Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells
Abstract
1. Introduction
2. Materials and Methods
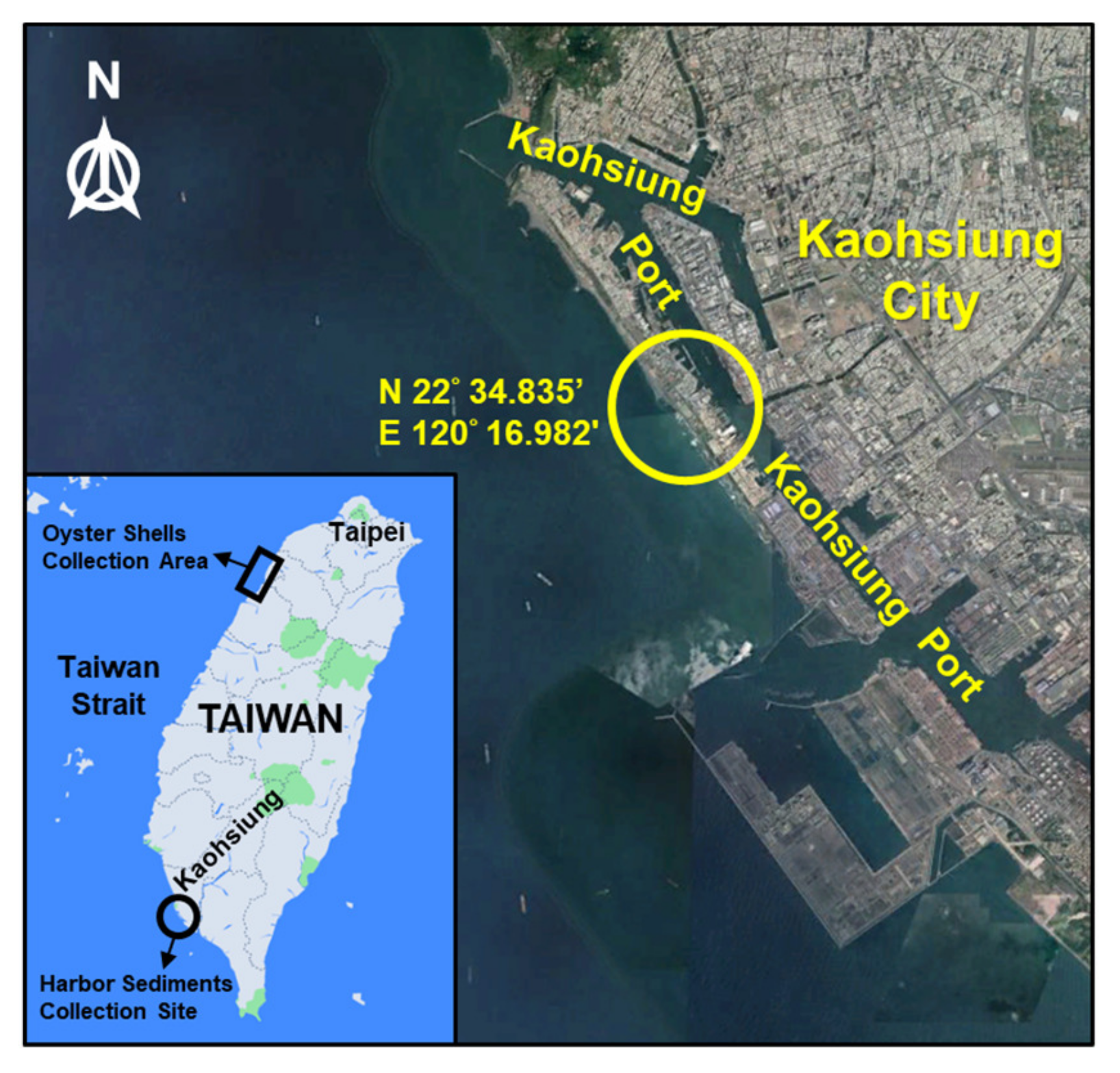
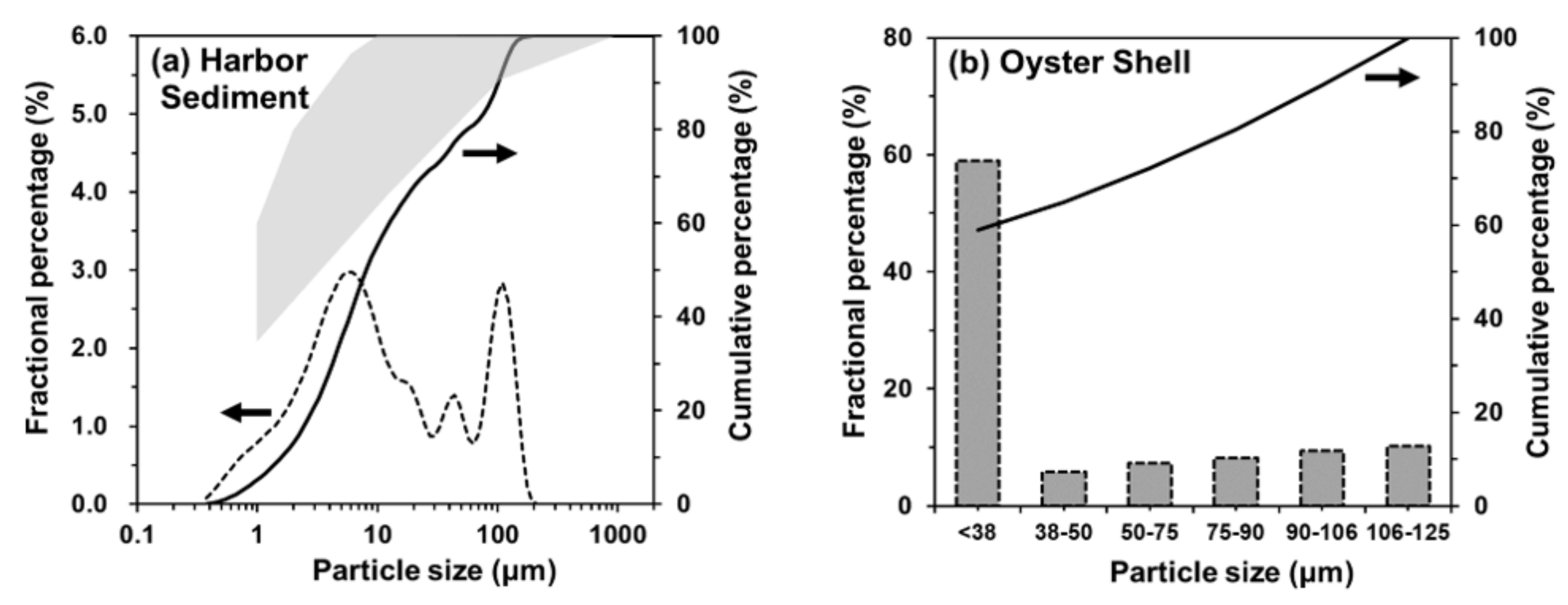
2.1. Raw Materials
2.2. Aggregate Preparation
2.3. Aggregate Performance Tests
2.4. Statistical Analysis
3. Results and Discussion
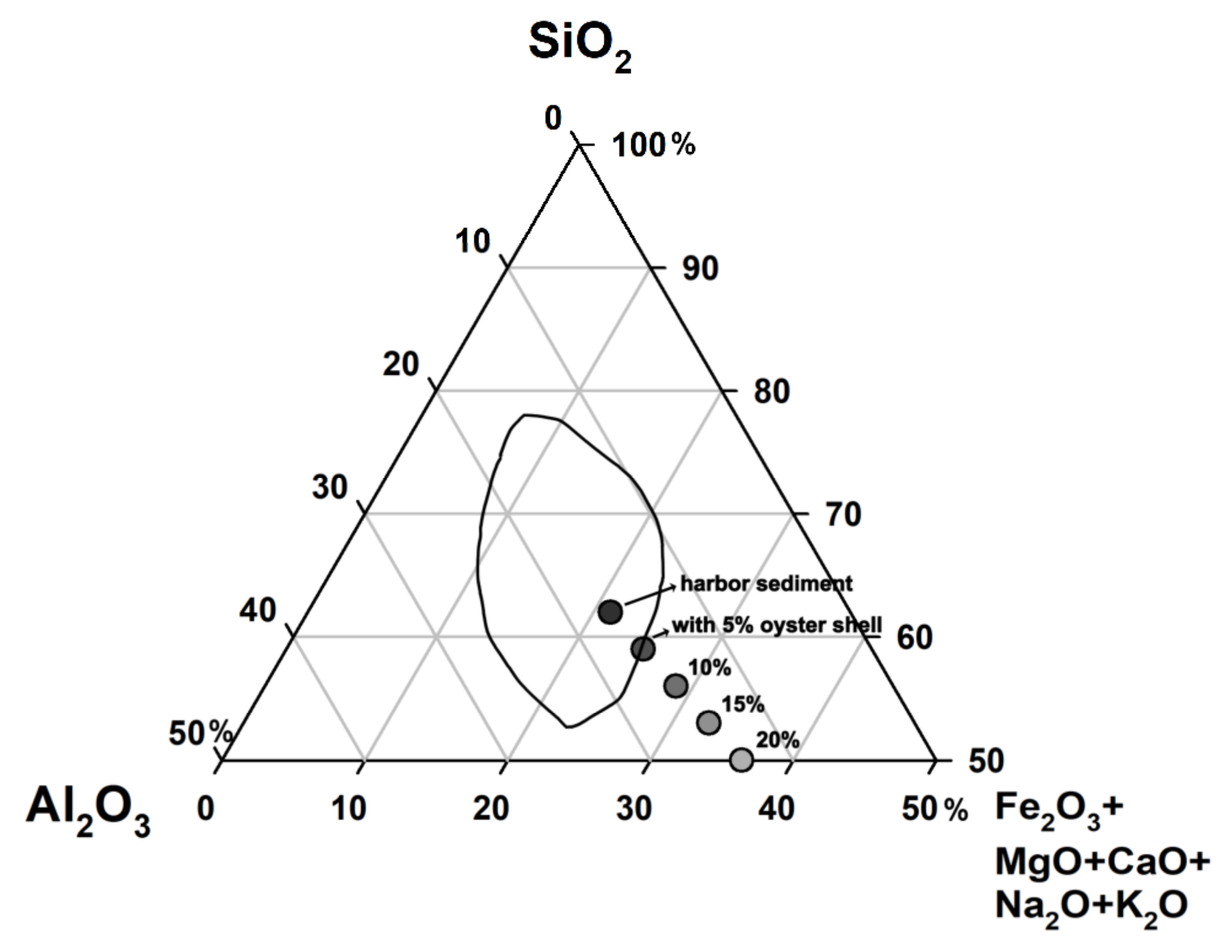
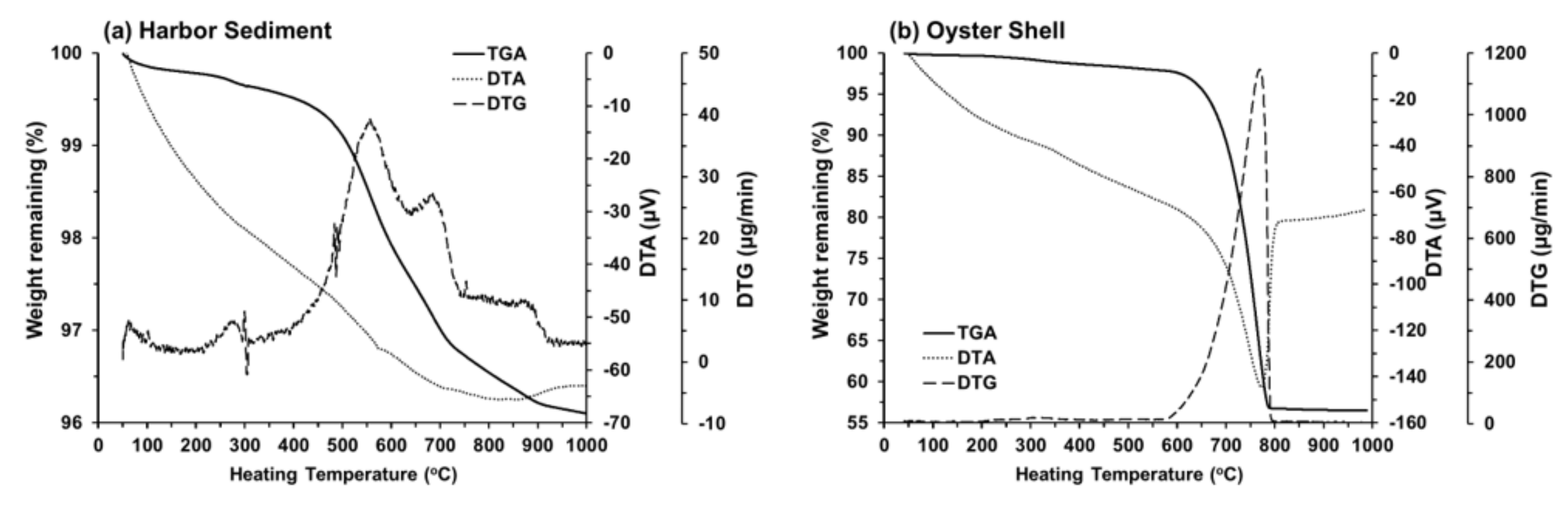
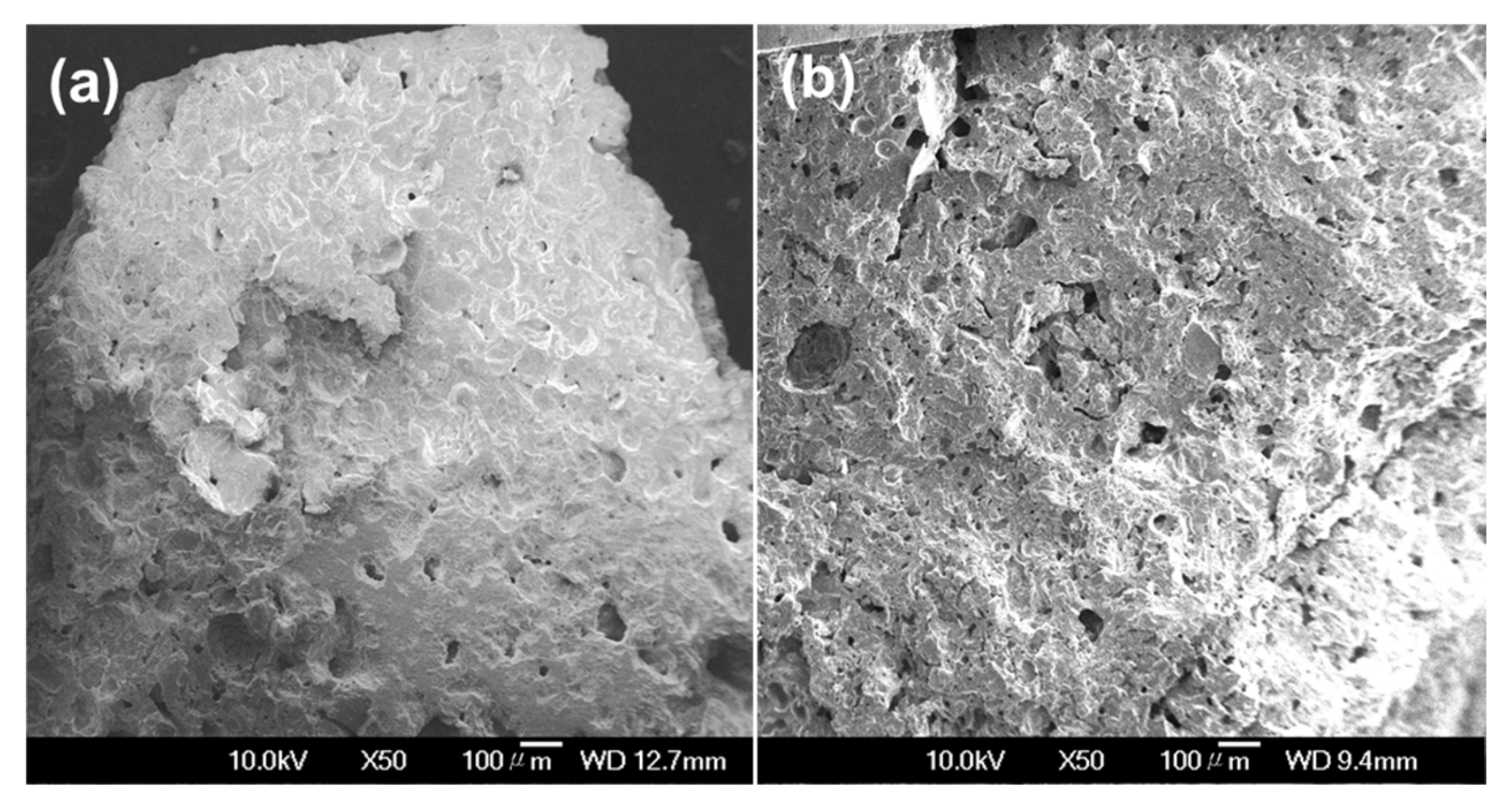
3.1. Effects of Sintering Temperature and Oyster Shell Content
3.2. Aggregate Performance and Possible Applications
3.3. Leachability of Toxic Metals and Chloride Ions
3.4. Economic and Environmental Considerations
4. Conclusions
- (1)
- Dredged harbor sediments admixed with oyster shells could feasibly be sintered into lightweight aggregates (LWAs) with suitable engineering properties for construction applications.
- (2)
- The recommended optimal oyster shell content and sintering temperature are 5–15% and 1125 °C, respectively.
- (3)
- The oyster shell content and sintering temperature firmly controlled the LWA’s engineering properties (particle density, water absorption, and crushing strength). Briefly, the addition of oyster shells was beneficial in reducing the particle density but was conducive to water absorption, and higher sintering temperatures could keep the water absorption low and increase the crushing strength.
- (4)
- The sintering process could effectively mitigate the leachability of chloride ions and metals in the sediment-based LWAs, making the LWAs safe for building material applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crocetti, P.; González-Camejo, J.; Li, K.; Foglia, A.; Eusebi, A.L.; Fatone, F. An overview of operations and processes for circular management of dredged sediments. Waste Manag. 2022, 146, 20–35. [Google Scholar] [CrossRef]
- Bray, N.; Cohen, M. Dredging for Development; International Association of Dredging Companies (IADC): Voorburg, The Netherlands; International Association of Ports and Harbors (IAPH): Tokyo, Japan, 2010. [Google Scholar]
- Chen, C.F.; Ju, Y.R.; Chen, C.W.; Dong, C.D. Changes in the total content and speciation patterns of metals in the dredged sediments after ocean dumping: Taiwan continental slope. Ocean Coast Manag. 2019, 181, 104893. [Google Scholar] [CrossRef]
- Chen, C.F.; Ju, Y.R.; Chen, C.W.; Dong, C.D. Vertical profile, contamination assessment, and source apportionment of heavy metals in sediment cores of Kaohsiung Harbor, Taiwan. Chemosphere 2016, 165, 67–79. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.F.; Chen, C.W. Vertical profile, sources, and equivalent toxicity of polycyclic aromatic hydrocarbons in sediment cores from the river mouths of Kaohsiung Harbor, Taiwan. Mar. Pollut. Bull. 2014, 85, 665–671. [Google Scholar] [CrossRef]
- Bortali, M.; Rabouli, M.; Yessari, M.; Hajjaji, A. Characterizing harbor dredged sediment for sustainable reuse as construction material. Sustainability 2023, 15, 1834. [Google Scholar] [CrossRef]
- Manap, N.; Voulvoulis, N. Environmental management for dredging sediments–The requirement of developing nations. J. Environ. Manag. 2015, 147, 338–348. [Google Scholar] [CrossRef]
- Slimanou, H.; Eliche-Quesada, D.; Kherbache, S.; Bouzidi, N. Harbor Dredged Sediment as raw material in fired clay brick production: Characterization and properties. J. Build. Eng. 2020, 28, 101085. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.; Li, J.S.; Baek, K.; Hou, D.; Ding, S.; Poon, C.S. Recycling dredged sediment into fill materials, partition blocks, and paving blocks: Technical and economic assessment. J. Clean. Prod. 2018, 199, 69–76. [Google Scholar] [CrossRef]
- Dubois, V.; Abriak, N.E.; Zentar, R.; Ballivy, G. The use of marine sediments as a pavement base material. Waste Manag. 2009, 29, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Dauji, S. Disposal of sediments for sustainability: A review. Int. J. Econ. Energy Environ. 2017, 2, 96–103. [Google Scholar] [CrossRef]
- Shi, J.; Liu, B.; He, Z.; Liu, Y.; Jiang, J.; Xiong, T.; Shi, J. A green ultra-lightweight chemically foamed concrete for building exterior: A feasibility study. J. Clean. Prod. 2021, 288, 125085. [Google Scholar] [CrossRef]
- El Mahdi Safhi, A.; Rivard, P.; Yahia, A.; Benzerzour, M.; Khayat, K.H. Valorization of dredged sediments in self-consolidating concrete: Fresh, hardened, and microstructural properties. J. Clean. Prod. 2020, 263, 121472. [Google Scholar] [CrossRef]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.E. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. Int. J. Sediment Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lin, S.K.; Ju, Y.R.; Wu, C.H.; Lin, Y.L.; Chen, C.W.; Dong, C.D. Reutilization of dredged harbor sediment and steel slag by sintering as lightweight aggregate. Process Saf. Environ. Prot. 2019, 126, 287–296. [Google Scholar] [CrossRef]
- Wei, Y.L.; Lin, C.Y.; Cheng, S.H.; Wang, H.P. Recycling steel-manufacturing slag and harbor sediment into construction materials. J. Hazard. Mater. 2014, 265, 253–260. [Google Scholar] [CrossRef]
- Lim, Y.C.; Chen, C.F.; Chen, C.W.; Dong, C.D. Application of basic oxygen furnace slag in increased utilization of dredged harbor sediment. J. Sustain. Metall. 2021, 7, 704–717. [Google Scholar] [CrossRef]
- Todaro, F.; De Gisi, S.; Notarnicola, M. Contaminated marine sediments: Waste or resource? An overview of treatment technologies. Procedia Environ. Sci. Eng. Manag 2016, 3, 157–164. [Google Scholar]
- Lim, Y.C.; Chen, C.F.; Chen, C.W.; Dong, C.D. Development of alternative disposals for waste rice husk and dredged harbor sediment by sintering as lightweight aggregates. Environ. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Bhairappanavar, S.; Liu, R.; Shakoor, A. Eco-friendly dredged material-cement bricks. Constr. Build. Mater. 2021, 271, 121524. [Google Scholar] [CrossRef]
- Hassanpour, M.; Shafigh, P.; Mahmud, H.B. Lightweight aggregate concrete fiber reinforcement–a review. Constr. Build. Mater. 2012, 37, 452–461. [Google Scholar] [CrossRef]
- Tala, S.; Al-Ajlouni, M.G.; Ayad, J.Y.; Othman, Y.A.; Hilaire, R.S. Performance of six different soilless green roof substrates for the Mediterranean region. Sci. Total Environ. 2020, 730, 139182. [Google Scholar]
- Ayati, B.; Ferrándiz-Mas, V.; Newport, D.; Cheeseman, C. Use of clay in the manufacture of lightweight aggregate. Constr. Build. Mater. 2018, 162, 124–131. [Google Scholar] [CrossRef]
- Dondi, M.; Capaggregateti, P.; D’Amore, M.; de Gennaro, R.; Graziano, S.F.; Langella, A.; Raimondo, M.; Zanelli, C. Lightweight aggregates from waste materials: Reappraisal of expansion behavior and prediction schemes for bloating. Constr. Build. Mater. 2016, 127, 394–409. [Google Scholar] [CrossRef]
- European Market Observatory for Fisheries and Aquaculture Products. Oysters in the EU. 2022. Available online: https://www.eumofa.eu/documents/20178/517783/PTAT+Oyster_EN.pdf (accessed on 31 January 2023).
- Taiwan Fisheries. Annual Report 2021. Available online: https://www.fa.gov.tw/view.php?theme=FS_AR&subtheme=&id=21 (accessed on 31 January 2023). (In Chinese)
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The potential use of oyster shell waste in new value-added by-product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- de Alvarenga, R.A.F.; Galindro, B.M.; de Fátima Helpa, C.; Soares, S.R. The recycling of oyster shells: An environmental analysis using Life Cycle Assessment. J. Environ. Manag. 2012, 106, 102–109. [Google Scholar] [CrossRef]
- Wei, Y.L.; Kuo, P.J.; Yin, Y.Z.; Huang, Y.T.; Chung, T.H.; Xie, X.Q. Co-sintering oyster shell with hazardous steel fly ash and harbor sediment into construction materials. Constr. Build. Mater. 2018, 172, 224–232. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, R.; Xu, Y.; Ye, F.; Xu, R.; Han, Y. Effect of SiO2, Al2O3 and CaO on characteristics of lightweight aggregates produced from MSWI bottom ash sludge (MSWI-BAS). Constr. Build. Mater. 2019, 205, 368–376. [Google Scholar] [CrossRef]
- Veit, U.; Rüssel, C. Density and Young s Modulus of ternary glasses close to the eutectic composition in the CaO–Al2O3–SiO2-system. Ceram. Int. 2016, 42, 5810–5822. [Google Scholar] [CrossRef]
- Riley, C.M. Relation of chemical properties to the bloating of clays. J. Am. Ceram. Soc. 1951, 34, 121–128. [Google Scholar] [CrossRef]
- Cougny, G. Specifications for clayey raw materials used to produce expanded lightweight aggregates. Bull. Eng. Geol. Environ. 1990, 41, 47–55. [Google Scholar]
- Qi, Y.; Yue, Q.; Han, S.; Yue, M.; Gao, B.; Yu, H.; Shao, T. Preparation and mechanism of ultra-lightweight ceramics produced from sewage sludge. J. Hazard. Mater. 2010, 176, 76–84. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, K.G.; Lee, Y.S.; Wie, Y.M. Manufacturing and application of artificial lightweight aggregate from water treatment sludge. J. Clean. Prod. 2021, 307, 127260. [Google Scholar] [CrossRef]
- Moreno-Maroto, J.M.; González-Corrochano, B.; Alonso-Azcárate, J.; Rodríguez, L.; Acosta, A. Manufacturing of lightweight aggregates with carbon fiber and mineral wastes. Cem. Concr. Compos. 2017, 83, 335–348. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodríguez, L.; Lorenzo, A.P.; Torío, M.F.; Ramos, J.J.T.; Corvinos, M.D.; Muro, C. Valorization of washing aggregate sludge and sewage sludge for lightweight aggregates production. Constr. Build. Mater. 2016, 116, 252–262. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Bai, Y.; Xu, G. Effects of sintering temperature on the characteristics of lightweight aggregate made from sewage sludge and river sediment. J. Alloys Compd. 2018, 748, 522–527. [Google Scholar] [CrossRef]
- Li, B.; Ling, T.C.; Qu, L.; Wang, Y. Effects of a two-step heating process on the properties of lightweight aggregate prepared with sewage sludge and saline clay. Constr. Build. Mater. 2016, 114, 119–126. [Google Scholar] [CrossRef]
- Lim, Y.C.; Shih, Y.J.; Tsai, K.C.; Yang, W.D.; Chen, C.W.; Dong, C.D. Recycling dredged harbor sediment to construction materials by sintering with steel slag and waste glass: Characteristics, alkali-silica reactivity and metals stability. J. Environ. Manag. 2020, 270, 110869. [Google Scholar] [CrossRef]
- Chen, H.J.; Wang, S.Y.; Tang, C.W. Reuse of incineration fly ashes and reaction ashes for manufacturing lightweight aggregate. Constr. Build. Mater. 2010, 24, 46–55. [Google Scholar] [CrossRef]
- Souza, M.M.; Anjos, M.A.S.; Sá, M.V.V.A.; Souza, N.S.L. Developing and classifying lightweight aggregates from sewage sludge and rice husk ash. Case Stud. Constr. Mater. 2020, 12, e00340. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Virdi, G.S. Properties and microstructure of lightweight aggregate produced from sintered sewage sludge ash. Resour. Conserv. Recycl. 2005, 45, 18–30. [Google Scholar] [CrossRef]
- Tang, C.W.; Chen, H.J.; Wang, S.Y.; Spaulding, J. Production of synthetic lightweight aggregate using reservoir sediments for concrete and masonry. Cem. Concr. Compos. 2011, 33, 292–300. [Google Scholar] [CrossRef]
- Mangialardi, T. Sintering of MSW fly ash for reuse as a concrete aggregate. J. Hazard. Mater. 2001, 87, 225–239. [Google Scholar] [CrossRef] [PubMed]
- González-Corrochano, B.; Alonso-Azcárate, J.; Rodas, M. Effect of thermal treatment on the retention of chemical elements in the structure of lightweight aggregates manufactured from contaminated mine soil and fly ash. Constr. Build. Mater. 2012, 35, 497–507. [Google Scholar] [CrossRef]
- Xu, G.; Liu, M.; Li, G. Stabilization of heavy metals in lightweight aggregate made from sewage sludge and river sediment. J. Hazard. Mater. 2013, 260, 74–81. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | K2O | CaO | Na2O | MgO | LOI | |
|---|---|---|---|---|---|---|---|---|
| Harbor sediment | 62.0 | 16.8 | 7.2 | 4.0 | 2.5 | 2.3 | 2.2 | 3.9 |
| Oyster shell | 2.1 | 0.8 | 0.4 | 0.2 | 92.6 | 0.9 | 0.8 | 43.5 |
| Ts | OS | PD | WA | CS | WL | BI | |
|---|---|---|---|---|---|---|---|
| PD | 0.41 * | −0.70 ** | |||||
| WA | −0.67 ** | 0.58 ** | −0.89 ** | ||||
| CS | 0.91 ** | −0.21 | 0.68 ** | −0.84 ** | |||
| WL | 0.11 | 0.98 ** | −0.66 ** | 0.52 ** | −0.12 | ||
| BI | −0.48 * | 0.60 ** | −0.78 ** | 0.80 ** | −0.63 ** | 0.53 ** | |
| Po | −0.71 ** | 0.52 ** | −0.84 ** | 0.98 ** | −0.85 ** | 0.45 ** | 0.79 ** |
| Group | Ts (°C) | OS (%) | PD (g/cm3) | WA (%) | CS (MPa) | Application |
|---|---|---|---|---|---|---|
| A-1 | 1000–1100 | 5–15 | 1.66–1.76 | 15.6–18.8 | 1.0–5.8 | Non-structural LWC |
| A-2 | 1000–1100 | 20 | 1.57–1.61 | 21.0–22.0 | 1.0–2.2 | Gardening and geotechnical uses |
| B-1 | 1125 | 5–15 | 1.73–1.83 | 12.2–15.1 | 7.2–10.4 | Structural LWC |
| B-2 | 1125 | 20 | 1.58 | 20.0 | 3.8 | Acoustic and thermal insulation |
| C-1 | 1150 | 5–15 | 1.87–2.05 | 3.5–8.0 | 17.5–40.1 | Do not belong to LWA category |
| C-2 | 1150 | 20 | 1.66 | 14.3 | 16.5 | High-strength LWC |
| Raw Materials | Sintered Aggregates | Limits 2 | ||||
|---|---|---|---|---|---|---|
| HS 1 | OS 1 | 0% OS | 10% OS | 20% OS | ||
| Cl− | 5700 | 3100 | 16, 4, 1 3 | 24, 7, 3 | 25, 12, 6 | 120 |
| Harbor Sediment | Sintered Aggregates with Ts 1 of 1100 °C | Limits 2 | |||
|---|---|---|---|---|---|
| 0% OS 1 | 10% OS | 20% OS | |||
| Ag | nd 3 | nd | nd | nd | 5.0 |
| As | nd | nd | nd | nd | 5.0 |
| Cd | 0.03 | nd | nd | nd | 1.0 |
| Cr | 0.05 | nd | 0.02 | 0.07 | 5.0 |
| Cu | 0.71 | 0.11 | 0.09 | nd | 15.0 |
| Hg | nd | nd | nd | nd | 0.2 |
| Ni | 0.28 | 0.03 | 0.02 | 0.02 | - |
| Pb | 0.05 | nd | nd | nd | 5.0 |
| Se | nd | nd | nd | nd | 1.0 |
| Zn | 6.75 | 0.23 | 0.08 | nd | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.C.; Chen, C.-F.; Chen, C.-W.; Dong, C.-D. Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells. Sustainability 2023, 15, 5466. https://doi.org/10.3390/su15065466
Lim YC, Chen C-F, Chen C-W, Dong C-D. Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells. Sustainability. 2023; 15(6):5466. https://doi.org/10.3390/su15065466
Chicago/Turabian StyleLim, Yee Cheng, Chih-Feng Chen, Chiu-Wen Chen, and Cheng-Di Dong. 2023. "Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells" Sustainability 15, no. 6: 5466. https://doi.org/10.3390/su15065466
APA StyleLim, Y. C., Chen, C.-F., Chen, C.-W., & Dong, C.-D. (2023). Valorization of Dredged Harbor Sediments through Lightweight Aggregate Production: Application of Waste Oyster Shells. Sustainability, 15(6), 5466. https://doi.org/10.3390/su15065466








