Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future
Abstract
:1. Introduction
| Fatty Acids | SP | CP | CV | CM | PMS | SES | RB | SFL | COC | PNT |
|---|---|---|---|---|---|---|---|---|---|---|
| Butyric acid (C4:0) | 0.12 | 0.38 | 0.20 | nd | nd | nd | nd | nd | nd | nd |
| Caproic acid (C6:0) | 0.28 | 4.91 | 2.77 | nd | nd | 0.38 | nd | nd | 0.52 | nd |
| Caprylic acid (C8:0) | 3.73 | 3.80 | 0.26 | nd | nd | 4.91 | nd | nd | 7.6 | nd |
| Capric acid (C10:0) | nd | nd | nd | nd | nd | 3.8 | nd | nd | 5.5 | nd |
| Undecanoic acid (C11:0) | 0.58 | 1.45 | 1.39 | nd | nd | nd | nd | nd | nd | nd |
| Undecenoic acid (C11:1) | 0.89 | 2.63 | 2.17 | nd | nd | nd | nd | nd | nd | nd |
| Lauric acid (C12:0) | 0.49 | 1.44 | 0.87 | nd | nd | 1.45 | nd | 0.02 | 47.7 | nd |
| Lauroleic acid (C12:1) | 0.39 | 0.45 | 0.41 | nd | nd | nd | nd | nd | nd | nd |
| Tridecanoic acid (C13:0) | 0.86 | 0.82 | 1.03 | nd | nd | nd | nd | nd | nd | nd |
| Myristic acid (C14:0) | 0.23 | 0.65 | 0.69 | 13.89 | 0.17 | 2.63 | 0.39 | 0.09 | 19.90 | 0.04 |
| Pentadecanoic acid (C15:0) | 1.53 | 0.88 | 1.70 | 0.67 | nd | 1.44 | nd | nd | nd | nd |
| Pentadecenoic acid (C15:1) | 3.16 | 1.79 | 3.53 | nd | nd | nd | nd | nd | nd | nd |
| Palmitic acid (C16:0) | 46.07 | 14.63 | 14.42 | 20.48 | 13.1 | 0.45 | 20 | 6.20 | nd | 7.50 |
| Palmitoleic acid (C16:1) | 1.26 | 3.7 | 4.04 | 38.39 | 0.12 | 14.63 | 0.19 | 0.12 | nd | 0.07 |
| Hexadecadienoic acid (C16:2) | 3.38 | 5.44 | 5.34 | nd | nd | nd | nd | nd | nd | nd |
| Hexadecatrienoic acid (C16:3) | 0.14 | 5.01 | 4.90 | nd | nd | nd | nd | nd | nd | nd |
| Margaric acid (C17:0) | 0.27 | 0.35 | 0.12 | 3.26 | 0.13 | 0.82 | nd | 0.02 | nd | 0.07 |
| Heptadecenoic acid (C17:1) | 0.27 | nd | 0.27 | nd | nd | 3.7 | nd | nd | nd | nd |
| Stearic acid (C18:0) | 1.41 | 1.4 | 1.57 | 1.8 | 5.7 | 0.65 | 2.10 | 2.80 | 2.70 | 2.10 |
| Oleic acid (C18:1) | 5.23 | 18.05 | 17.62 | 1.42 | 24.9 | 10.45 | 42.7 | 28.00 | 6.20 | 71.10 |
| Linoleic acid (C18:2-6) | 17.43 | 12.26 | 11.97 | 1.6 | 54.2 | nd | 33.10 | 62.2 | 1.6 | 18.20 |
| ϒ-linolenic acid (C18:3-6) | 8.87 | nd | nd | 1.7 | nd | 18.05 | nd | nd | nd | nd |
| α-linolenic acid (C18:3-3) | nd | 15.75 | 15.79 | nd | 0.12 | 1.4 | 0.45 | 0.16 | nd | nd |
| Arachidic acid (C20:0) | nd | nd | nd | nd | 0.47 | 0.88 | nd | 0.21 | nd | 1.01 |
| Docosanoic acid (C22:0) | nd | nd | nd | nd | nd | 1.79 | nd | nd | nd | nd |
| References | [36] | [37] | [38] | [39] | [40] | [41] | [27,42] | [43] | [44] | [42] |
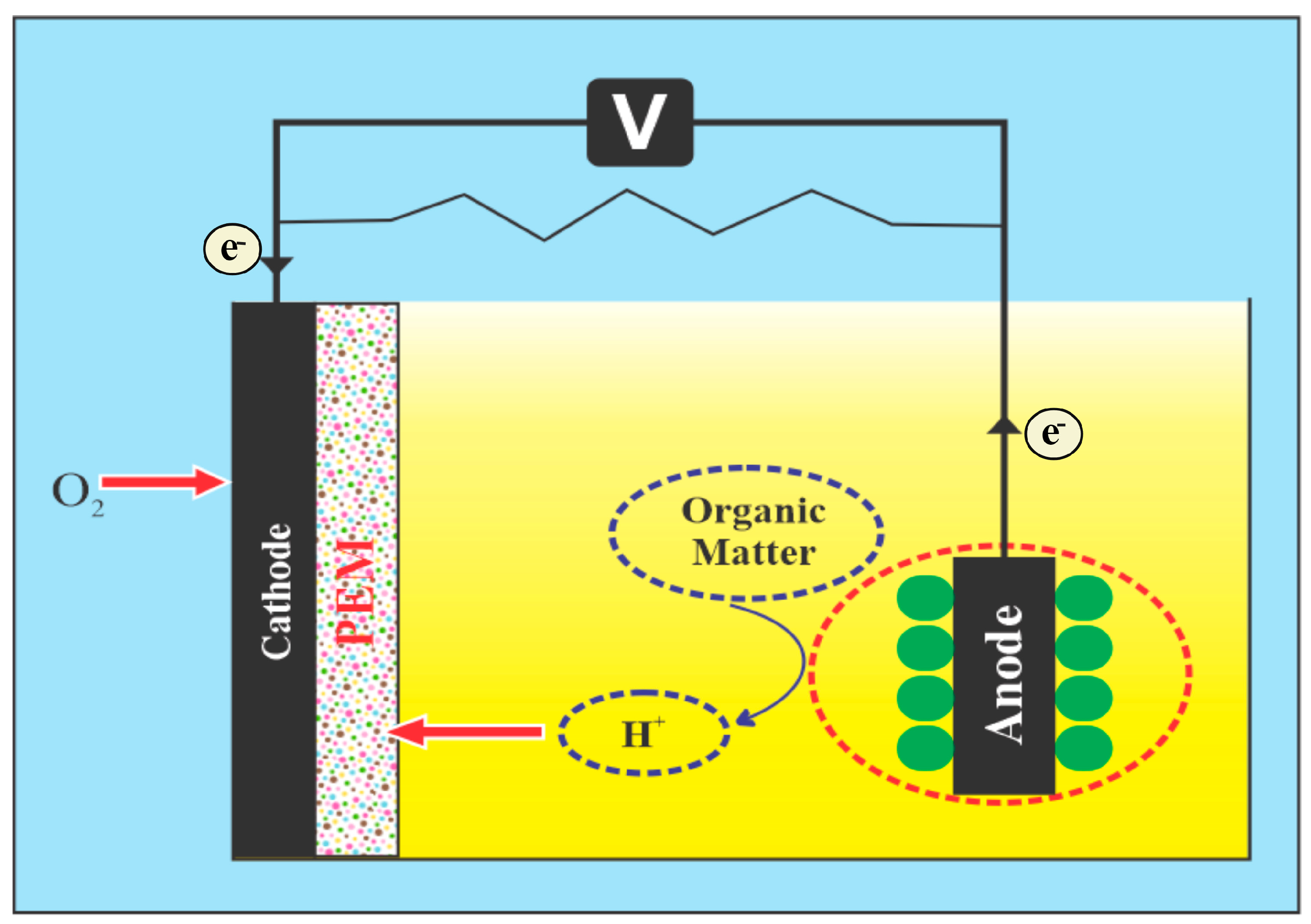
2. Configuration of an Algae Fuel Cell
2.1. Oxidation Occurs at the Anode
2.2. Photosynthesis and Carbon Dioxide Transfer
2.3. Cathodic Reduction Process
3. Bioactive Organisms: Harnessing the Potential of Microalgae
| Species | EPA and DHA in % | References |
|---|---|---|
| Nannochloropsis sp. | 26.7 EPA + DHA | [74] |
| Nannochloropsis oceanica | 23.4 EPA | [75] |
| Nannochloropsis salina | ~28 EPA | [76] |
| Pinguiococcus pyrenoidosus | 22.03 EPA + DHA | [72] |
| Thraustochytrium sp. | 45.1 EPA + DHA | [72] |
| Chlorella minutissima | 39.9 EPA | [77] |
| Dunaliella salina | 21.4 EPA | [78] |
| Pavlova viridis | 36.0 EPA + DHA | [79] |
| Pavlova lutheri | 41.5 EPA +DHA | [80] |
| Isocrysis galbana | ~28.0 EPA + DHA | [81] |
| Species | Oil Content (% wt.) | References |
|---|---|---|
| Chlamydomonas sp. | 22.7 | [82] |
| Chaetoceros muelleri | 13–24 | [83] |
| Parietochloris incise | 62 | [66] |
| Tetraselmis tetrathele | 25–30 | [84] |
| Nostoc commune | 22 | [84] |
| Emiliania huxleyi | 43.8 | [84] |
| Chroomonas salina | 12–14.5 | [84] |
| Mesotaenium sp. | 19–35 | [66] |
| Spirulina Platensis | 4–11 | [84] |
| Synechocystis sp. | 11 | [85] |
| Nannochloris sp. | 25–56 | [86] |
| Neochloris oleoabundans | 35–65 | [86] |
| Chlorella sp. | 28–53 | [86] |
| Schizochytrium sp. | 50–77 | [87] |
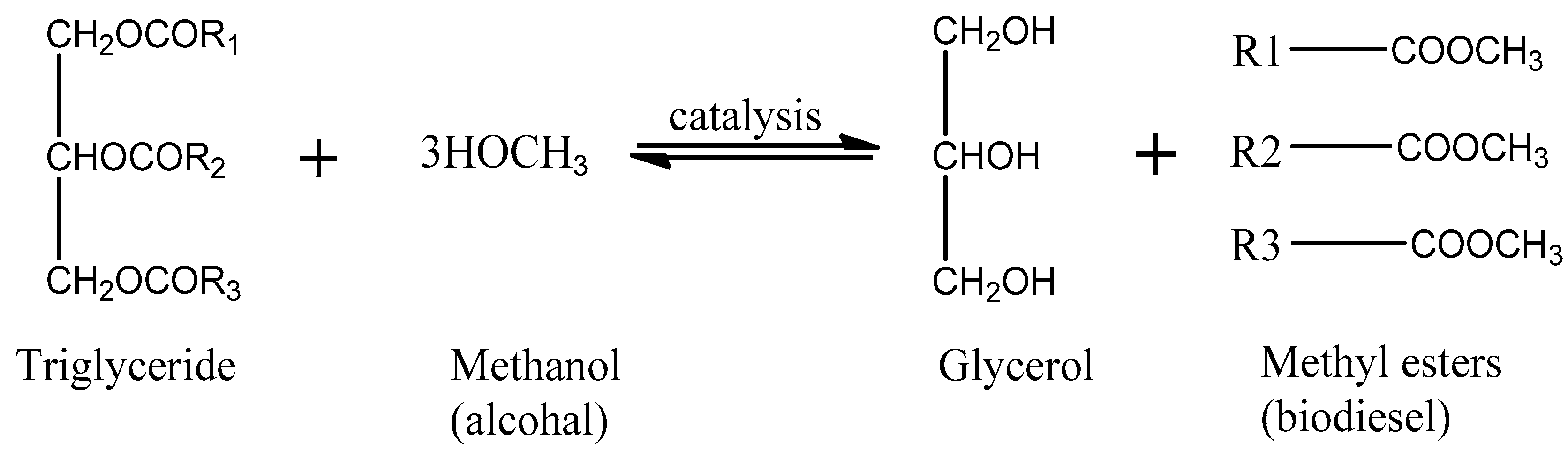
4. Microalgae as Third-Generation Biofuel Feedstock: A Sustainable Solution
5. Photosynthesis in Microalgae Cells: Harnessing Light Energy
6. Biomass Pre-Treatment for Microbial Fuel Cells (MFCs): Enhancing Efficiency
6.1. Physical Pre-Treatment for Biomass
6.2. Acid Pre-Treatment for Biomass
6.3. Alkali Pre-Treatment for Biomass
6.4. Enzymatic Pre-Treatment for Biomass
7. Methods for Increasing Power Production through Improved Reactor Design
7.1. Reactor Designs and Components
7.2. Reactor Designs
7.2.1. Single-Chambered Reactor
7.2.2. Double Chambered Reactor
7.3. Reactor Components
7.3.1. Electrodes
7.3.2. Proton Exchange Membrane
7.3.3. Carbon Dioxide Chamber
8. Factors That Influence Microalgae Growth
8.1. Light
8.2. Carbon Dioxide
8.3. Temperature
8.4. Nutrients
8.5. Culture Medium
9. Global Microalgae Oil Market
10. Global Microalgae Market 2020–2026
11. Worldwide Technologies Used for Microalgae Biofuel Production by Companies
12. Application of Nanomaterials in Microalgae Cultivation
13. Factors Affecting Microalgae Growth and Their Impact on Fuel Cell
14. Potential Applications and Important Findings
- Certain species of algae have demonstrated the ability to effectively remove heavy metals from streams, offering an environmentally advantageous solution [178]. Moreover, these algae species have a higher capacity for synthesizing carbohydrates as conserved polymers rather than lipids. This makes them promising candidates for bioethanol production, as the isolated carbohydrates can be converted into fermentable sugars [45].
- Algal biomass possesses great versatility and is capable of being used to produce proteins, pigments, polymers, and other valuable compounds alongside bioethanol [104,179]. Additionally, algal biomass contributes to nutrient recycling in nearby waters and coastal areas while also serving as a substitute for fossil fuels, thereby mitigating climate change [180].
- Macroalgae, as a potential feedstock for cellulosic ethanol, exhibit a high biomass yield surpassing many terrestrial crops [23].
- The vast oceanic expanse in coastal regions, which falls within the exclusive economic zone, presents an opportunity for the cultivation of algae biomass. Utilizing seawater for algal growth shows promise in addressing water scarcity issues. Ecologically, macroalgae play a significant role in reducing atmospheric carbon dioxide levels and oxygenating water bodies [181].
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, B.E.; Regan, J.M. Microbial Fuel Cells—Challenges and Applications. Environ. Sci. Technol. 2006, 40, 5172–5180. [Google Scholar] [CrossRef]
- Yang, C.; Gao, X.; Jiang, Y.; Sun, B.; Gao, F.; Yang, S. Synergy between Methylerythritol Phosphate Pathway and Mevalonate Pathway for Isoprene Production in Escherichia Coli. Metab. Eng. 2016, 37, 79–91. [Google Scholar] [CrossRef]
- Mehedintu, A.; Sterpu, M.; Soava, G. Estimation and Forecasts for the Share of Renewable Energy Consumption in Final Energy Consumption by 2020 in the European Union. Sustainability 2018, 10, 1515. [Google Scholar] [CrossRef]
- Contescu, C.; Adhikari, S.; Gallego, N.; Evans, N.; Biss, B. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. C 2018, 4, 51. [Google Scholar] [CrossRef]
- Oil Market Report—October 2020—Analysis. Available online: https://www.iea.org/reports/oil-market-report-october-2020 (accessed on 14 September 2023).
- Pandey, A.; Srivastava, S.; Kumar, S. Carbon Dioxide Fixation and Lipid Storage of Scenedesmus sp. ASK22: A Sustainable Approach for Biofuel Production and Waste Remediation. J. Environ. Manag. 2023, 332, 117350. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. AP-42: Compilation of Air Emissions Factors. Available online: https://www.epa.gov/air-emissions-factors-and-quantification/ap-42-compilation-air-emissions-factors (accessed on 14 September 2023).
- Pope, C.A.; Ezzati, M.; Dockery, D.W. Fine-Particulate Air Pollution and Life Expectancy in the United States. N. Engl. J. Med. 2009, 360, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Srivastava, S.; Kumar, S. Scenedesmus sp. ASK22 Cultivation Using Simulated Dairy Wastewater for Nutrient Sequestration and Biofuel Production: Insight into Fuel Properties and Their Blends. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Li, Y.; Rezgui, Y.; Zhu, H. District Heating and Cooling Optimization and Enhancement—Towards Integration of Renewables, Storage and Smart Grid. Renew. Sustain. Energy Rev. 2017, 72, 281–294. [Google Scholar] [CrossRef]
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of Renewable Energy Sources Market and Biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Misra, R.; Rawat, I.; Bux, F. Trends and Novel Strategies for Enhancing Lipid Accumulation and Quality in Microalgae. Renew. Sustain. Energy Rev. 2016, 55, 1–16. [Google Scholar] [CrossRef]
- Anderson, T.R.; Hawkins, E.; Jones, P.D. CO2, the Greenhouse Effect and Global Warming: From the Pioneering Work of Arrhenius and Callendar to Today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of Iron on Growth and Lipid Accumulation in Chlorella Vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Development and Cost-Benefit Analysis of a Novel Process for Biofuel Production from Microalgae Using Pre-Treated High-Strength Fresh Cheese Whey Wastewater. Environ. Sci. Pollut. Res. 2020, 27, 23963–23980. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J. Grand Challenges in Advanced Fossil Fuel Technologies. Front. Energy Res. 2014, 2. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Awaworyi Churchill, S.; Paramati, S.R. The Dynamic Impact of Renewable Energy and Institutions on Economic Output and CO2 Emissions across Regions. Renew. Energy 2017, 111, 157–167. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Craggs, R.J.; Heubeck, S.; Lundquist, T.J.; Benemann, J.R. Algal Biofuels from Wastewater Treatment High Rate Algal Ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar] [CrossRef]
- Di Termini, I.; Prassone, A.; Cattaneo, C.; Rovatti, M. On the Nitrogen and Phosphorus Removal in Algal Photobioreactors. Ecol. Eng. 2011, 37, 976–980. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and Recent Trends in Biofuels. Progress in Energy and Combustion. Science 2007, 33, 1–18. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Sequential Optimization of Essential Nutrients Addition in Simulated Dairy Effluent for Improved Scenedesmus sp. ASK22 Growth, Lipid Production and Nutrients Removal. Biomass Bioenergy 2019, 128, 105319. [Google Scholar] [CrossRef]
- Chisti, Y.; Yan, J. Energy from Algae: Current Status and Future Trends. Appl. Energy 2011, 88, 3277–3279. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A Review on Algal-Bacterial Symbiotic System for Effective Treatment of Wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Schmid, M.; Kraft, L.G.K.; Van Der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian Seaweeds: A Promising Resource for Omega-3 Fatty Acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae Beats Bioethanol. Trends Biotechnol. 2008, 26, 126–131. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel Production: Challenges and Opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Moshood, T.; Nawanir, G.; Sorooshian, S.; Mahmud, F.; Adeleke, A. Barriers and Benefits of ICT Adoption in the Nigerian Construction Industry. A Comprehensive Literature Review. ASI 2020, 3, 46. [Google Scholar] [CrossRef]
- Rajeev, A.; Pati, R.K.; Padhi, S.S.; Govindan, K. Evolution of Sustainability in Supply Chain Management: A Literature Review. J. Clean. Prod. 2017, 162, 299–314. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Chisti, Y. Biotechnology—a Sustainable Alternative for Chemical Industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef]
- IEA (International Energy Agency). Biofuels for Transport: An International Perspective; IEA: Paris, France, 2004; Available online: https://www.iea.org/reports/biofuels-for-transport-an-international-perspective (accessed on 14 September 2023).
- Malyan, S.K.; Bhatia, A.; Tomer, R.; Harit, R.C.; Jain, N.; Bhowmik, A.; Kaushik, R. Mitigation of Yield-Scaled Greenhouse Gas Emissions from Irrigated Rice through Azolla, Blue-Green Algae, and Plant Growth–Promoting Bacteria. Environ. Sci. Pollut. Res. 2021, 28, 51425–51439. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Ilango, K.; Praveena, N.; Sircar, A.; Balasubramanian, R.; Sakthisaravanan, A.; Kannan, R. Algal Fuel Cell. In Microalgal Biotechnology; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-332-2. [Google Scholar]
- Sara, L.; Konda, S.; Nikitha, B.; Palupanuri, N. Phospholipid Fatty Acid Profile of Spirulina Platensis. In GeNeDis 2020; Vlamos, P., Ed.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1339, pp. 161–167. ISBN 978-3-030-78786-8. [Google Scholar]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 Biofixation and Fatty Acid Composition of Scenedesmus Obliquus and Chlorella Pyrenoidosa in Response to Different CO2 Levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, Fatty Acid, and Pigment Content of Chlorella Vulgaris under Different Light Regimes. J. Appl. Phycol 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Wang, X.-W.; Liang, J.-R.; Luo, C.-S.; Chen, C.-P.; Gao, Y.-H. Biomass, Total Lipid Production, and Fatty Acid Composition of the Marine Diatom Chaetoceros Muelleri in Response to Different CO2 Levels. Bioresour. Technol. 2014, 161, 124–130. [Google Scholar] [CrossRef]
- Siano, F.; Straccia, M.C.; Paolucci, M.; Fasulo, G.; Boscaino, F.; Volpe, M.G. Physico-Chemical Properties and Fatty Acid Composition of Pomegranate, Cherry and Pumpkin Seed Oils: Properties and Fatty Acids of Three Seed Oils. J. Sci. Food Agric. 2016, 96, 1730–1735. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Vick, B.A.; Baldwin, B.S.; Buehring, N.; Astatkie, T.; Johnson, B. Oil Content and Saturated Fatty Acids in Sunflower as a Function of Planting Date, Nitrogen Rate, and Hybrid. Agron. J. 2009, 101, 1003–1011. [Google Scholar] [CrossRef]
- Saunders, R.M. Rice Bran: Composition and Potential Food Uses. Food Rev. Int. 1985, 1, 465–495. [Google Scholar] [CrossRef]
- Yen, H.-W.; Hu, I.-C.; Chen, C.-Y.; Ho, S.-H.; Lee, D.-J.; Chang, J.-S. Microalgae-Based Biorefinery—From Biofuels to Natural Products. Bioresour. Technol. 2013, 135, 166–174. [Google Scholar] [CrossRef]
- Boateng, L.; Ansong, R.; Owusu, W.B.; Steiner-Asiedu, M. Coconut Oil and Palm Oil’s Role in Nutrition, Health, and National Development: A Review. Ghana Med. J. 2016, 50, 189–196. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, G.; Xu, M. Microbial Fuel Cells Come of Age. J. Chem. Technol. Biotechnol. 2011, 86, 625–632. [Google Scholar] [CrossRef]
- Chandra, R.; Venkata Subhash, G.; Venkata Mohan, S. Mixotrophic Operation of Photo-Bioelectrocatalytic Fuel Cell under Anoxygenic Microenvironment Enhances the Light Dependent Bioelectrogenic Activity. Bioresour. Technol. 2012, 109, 46–56. [Google Scholar] [CrossRef]
- Nishio, K.; Hashimoto, K.; Watanabe, K. Light/Electricity Conversion by Defined Cocultures of Chlamydomonas and Geobacter. J. Biosci. Bioeng. 2013, 115, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Kannan, N.; Donnellan, P. Algae-Assisted Microbial Fuel Cells: A Practical Overview. Bioresour. Technol. Rep. 2021, 15, 100747. [Google Scholar] [CrossRef]
- Kakarla, R.; Min, B. Photoautotrophic Microalgae Scenedesmus Obliquus Attached on a Cathode as Oxygen Producers for Microbial Fuel Cell (MFC) Operation. Int. J. Hydrogen Energy 2014, 39, 10275–10283. [Google Scholar] [CrossRef]
- Gaj, T.; Sirk, S.J.; Shui, S.; Liu, J. Genome-Editing Technologies: Principles and Applications. Cold Spring Harb. Perspect. Biol. 2016, 8, a023754. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-M.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Mohamed, A.R.; Lim, J.W. Ameliorating Cu2+ Reduction in Microbial Fuel Cell with Z-Scheme BiFeO3 Decorated on Flower-like ZnO Composite Photocathode. Chemosphere 2022, 287, 132384. [Google Scholar] [CrossRef]
- Xu, C.; Poon, K.; Choi, M.M.F.; Wang, R. Using Live Algae at the Anode of a Microbial Fuel Cell to Generate Electricity. Environ. Sci. Pollut. Res. 2015, 22, 15621–15635. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Zhou, M. A Photosynthetic Algal Microbial Fuel Cell for Treating Swine Wastewater. Environ. Sci. Pollut. Res. 2019, 26, 6182–6190. [Google Scholar] [CrossRef]
- Li, Y.; Horsman, M.; Wu, N.; Lan, C.Q.; Dubois-Calero, N. Biofuels from Microalgae. Biotechnol. Prog. 2008, 24, 815–820. [Google Scholar] [CrossRef]
- Zhou, M.; He, H.; Jin, T.; Wang, H. Power Generation Enhancement in Novel Microbial Carbon Capture Cells with Immobilized Chlorella Vulgaris. J. Power Sources 2012, 214, 216–219. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate Metabolism and Respiration in Algae. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2003; Volume 14, pp. 205–224. ISBN 978-94-010-3772-3. [Google Scholar]
- Logroño, W.; Pérez, M.; Urquizo, G.; Kadier, A.; Echeverría, M.; Recalde, C.; Rákhely, G. Single Chamber Microbial Fuel Cell (SCMFC) with a Cathodic Microalgal Biofilm: A Preliminary Assessment of the Generation of Bioelectricity and Biodegradation of Real Dye Textile Wastewater. Chemosphere 2017, 176, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Bazdar, E.; Roshandel, R.; Yaghmaei, S.; Mardanpour, M.M. The Effect of Different Light Intensities and Light/Dark Regimes on the Performance of Photosynthetic Microalgae Microbial Fuel Cell. Bioresour. Technol. 2018, 261, 350–360. [Google Scholar] [CrossRef] [PubMed]
- de Morais, M.G.; Costa, J.A.V. Carbon Dioxide Fixation by Chlorella Kessleri, C. Vulgaris, Scenedesmus Obliquus and Spirulina sp. Cultivated in Flasks and Vertical Tubular Photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Luo, J.; Tan, C.; Tian, X.; Su, P.; Gu, T. Carbon Dioxide Sequestration Accompanied by Bioenergy Generation Using a Bubbling-Type Photosynthetic Algae Microbial Fuel Cell. Bioresour. Technol. 2019, 280, 95–103. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Min, B. Leachate Treatment and Electricity Generation Using an Algae-Cathode Microbial Fuel Cell with Continuous Flow through the Chambers in Series. Sci. Total Environ. 2020, 723, 138054. [Google Scholar] [CrossRef]
- Kakarla, R.; Kim, J.R.; Jeon, B.-H.; Min, B. Enhanced Performance of an Air–Cathode Microbial Fuel Cell with Oxygen Supply from an Externally Connected Algal Bioreactor. Bioresour. Technol. 2015, 195, 210–216. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Church, J.; Lee, S.-J.; Park, J.; Lee, W.H. Use of Microalgae for Advanced Wastewater Treatment and Sustainable Bioenergy Generation. Environ. Eng. Sci. 2016, 33, 882–897. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Cabello, J.; Toledo-Cervantes, A.; Sánchez, L.; Revah, S.; Morales, M. Effect of the Temperature, pH and Irradiance on the Photosynthetic Activity by Scenedesmus Obtusiusculus under Nitrogen Replete and Deplete Conditions. Bioresour. Technol. 2015, 181, 128–135. [Google Scholar] [CrossRef]
- González-Fernández, C.; Mahdy, A.; Ballesteros, I.; Ballesteros, M. Impact of Temperature and Photoperiod on Anaerobic Biodegradability of Microalgae Grown in Urban Wastewater. Int. Biodeterior. Biodegrad. 2016, 106, 16–23. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Phyco-Remediation of Dairy Effluents and Biomass Valorization: A Sustainable Approach. In Application of Microalgae in Wastewater Treatment; Gupta, S.K., Bux, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 195–213. ISBN 978-3-030-13908-7. [Google Scholar]
- García, D.; Posadas, E.; Grajeda, C.; Blanco, S.; Martínez-Páramo, S.; Acién, G.; García-Encina, P.; Bolado, S.; Muñoz, R. Comparative Evaluation of Piggery Wastewater Treatment in Algal-Bacterial Photobioreactors under Indoor and Outdoor Conditions. Bioresour. Technol. 2017, 245, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-B.; Yang, L.-B.; Zhang, Y.-L.; Zhao, F.-C.; Chu, H.-Q.; Guo, J. Chlorella Pyrenoidosa Cultivation in Outdoors Using the Diluted Anaerobically Digested Activated Sludge. Bioresour. Technol. 2015, 198, 340–350. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Vanbroekhoven, K.; Pant, D. Techno-Productive Potential of Photosynthetic Microbial Fuel Cells through Different Configurations. Renew. Sustain. Energy Rev. 2014, 39, 617–627. [Google Scholar] [CrossRef]
- Krishnaraj, R.N.; Berchmans, S.; Pal, P. The Three-Compartment Microbial Fuel Cell: A New Sustainable Approach to Bioelectricity Generation from Lignocellulosic Biomass. Cellulose 2015, 22, 655–662. [Google Scholar] [CrossRef]
- Scott, S.D.; Armenta, R.E.; Berryman, K.T.; Norman, A.W. Use of Raw Glycerol to Produce Oil Rich in Polyunsaturated Fatty Acids by a Thraustochytrid. Enzym. Microb. Technol. 2011, 48, 267–272. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Kennedy, S.-J.; Güttler, J.; Wood, S.A.; Williams, D.E.; Packer, M.A. A Cost-Effective Microbial Fuel Cell to Detect and Select for Photosynthetic Electrogenic Activity in Algae and Cyanobacteria. J. Appl. Phycol. 2014, 26, 15–23. [Google Scholar] [CrossRef]
- Tan, X.B.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Wong, C.Y.; Ramli, A.; Kiew, P.L.; Lee, K.T. Semi-Continuous Cultivation of Chlorella Vulgaris Using Chicken Compost as Nutrients Source: Growth Optimization Study and Fatty Acid Composition Analysis. Energy Convers. Manag. 2018, 164, 363–373. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty Acid Composition of 12 Microalgae for Possible Use in Aquaculture Feed. Aquacult. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Sang, M.; Wang, M.; Liu, J.; Zhang, C.; Li, A. Effects of Temperature, Salinity, Light Intensity, and pH on the Eicosapentaenoic Acid Production of Pinguiococcus Pyrenoidosus. J. Ocean. Univ. China 2012, 11, 181–186. [Google Scholar] [CrossRef]
- Yongmanitchai, W.; Ward, O.P. Growth of and Omega-3 Fatty Acid Production by Phaeodactylum Tricornutum under Different Culture Conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [CrossRef]
- Bhosale, R.A.; Rajabhoj, M.P.; Chaugule, B.B. Dunaliella Salina Teod. as a Prominent Source of Eicosapentaenoic Acid. Int. J. Algae 2010, 12, 185–189. [Google Scholar] [CrossRef]
- Hu, C.; Li, M.; Li, J.; Zhu, Q.; Liu, Z. Variation of Lipid and Fatty Acid Compositions of the Marine Microalga Pavlova Viridis (Prymnesiophyceae) under Laboratory and Outdoor Culture Conditions. World J. Microbiol. Biotechnol. 2008, 24, 1209–1214. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined Effects of Irradiance Level and Carbon Source on Fatty Acid and Lipid Class Composition in the Microalga Pavlova Lutheri Commonly Used in Mariculture. J. Exp. Mar. Biol. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Yago, T.; Arakawa, H.; Morinaga, T.; Yoshie-Stark, Y.; Yoshioka, M. Effect of Wavelength of Intermittent Light on the Growth and Fatty Acid Profile of the Haptophyte Isochrysis Galbana. In Global Change: Mankind-Marine Environment Interactions; Ceccaldi, H.-J., Dekeyser, I., Girault, M., Stora, G., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 43–45. ISBN 978-90-481-8629-7. [Google Scholar]
- Jeon, S.-M.; Kim, J.H.; Kim, T.; Park, A.; Ko, A.-R.; Ju, S.-J.; Heo, S.-J.; Oh, C.; Affan, M.A.; Shim, W.-B.; et al. Morphological, Molecular, and Biochemical Characterization of Monounsaturated Fatty Acids-Rich Chlamydomonas sp. KIOST-1 Isolated from Korea. J. Microbiol. Biotechnol. 2015, 25, 723–731. [Google Scholar] [CrossRef]
- Rodríguez, E.; López-Elías, J.; Aguirre-Hinojosa, E.; Del, G.-A.; Constantino-Franco, F.; Miranda-Baeza, A.; Nieves-Soto, M. Evaluation of the Nutritional Quality of Chaetoceros Muelleri Schütt (Chaetocerotales: Chaetocerotaceae) and Isochrysis sp. (Isochrysidales: Isochrysidaceae) Grown Outdoors for the Larval Development of Litopenaeus Vannamei (Boone, 1931) (Decapoda: Penaeidae). Arch. Biol. Sci. 2012, 64, 963–970. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and Lipid Induction Strategies in Microalgae for Biofuel Production and Other Applications. Microb. Cell. Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Idrissi Abdelkhalek, E.A.; Mohamed, B.; Mohammed, A.M.; Lotfi, A. Growth Performance and Biochemical Composition of Nineteen Microalgae Collected from Different Moroccan Reservoirs. Medit. Mar. Sci. 2016, 17, 323. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel Production—Current State of the Art and Challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421. [Google Scholar] [CrossRef]
- Sun, L.; Ren, L.; Zhuang, X.; Ji, X.; Yan, J.; Huang, H. Differential Effects of Nutrient Limitations on Biochemical Constituents and Docosahexaenoic Acid Production of Schizochytrium sp. Bioresour. Technol. 2014, 159, 199–206. [Google Scholar] [CrossRef]
- Pandey, A.; Srivastava, S.; Kumar, S. Isolation, Screening and Comprehensive Characterization of Candidate Microalgae for Biofuel Feedstock Production and Dairy Effluent Treatment: A Sustainable Approach. Bioresour. Technol. 2019, 293, 121998. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from Microalgae—A Review of Technologies for Production, Processing, and Extractions of Biofuels and Co-Products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Yap, Y.J.; Mohd Zaid, H.F.; Lam, M.K.; Lim, J.W.; Ho, Y.-C.; Tao, Y. Enhancing Microalga Chlorella Sorokiniana CY-1 Biomass and Lipid Production in Palm Oil Mill Effluent (POME) Using Novel-Designed Photobioreactor. Bioengineered 2020, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Escobar, R.; Antonaya-Baena, M.F.; Sánchez-Zamora, M.A.; Molina-Soria, C. The Amount of Nitrogen Applied and Nutritional Status of Olive Plants Affect Nitrogen Uptake Efficiency. Sci. Hortic. 2014, 167, 1–4. [Google Scholar] [CrossRef]
- Groth, T.; Bentzen, J. Prices of Agricultural Commodities, Biofuels and Fossil Fuels in Long-Run Relationships: A Comparative Study for the USA and Europe. Food Econ. 2013, 9, 27–36. [Google Scholar] [CrossRef]
- Lee, K.; Eisterhold, M.; Rindi, F.; Palanisami, S.; Nam, P. Isolation and Screening of Microalgae from Natural Habitats in the Midwestern United States of America for Biomass and Biodiesel Sources. J. Nat. Sci. Biol. Med. 2014, 5, 333. [Google Scholar] [CrossRef] [PubMed]
- Rosli, S.S.; Wong, C.Y.; Yunus, N.M.; Lam, M.K.; Show, P.L.; Cheng, C.K.; Wang, D.K.; Oh, W.D.; Lim, J.W. Optimum Interaction of Light Intensity and CO2 Concentration in Bioremediating N-Rich Real Wastewater via Assimilation into Attached Microalgal Biomass as the Feedstock for Biodiesel Production. Process Saf. Environ. Prot. 2020, 141, 355–365. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of Micro and Macro Algae for Biofuel Production: A Brief Review. BioResources 2013, 9, 1606–1633. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second Generation Biofuels: High-Efficiency Microalgae for Biodiesel Production. Bioenerg. Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Hossain, A.B.M.S.; Salleh, A.; Boyce, A.N.; Chowdhury, P.; Naqiuddin, M. Biodiesel Fuel Production from Algae as Renewable Energy. Am. J. Biochem. Biotechnol. 2008, 4, 250–254. [Google Scholar] [CrossRef]
- Behera, S.; Ray, R.C. Batch Ethanol Production from Cassava (Manihot Esculenta Crantz.) Flour Using Saccharomyces Cerevisiae Cells Immobilized in Calcium Alginate. Ann. Microbiol. 2015, 65, 779–783. [Google Scholar] [CrossRef]
- Marques, A.E.; Barbosa, A.T.; Jotta, J.; Coelho, M.C.; Tamagnini, P.; Gouveia, L. Biohydrogen Production by Anabaena sp. PCC 7120 Wild-Type and Mutants under Different Conditions: Light, Nickel, Propane, Carbon Dioxide and Nitrogen. Biomass Bioenergy 2011, 35, 4426–4434. [Google Scholar] [CrossRef]
- Hughes, A.D.; Kelly, M.S.; Black, K.D.; Stanley, M.S. Biogas from Macroalgae: Is It Time to Revisit the Idea? Biotechnol. Biofuels 2012, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Behera, S.; Yadav, Y.K.; Kumar, S. Potential of Wheat Straw for Biogas Production Using Thermophiles. In Recent Advances in Bioenergy Research; Sardar Swaran Singh National Institute of Renewable Energy: New Delhi, India, 2014. [Google Scholar] [CrossRef]
- Saqib, A.; Rizwan, M.; Rashid, U.; Ibrahim, M.; Gill, S.; Mehmood, M. Marine Macro Algae Ulva: A Potential Feed-Stock for Bio-Ethanol and Biogas Production. Asian J. Agric. Biol. 2013, 1, 155–163. [Google Scholar]
- Goldemberg, J. Ethanol for a Sustainable Energy Future. Science 2007, 315, 808–810. [Google Scholar] [CrossRef]
- Kraan, S. Mass-Cultivation of Carbohydrate Rich Macroalgae, a Possible Solution for Sustainable Biofuel Production. Mitig Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Demirbaş, A. Oily Products from Mosses and Algae via Pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2006, 28, 933–940. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of Liquid Biofuels from Renewable Resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Severes, A.; Hegde, S.; D’Souza, L.; Hegde, S. Use of Light Emitting Diodes (LEDs) for Enhanced Lipid Production in Micro-Algae Based Biofuels. J. Photochem. Photobiol. B Biol. 2017, 170, 235–240. [Google Scholar] [CrossRef]
- Patle, D.S.; Pandey, A.; Srivastava, S.; Sawarkar, A.N.; Kumar, S. Ultrasound-Intensified Biodiesel Production from Algal Biomass: A Review. Environ. Chem. Lett. 2021, 19, 209–229. [Google Scholar] [CrossRef]
- Zuorro, A.; García-Martínez, J.B.; Barajas-Solano, A.F. The Application of Catalytic Processes on the Production of Algae-Based Biofuels: A Review. Catalysts 2020, 11, 22. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Li, D.; Chu, H.; Zhou, X.; Zhang, Y.; Yu, H. Nutrients Removal and Lipids Production by Chlorella Pyrenoidosa Cultivation Using Anaerobic Digested Starch Wastewater and Alcohol Wastewater. Bioresour. Technol. 2015, 181, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Serrano, C.; Morales-Amaral, M.M.; Acién, F.G.; Escudero, R.; Fernández-Sevilla, J.M.; Molina-Grima, E. Utilization of Secondary-Treated Wastewater for the Production of Freshwater Microalgae. Appl. Microbiol. Biotechnol. 2015, 99, 6931–6944. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Pancha, I.; Desai, C.; Chokshi, K.; Paliwal, C.; Ghosh, T.; Mishra, S. Effects of Different Media Composition, Light Intensity and Photoperiod on Morphology and Physiology of Freshwater Microalgae Ankistrodesmus Falcatus–A Potential Strain for Bio-Fuel Production. Bioresour. Technol. 2014, 171, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Suriya Narayanan, G.; Kumar, G.; Seepana, S.; Elankovan, R.; Arumugan, S.; Premalatha, M. Isolation, Identification and Outdoor Cultivation of Thermophilic Freshwater Microalgae Coelastrella sp. FI69 in Bubble Column Reactor for the Application of Biofuel Production. Biocatal. Agric. Biotechnol. 2018, 14, 357–365. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Hu, Q.; Cheng, P.; Ji, B.; Liu, J.; Chen, Y.; Zhang, W.; Chen, X.; Chen, L.; et al. Attached Cultivation Technology of Microalgae for Efficient Biomass Feedstock Production. Bioresour. Technol. 2013, 127, 216–222. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint Production of Biodiesel and Bioethanol from Filamentous Oleaginous Microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef]
- Randrianarison, G.; Ashraf, M.A. Microalgae: A Potential Plant for Energy Production. Geol. Ecol. Landsc. 2017, 1, 104–120. [Google Scholar] [CrossRef]
- He, Y.; Hong, Y.; Liu, X.; Zhang, Q.; Liu, P.; Wang, S. Influences of Carbon and Nitrogen Sources and Metal Ions on the Heterotrophic Culture of Scenedesmus Sp. LX1. Environ. Sci. Pollut. Res. 2019, 26, 13381–13389. [Google Scholar] [CrossRef]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for Oil: Strain Selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a Low-Cost Photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Lam, M.K.; Uemura, Y.; Mansor, N.; Lim, J.W.; Show, P.L.; Tan, I.S.; Lim, S. High Biodiesel Yield from Wet Microalgae Paste via In-Situ Transesterification: Effect of Reaction Parameters towards the Selectivity of Fatty Acid Esters. Fuel 2020, 272, 117718. [Google Scholar] [CrossRef]
- Scott, S.A.; Davey, M.P.; Dennis, J.S.; Horst, I.; Howe, C.J.; Lea-Smith, D.J.; Smith, A.G. Biodiesel from Algae: Challenges and Prospects. Curr. Opin. Biotechnol. 2010, 21, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Srikanth, S.; Chiranjeevi, P.; Arora, S.; Chandra, R. Algal Biocathode for in Situ Terminal Electron Acceptor (TEA) Production: Synergetic Association of Bacteria–Microalgae Metabolism for the Functioning of Biofuel Cell. Bioresour. Technol. 2014, 166, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Trissl, H.-W. Modeling the Excitation Energy Capture in Thylakoid Membranes. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2003; Volume 14, pp. 245–276. ISBN 978-94-010-3772-3. [Google Scholar]
- Raven, J.A.; Geider, R.J. Adaptation, Acclimation and Regulation in Algal Photosynthesis. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2003; Volume 14, pp. 385–412. ISBN 978-94-010-3772-3. [Google Scholar]
- Kern, J.; Renger, G. Photosystem II: Structure and Mechanism of the Water:Plastoquinone Oxidoreductase. Photosynth. Res. 2007, 94, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Ostendorf, E.; Kohzuma, K.; Hoh, D.; Strand, D.D.; Sato-Cruz, M.; Savage, L.; Cruz, J.A.; Fisher, N.; Froehlich, J.E.; et al. Chloroplast ATP Synthase Modulation of the Thylakoid Proton Motive Force: Implications for Photosystem I and Photosystem II Photoprotection. Front. Plant Sci. 2017, 8, 719. [Google Scholar] [CrossRef] [PubMed]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and Characterization of Bio-Oil from Hydrothermal Liquefaction of Microalgae Dunaliella Tertiolecta Cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Singh, J.S. Microbes: The Chief Ecological Engineers in Reinstating Equilibrium in Degraded Ecosystems. Agric. Ecosyst. Environ. 2015, 203, 80–82. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine Macroalgae: An Untapped Resource for Producing Fuels and Chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef]
- Al-lwayzy, S.; Yusaf, T.; Al-Juboori, R. Biofuels from the Fresh Water Microalgae Chlorella Vulgaris (FWM-CV) for Diesel Engines. Energies 2014, 7, 1829–1851. [Google Scholar] [CrossRef]
- Cancela, Á.; Maceiras, R.; Sánchez, Á.; Alfonsin, V.; Urrejola, S. Transesterification of Marine Macroalgae Using Microwave Technology. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 1598–1603. [Google Scholar] [CrossRef]
- Nugent, J.H.A.; Purton, S.; Evans, M.C.W. Oxygenic Photosynthesis in Algae and Cyanobacteria: Electron Transfer in Photosystems I and II. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2003; Volume 14, pp. 133–156. ISBN 978-94-010-3772-3. [Google Scholar]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A Review of the Substrates Used in Microbial Fuel Cells (MFCs) for Sustainable Energy Production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Wegner, T.H.; Jones, E.P. A Fundamental Review of the Relationships between Nanotechnology and Lignocellulosic Biomass. In The Nanoscience and Technology of Renewable Biomaterials; Lucia, L.A., Rojas, O.J., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 1–41. ISBN 978-1-4051-6786-4. [Google Scholar]
- Magdalena, J.; Ballesteros, M.; González-Fernandez, C. Efficient Anaerobic Digestion of Microalgae Biomass: Proteins as a Key Macromolecule. Molecules 2018, 23, 1098. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Shen, Y.; Gao, L.; Liao, Z.-H.; Sun, J.-Z.; Yong, Y.-C. Improving the Extracellular Electron Transfer of Shewanella Oneidensis MR-1 for Enhanced Bioelectricity Production from Biomass Hydrolysate. RSC Adv. 2017, 7, 30488–30494. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment Methods of Lignocellulosic Biomass for Anaerobic Digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Hou, S.; Zhang, J.; Wang, H.; Xie, J. Production of Electricity from Rice Straw with Different Pretreatment Methods Using a Sediment Microbial Fuel Cell. Int. J. Electrochem. Sci. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Xiao, B.; Yang, F.; Liu, J. Evaluation of Electricity Production from Alkaline Pretreated Sludge Using Two-Chamber Microbial Fuel Cell. J. Hazard. Mater. 2013, 254–255, 57–63. [Google Scholar] [CrossRef]
- Wagner, A.; Lackner, N.; Mutschlechner, M.; Prem, E.; Markt, R.; Illmer, P. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Resources to Enhance Biogas Production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass Pretreatment: Fundamentals toward Application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Saba, B.; Christy, A.D.; Yu, Z.; Co, A.C. Sustainable Power Generation from Bacterio-Algal Microbial Fuel Cells (MFCs): An Overview. Renew. Sustain. Energy Rev. 2017, 73, 75–84. [Google Scholar] [CrossRef]
- Hou, Q.; Nie, C.; Pei, H.; Hu, W.; Jiang, L.; Yang, Z. The Effect of Algae Species on the Bioelectricity and Biodiesel Generation through Open-Air Cathode Microbial Fuel Cell with Kitchen Waste Anaerobically Digested Effluent as Substrate. Bioresour. Technol. 2016, 218, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Logan, B.E. Ammonia Treatment of Carbon Cloth Anodes to Enhance Power Generation of Microbial Fuel Cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Wei, J.; Liang, P.; Huang, X. Recent Progress in Electrodes for Microbial Fuel Cells. Bioresour. Technol. 2011, 102, 9335–9344. [Google Scholar] [CrossRef]
- Commault, A.S.; Lear, G.; Weld, R.J. Maintenance of Geobacter -Dominated Biofilms in Microbial Fuel Cells Treating Synthetic Wastewater. Bioelectrochemistry 2015, 106, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Yang, S.; Li, H.; Li, X. Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells. IJERPH 2018, 15, 1349. [Google Scholar] [CrossRef] [PubMed]
- Thorne, R.; Hu, H.; Schneider, K.; Bombelli, P.; Fisher, A.; Peter, L.M.; Dent, A.; Cameron, P.J. Porous Ceramic Anode Materials for Photo-Microbial Fuel Cells. J. Mater. Chem. 2011, 21, 18055. [Google Scholar] [CrossRef]
- Schneider, K.; Thorne, R.J.; Cameron, P.J. An Investigation of Anode and Cathode Materials in Photomicrobial Fuel Cells. Phil. Trans. R. Soc. A 2016, 374, 20150080. [Google Scholar] [CrossRef]
- Li, J.; Hu, J.; Yang, C.; Pu, W.; Hou, H.; Xu, J.; Liu, B.; Yang, J. Enhanced Detection of Toxicity in Wastewater Using a 2D Smooth Anode Based Microbial Fuel Cell Toxicity Sensor. RSC Adv. 2019, 9, 8700–8706. [Google Scholar] [CrossRef]
- Hou, Q.; Pei, H.; Hu, W.; Jiang, L.; Yu, Z. Mutual Facilitations of Food Waste Treatment, Microbial Fuel Cell Bioelectricity Generation and Chlorella Vulgaris Lipid Production. Bioresour. Technol. 2016, 203, 50–55. [Google Scholar] [CrossRef]
- Douglas, S.E.; Raven, J.A.; Larkum, A.W.D. The Algae and Their General Characteristics. In Photosynthesis in Algae; Larkum, A.W.D., Douglas, S.E., Raven, J.A., Eds.; Advances in Photosynthesis and Respiration; Springer Netherlands: Dordrecht, The Netherlands, 2003; Volume 14, pp. 1–10. ISBN 978-94-010-3772-3. [Google Scholar]
- Choi, S.; Chae, J. Optimal Biofilm Formation and Power Generation in a Micro-Sized Microbial Fuel Cell (MFC). Sens. Actuators A Phys. 2013, 195, 206–212. [Google Scholar] [CrossRef]
- Choudhary, P.; Prajapati, S.K.; Kumar, P.; Malik, A.; Pant, K.K. Development and Performance Evaluation of an Algal Biofilm Reactor for Treatment of Multiple Wastewaters and Characterization of Biomass for Diverse Applications. Bioresour. Technol. 2017, 224, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Del Campo, A.; Perez, J.F.; Cañizares, P.; Rodrigo, M.A.; Fernandez, F.J.; Lobato, J. Characterization of Light/Dark Cycle and Long-Term Performance Test in a Photosynthetic Microbial Fuel Cell. Fuel 2015, 140, 209–216. [Google Scholar] [CrossRef]
- Microalgae: Global Strategic Business Report. Available online: https://www.researchandmarkets.com/reports/5140359/microalgae-global-strategic-business-report (accessed on 18 September 2023).
- González Del Campo, A.; Cañizares, P.; Rodrigo, M.A.; Fernández, F.J.; Lobato, J. Microbial Fuel Cell with an Algae-Assisted Cathode: A Preliminary Assessment. J. Power Sources 2013, 242, 638–645. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Species Selection for the Design of Gold Nanobioreactor by Photosynthetic Organisms. J. Nanopart. Res. 2012, 14, 883. [Google Scholar] [CrossRef]
- Hill, C.B.; Li, Y.; Hartman, G.L. Soybean Aphid Resistance in Soybean Jackson Is Controlled by a Single Dominant Gene. Crop Sci. 2006, 46, 1606–1608. [Google Scholar] [CrossRef]
- De Gorter, H.; Just, D.R. The Social Costs and Benefits of Biofuels: The Intersection of Environmental, Energy and Agricultural Policy. Appl. Econ. Perspect. Policy 2010, 32, 4–32. [Google Scholar] [CrossRef]
- Khanna, M.; Ando, A.W.; Taheripour, F. Welfare Effects and Unintended Consequences of Ethanol Subsidies. Rev. Agric. Econ. 2008, 30, 411–421. [Google Scholar] [CrossRef]
- Balu, A.M.; Budarin, V.; Shuttleworth, P.S.; Pfaltzgraff, L.A.; Waldron, K.; Luque, R.; Clark, J.H. Valorisation of Orange Peel Residues: Waste to Biochemicals and Nanoporous Materials. ChemSusChem 2012, 5, 1694–1697. [Google Scholar] [CrossRef]
- Kleinert, M.; Barth, T. Towards a Lignincellulosic Biorefinery: Direct One-Step Conversion of Lignin to Hydrogen-Enriched Biofuel. Energy Fuels 2008, 22, 1371–1379. [Google Scholar] [CrossRef]
- Volk, T. CO2 Rising: The World’s Greatest Environmental Challenge; MIT Press: Cambridge, MA, USA, 2008; ISBN 978-0-262-22083-5. [Google Scholar]
- Prabhakar, S.V.R.K.; Elder, M. Biofuels and Resource Use Efficiency in Developing Asia: Back to Basics. Appl. Energy 2009, 86, S30–S36. [Google Scholar] [CrossRef]
- Fischer, G.; Hizsnyik, E.; Prieler, S.; Shah, M.; van Velthuizen, H.T. Biofuels and Food Security: Implications of an Accelerated Biofuels Production. Available online: https://pure.iiasa.ac.at/id/eprint/8984/ (accessed on 14 September 2023).
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Polymeric Nanocomposites: Structure, Manufacture, And Properties. In Engineering of Polymers and Chemical Complexity; Focke, W.W.; Radusch, H.-J. (Eds.) Apple Academic Press: Palm Bay, FL, USA, 2014; Volume II, pp. 21–52. ISBN 978-0-429-15631-1. [Google Scholar]
- Windt, W.D.; Aelterman, P.; Verstraete, W. Bioreductive Deposition of Palladium (0) Nanoparticles on Shewanella Oneidensis with Catalytic Activity towards Reductive Dechlorination of Polychlorinated Biphenyls. Environ. Microbiol 2005, 7, 314–325. [Google Scholar] [CrossRef] [PubMed]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A Biorefinery Processing Perspective: Treatment of Lignocellulosic Materials for the Production of Value-Added Products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Cabral, E.M.; Mondala, J.R.M.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of Molecular Weight Fractionation on the Antimicrobial and Anticancer Properties of a Fucoidan Rich-Extract from the Macroalgae Fucus Vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Saeman, J.F. Kinetics of Wood Saccharification—Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Aderhold, D.; Williams, C.J.; Edyvean, R.G.J. The Removal of Heavy-Metal Ions by Seaweeds and Their Derivatives. Bioresour. Technol. 1996, 58, 1–6. [Google Scholar] [CrossRef]
- Reith, J.H.; Deurwaarder, E.P.; Hemmes, K.; Curvers, A.P.W.M.; Kamermans, P.; Brandenburg, W.; Zeeman, G. Bio-Offshore. Large-Scale Cultivation of Seaweeds in Combination with Offshore Wind Farms in the North Sea; Bio-Offshore. Grootschalige Teelt van Zeewieren in Combinatie met Offshore Windparken in de Noordzee. 2005. Available online: https://www.osti.gov/etdeweb/biblio/20653933 (accessed on 14 September 2022).
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from Microalgae: A Critical Evaluation from Laboratory to Large Scale Production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Afzal, S.; Yadav, A.K.; Poonia, A.K.; Choure, K.; Yadav, A.N.; Pandey, A. Antimicrobial Therapeutics Isolated from Algal Source: Retrospect and Prospect. Biologia 2022, 78, 291–305. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the Production of Bulk Chemicals and Biofuels. Biofuels Bioprod. Bioref. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and Macroalgal Biomass: A Renewable Source for Bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef]
- Goh, C.S.; Lee, K.T. A Visionary and Conceptual Macroalgae-Based Third-Generation Bioethanol (TGB) Biorefinery in Sabah, Malaysia as an Underlay for Renewable and Sustainable Development. Renew. Sustain. Energy Rev. 2010, 14, 842–848. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.W.R.; Khoo, K.S.; Yew, G.Y.; Leong, W.H.; Lim, J.W.; Lam, M.K.; Ho, Y.-C.; Ng, H.S.; Munawaroh, H.S.H.; Show, P.L. Advances in Production of Bioplastics by Microalgae Using Food Waste Hydrolysate and Wastewater: A Review. Bioresour. Technol. 2021, 342, 125947. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and Antimicrobial Activity of Biosynthesized Red Sea Marine Algal Silver Nanoparticles. Sci. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef]
- Xing, C.; Yuan, X.; Wu, X.; Shao, X.; Yuan, J.; Yan, W. Chemometric Classification and Quantification of Sesame Oil Adulterated with Other Vegetable Oils Based on Fatty Acids Composition by Gas Chromatography. LWT 2019, 108, 437–445. [Google Scholar] [CrossRef]
- Posten, C. Design Principles of Photo-Bioreactors for Cultivation of Microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Sun, C.-H.; Fu, Q.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X.; Reungsang, A.; Chang, H.-X. Life-Cycle Assessment of Biofuel Production from Microalgae via Various Bioenergy Conversion Systems. Energy 2019, 171, 1033–1045. [Google Scholar] [CrossRef]





| Algal Strain | Power/Voltage | Reactor Type | Electrode |
|---|---|---|---|
| Chlorella pyrenoidosa | 6400 mW/m3 | DCMFC/Mixed anaerobic sewage sludge in synthetic WW/BG-11 | Carbon felt |
| Chlorella vulgaris | 36.4 mW/m2 | DCMFC/Activated sludge/BG-11 | Nickel foam/Graphene |
| Chlorella vulgaris | 123.2 ± 27.5 mW/m3 | Textile wastewater/No catholyte, algal biofilm on air cathode | Carbon fibre brush |
| Chlorella vulgaris | 126 mW/m3 | DCMFC/Municipal Wastewater/BG-11; SCMFC/Real dye | Stainless Steel Mesh |
| Chlorella vulgaris | 3720 mW/m3 | PAMFC/Swine WW/Diluted swine WW | Carbon felt/carbon fibre |
| Chlorella vulgaris | 1108.9 mW/m3 | Bubbling PAMFC/Anaerobic sludge with phosphate buffer supplemented with glucose/BG-11 | Carbon felt/Carbon fibre cloth |
| Mixed Algal Culture | 3300 mW/m3 | PAMFC/Anaerobic sludge/Anolyte effluent anaerobic sludge | Carbon brush/Carbon cloth |
| Mixed Algal Culture | 268 mW/m2 | PAMFC/Anaerobic sludge/Anolyte effluent anaerobic sludge | Carbon fibre brush/Carbon cloth |
| Scenedesmus obliquus | 153 mW/m2 | DCMFC/Municipal wastewater/BBM | Plain carbon paper/carbon paper |
| Scenedesmus obliquus | 951 mW/m3 | DCMFC/Municipal wastewater replaced later with BBM/Ferricyanide | Toray carbon paper |
| Biomass | Pre-Treatment | Increased Biogas Yield |
|---|---|---|
| Chlorella vulgaris | Proteases (86–96% solubilization) | 51% |
| Scenedesmus sp. | Thermal (75 °C for 10 h) | 58% |
| Stigeoclonium sp. | Thermal (130 °C for 15–30 min) | 28% |
| Nitzschia sp | Thermal (130 °C for 15–30 min) | 28% |
| Scenedesmus sp. | Thermal (95 °C for 10 h) | 69% |
| Chlorella sp. | Thermal (70 °C for 30 min) | 37–48% |
| Chlamydomonas reinhardtii | Proteases (86–96% solubilization) | 7% |
| Chlorella sp. and Scenedesmus sp. | Chemical (CaO; 4 and 10% w/w) at 25, 55 and 72 °C | 25% |
| Monoraphidium sp. and Stigeoclonium sp. | Mechanical (26.7 KJ/g TS for 30 min) | 85% |
| Scenedesmus sp. | Proteases (30% solubilization) | 1.53-fold |
| Chlorella vulgaris and Scenedesmus sp. | Carbohydrases (84% and 36% solubilization) | 1.2-fold |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, I.; Pandey, A.; Shangdiar, S.; Rai, P.K.; Kumar, A.; Amesho, K.T.T.; Bux, F. Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future. Sustainability 2023, 15, 14029. https://doi.org/10.3390/su151814029
Singh I, Pandey A, Shangdiar S, Rai PK, Kumar A, Amesho KTT, Bux F. Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future. Sustainability. 2023; 15(18):14029. https://doi.org/10.3390/su151814029
Chicago/Turabian StyleSingh, Indrajeet, Ashutosh Pandey, Sumarlin Shangdiar, Piyush Kant Rai, Ajay Kumar, Kassian T. T. Amesho, and Faizal Bux. 2023. "Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future" Sustainability 15, no. 18: 14029. https://doi.org/10.3390/su151814029
APA StyleSingh, I., Pandey, A., Shangdiar, S., Rai, P. K., Kumar, A., Amesho, K. T. T., & Bux, F. (2023). Towards Sustainable Energy: Harnessing Microalgae Biofuels for a Greener Future. Sustainability, 15(18), 14029. https://doi.org/10.3390/su151814029










