Abstract
Large-scale hydrogen (H2) production is an essential gear in the future bioeconomy. Hydrogen production through electrocatalytic seawater splitting is a crucial technique and has gained considerable attention. The direct seawater electrolysis technique has been designed to use seawater in place of highly purified water, which is essential for electrolysis, since seawater is widely available. This paper offers a structured approach by briefly describing the chemical processes, such as competitive chloride evolution, anodic oxygen evolution, and cathodic hydrogen evolution, that govern seawater electrocatalytic reactions. In this review, advanced technologies in transition metal phosphide-based seawater electrolysis catalysts are briefly discussed, including transition metal doping with phosphorus, the nanosheet structure of phosphides, and structural engineering approaches. Application progress, catalytic process efficiency, opportunities, and problems related to transition metal phosphides are also highlighted in detail. Collectively, this review is a comprehensive summary of the topic, focusing on the challenges and opportunities.
1. Introduction
Since high-purity water is required for electrolysis and seawater is widely available for hydrogen (H2) production, extensive research has been performed to create direct seawater electrolysis technology. The quest for pure, renewable, and affordable energy has become essential to ensuring worldwide socioeconomic growth in light of the energy shortage and the need to protect the ecosystem [1,2,3,4]. Although freshwater makes up only 3.5% of the world’s water resources, freshwater electrocatalytic splitting is regarded as a safe and effective way to generate adulterated H2 [5]. Therefore, piezoelectric catalytic wastewater degradation may be achieved by utilizing the energy generated by water flow and low-frequency mechanical energy [6,7]. Several approaches for H2 production and H2 chemical materials storage have been established, but discovering an effective and secure method for H2 production is still required. Asim et al. [8] developed the solid material ammonia borane (NH3BH3) as the most promising H2 storage material. The same authors developed an amalgamation of gold nanoparticles with metal phosphides and speculated them to be effective catalysts (e.g., Au/Ni2P and Au/CoP) to improve the H2 evolution rate [9]. Moreover, in the long-term, the electrolysis of water processes that lead to H2 generation may increase the likelihood of seawater electrolysis [10]. Simultaneously, seawater electrolysis encounters obstacles from the chlorine evolving reaction (CER) and the kinetically slow evolution reaction of oxygen (OER) when the total salinity is typically 3.5 weight percent and the pH is around 8 [11].
In addition, there are highly promising opportunities for developing inexpensive, effective, and efficient transition metals and their compounds to substitute for catalysts, based on noble metals. In particular, several transition metals, including transition metal phosphides (TMPs), transition metal oxides (TMOs), transition metal dihalides (TMDs), transition metal carbides (TMCs), and transition metal nitrides (TMNs), have shown high activity and stability. TMPs are considered a good alternative to rare metals because of their high electrical conductivity, strong durability against corrosion, as well as substantial catalytic activity. Therefore, due to their distinctive physicochemical properties, TMPs are one of the most intriguing potential electrocatalysts that break through the limits of being suitable. They have been extensively employed in numerous catalytic reactions in the fields of energy transformation and catalysis, such as photocatalytic hydrogen evolution [12]. Because of the availability of natural resources and their excellent conductivity, stability, and metal atom coordination effects, TMPs have received much attention recently [13,14]. Nevertheless, the working efficiency of TMP-based hydrogen evolution reaction (HER) catalysts continues to be impaired by some challenging and unresolved issues. It is still unclear whether active dopants deliver more active sites to enhance the activity of TMPs’ occupant sites [15]. Recent research has revealed that seawater electrolysis has good characteristics and stability [16]. For instance, Wu et al. [11] and Liu et al. [16] constructed catalysts that showed outstanding activity and stability, such as (Ni2P-Fe2P). The same authors developed a CoPx@FeOOH catalyst, which was also stable for 80 hours at a high-level current of about 500 mA/cm2 in 1.0 M KOH seawater with an overpotential of 283 mV for 100 mA/cm2. Chang et al. [17] developed a Fe, P-NiSe2 NF catalyst for gas phase chemical deposition, which displayed stability over eight days with a significant current density of about 800 mA/cm2 at 1.8 V. Moreover, during unrestricted seawater circumstances, the open-circuit voltage for HER at 10 mA/cm2 was 290 mV for the CoNiP/CoxP/NF catalyst [18].
Furthermore, TMPs are also potentially useful, non-noble electrochemical catalysts for the evolution reaction of H2. Due to their excellent HER electrocatalytic efficiency, high conductivity, and durability against corrosion, TMPs have sparked great interest. TMPs are recognized as desirable HER catalyst components compared to other transition metal elements (e.g., metal sulfides) because of their abundant reserves, unique framework, variable composition, and outstanding electrical conductivity [15]. Recent studies have found that transition metal phosphides have exceptional stability and activity in seawater electrolysis. Table 1 provides a quick overview of the characteristics of various recently developed TMP-based electrocatalysts. Although extensive work has been performed, there is still vast room to develop a stable, high-potential catalyst in order to produce sustainable hydrogen from seawater in the long-term.

Table 1.
Transition metal-based electrocatalysts.
Some recently published reviews have summarized the progress in water splitting and H2 evolution reactions. The literature summarized by Shah et al. [36] discussed the structure, mechanism, and potential of transition metal tellurides (TMTs) and phosphides (TMPs) for HER. This article comprehensively reviewed the progress until 2022, with emphasis on TMTs and HERs. Ding et al. [37] summarized the potential of asymmetrically coordinated atoms for HERs. Fe, Ni, and Co are excellent materials with low cost, good availability, and improved water-splitting efficiency. The work summarized by Li et al. [38] focused on advances in the fabrication of layered double hydroxide (LDH) two-dimensional (2D) materials using these economical materials. Experts are emphasizing the in situ evaluation of various exciting parameters and the impact of the reaction environment on the water-splitting efficiency. The incorporation of simulation techniques with experimental investigation can help to improve the process efficiency and H2 yield. Although some reviews have been published focusing on water splitting and HERs, there is still no critical discussion about the application of TMPs in seawater splitting. Therefore, this review was designed to give a quick overview of current developments regarding seawater electrolysis for H2 production and the progress of HER electrocatalysts based on transition metal phosphides (TMPs). The most current achievements in TMPs utilized in seawater electrolysis are addressed, since new information has been discovered when designing highly effective and inexpensive TMP-based catalysts. The development of TMPs in seawater electrolysis is examined from many angles, including phosphorus-doped transition metals, bimetal phosphide phase structure, and TMP compounds, all of which are anticipated to advance electrolysis in the future.
2. A synopsis of TMPs
Structure: Fundamental Concepts
Phosphides are the products created when phosphorus is combined with any d- (such as nickel (Ni), molybdenum (Mo), tungsten (W), cobalt (Co), and iron (Fe)) or f-metal. TMPs are resistant metallic substances with acidic as well as metallic sites [39,40]. The metal phosphides react quickly with water and moisture in the air or stored grain to form phosphine gas. Additionally, these materials have complicated structural characteristics and special chemical, physical, and electrical properties because of the crystal lattice interactions between the metal and phosphorus. Previous research on TMP structures can be found in the literature, as reported by [41,42].
TMPs have diverse characteristics that are influenced by several important criteria, including preparation method, P source, capping agent, heating rate, and so on, in addition to their morphology and particle size. TMPs have been used in a wide variety of catalytic reactions because of the versatile nature of their structures [43]. There are numerous methods for synthesizing TMPs outlined in the literature. These methods have been grouped into four different groups: (i) P solvothermal reactions, (ii) solution-phase reactions, (iii) gas–solid reactions, and (iv) other methods [44]. As described by Bhunia et al. [45], different TMP synthesis methods have distinct benefits and drawbacks based on various comparison criteria, including electrocatalyst surface area, TMP conductivity, and other parameters, as shown in Table 2.

Table 2.
A brief review of the latest and most recent advanced methods for synthesizing transition metal phosphide-based catalysts.
Additionally, TMPs have a formula of MxPy due to P’s somewhat stronger electronegativity than metal, which inhibits electron dispersion around the metal atom while enhancing metal-to-P electron transport. The difference between the M–P electronegativity and the M:P ratio determines the properties of the metal phosphide. TMPs, on the other hand, display a mix of covalent and ionic nature bonds due to minor differences in electronegativity. This results in a little positive charge (+) for the metal and a tiny negative charge (−) for the phosphorus. As a result, this unique bond has exceptional thermal and chemical stability as well as strength [46]. On the other hand, TMPs have high activities and good stability due to their earth-abundant resources [47,48,49].
Due to the stoichiometric ratio of metal to phosphorus in their chemical formula (metal-rich phosphides such as M3P and M2P, mono-metal phosphides such as MP, and phosphorus-rich metal phosphides such as MP2 and MP3), an additional approach to classifying TMPs is to split them into three categories: binary, ternary, and supported types [50], as shown in Table 3. In the open-source literature, several different nanostructures of binary TMPs have been discovered, involving CoP in the form of nanotubes, nanoparticles, nanosheets, and nanorods [51,52]; Co2P in the form of nanoflowers, nanoparticles, and nanosheets [53,54]; Cu3P in the form of nanoarrays and nanowires [55,56]; and MoP in the form of nanoflakes and nanoparticles [57,58]. Ternary phosphides are outstanding catalysts for various chemical reactions and exhibit interesting structures. They might be present in the form of nanowires NiCo2Px [59,60], porous NiCu-P [61,62,63], and core–shell CoMoP [64]. Several studies have addressed the use of supported TMPs and the difficulties associated with their chemistry in their use. These catalysts can behave in diverse forms, for example, alumina, silica, and carbon [65,66,67].

Table 3.
Other Classifications of TMPs with their Performances.
3. Electrolysis of Seawater
Many catalysts have been investigated in seawater electrolysis up to this point [22,27,39,48].
A complete overview and in-depth knowledge of the reaction process are needed to implement industrial seawater electrolysis and achieve high-efficiency hydrogen production. Notably, the significant water electrolysis reaction is formed by two half-cell reactions, the evolution reaction of hydrogen (HER) and the evolution reaction of oxygen (OER) [71,72], and both depend on the electrolyte’s pH [73]. This means that OER refers to oxidizing water at the anode, while HER refers to reducing water at the cathode to yield H2. Additionally, water electrolysis, a thermodynamic chemical process, has an overall Gibbs free energy for hydrogen adsorption (ΔGH*) value of about 237.2 Kj mol−1 [74].
In different pH environments, water decomposes according to Equations (1)–(4).
In acidic pH:
Anode: 2H2O→O2 + 4H+ + 4e−
Cathode: 2H+ + 2e−→H2
In basic pH:
Anode: 4OH−→O2 + 2H2O + 4e−
Cathode: 2H2O + 2e−→H2 + 2OH−
3.1. Characteristics of Seawater Catalytic Reaction
Seawater is generally rich in salts compared to freshwater, which complicates the electrolytic process [10,75]. The effects of different chemical elements (anions and cations) present in seawater on water electrolysis are discussed in the following paragraphs.
3.2. Complementary Effects of Complex Ions
Due to the presence of up to 3.5 wt% salts, seawater has strong ionic conductivity [76]. In seawater, ions of magnesium, sodium, chloride, potassium, calcium, and sulfate account for >99% of the total seawater ion content [77,78]. It is also estimated that artificial seawater has a dissolved solids content totaling approx. 35,000 ppm, of which sodium chloride (NaCl) makes up about 30,000 ppm. The composition of seawater is presented in Table 4 [79,80].

Table 4.
Main components (in ppm) of seawater.
Nevertheless, to simulate real seawater, it has also reportedly been claimed that some Mg2+, Ca2+, K+, and SO42− are added. Seawater’s complex ion composition can boost its ionic conductivity, making seawater electrolysis more challenging. As the H+ is depleted, for example, the resultant OH combines with both cations (Ca2+ and Mg2+) to produce insoluble precipitates of calcium oxide and magnesium oxide, respectively. These insoluble precipitates on the electrode surface could obstruct the reaction sites [73,81,82].
3.3. Effects of Complex Ions
Chloride ions can damage both anodes and cathodes in seawater. The active cores of the cathode side’s catalysts are inhibited by chloride ions, slowing down the reaction and hastening the catalysts’ deterioration [83]. However, because the chloride ions may take part in reduction events that are harmful to OER, considerable amounts of chlorine or hypochlorite may develop on the anode side [84]. The reduction processes of Cl− at the anode side are described by Equations (5) and (6) [84,85].
In acid pH medium: 2Cl−→Cl2 + 2e−
In basic pH medium: Cl− + 2OH−→ClO− + H2O + 2e−
On the other hand, OER is more beneficial kinetically than CER, as illustrated by the Pourbaix diagram in Figure 1b [86]. The volt differential between CER and OER in the basic environment (blue area, Figure 1b) is 0.490 V in the pH range of 7.5 to 14, but it decreases under acidic conditions. In these circumstances, OER should have an overpotential in an alkaline medium significantly lower than 0.49 V in an attempt to develop O2 and prevent CER from producing hypochlorite ions (ClO−).
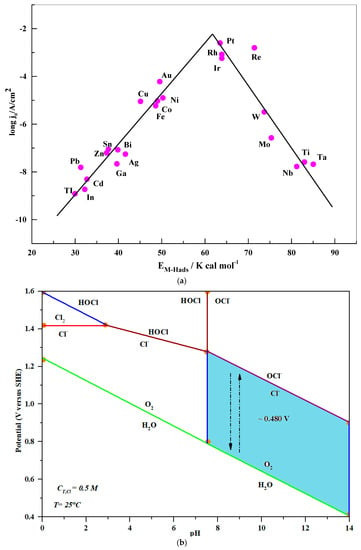
Figure 1.
(a) Volcano plot for HER [86]. (b) Pourbaix diagram of seawater simulated with 0.5 M NaCl solution, adopted with permission from Ref. [84] ©2013 RSC.
4. Seawater Challenge and Perspectives
The chemical drawbacks include high activation energies, slow ion and gas diffusion rates, and electrolytic system-related issues like the amount of dissolved solute, electrode resistance, and barriers to electrolyte transport. These factors might lead to high cell voltage or poor durability for water splitting. Researchers are currently concentrating on constructing a series of stable and highly efficient catalysts via morphology structure adjustment, heterostructure design, and synergistic effects. Furthermore, electrolyzers with excellent efficiency and low cost are going to overcome several issues in the seawater electrolysis process [87]. Moreover, the presence of various dispersed cations (e.g., Ca2+, Na+, Mg2+, etc.), microorganisms, and fine particles on the negative electrode (cathode) is the major challenge in direct HER seawater splitting. These cations have the potential to contaminate electrodes and catalysts in the electrolytic system or speed up their deterioration, which would reduce their durability [88].
Furthermore, Dresp, Dionigi, Klingenhof and Strasser [10] have discussed that seawater has a significant number of electrochemically active anions (such as Cl−) that will interact with and undermine the anodic OER. During seawater electrolysis, all of these anions may keep competing with the OER at the anode with their matching conventional redox potentials.
Bennett first recognized and highlighted this significant issue in 1980 [89]. He discovered that direct seawater electrolysis might lead to high current efficiency during the cathodic evolution of H2. However, a wide range of chloride ions are generally produced at the anode as part of a hypochlorite solution (ClO−). According to what was seen, the chemistry of chloride ions in aqueous solutions is complex, and chemical effects are possible with respect to pH, applied potential, and chloride ion concentration. Meanwhile, the connection between the exchange current densities (jo) of metal electrodes in an acidic solution and the active sites on the metal catalysts’ surfaces is being investigated, as shown in Figure 1a. According to [10,75,83,86], detailed analysis of the anodic seawater yielded a computed Pourbaix diagram that comprised the OER and chloride chemistry (Figure 1b).
Numerous studies on creating high-performance catalysts for seawater electrolysis have been conducted to address these problems. For direct seawater electrolysis, the seawater must first be pre-filtered [75]. It was noted that the problem linked to the physical obstructions caused by microbiological contamination and solid impurities, which hastens the impact on the catalysts, might be resolved through membrane filtration processes, e.g., micro-filtration or ultra-filtration. Sharif et al. [90] carried out extensive work on the developing electrode and membrane materials, which substantially contribute to bio-energy production and performance. Seawater electrolysis can be made more effective and long-lasting by filtering and pretreating natural seawater.
Many other technologies are included among electrochemical-based H2 production technologies, but due to their uniqueness, some of them cannot be directly applied to the use of seawater (e.g., simultaneous release of oxygen and chlorine at the anode and dissolved salts). Table 5 summarizes the list of technologies that can be used for seawater electrolysis, such as membrane electrolyzers, unutilized regenerative technology, battolyser technology, and anion exchange membrane (AEM). These electrochemical technologies have the potential to reduce costs and improve the efficiency of H2 production [91].

Table 5.
Emergent technologies for seawater electrolysis.
Consequently, current research on H2 production via seawater electrolysis is still in its formative stages. Instead, developing catalytic materials with high OER selectivity has remained a significant challenge in meeting the needs of advanced manufacturing of H2 production. Despite the funds and time invested in its progress, direct seawater splitting is still in its developmental stage and is a long way from market potential. Consequently, it is a challenge to make the transition to a hydrogen economy, since it requires cost reductions and improvements in hydrogen production and storage technologies [103].
5. Seawater Electrolysis with TMP-Based Catalysts
Improving the selectivity of TMPs against corrosion and oxidation is a significant design challenge. This problem might attack the catalyst, producing toxic chlorine or hypochlorite and drastically reducing efficiency. As discussed above, chloride ions on the cathode side may inhibit HER by blocking the active center of the catalyst, thereby accelerating the degradation of the cathode catalyst for the reaction. Previous investigations reported in the literature demonstrated good performance for a membrane electrode assembly (MEA) with a NiFe LDH (NiFe-layered double hydroxide) anode catalyst, AEM membrane, and Raney nickel catalyst. Xing et al. [104] prepared AEM electrolyzer cells that performed better than those previously documented by the scientific community, with over 1000 h of operation at a constant current density of 300 mA cm−2. Soren et al. [10] created a seawater electrolyzer with an anode catalyst of nanostructured NiFe-layered double hydroxide and a cathode catalyst of Pt nanoparticles.
Three different types of electrolytes are commonly used for seawater electrolysis: an alkaline solution with NaCl, an alkaline solution with seawater, and seawater. The performance of HER-type solutions was improved by the electrolyte introduced into the alkaline environment, and the reported overpotential values were also enhanced, as shown in Figure 2.
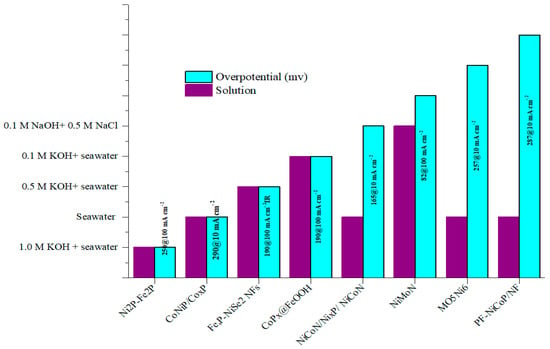
Figure 2.
HER-type solutions used and attained overpotential of typical materials reported in the literature [16,17,18,35,84,88,105,106].
5.1. Dopants
In general, element doping can be divided into metal and non-metal doping. It is worth noting that non-metal doping is an efficient strategy for optimizing the HER electrocatalytic activity of TMPs [107]. For example, non-metal doping on carbon-based materials may also improve C/TMP heterostructure performance [108]. Moreover, non-metal doping could also improve the electrocatalytic activity for HER. In this case, Niu et al. synthesized P-doped cobalt oxide nanoarrays with NF (CoRuPO/NF) [109]. Meanwhile, doping is a common but useful way to modulate the electronic structure. For instance, the foreign substances that are included in catalysts have the potential to modify their electronic structure, leading to considerable alterations in their physicochemical characteristics. It was recently noticed that incorporating another metal element into TMPs can efficiently modify the adsorption strength of hydrogen intermediates, strengthening the TMPs’ catalytic performance. In this case, elemental doping is a flexible method to increase the intrinsic activity of the catalysts [110,111].
Moreover, switching the electrocatalyst’s surface bulk and heteroatoms is an exciting option to enhance the effectiveness of seawater electrolysis. Controlling electrolyte diffusion, speeding up gas adsorption and desorption, and improving catalysis would be possible [112,113]. Numerous research teams [17,105,114,115,116] have looked into adding foreign atoms to single TMPs to address the drawbacks.
For instance, in [17], three steps including anodic processing, vapor deposition, and electrodeposition were applied for NiSe2 nanoporous film synthesis. As a result, the as-prepared nanocatalysts demonstrated impressive electrocatalytic performance and catalytic activity. In addition, Figure 3a presents the results of a simulative study of a crystal lattice for Fe, P-NiSe2 NF, and Figure 3b,c shows that the highly permeable films existed as part of Fe, P-NiSe2 NF and an FeNi alloy substrate on the surface. Consequently, in both the ABF and HAADF STEM images, NiSe2’s crystallized structure and lattice fringe were visible (Figure 3d–f). Likewise, four compounds were observed with homogeneous distributions, such as P, Ni, Fe, and Se, as presented in Figure 3g,h. Additionally, an overpotential of 120 and 180 mV was considered necessary in the basic solution (0.5 M KOH) with 90% infrared adjustment to produce current densities of 100 and 500 mA cm−2.
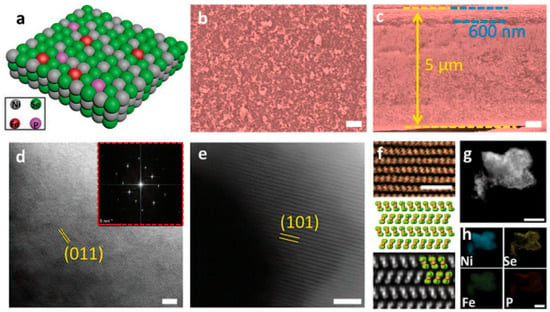
Figure 3.
(a) The dual-doped structure. (b,c) Top and cross-sectional SEM images. (d) ABF-STEM image. (e) HAADF-STEM image. (f) The simulated NiSe2. (g) STEM image. (h) EDS mapping of different elements [17].
Using four distinct feeding mechanisms, the Fe, P-NiSe2 NF catalyst exhibited excellent electrolytic efficiency in the seawater electrolyte compared to PGM-free catalysts. Moreover, the electrocatalyst exhibited about 800 mA cm−2 of current density with 1.8 V of voltage [17]. It maintained system stability for 200 h in an electrolytic cell with seawater electrolyte. During the doping process, this phenomenon clarified the role of the iron atom (Fe) in delivering primary active sites for the HER methodology. However, the selenide (Se) dissolution was retarded and the electronic conductivity was improved by doping with the phosphorus atom (P), which formed a passivation layer that preserved the P–O bonds.
Because of the powerful P–O bond environment, the bifunctionality of phosphide catalysts was attributed to the metal sites (e.g., metal atoms or metal–P groups) and P–OH groups (acidic sites) at the surface of phosphides [117]. This means that the bimetal phosphides enhance the electrocatalytic activity and long-term stability through the simultaneous influence of the different metals. Moreover, the population of OH groups has been linked to the selectivity through the reactions of hydrogenolysis and hydrogenation [118].
Compared to previous studies published in the scientific literature, adding Fe to CoP may improve the catalyst’s HER performance. For example, the authors in [119] demonstrated in a volcano diagram that the H2 adsorption energies of the intermediates exhibited a relationship with the catalytic activity. This means, after adding Fe to the CoP catalyst, the ΔG* of CoP was optimized and the ΔG* of FeCoP was closer to thermal neutrality than that of CoP. This was because Fe substituted for the Co atoms, reducing the link between the catalyst and hydrogen to the point that the free energy of hydrogen adsorption of CoP was closer to 0 eV.
Likewise, it was found that the doping amount had a great effect on catalyst performance. In this case, the electrochemical performance of the catalyst raised and then dropped as the amount of doped Fe increased. In addition to Fe, some other metal doping elements (e.g., Mn [120], Ni [121], and Al [122]) are also effective in improving catalyst performance. For example, the doping of Mn was performed by developing Mn-CoP nanosheets on Ti plates, and the catalyst exhibited a good HER activity in 1 M KOH with an overpotential of about 76 mV at 10 mA/cm2. For Ni-doped FeP/C, it is important to note that the HER overpotentials of NFP/C were significantly lower than those of undoped nanorod arrays, which could be the outcome of the charge transfer following Ni doping in Fe. However, as a way to raise the effectiveness of the catalyst, doping non-metal elements (e.g., N, B, F, etc.) has also been introduced. A detailed description of the various types of electrocatalysts used for HER has been provided, including noble metal catalysts, non-precious metal catalysts, and carbon-based electrocatalysts.
Current study evidence has shown that doping non-metals (N) into a catalyst can improve HER electrocatalytic performance [123]. Indeed, the purpose of this study was to look into the effect of non-metal element doping on the acidic HER catalytic properties of TMPs. Zhang et al. transformed rigid NiCo alloy foam into the corresponding hydroxides before doping N and P with N2/PH3 plasma [124]. The subsequently formed N-doped NiCoP encountered abundant heterointerfaces and polycrystalline phases, being advantageous to HER reaction kinetics. Another study reported by Anjum demonstrated that sulfur-doped CoP could significantly exhibit better electrocatalytic performance for HER than CoP and NiFeP [107]. Meanwhile, the incorporation of a strongly electronegative fluorine (F) atom may have resulted in significant modifications to the electronic structure, which enhanced the productivity of electrocatalytic HER. In this case, with smaller overpotentials of 99 and 106 mV at a current density of 10 mA cm−2, the developed F-CoP/Ni2P/NF catalyst demonstrated excellent HER activity in both alkaline and acidic electrolytes and possessed good durability [125]. Finally, non-metal element doping may successfully preserve the host catalyst’s highly conductive metal features while additionally functioning in conjunction with metal dopants to speed up charge transfer [126]. Non-metal doping can also modify the outside of the crystal arrangement and create further active sites, which enhances HER catalytic processes and H2 adsorption [127]. The non-metals’ smaller atomic weights and heterogeneous doping may call for a challenging and precise description of the dopant framework. This may present further difficulties when figuring out the HER mechanisms and the specific impact of non-metal dopants.
Herein, oxides, nitrides, phosphides, borides, and hybrid catalysts (Table 6) are successful examples that have been used as OER catalysts. Instead, challenging the oxidation of chloride ions in seawater not only minimizes OER effectiveness but also creates chlorine-containing species that affect the electrolyzer and lead to environmental issues [128,129].

Table 6.
Main highlights of the OER performance of the electrocatalysts that have been reported.
Likewise, for HER electrocatalysts, previous research has discovered few competitive reactions to HER in seawater electrolytes, as opposed to those in the anode compartment. At this point, the most commonly reported electrocatalysts for HER in-seawater electrolysis are noble metal alloys, carbon-supported noble metals, MXene-based complexes, metal phosphides, metal oxides, metal hydroxides, metal nitrides, and hybrid electrocatalysts, as shown in Table 7.

Table 7.
Main highlights of the HER performance of the electrocatalysts that have been reported.
5.2. Structure of Bimetal Phosphide Phases
Previous studies showed the advancement of an in situ nickel foam (NF) nickel–exchange phosphorylation electrocatalyst. It can change the local distribution of electrons for the central metal, making it easier to create low-cost, high-performance seawater electrolytic catalysts. For instance, the authors in [11] used phosphatization and acidification to create an Ni2P-Fe2P/NF electrocatalyst with a 2D nanosheet structure. Similarly, the results in [39] demonstrated that the Ni2P-Fe2P/NF catalyst with a two-dimensional nanosheet structure, prepared using acidification and phosphating methods, exhibited excellent intrinsic activity (Figure 4a).
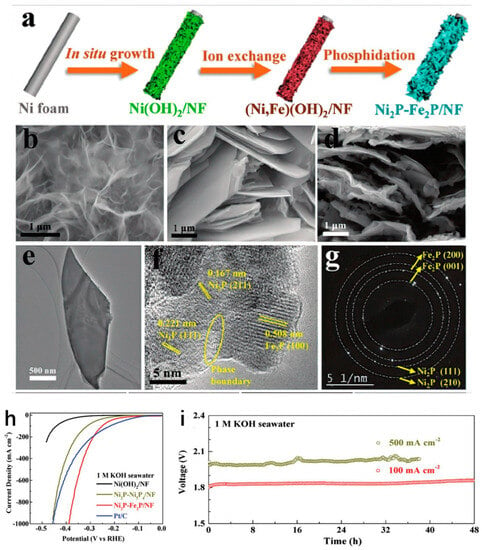
Figure 4.
(a) Schematic illustration of Ni2P-Fe2P/NF. (b–d) SEM images of Ni (OH)2/NF, Ni2P-Ni5P4/NF, and Ni2P-Fe2P/NF, respectively. (e) TEM image of Ni2P-Fe2P/ NF. (f) HRTEM image of Ni2P-Fe2P/NF. (g) SEAD pattern of Ni2P-Fe2P/NF. (h) LSV in 1.0 M KOH seawater. (i) Stability was tested at 0 and 500 mA cm−2 in 1.0 M KOH seawater [16].
In this case, the Ni-based precursors’ ultra-thin two-dimensional structures were significantly linked with the morphological outcome of the Ni2P-Fe2P/NF catalyst, as seen in Figure 4b–d. In this study, the framework of the bimetallic phosphide crystal that established the heterostructure between the Fe2P and Ni2P phases is presented in Figure 4e–g. Furthermore, in 1.0 M KOH of seawater controlled for 48 h, the catalyst demanded overpotentials of 252/261 mV and 389/337 mV for HER/OER, as depicted in Figure 4h,i. However, significant progress has been made on cobalt-doped Fe2P (Co-Fe2P), which was assessed in a simulated seawater electrolyte with two molarity solutions of 0.5 M NaCl and 1.0 M KOH [150]. Instead, the same group demonstrated overpotential values of about 460 mV at 100 mA cm−2 and strong electrochemical durability up to 22 h operation. Moreover, through the aid of a self-supported heterogeneous bimetallic phosphide array electrode (Ni2P-FeP), successful hydrogen evolution from seawater splitting could be accomplished due to chloride corrosion [151]. Another self-supported bimetallic phosphide heterostructure electrode (C@CoP-FeP/FF) with a strong connection to an exceptionally thin carbon layer was developed that enabled overall simulated seawater splitting [152]. Other substantial advancements have been made on Ce-doped ordered mesoporous cobalt ferrite phosphide (CoP/Fe2P) as robust catalysts for water oxidation, which revealed a highly active electrocatalyst for OER [153]. There was also a structural engineering strategy involving Fe-doped Ni2P nanosheet arrays supported on Ni foam that was designed to enhance the electrocatalytic performance for both OER and HER [154]. The performance of the electrocatalyst was enhanced because there were extra active sites available. It was shown that increasing the specific surface area of the electrocatalyst improved the performance. This boosted not only particular surface area scattering but also the entire interaction between both the electrolyte and active sites. As a result, Ni-Fe effectively contributed to increased stability and corrosion resistance, which was advantageous for seawater electrolysis.
5.3. Compounds of TMPs
It has been acknowledged that heterostructured electrocatalysts are an intriguing place to start when trying to boost activity by raising the concentration of active sites and the mass transfer rate. In [144], the authors used chemical vapor deposition and electrochemical deposition to create CoNiP/CoxP/NF electrocatalysts. Figure 5 illustrates the first step in synthesizing CoNiP/CoxP/NF, using NF as a substrate with a 3D structure, and high conductivity was obtained. Then, CoNiP/CoxP/NF was synthesized by chemical vapor deposition, followed by the formation of CoNi alloy/NF through electrodeposition. Instead, it was demonstrated that the open-circuit voltage of the CoNiP/CoxP/NF electrocatalyst was around 290 mV for HER and indicated stability for 500 h in seawater (Figure 5). Because of the high concentration of exposed active sites and excellent corrosion resistance in this structure, the electrolytic performance was better and became more stable over time. DFT simulations revealed that CoNiP had an appropriate thermodynamic activity for the desorption and adsorption of H2 [155].
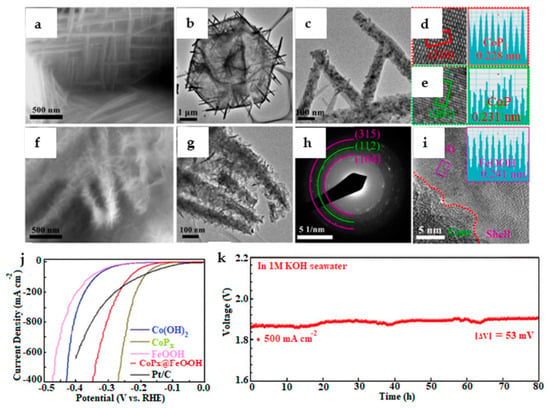
Figure 5.
(a,f) SEM image of CoPx and CoPx@FeOOH. (b,c,g) TEM images of CoPx and CoPx@FeOOH. (d,e,i) HRTEM images of CoPx and CoPx@FeOOH. (h) SAED pattern. (j) HER polarization curves of different catalysts. (k) Chronopotentiometric curve recorded over CoPx||CoPx@FeOOH in 1.0 M KOH seawater electrolyte [46].
Further, the authors in [18] focused on the preparation of a catalyst with a core–shell structure manufactured using a three-step process of hydrothermal-phosphatization-electrodeposition. It is widely known that CoPx precursors present smooth straight NWs and diverse CoP2 distributions, as shown in Figure 5a–e. According to the SAED and HR-TEM images shown in Figure 5f–i, the core–shell structure remained with CoPx as the core and FeOOH as the shell. Furthermore, with current densities of 100 and 500 microamps, the catalyst had a lower overpotential and long-lasting durability over 80 h in seawater containing 1 M KOH (Figure 5j,k). In this study, the catalyst was active and stable due to the corrosion-resistant layer of its basic structure, demonstrating that structural design is critical for catalyst performance.
6. Conclusions and Outlook
Seawater is a resource that will never run out, so producing hydrogen via electrolysis may solve the world’s energy issues. However, due to the cations and anions, seawater electrolysis is undoubtedly more complicated than freshwater electrolysis. In this review article, most of the discussion about the production of H2, as an arising energy carrier, has been illustrated and the methods used for this goal have been highlighted. A great deal of progress has been achieved in the construction of electrocatalysts for seawater splitting for H2 production by employing atomic, molecular, and nanoscale material engineering approaches. However, the catalytic activity and stability of the most developed materials do not match the criteria for practical application.
This report focused on the catalysts developed for seawater electrolysis as well as the latest research findings for several TMPs. As previously stated, creating novel TMPs and preventing excessive costs will contribute to the expansion of seawater electrolysis. TMPs are now created in small-scale procedures using various techniques, while scale-up manufacturing with consistent morphologies and structures has recently received attention.
For seawater electrolysis, TMP-based electrocatalysts have demonstrated favorable activity, and suitable ones have shown high selectivity and durability as well. In summary, the following factors can be used to design a perfect TMP-based HER electrocatalyst:
- i.
- After more evaluation, TMPs can be anticipated to be an essential tool for the hydrogen energy sector. Since oxidation products can considerably damage and even ruin TMP-based catalyst activity and durability, seawater electrolysis without CER is more advantageous.
- ii.
- More research must be conducted to determine the exact mechanism(s) underlying TMP-based catalysts. Furthermore, one of the remaining barriers to the commercial viability of TMPs and multi-elemental TMPs as catalysts is the development of a scalable, cost-effective synthesis methodology.
- iii.
- It is exceedingly important to develop catalysts that are more selective for HER and OER than other competing reactions.
- iv.
- Even though TMPs have sophisticated chemical and physical features, their ability to catalyze is inadequate as a result of their poor electrical conductivity and the high absorption energies of the hydrogen intermediates.
- v.
- Despite significant progress in explaining the OER and HER electrocatalytic methodologies, several issues remain in the direction of commercial large-scale hydrogen production via seawater splitting by electrolysis.
- vi.
- Consequently, it is anticipated that electronic structure modulation, microstructure regulation, and multi-component hybrid engineering will be helpful resources for designing effective TMP-based HER electrocatalysts.
- vii.
- TMP-based HER electrocatalysts can now compete with exceptionally advanced noble metal catalysts. We, therefore, compared common electrocatalysts made using the aforementioned multiscale strategies from the standpoint of HER catalytic activity and stability.
- viii.
- TMP-based catalysts should also benefit from being inexpensive and simple to prepare in order to ensure their wide-scale commercial application.
Author Contributions
W.T.: conceptualization, data curation, data analysis, writing—original draft, writing—review and editing; X.Z.: project administration, investigation, funding acquisition; R.K.: writing—review and editing, data analysis and curation, methodology; M.S.: writing—review & editing, resources, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of Sichuan Province of China (No 2018JY0327).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
TMPs: Transition Metal Phosphides, TMOs: Transition Metal Oxides, TMDs: Transition Metal Dihalides, TMCs: Transition Metal Carbides, TMNs: Transition Metal Nitrides, HER: Hydrogen Evolution Reaction, OER: Oxygen Evolution Reaction, CER: Chloride Evolving Reaction, H: Hydrogen, Cl−: Chloride, P: Phosphorous, M: Metal, ΔG*: Standard Gibbs Free Energy Change (kJ·mol−1), HRTEM: High-Resolution Transmission Electron Microscopy, SAED: Selected-Area Electron Diffraction, TEM: Transition Electron Microscopy, HAADF-STEM: High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy, ABF-STEM: Annular Bright Field-Scanning Transmission Electron Microscope, MEA: Membrane Electrode Assembly, NiFe LDH: NiFe-layered Double Hydroxide.
References
- Tian, X. Engineering bunched Pt-Ni alloy nanocages for efficient oxygen reduction in practical fuel cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Lu, X.F.; Xia, B.Y.; Lou, X.W.D. Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule 2020, 4, 45–68. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Li, J.; Chen, Q.; Du, Y.; Rao, P.; Li, R.; Jia, C.; Kang, Z.; Deng, P. Engineering ruthenium-based electrocatalysts for effective hydrogen evolution reaction. Nanomicro Lett. 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Huang, W.; Luo, J.; Deng, P.J. Progress in the development of heteroatom-doped nickel phosphates for electrocatalytic water splitting. J. Colloid. Interface Sci. 2022, 607, 1091–1102. [Google Scholar] [CrossRef]
- Feng, S.; Yu, Y.; Li, J.; Luo, J.; Deng, P.; Jia, C.; Shen, Y.; Tian, X. Recent progress in seawater electrolysis for hydrogen evolution by transition metal phosphides. Catal. Commun. 2022, 162, 106382. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Hao, J.; Wang, Z.; Huo, B.; Qi, J.; Wang, Y.; Meng, F. Sustainable self-powered degradation of antibiotics using Fe3O4@MoS2/PVDF modified pipe with superior piezoelectric activity: Mechanism insight, toxicity assessment and energy consumption. Appl. Catal. B 2023, 331, 122655. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, M.; Huo, B.; Wang, J.; Yang, L.; Ma, W.; Qi, J.; Wang, Y.; Zhu, Z.; Meng, F. A novel ZnO/CQDs/PVDF piezoelectric system for efficiently degradation of antibiotics by using water flow energy in pipeline: Performance and mechanism. Nano Energy 2023, 107, 108162. [Google Scholar] [CrossRef]
- Asim, M.; Zhang, S.; Wang, Y.; Maryam, B.; Sajid, M.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.-J. Self-supporting NiCoP for hydrogen generation via hydrolysis of ammonia borane. Fuel 2022, 318, 123544. [Google Scholar] [CrossRef]
- Asim, M.; Maryam, B.; Zhang, S.; Sajid, M.; Kurbanov, A.; Pan, L.; Zou, J.-J. Synergetic effect of Au nanoparticles and transition metal phosphides for enhanced hydrogen evolution from ammonia-borane. J. Colloid. Interface Sci. 2023, 638, 14–25. [Google Scholar] [CrossRef]
- Dresp, S.R.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 2021, 31, 2006484. [Google Scholar] [CrossRef]
- Hong, L.F.; Guo, R.T.; Yuan, Y.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. Recent progress of transition metal phosphides for photocatalytic hydrogen evolution. ChemSusChem 2021, 14, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A., III; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Commun. 2018, 9, 2551. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xu, G.; Yang, H.; Wang, H.; Xia, B.Y. Rational Design of Transition Metal Phosphide-Based Electrocatalysts for Hydrogen Evolution. Adv. Funct. Mater. 2023, 33, 2208358. [Google Scholar] [CrossRef]
- Liu, D.; Ai, H.; Chen, M.; Zhou, P.; Li, B.; Liu, D.; Du, X.; Lo, K.H.; Ng, K.W.; Wang, S.P. Multi-phase heterostructure of CoNiP/CoxP for enhanced hydrogen evolution under alkaline and seawater conditions by promoting H2O dissociation. Small 2021, 17, 2007557. [Google Scholar] [CrossRef]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G. Dual-doping and synergism toward high-performance seawater electrolysis. J. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@FeOOH for efficient seawater electrolysis. Appl. Catal. B 2021, 294, 120256. [Google Scholar] [CrossRef]
- Han, L.; Dong, S.; Wang, E. Transition-metal (Co, Ni and Fe)-based electrocatalysts for the water oxidation reaction. J. Adv. Mater. 2016, 28, 9266–9291. [Google Scholar] [CrossRef]
- Liu, G.; Wang, M.; Xu, Y.; Wang, X.; Li, X.; Liu, J.; Cui, X.; Jiang, L. Porous CoP/Co2P heterostructure for efficient hydrogen evolution and application in magnesium/seawater battery. J. Power Source 2021, 486, 229351. [Google Scholar] [CrossRef]
- Weng, C.C.; Ren, J.T.; Yuan, Z.Y. Transition metal phosphide-based materials for efficient electrochemical hydrogen evolution: A critical review. J. Catal. 2020, 13, 3357–3375. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xu, Y.; Lv, H.; Wang, M.; Cui, X.; Liu, G.; Jiang, L.J. Triggering the intrinsic catalytic activity of Ni-doped molybdenum oxides via phase engineering for hydrogen evolution and application in Mg/seawater batteries. ACS Sustain. Chem. Eng. 2021, 9, 13106–13113. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Amorphous catalysts and electrochemical water splitting: An untold story of harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect engineering in photocatalytic materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Shi, R.; Zhao, Y.; Waterhouse, G.I.; Zhang, S.; Zhang, T. Defect engineering in photocatalytic nitrogen fixation. ACS Catal. 2019, 9, 9739–9750. [Google Scholar] [CrossRef]
- Song, G.; Cong, S.; Zhao, Z. Defect engineering in semiconductor-based SERS. J. Chem. Eng. 2022, 13, 1210–1224. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y.; Deng, D.; Yang, H.; Tian, H.; Li, J.; Ren, P.; Bao, X. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Peng, H.; Hong, X.; Chan, K.; Nørskov, J.K. Understanding trends in electrochemical carbon dioxide reduction rates. Nat. Commun. 2017, 8, 15438. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, L.; Han, Y.; Xu, M.; Dong, S. Nitrogen-doped carbon encapsulating γ-MoC/Ni heterostructures for efficient oxygen evolution electrocatalysts. Nanoscale 2017, 9, 5583–5588. [Google Scholar] [CrossRef] [PubMed]
- Vrubel, H.; Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. Engl. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Luo, J.; Nan, H.; Zou, H.; Chen, R.; Shu, T.; Li, X.; Li, Y.; Song, H.; Liao, S. Transition metal nitride coated with atomic layers of Pt as a low-cost, highly stable electrocatalyst for the oxygen reduction reaction. J. Am. Chem. Soc. 2016, 138, 1575–1583. [Google Scholar] [CrossRef]
- Tian, X.; Tang, H.; Luo, J.; Nan, H.; Shu, T.; Du, L.; Zeng, J.; Liao, S.; Adzic, R.R. High-performance core–shell catalyst with nitride nanoparticles as a core: Well-defined titanium copper nitride coated with an atomic Pt layer for the oxygen reduction reaction. ACS Catal. 2017, 7, 3810–3817. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.-Z. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12, 12761–12769. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Khan, N.A.; Imran, M.; Rashid, M.; Tufail, M.K.; Rehman, A.U.; Balkourani, G.; Sohail, M.; Najam, T.; Tsiakaras, P. Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis. Menbranes 2023, 13, 113. [Google Scholar] [CrossRef]
- Ding, J.; Liu, W.; Zhang, S.; Luo, J.; Liu, X. A mini review: Recent. advances in asymmetrically coordinated atom sites for high-efficiency hydrogen evolution reaction. Energies 2023, 16, 2664. [Google Scholar] [CrossRef]
- Li, C.; Bao, Y.; Liu, E.; Zhao, B.; Sun, T. Recent Advances of Modified Ni (Co, Fe)-Based LDH 2D Materials for Water Splitting. Molecules 2023, 28, 1475. [Google Scholar] [CrossRef]
- Ren, T.; Li, M.; Chu, Y.; Chen, J. Thioetherification of isoprene and butanethiol on transition metal phosphides. J. Energy Chem. 2018, 27, 930–939. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Bussell, M.E. New methods for the preparation of nanoscale nickel phosphide catalysts for heteroatom removal reactions. React. Chem. Eng. 2017, 2, 628–635. [Google Scholar] [CrossRef]
- Golubeva, M.; Zakharyan, E.; Maximov, A. Transition metal phosphides (Ni, Co, Mo, W) for hydrodeoxygenation of biorefinery products (a review). Pet. Chem. 2020, 60, 1109–1128. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, T.; Amiinu, I.S.; Cheng, R.; Wang, P.; Zhang, C.; Ji, P.; Hu, W.; Liu, J.; Mu, S. Transition-metal phosphides: Activity origin, energy-related electrocatalysis applications, and synthetic strategies. Adv. Funct. Mater. 2020, 30, 2004009. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Campos-Martin, J.M.; Fierro, J. Transition metal phosphides for the catalytic hydrodeoxygenation of waste oils into green diesel. J. Catal. 2019, 9, 293. [Google Scholar] [CrossRef]
- Bhunia, K.; Chandra, M.; Sharma, S.K.; Pradhan, D.; Kim, S.-J. A critical review on transition metal phosphide based catalyst for electrochemical hydrogen evolution reaction: Gibbs free energy, composition, stability, and true identity of active site. Coord. Chem. Rev. 2023, 478, 214956. [Google Scholar] [CrossRef]
- Shi, Y.; Li, M.; Yu, Y.; Zhang, B. Recent advances in nanostructured transition metal phosphides: Synthesis and energy-related applications. Energy Environ. Sci. 2020, 13, 4564–4582. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Liu, R.; Hu, Y.; Xiong, T.; Qiu, W.; Balogun, M.-S.J.T.; Pan, A.; Tong, Y. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr (oxy) oxides for efficient hydrogen production in alkaline, seawater and acidic media. Appl. Catal. B 2019, 251, 181–194. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Wu, X.; Niu, Y.; Feng, B.; Li, W.; Hu, W.; Li, C. Molybdenum carbide/phosphide hybrid nanoparticles embedded P, N co-doped carbon nanofibers for highly efficient hydrogen production in acidic, alkaline solution and seawater. Electrochim. Acta 2018, 281, 710–716. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Pei, Y.; Cheng, Y.; Chen, J.; Smith, W.; Dong, P.; Ajayan, P.M.; Ye, M.; Shen, J. Recent developments of transition metal phosphides as catalysts in the energy conversion field. J. Mater. Chem. 2018, 6, 23220–23243. [Google Scholar] [CrossRef]
- Yu, S.H.; Chua, D.H. Toward high-performance and low-cost hydrogen evolution reaction electrocatalysts: Nanostructuring cobalt phosphide (CoP) particles on carbon fiber paper. ACS Appl. Mater. Interfaces 2018, 10, 14777–14785. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Huang, G.; Chen, C.; Huang, L.; Chen, R.; Wang, S. Porous CoP nanosheets converted from layered double hydroxides with superior electrochemical activity for hydrogen evolution reactions at wide pH ranges. Chem. Commun. 2018, 54, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-B.; Luo, S.-H.; Liu, C.-L.; Yi, T.-F.; Zhai, Y.-C. High-surface-area and porous Co2P nanosheets as cost-effective cathode catalysts for Li–O2 batteries. ACS Appl. Mater. Interfaces 2018, 10, 21281–21290. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Wang, M.Q.; Chen, G.; Deng, Y.H.; Li, L.J.; Luo, H.Q.; Li, N.B. One-step CVD synthesis of carbon framework wrapped Co2P as a flexible electrocatalyst for efficient hydrogen evolution. J. Mater. Chem. 2017, 5, 7791–7795. [Google Scholar] [CrossRef]
- Fan, M.; Chen, Y.; Xie, Y.; Yang, T.; Shen, X.; Xu, N.; Yu, H.; Yan, C. Half-cell and full-cell applications of highly stable and binder-free sodium ion batteries based on Cu3P nanowire anodes. Adv. Funct. Mater. 2016, 26, 5019–5027. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, R.; Zhang, L.; Liu, D.; Hao, S.; Du, G.; Asiri, A.M.; Kong, R.; Sun, X. Energy-efficient electrolytic hydrogen generation using a Cu3P nanoarray as a bifunctional catalyst for hydrazine oxidation and water reduction. Inorg. Chem. Front. 2017, 4, 420–423. [Google Scholar] [CrossRef]
- Jiang, Y.; Lu, Y.; Lin, J.; Wang, X.; Shen, Z. A hierarchical MoP nanoflake array supported on Ni foam: A bifunctional electrocatalyst for overall water splitting. Small Methods 2018, 2, 1700369. [Google Scholar] [CrossRef]
- Lan, K.; Wang, X.; Yang, H.; Iqbal, K.; Zhu, Y.; Jiang, P.; Tang, Y.; Yang, Y.; Gao, W.; Li, R. Ultrafine MoP nanoparticles well embedded in carbon nanosheets as electrocatalyst with high active site density for hydrogen evolution. ChemElectroChem 2018, 5, 2256–2262. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Yu, S.; Wen, T.; Zhu, X.; Yang, F.; Sun, X.; Wang, X.; Hu, W. Ternary NiCo2Px nanowires as pH-universal electrocatalysts for highly efficient hydrogen evolution reaction. J. Adv. Mater. 2017, 29, 1605502. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, G.; Yu, L.; Qu, J.; Liu, H. Oxygen doping to optimize atomic hydrogen binding energy on NiCoP for highly efficient hydrogen evolution. Small 2018, 14, 1800421. [Google Scholar] [CrossRef]
- Asnavandi, M.; Suryanto, B.H.; Yang, W.; Bo, X.; Zhao, C. Engineering, Dynamic hydrogen bubble templated NiCu phosphide electrodes for pH-insensitive hydrogen evolution reactions. ACS Sustain. Chem. Eng. 2018, 6, 2866–2871. [Google Scholar]
- Guan, Q.; Sun, C.; Li, R.; Li, W. The synthesis and investigation of ruthenium phosphide catalysts. Catal. Commun. 2011, 14, 114–117. [Google Scholar] [CrossRef]
- Park, J.; Koo, B.; Yoon, K.Y.; Hwang, Y.; Kang, M.; Park, J.-G.; Hyeon, T.J. Generalized synthesis of metal phosphide nanorods via thermal decomposition of continuously delivered metal− phosphine complexes using a syringe pump. J. Am. Chem. Soc. 2005, 127, 8433–8440. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Berenguer, A.; Sankaranarayanan, T.; Gómez, G.; Moreno, I.; Coronado, J.; Pizarro, P.; Serrano, D. Evaluation of transition metal phosphides supported on ordered mesoporous materials as catalysts for phenol hydrodeoxygenation. Green. Chem. 2016, 18, 1938–1951. [Google Scholar] [CrossRef]
- Korányi, T.I.; Vít, Z.; Poduval, D.G.; Ryoo, R.; Kim, H.S.; Hensen, E.J. SBA-15-supported nickel phosphide hydrotreating catalysts. J. Catal. 2008, 253, 119–131. [Google Scholar] [CrossRef]
- Moon, J.-S.; Lee, Y.-K. Support effects of Ni2P catalysts on the hydrodeoxygenation of guaiacol: In situ XAFS studies. Top. Catal. 2015, 58, 211–218. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous structured Ni–Fe–P nanocubes derived from a prussian blue analogue as an electrocatalyst for efficient overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M. In-situ regeneration of activated carbon using electric potential swing desorption (EPSD) for carbon dioxide and hydrogen sulphide removal from biogas. Eng. Phys. Sci. Bioresour. Technol. 2018, 249, 125–131. [Google Scholar] [CrossRef]
- Wu, S.-K.; Lai, P.-C.; Lin, Y.-C.; Wan, H.-P.; Lee, H.-T.; Chang, Y.-H. Atmospheric hydrodeoxygenation of guaiacol over alumina-, zirconia-, and silica-supported nickel phosphide catalysts. ACS Sustain. Chem. Eng. 2013, 1, 349–358. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Balamurugan, M.; Park, S.; Ha, H.; Jin, K.; Seo, H.; Nam, K.T. Importance of entropic contribution to electrochemical water oxidation catalysis. ACS Energy Lett. 2019, 4, 1918–1929. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Sadeghi Erami, R.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Niu, X.; Tang, Q.; He, B.; Yang, P. Robust and stable ruthenium alloy electrocatalysts for hydrogen evolution by seawater splitting. Electrochim. Acta 2016, 208, 180–187. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Chhetri, M.; Gupta, U.; Roy, A.; Rao, C. Photochemical and photoelectrochemical hydrogen generation by splitting seawater. Chem. Eur. J. 2018, 24, 18455–18462. [Google Scholar] [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep Sea Res. I. Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Esmaeilion, F. Hybrid renewable energy systems for desalination. Appl. Water Sci. 2020, 10, 84. [Google Scholar] [CrossRef]
- Kucera, J. Desalination: Water from Water; John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: https://www.perlego.com/book/992822/desalination-water-from-water-pdf (accessed on 22 May 2023).
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef] [PubMed]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel–iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Khatun, S.; Hirani, H.; Roy, P. Seawater electrocatalysis: Activity and selectivity. J. Mater. Chem. 2021, 9, 74–86. [Google Scholar] [CrossRef]
- Okada, T.; Abe, H.; Murakami, A.; Shimizu, T.; Fujii, K.; Wakabayashi, T.; Nakayama, M. A bilayer structure composed of Mg|Co-MnO2 deposited on a Co(OH)2 film to realize selective oxygen evolution from chloride-containing water. Langmuir 2020, 36, 5227–5235. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Li, Z.; Meng, X. Recent advances in electrocatalysts for seawater splitting in hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 69, 29685–29697. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen generation from seawater electrolysis over a sandwich-like NiCoN|NixP|NiCoN microsheet array catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Bennett, J. Electrodes for generation of hydrogen and oxygen from seawater. Int. J. Hydrogen Energy 1980, 5, 401–408. [Google Scholar] [CrossRef]
- Sharif, H.M.A.; Farooq, M.; Hussain, I.; Ali, M.; Mujtaba, M.; Sultan, M.; Yang, B. Recent innovations for scaling up microbial fuel cell systems: Significance of physicochemical factors for electrodes and membranes materials. J. Taiwan Inst. Chem. Eng. 2021, 129, 207–226. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Margallo, M.; Onandía, R.; Aldaco, R.; Irabien, A.J.C.T. Connecting wastes to resources for clean technologies in the chlor-alkali industry: A life cycle approach. Clean Technol. Environ. Policy 2018, 20, 229–242. [Google Scholar] [CrossRef]
- Meier, K. Hydrogen production with sea water electrolysis using Norwegian offshore wind energy potentials: Techno-economic assessment for an offshore-based hydrogen production approach with state-of-the-art technology. J. Energy Environ. Eng. 2014, 5, 104. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.A.H.; Rooney, D. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, J.; Lior, N. Thermodynamic analysis of a solid oxide fuel cell based combined cooling, heating, and power system integrated with biomass gasification. Entropy 2021, 23, 1029. [Google Scholar] [CrossRef] [PubMed]
- Singla, M.K.; Nijhawan, P.; Oberoi, A.S. Hydrogen fuel and fuel cell technology for cleaner future: A review. Environ. Sci. Pollut. 2021, 28, 15607–15626. [Google Scholar] [CrossRef] [PubMed]
- Gui, Q.; Ba, D.; Li, L.; Liu, W.; Li, Y.; Liu, J. Recent advances in materials and device technologies for aqueous hybrid supercapacitors. Sci. China Mater. 2022, 65, 10–31. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, C.; Sun, S.; Huang, Y.; Meng, G.; Han, A.; Liu, J. Mesoporous Fe3O4@C nanoarrays as high-performance anode for rechargeable Ni/Fe battery. Sci. China Mater. 2021, 64, 1105. [Google Scholar] [CrossRef]
- Rasul, M.; Hazrat, M.; Sattar, M.; Jahirul, M.; Shearer, M. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Siddiqui, S.; Bhatnagar, P.; Dhingra, S.; Upadhyay, U.; Sreedhar, I. Wastewater treatment and energy production by microbial fuel cells. Biomass Convers. Biorefinery 2021, 13, 3569–3592. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Weng, C.-C.; Ren, J.-T.; Yuan, Z.-Y. An overview and recent advances in electrocatalysts for direct seawater splitting. Front. Chem. Sci. Eng. 2021, 15, 1408–1426. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Liu, P.; Liang, J.; Luo, Y.; Cui, G.; Tang, B.; Liu, Q.; Yan, X.; Hao, H. Ni(OH)2 nanoparticles encapsulated in conductive nanowire array for high-performance alkaline seawater oxidation. Nano Res. 2022, 15, 6084–6090. [Google Scholar] [CrossRef]
- Aralekallu, S.; Sannegowda, L.K.; Singh, V. Developments in electrocatalysts for electrocatalytic hydrogen evolution reaction with reference to bio-inspired phthalocyanines. Int. J. Hydrogen Energy 2023, 48, 16569–16592. [Google Scholar] [CrossRef]
- Xing, J.; Zeng, Z.; Best, W.; Liu, Z.; Bonville, L.; Maric, R.; Bliznakov, S. Long-term durability test of highly efficient membrane electrode assemblies for anion exchange membrane seawater electrolyzers. J. Power Source 2023, 558, 232564. [Google Scholar] [CrossRef]
- Lv, Q.; Han, J.; Tan, X.; Wang, W.; Cao, L.; Dong, B. Featherlike NiCoP holey nanoarrys for efficient and stable seawater splitting. ACS Appl. Energy Mater. 2019, 2, 3910–3917. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 5106. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.A.R.; Okyay, M.S.; Kim, M.; Lee, M.H.; Park, N.; Lee, J.S. Bifunctional sulfur-doped cobalt phosphide electrocatalyst outperforms all-noble-metal electrocatalysts in alkaline electrolyzer for overall water splitting. Nano Energy 2018, 53, 286–295. [Google Scholar] [CrossRef]
- Zhang, L.; Chang, C.; Hsu, C.-W.; Chang, C.-W.; Lu, S.-Y. Hollow nanocubes composed of well-dispersed mixed metal-rich phosphides in N-doped carbon as highly efficient and durable electrocatalysts for the oxygen evolution reaction at high current densities. J. Mater. Chem. 2017, 5, 19656–19663. [Google Scholar] [CrossRef]
- Niu, J.; Yang, J.; Channa, A.I.; Ashalley, E.; Yang, J.; Jiang, J.; Li, H.; Ji, H.; Niu, X. Enhancing the water splitting performance via decorating Co3O4 nanoarrays with ruthenium doping and phosphorization. RSC Adv. 2020, 10, 27235–27241. [Google Scholar] [CrossRef]
- Niu, S.; Jiang, W.-J.; Wei, Z.; Tang, T.; Ma, J.; Hu, J.-S.; Wan, L.-J. Se-doping activates FeOOH for cost-effective and efficient electrochemical water oxidation. J. Am. Chem. Soc. 2019, 141, 7005–7013. [Google Scholar] [CrossRef]
- Song, J.; Qiu, S.; Hu, F.; Ding, Y.; Han, S.; Li, L.; Chen, H.Y.; Han, X.; Sun, C.; Peng, S. Sub-2 nm thiophosphate nanosheets with heteroatom doping for enhanced oxygen electrocatalysis. Adv. Funct. Mater. 2021, 31, 2100618. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported transition metal phosphides: Activity survey for HER, ORR, OER and corrosion resistance in acid and alkaline electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Read, C.G.; Callejas, J.F.; Holder, C.F.; Schaak, R.E. General strategy for the synthesis of transition metal phosphide films for electrocatalytic hydrogen and oxygen evolution. ACS Appl. Mater. Interfaces 2016, 8, 12798–12803. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ding, X.; Han, M.; Zhu, M. Morphology and Composition Regulation of FeCoNi Prussian Blue Analogues to Advance in the Catalytic Performances of the Derivative Ternary Transition-Metal Phosphides for OER. ChemCatChem 2020, 12, 4339–4345. [Google Scholar] [CrossRef]
- Shin, D.; Kim, H.J.; Kim, M.; Shin, D.; Kim, H.; Song, H.; Choi, S.-I. FexNi2–xP Alloy Nanocatalysts with Electron-Deficient Phosphorus Enhancing the Hydrogen Evolution Reaction in Acidic Media. ACS Catal. 2020, 10, 11665–11673. [Google Scholar] [CrossRef]
- Wu, R.; Xiao, B.; Gao, Q.; Zheng, Y.R.; Zheng, X.S.; Zhu, J.F.; Gao, M.R.; Yu, S.H. A janus nickel cobalt phosphide catalyst for high-efficiency neutral-pH water splitting. Angew. Chem. 2018, 130, 15671–15675. [Google Scholar] [CrossRef]
- Oyama, S.T.; Lee, Y.-K. Mechanism of hydrodenitrogenation on phosphides and sulfides. J. Phys. Chem. B 2005, 109, 2109–2119. [Google Scholar] [CrossRef]
- Robinson, A.M.; Hensley, J.E.; Medlin, J. Bifunctional catalysts for upgrading of biomass-derived oxygenates: A review. ACS Catal. 2016, 6, 5026–5043. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Nørskov, J.K.; Abild-Pedersen, F.; Jaramillo, T. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Liu, D.; Hao, S.; Du, G.; Ma, Y.; Asiri, A.M.; Sun, X.; Chen, L. Mn doping of CoP nanosheets array: An efficient electrocatalyst for hydrogen evolution reaction with enhanced activity at all pH values. ACS Catal. 2017, 7, 98–102. [Google Scholar] [CrossRef]
- Lu, X.F.; Yu, L.; Lou, X. Highly crystalline Ni-doped FeP/carbon hollow nanorods as all-pH efficient and durable hydrogen evolving electrocatalysts. Sci. Adv. 2019, 5, eaav6009. [Google Scholar] [CrossRef]
- Lv, X.; Hu, Z.; Ren, J.; Liu, Y.; Wang, Z.; Yuan, Z.-Y. Self-supported Al-doped cobalt phosphide nanosheets grown on three-dimensional Ni foam for highly efficient water reduction and oxidation. Inorg. Chem. Front. 2019, 6, 74–81. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, J.; Chen, G.; Chen, W.; Song, C.; Li, C.; Ostrikov, K. In situ engineering bi-metallic phospho-nitride bi-functional electrocatalysts for overall water splitting. Appl. Catal. B 2019, 254, 414–423. [Google Scholar] [CrossRef]
- Yang, Y.; Kang, M.; Guo, X.; An, X.; Guo, F.; Zhang, L.; Wang, D.; Sun, D.; Zhou, X.; Yang, Z.J. Fluorine-regulated binary cobalt nickel phosphides nanoarrays on nickel foam for enhanced hydrogen evolution reaction. J. Mater. Sci. 2023, 58, 6407–6418. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Z.; Wu, H.; Yu, P.; Wang, Y.; Xiao, F.; Chen, S.; Du, S.; Xie, Y.; Wu, Y. Boron-induced electronic-structure reformation of CoP nanoparticles drives enhanced pH-universal hydrogen evolution. Angew. Chem. Int. Ed. 2020, 59, 4154–4160. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Su, H.; Hong, A.N.; Wang, Y.; Yang, H.; Ge, L.; Song, W.; Liu, J.; Ma, T. Electron Redistributed S-Doped Nickel Iron Phosphides Derived from One-Step Phosphatization of MOFs for Significantly Boosting Electrochemical Water Splitting. Adv. Funct. Mater. 2022, 32, 2200733. [Google Scholar] [CrossRef]
- Sohrabnejad-Eskan, I.; Goryachev, A.; Exner, K.S.; Kibler, L.A.; Hensen, E.J.; Hofmann, J.P.; Over, H. Temperature-dependent kinetic studies of the chlorine evolution reaction over RuO2 (110) model electrodes. ACS Catal. 2017, 7, 2403–2411. [Google Scholar] [CrossRef]
- Vos, J.; Koper, M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268. [Google Scholar] [CrossRef]
- Tu, Q.; Liu, W.; Jiang, M.; Wang, W.; Kang, Q.; Wang, P.; Zhou, W.; Zhou, F. Preferential adsorption of hydroxide ions onto partially crystalline NiFe-layered double hydroxides leads to efficient and selective OER in alkaline seawater. ACS Appl. Energy Mater. 2021, 4, 4630–4637. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy) hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Petrulevičienė, M.; Butkutė, S.; Jasulaitienė, V.; Selskis, A.; Ramanauskas, R. Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution. Int. J. Hydrogen Energy 2019, 44, 5929–5939. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Ramani, V. Selective seawater splitting using pyrochlore electrocatalyst. ACS Appl. Energy Mater. 2020, 3, 3978–3983. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Fan, J.; Lv, H.; Hao, W. Preparation of Ti@NiB electrode via electroless plating toward high-efficient alkaline simulated seawater splitting. J. Electroanal. Chem. 2021, 901, 115761. [Google Scholar] [CrossRef]
- Gupta, S.; Forster, M.; Yadav, A.; Cowan, A.J.; Patel, N.; Patel, M. Highly efficient and selective metal oxy-boride electrocatalysts for oxygen evolution from alkali and saline solutions. ACS Appl. Energy Mater. 2020, 3, 7619–7628. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Chen, H.; Zhang, Z.; Zou, X. Design of a multilayered oxygen-evolution electrode with high catalytic activity and corrosion resistance for saline water splitting. Adv. Funct. Mater. 2021, 31, 2101820. [Google Scholar] [CrossRef]
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Yu, K.; Feng, Y.; Zhu, Z. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Zhang, X.; Kong, W.; Peng, O.; Yang, Y.; Wang, S.; Cheng, J.; He, T.; Amini, A. Polyoxometalate-derived hexagonal molybdenum nitrides (MXenes) supported by boron, nitrogen codoped carbon nanotubes for efficient electrochemical hydrogen evolution from seawater. Adv. Funct. Mater. 2019, 29, 1805893. [Google Scholar] [CrossRef]
- Tian, F.; Geng, S.; He, L.; Huang, Y.; Fauzi, A.; Yang, W.; Liu, Y.; Yu, Y. Interface engineering: PSS-PPy wrapping amorphous Ni-Co-P for enhancing neutral-pH hydrogen evolution reaction performance. J. Chem. Eng. 2021, 417, 129232. [Google Scholar] [CrossRef]
- Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 31, 2107333. [Google Scholar] [CrossRef]
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru incorporated amorphous cobalt-based oxides for high-current-density overall water splitting in alkaline and seawater media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. J. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, Q.; He, B.; Yang, P. Robust electrocatalysts from an alloyed Pt–Ru–M (M=Cr, Fe, Co, Ni, Mo)-decorated Ti mesh for hydrogen evolution by seawater splitting. J. Mater. Chem. A 2016, 4, 6513–6520. [Google Scholar] [CrossRef]
- Zheng, J. Seawater splitting for high-efficiency hydrogen evolution by alloyed PtNix electrocatalysts. Appl. Surf. Sci. 2017, 413, 360–365. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Y.; Xi, H.; Li, C. Seawater splitting for hydrogen evolution by robust electrocatalysts from secondary M (M=Cr, Fe, Co, Ni, Mo) incorporated Pt. RSC Adv. 2018, 8, 9423–9429. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Yang, X.; Fa, W.; Ge, S. High-efficiency and stable alloyed nickel based electrodes for hydrogen evolution by seawater splitting. J. Alloys Compd. 2018, 732, 248–256. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Huang, B.; Xie, Z. Surface engineering of Rh catalysts with N/S-codoped carbon nanosheets toward high-Performance hydrogen evolution from seawater. ACS Sustain. Chem. Eng. 2019, 7, 18835–18843. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel hollow MXene tailored low-Pt catalyst for efficient hydrogen evolution in full-pH range and seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B 2021, 297, 120386. [Google Scholar] [CrossRef]
- Li, J.; Song, M.; Hu, Y.; Zhang, C.; Liu, W.; Huang, X.; Zhang, J.; Zhu, Y.; Zhang, J.; Wang, D. A self-supported heterogeneous bimetallic phosphide array electrode enables efficient hydrogen evolution from saline water splitting. Nano Res. 2023, 16, 3658–3664. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Huang, X.; Zhu, Y.; Wang, D. Bimetallic phosphide heterostructure coupled with ultrathin carbon layer boosting overall alkaline water and seawater splitting. Small 2023, 19, 2206533. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, M.; Yang, W.; Yu, Y.; Hao, S. Ce-Doped Ordered Mesoporous Cobalt Ferrite Phosphides as Robust Catalysts for Water Oxidation. Eur. J. Chem. 2020, 26, 13305–13310. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, J.; Guo, X.; Li, J.; Huang, Y.; Geng, S.; Yu, Y.; Liu, Y.; Yang, W. Structural engineering of Fe-doped Ni2P nanosheets arrays for enhancing bifunctional electrocatalysis towards overall water splitting. Appl. Surf. Sci. 2021, 536, 147909. [Google Scholar] [CrossRef]
- Feng, H.; Tang, L.; Zeng, G.; Yu, J.; Deng, Y.; Zhou, Y.; Wang, J.; Feng, C.; Luo, T.; Shao, B. Electron density modulation of Fe1-xCoxP nanosheet arrays by iron incorporation for highly efficient water splitting. Nano Energy 2020, 67, 104174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).