cm−1 (cadmium). This result may be due to lead and cadmium’s preferred interaction with amine groups [76]. As a result of these findings, the hydroxyl and amine groups on the surface of Scenedesmus sp. were found to be involved in the biosorption process. Previous research using microalgae has validated these findings [63].
SEM was used to examine the morphology of Scenedesmus sp. before and after lead and cadmium adsorption (Figure 12a–c). The cells were smooth and had specific dimensions before exposure; however, after the adsorption of metal ions, they were damaged and enlarged. This could be because of the lead and cadmium ions precipitating on the cell surface and becoming attached to the functional groups [77].
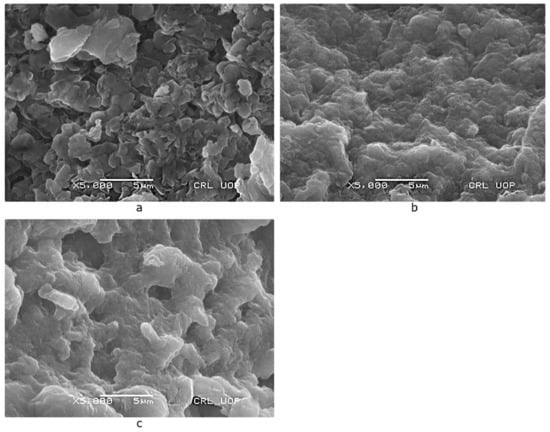
Figure 12.
SEM micrograph of Scenedesmus sp. (a) before (b) after lead (c) cadmium adsorption.
Furthermore, these alterations were most likely induced by the adsorbents being treated with a metal solution; some of the positive ions that were initially present in the cell wall matrix were replaced by metal ions, resulting in greater crosslinking. Lead and cadmium ions occupied the available free binding sites due to the ion-exchange process, as shown in Figure 13 [78].
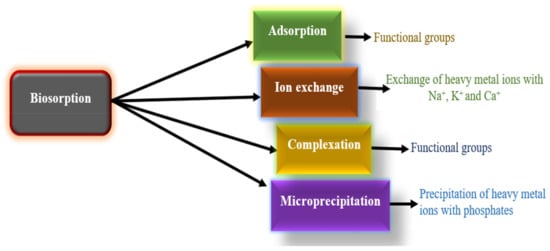
Figure 13.
Different types of biosorption mechanisms.
3.10. Implications
This work could be interpreted as a multidisciplinary approach to large-scale heavy metal remediation. Furthermore, cultivating microalgae in wastewater generates biodiesel and animal feed. When compared to other conventional strategies for environmental remediation and the production of valuable products, the employment of microalgae is an efficient and cost-effective technique.
4. Conclusions
Experimental evidence suggests that Scenedesmus sp. could be used as an efficient sorbent for the removal of metal ions. The initial pH had a substantial impact on metal uptake. Ninety and 60 min were the equilibrium times for lead and cadmium. Adsorption kinetics were described by a pseudo-second-order model at optimized conditions. Langmuir, Freundlich, and Temkin isotherm models were used to examine experimental data, and it was observed that the Langmuir model provided a better fit. Heavy metal ion removal by Scenedesmus sp. appears to be an efficient and low-cost alternative method to explore in industrial wastewater treatment. This is the first time that Poonch Azad Kashmir microalgae have been studied for their ability to adsorb heavy metals, indicating their ability to remove lead and cadmium. This understanding contributes to the development of new, inexpensive, and efficient biobased innovative solutions for the bioremediation of heavy-metal-polluted industrial wastewater.
Author Contributions
R.W. and A.S.M. generated the idea. Supervision was provided by A.S.M. Experimental facilities were provided by A.S.M. Experimental study was performed by R.W. R.W. and J.I. wrote the manuscript. M.K., L.A.M., M.H., J.I. and W.C., helped with software, manuscript editing, and revision. A.A. reviewed the manuscript and provided funds. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R350), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the raw data of this research can be obtained from the corresponding authors upon reasonable request.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2023R350), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae—A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Macro and micro algae in pollution control and biofuel production—A review. ChemBioEng Rev. 2020, 7, 18–33. [Google Scholar] [CrossRef]
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2021, 75, 701–717. [Google Scholar] [CrossRef]
- Koller, M.; Saleh, H.M. Introductory chapter: Introducing heavy metals. Heavy Met. 2018, 1, 3–11. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Naja, G.; Volesky, B. The mechanism of metal cation and anion biosorption. In Microbial Biosorption of Metals; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–58. [Google Scholar]
- Ianăşi, C.; Picioruş, M.; Nicola, R.; Ciopec, M.; Negrea, A.; Nižňanský, D.; Len, A.; Almásy, L.; Putz, A.-M. Removal of cadmium from aqueous solutions using inorganic porous nanocomposites. Korean J. Chem. Eng. 2019, 36, 688–700. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I. Silica-coated magnetic nanocomposites for pb2+ removal from aqueous solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Khanday, W.A.; Khanday, S.A.; Danish, M. Application of erionite as an adsorbent for Cd2+, Cu2+, and Pb2+ ions in water. Desalin Water Treat 2020, 205, 328–335. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Rafatullah, M.; Sulaiman, O.; Ahmad, A. Adsorption of Pb (II) ions from aqueous solutions by date bead carbon activated with ZnCl2. CLEAN–Soil Air Water 2011, 39, 392–399. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, A.K.; Sikandar, M. Study of sorption and desorption of Cd (II) from aqueous solution using isolated green algae Chlorella vulgaris. Appl. Water Sci. 2018, 8, 225. [Google Scholar] [CrossRef]
- Jordao, C.; Nascentes, C.; Cecon, P.; Fontes, R.; Pereira, J. Heavy metal availability in soil amended with composted urban solid wastes. Environ. Monit. Assess. 2006, 112, 309–326. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Khanna, K.; Arora, S.; Sharma, A.; Bhardwaj, R. Current scenario of Pb toxicity in plants: Unraveling plethora of physiological responses. In Reviews of Environmental Contamination and Toxicology Volume 249; Springer: Berlin/Heidelberg, Germany, 2020; pp. 153–197. [Google Scholar]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Módenes, A.N.; Espinoza-Quiñones, F.R.; Colombo, A.; Geraldi, C.L.; Trigueros, D.E. Inhibitory effect on the uptake and diffusion of Cd2+ onto soybean hull sorbent in Cd–Pb binary sorption systems. J. Environ. Manag. 2015, 154, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Yum, S.; Park, H.-S.; Lee, T.-K.; Ryu, J.-C. Effects of heavy metals on antioxidants and stress-responsive gene expression in Javanese medaka (Oryzias javanicus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 289–299. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Yadav, M.; Gupta, R.; Arora, G.; Yadav, P.; Srivastava, A.; Sharma, R.K. Current status of heavy metal contaminants and their removal/recovery techniques. In Contaminants in Our Water: Identification and Remediation Methods; ACS Publications: New York, NY, USA, 2020; pp. 41–64. [Google Scholar]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Khalili, A.; Najafpour, G.D.; Amini, G.; Samkhaniyani, F. Influence of nutrients and LED light intensities on biomass production of microalgae Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2015, 20, 284–290. [Google Scholar] [CrossRef]
- Jalilian, N.; Najafpour, G.; Khajouei, M. Enhanced vitamin B12 production using Chlorella vulgaris. Int. J. Eng. 2019, 32, 1–9. [Google Scholar]
- Ziolkowska, J.R. Prospective technologies, feedstocks and market innovations for ethanol and biodiesel production in the US. Biotechnol. Rep. 2014, 4, 94–98. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN–Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Mirghaffari, N.; Moeini, E.; Farhadian, O. Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J. Appl. Phycol. 2015, 27, 311–320. [Google Scholar] [CrossRef]
- Manzoor, F.; Karbassi, A.; Golzary, A. Removal of heavy metal contaminants from wastewater by using Chlorella vulgaris beijerinck: A review. Curr. Environ. Manag. 2019, 6, 174–187. [Google Scholar] [CrossRef]
- Ardal, E. Phycoremediation of Pesticides Using Microalgae. Master’s Thesis, Degree Project for MSc Thesis in Horticulture. Faculty of Landscape Architecture, Horticulture and Crop Production Science, Swedish University of Agriculture, Uppsala, Sweden, 2014. Available online: http://stud.epsilon.slu.se (accessed on 1 February 2023).
- Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M. Biosorption: An interplay between marine algae and potentially toxic elements—A review. Mar. Drugs 2018, 16, 65. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro algae Gracilaria verrucosa as a biosorbent: A study of sorption mechanisms. Algal Res. 2012, 1, 194–204. [Google Scholar] [CrossRef]
- Hammed, A.M.; Prajapati, S.K.; Simsek, S.; Simsek, H. Growth regime and environmental remediation of microalgae. Algae 2016, 31, 189–204. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Vázquez, N.C.; Noriega-Hevia, G.; Otero, M. Utilization of non-living microalgae biomass from two different strains for the adsorptive removal of diclofenac from water. Water 2018, 10, 1401. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B. Potential use of green algae as a biosorbent for hexavalent chromium removal from aqueous solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef]
- Chu, W.-L.; Phang, S.-M. Biosorption of heavy metals and dyes from industrial effluents by microalgae. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Singapore, 2019; pp. 599–634. [Google Scholar]
- Gu, S.; Lan, C.Q. Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef]
- Ranitha, M.; Nurlidia, M.; Muhammad Rashid, S.; Yoshimitsu, U. Bioremoval of lead in industrial wastewater by microalgae. J. Eng. Sci. Technol. 2016, 11, 43–49. [Google Scholar]
- Wang, G.; Zhang, S.; Yao, P.; Chen, Y.; Xu, X.; Li, T.; Gong, G. Removal of Pb (II) from aqueous solutions by Phytolacca americana L. biomass as a low cost biosorbent. Arab. J. Chem. 2018, 11, 99–110. [Google Scholar] [CrossRef]
- Mustapha, S.; Shuaib, D.; Ndamitso, M.; Etsuyankpa, M.; Sumaila, A.; Mohammed, U.; Nasirudeen, M. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb (II), Cd (II), Zn (II) and Cu (II) ions from aqueous solutions using Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 142. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Żyłkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.M.; Ammar, N.S.; Ghafar, H.H.A.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Munim, S.A.; Saddique, M.T.; Raza, Z.A.; Majeed, M.I. Preparation and physico-chemical characterization of β-cyclodextrin incorporated chitosan biosorbent beads with potential environmental applications. Mater. Res. Express 2018, 5, 065503. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Çolak, F.; Atar, N.; Yazıcıoğlu, D.; Olgun, A. Biosorption of lead from aqueous solutions by Bacillus strains possessing heavy-metal resistance. Chem. Eng. J. 2011, 173, 422–428. [Google Scholar] [CrossRef]
- Chinedu, O.J.; Charles, M.; Onyema, A.M. Equilibrium, kinetic, thermodynamic and thermal stability studies on sorption of Ni (II) ions from aqueous solution using dead biomass of fresh water green algae Cosmarium panamense. Der Chem. Sin. 2012, 3, 38–51. [Google Scholar]
- Öztürk, A. Removal of nickel from aqueous solution by the bacterium Bacillus thuringiensis. J. Hazard. Mater. 2007, 147, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Areco, M.M.; dos Santos Afonso, M. Copper, zinc, cadmium and lead biosorption by Gymnogongrus torulosus. Thermodynamics and kinetics studies. Colloids Surf. B Biointerfaces 2010, 81, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Tüzün, I.; Bayramoğlu, G.; Yalçın, E.; Başaran, G.; Celik, G.; Arıca, M.Y. Equilibrium and kinetic studies on biosorption of Hg (II), Cd (II) and Pb (II) ions onto microalgae Chlamydomonas reinhardtii. J. Environ. Manag. 2005, 77, 85–92. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.-S. Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.S.; Rodrigues, M.S.; De Carvalho, J.C.M.; Lodi, A.; Finocchio, E.; Perego, P.; Converti, A. Adsorption of Ni2+, Zn2+ and Pb2+ onto dry biomass of Arthrospira (Spirulina) platensis and Chlorella vulgaris. I. Single metal systems. Chem. Eng. J. 2011, 173, 326–333. [Google Scholar] [CrossRef]
- Yun, Y.-S.; Park, D.; Park, J.M.; Volesky, B. Biosorption of trivalent chromium on the brown seaweed biomass. Environ. Sci. Technol. 2001, 35, 4353–4358. [Google Scholar] [CrossRef]
- Kaewsarn, P. Biosorption of copper (II) from aqueous solutions by pre-treated biomass of marine algae Padina sp. Chemosphere 2002, 47, 1081–1085. [Google Scholar] [CrossRef]
- Al-Rub, F.A.; El-Naas, M.; Ashour, I.; Al-Marzouqi, M. Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process Biochem. 2006, 41, 457–464. [Google Scholar] [CrossRef]
- Molazadeh, P.; Khanjani, N.; Rahimi, M.R.; Nasiri, A. Adsorption of lead by microalgae Chaetoceros sp. and Chlorella sp. from aqueous solution. J. Community Health Res. 2015, 4, 114–127. [Google Scholar]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of methylene blue from aqueous solution with magnetite loaded multi-wall carbon nanotube: Kinetic, isotherm and mechanism analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Zafar, S.I. FTIR spectrophotometry, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. J. Hazard. Mater. 2009, 164, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Chen, C.; Chen, Q.; Ni, J. Adsorption of Pb (II) and Cd (II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method. J. Hazard. Mater. 2011, 189, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Mitrogiannis, D.; Çelekli, A.; Bozkurt, H.; Georgakakis, D.; Chrysikopoulos, C.V. Biosorption of Cu2+ and Ni2+ by Arthrospira platensis with different biochemical compositions. Chem. Eng. J. 2015, 259, 806–813. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H. Bio-sorption of cadmium and nickel ions using Spirulina platensis: Kinetic and equilibrium studies. Desalination 2011, 275, 141–147. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhu, Y.; Du, Y.; Yao, L.; Jiang, X.; Gao, P. Adsorption of Pb2+ and Cd2+ onto Spirulina platensis harvested by polyacrylamide in single and binary solution systems. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123926. [Google Scholar] [CrossRef]
- Katırcıoğlu, H.; Aslım, B.; Türker, A.R.; Atıcı, T.; Beyatlı, Y. Removal of cadmium (II) ion from aqueous system by dry biomass, immobilized live and heat-inactivated Oscillatoria sp. H1 isolated from freshwater (Mogan Lake). Bioresour. Technol. 2008, 99, 4185–4191. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Salman, M.; Dar, A.; Anwar, S. Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour. Technol. 2010, 101, 1752–1755. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Tezer, S. Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopus arrhizus in a batch system: Effect of temperature. Process Biochem. 2000, 36, 431–439. [Google Scholar] [CrossRef]
- Akar, T.; Demir, T.A.; Kiran, I.; Ozcan, A.; Ozcan, A.S.; Tunali, S. Biosorption potential of Neurospora crassa cells for decolorization of Acid Red 57 (AR57) dye. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2006, 81, 1100–1106. [Google Scholar]
- Mahamadi, C.; Nharingo, T. Competitive adsorption of Pb2+, Cd2+ and Zn2+ ions onto Eichhornia crassipes in binary and ternary systems. Bioresour. Technol. 2010, 101, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, W.; Cai, P.; Rong, X.; Feng, X.; Huang, Q. Competitive adsorption of Pb and Cd on bacteria–montmorillonite composite. Environ. Pollut. 2016, 218, 168–175. [Google Scholar] [CrossRef]
- Anayurt, R.A.; Sari, A.; Tuzen, M. Equilibrium, thermodynamic and kinetic studies on biosorption of Pb (II) and Cd (II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 2009, 151, 255–261. [Google Scholar] [CrossRef]
- Sarı, A.; Tuzen, M. Biosorption of Pb (II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T. Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res. 2003, 37, 4486–4496. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Use of alga for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef]
- Sheng, P.X.; Ting, Y.-P.; Chen, J.P.; Hong, L. Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J. Colloid Interface Sci. 2004, 275, 131–141. [Google Scholar] [CrossRef]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Moselhy, K.M.; Amer, A.; Abou El-Naga, E.H.; Mohamedein, L.I.; Al-Prol, A.E. Removal of cadmium from aqueous solution using marine green algae, Ulva lactuca. Egypt. J. Aquat. Res. 2014, 40, 235–242. [Google Scholar] [CrossRef]
- Saravanan, A.; Brindha, V.; Krishnan, S. Studies on the structural changes of the biomass Sargassum sp. on metal adsorption. J. Adv. Bioinf 2011, 2, 193–196. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).