Individual Pattern Response to CO2-Induced Acidification Stress in Haliotis rufescens Suggests Stage-Specific Acclimatization during Its Early Life History
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acclimation and Bioassay Overview
2.2. Water Chemistry Analyses
2.3. Hatching Success
2.4. Veliger Maturation Success
2.5. Morphometric Analyses
2.6. Birefringence
2.7. Competence Success
2.8. Settlement: Short- and Long-Term Acidification
2.9. Data Analysis
3. Results
3.1. pH Control and Water Chemistry
3.2. Larval Developmental Success
3.2.1. Hatching Success and Normal Shelled Larvae
3.2.2. Veliger Maturation Impairment
3.2.3. Competence Success
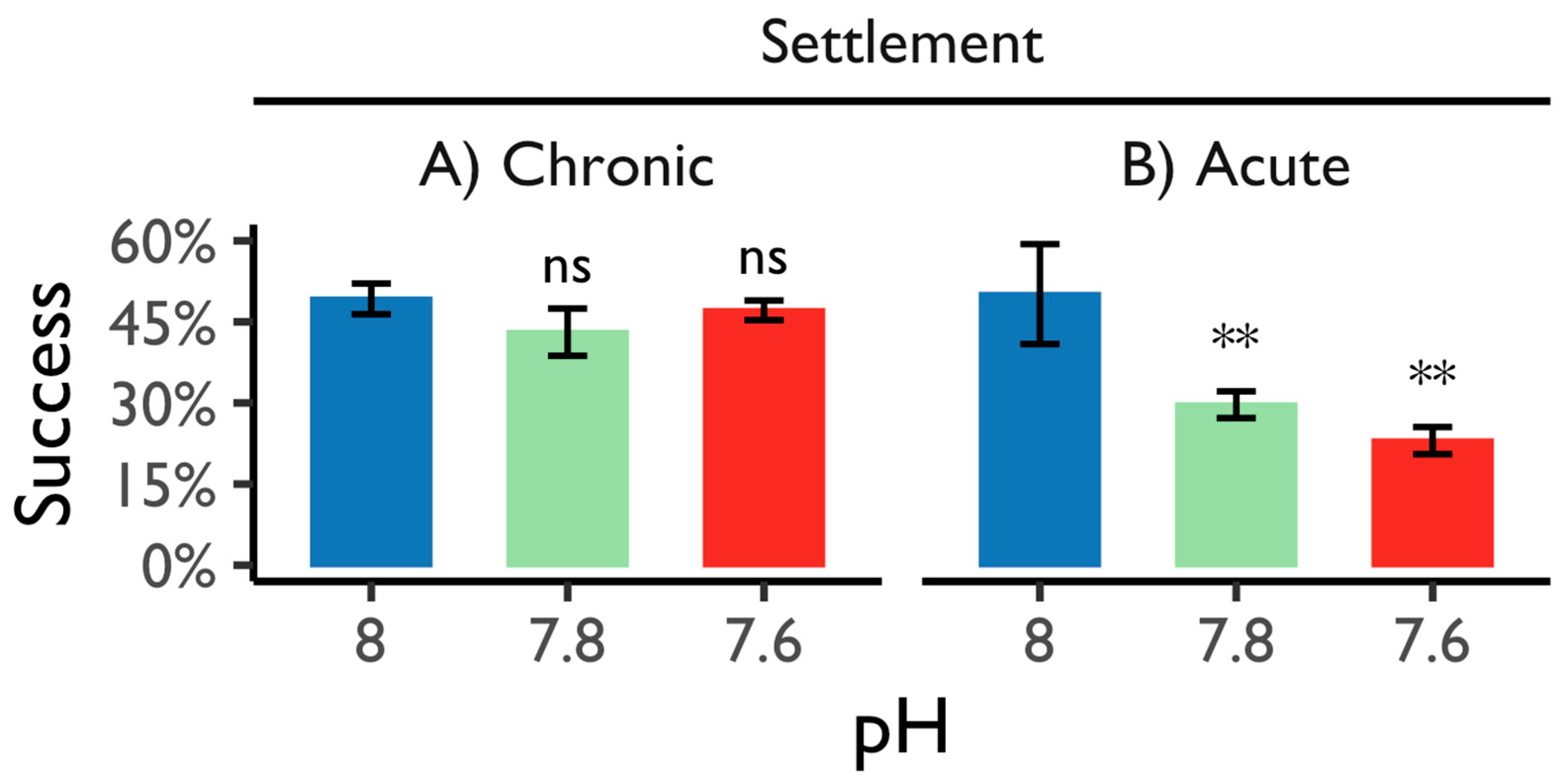
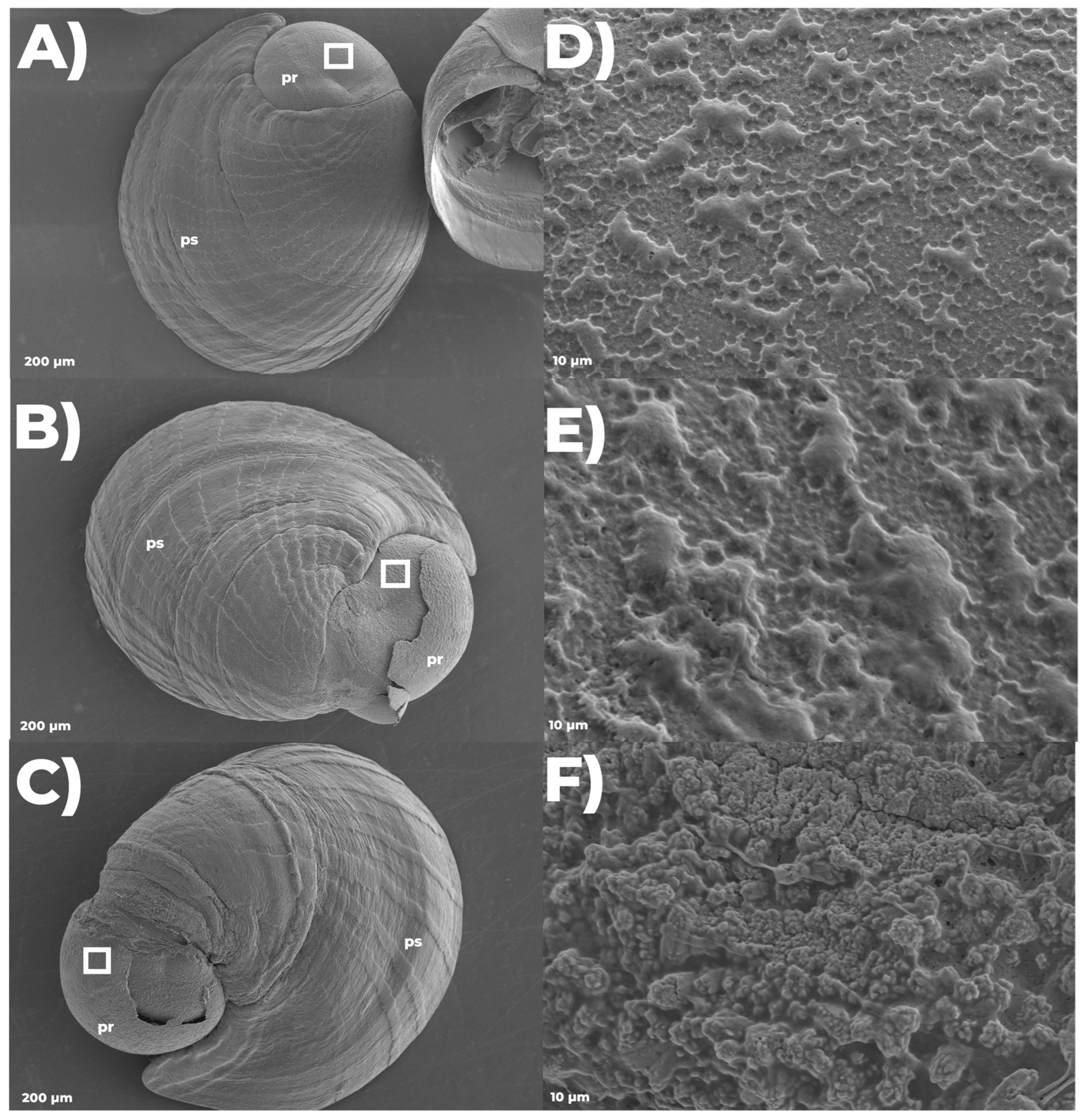
3.2.4. Settlement Success and Scanning Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zeebe, R.E. History of seawater carbonate chemistry, atmospheric CO2, and ocean acidification. Annu. Rev. Earth Planet. Sci. 2012, 40, 141–165. [Google Scholar] [CrossRef]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global carbon budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Millero, F.J. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science 2004, 305, 362–366. [Google Scholar] [CrossRef]
- Geiger, D.L.; Owen, B. Abalone: World-Wide Haliotidae; ConchBooks: Hackenhein, Germany, 2012. [Google Scholar]
- FAO. The State of the World’s Aquatic Genetic Resources For food and Agriculture; FAO Commission on Genetic Resources for Food and Agriculture Assessments: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Nielsen, C. Origin and diversity of marine larvae. In Evolutionary Ecology of Marine Invertebrate Larvae; Carrier, T.J., Reitzel, A.M., Heyland, A., Eds.; Oxford University Press: Oxford, UK, 2018; pp. 3–15. [Google Scholar] [CrossRef]
- Peters, H.; Rogers-Bennett, L.; De Shields, R.M. Haliotis rufescens. IUCN Red List. Threat. Species 2021, 2021, e.T78771583A78772573. [Google Scholar] [CrossRef]
- Feely, R.A.; Sabine, C.L.; Hernandez-Ayon, J.M.; Ianson, D.; Hales, B. Evidence for upwelling of corrosive “acidified” water onto the Continental Shelf. Science 2008, 320, 1490–1492. [Google Scholar] [CrossRef]
- Siedlecki, S.A.; Pilcher, D.; Howard, E.M.; Deutsch, C.; MacCready, P.; Norton, E.L.; Frenzel, H.; Newton, J.; Feely, R.A.; Alin, S.R.; et al. Coastal processes modify projections of some climate-driven stressors in the California Current System. Biogeosciences 2021, 18, 2871–2890. [Google Scholar] [CrossRef]
- Gazeau, F.; Parker, L.M.; Comeau, S.; Gattuso, J.P.; O’Connor, W.A.; Martin, S.; Pörtner, H.O.; Ross, P.M. Impacts of ocean acidification on marine shelled molluscs. Mar. Biol. 2013, 160, 2207–2245. [Google Scholar] [CrossRef]
- Byrne, M.; Ho, M.; Wong, E.; Soars, N.A.; Selvakumaraswamy, P.; Shepard-Brennand, H.; Dworjanyn, S.A.; Davis, A.R. Unshelled abalone and corrupted urchins: Development of marine calcifiers in a changing ocean. Proc. R. Soc. B Biol. Sci. 2010, 278, 2376–2383. [Google Scholar] [CrossRef]
- Crim, R.N.; Sunday, J.M.; Harley, C.D.G. Elevated seawater CO2 concentrations impair larval development and reduce larval survival in endangered northern abalone (Haliotis kamtschatkana). J. Exp. Mar. Biol. Ecol. 2011, 400, 272–277. [Google Scholar] [CrossRef]
- Kimura, R.; Takami, H.; Ono, T.; Onitsuka, T.; Nojiri, Y. Effects of elevated pCO2 on the early development of the commercially important gastropod, Ezo abalone Haliotis discus hannai. Fish. Oceanogr. 2011, 20, 357–366. [Google Scholar] [CrossRef]
- Guo, X.; Huang, M.; Pu, F.; You, W.; Ke, C. Effects of ocean acidification caused by rising CO2 on the early development of three mollusks. Aquat. Biol. 2015, 23, 147–157. [Google Scholar] [CrossRef]
- Onitsuka, T.; Takami, H.; Muraoka, D.; Matsumoto, Y.; Nakatsubo, A.; Kimura, R.; Ono, T.; Nojiri, Y. Effects of ocean acidification with pCO2 diurnal fluctuations on survival and larval shell formation of Ezo abalone, Haliotis discus hannai. Mar. Environ. Res. 2018, 134, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wessel, N.; Martin, S.; Badou, A.; Dubois, P.; Huchette, S.; Julia, V.; Nunes, F.; Harney, E.; Paillard, C.; Auzoux-Bordenave, S. Effect of CO2–induced ocean acidification on the early development and shell mineralization of the European abalone (Haliotis tuberculata). J. Exp. Mar. Biol. Ecol. 2018, 508, 52–63. [Google Scholar] [CrossRef]
- Auzoux-Bordenave, S.; Wessel, N.; Badou, A.; Martin, S.; M’Zoudi, S.; Avignon, S.; Roussel, S.; Huchette, S.; Dubois, P. Ocean acidification impacts growth and shell mineralization in juvenile abalone (Haliotis tuberculata). Mar. Biol. 2020, 167, 11. [Google Scholar] [CrossRef]
- Kavousi, J.; Roussel, S.; Martin, S.; Gaillard, F.; Badou, A.; Di Poi, C.; Huchette, S.; Dubois, P.; Auzoux-Bordenave, S. Combined effects of ocean warming and acidification on the larval stages of the European abalone Haliotis tuberculata. Mar. Pollut. Bull. 2022, 175, 113131. [Google Scholar] [CrossRef]
- Swezey, D.S.; Boles, S.E.; Aquilino, K.M.; Stott, H.K.; Bush, D.; Whitehead, A.; Rogers-Bennett, L.; Hill, T.M.; Sanford, E. Evolved differences in energy metabolism and growth dictate the impacts of ocean acidification on abalone aquaculture. Proc. Natl. Acad. Sci. USA 2020, 117, 26513–26519. [Google Scholar] [CrossRef]
- Guo, X.; Huang, M.; Luo, X.; You, W.; Ke, C. Effects of one-year exposure to ocean acidification on two species of abalone. Sci. Total Environ. 2022, 852, 158144. [Google Scholar] [CrossRef]
- Auzoux-Bordenave, S.; Ledoux, A.; Martin, S.; Di Poi, C.; Suquet, M.; Badou, A.; Gaillard, F.; Servili, A.; Le Goïc, N.; Huchette, S.; et al. Responses of early life stages of European abalone (Haliotis tuberculata) to ocean acidification after parental conditioning: Insights from a transgenerational experiment. Mar. Environ. Res. 2022, 181, 105753. [Google Scholar] [CrossRef]
- Prince, J.D.; Sellers, T.L.; Ford, W.B.; Talbot, S.R. Experimental evidence for limited dispersal of haliotid larvae (genus Halio- tis; Mollusca: Gastropoda). J. Exp. Mar. Biol. Ecol. 1987, 106, 243–263. [Google Scholar] [CrossRef]
- Burton, R.S.; Tegner, M.J. Enhancement of red abalone Haliotis rufescens stocks at San Miguel Island: Reassessing a success story. Mar. Ecol. Prog. Ser. 2000, 202, 303–308. [Google Scholar] [CrossRef]
- De Wit, P.; Palumbi, S.R. Transcriptome-wide polymorphisms of red abalone (Haliotis rufescens) reveal patterns of gene flow and local adaptation. Mol. Ecol. 2013, 22, 2884–2897. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Fabry, V.J.; Hansson, L.; Gattuso, J.P. Guide to Best Practices for Ocean Acidification Research and Data Reporting; reprinted edition including erratum; Publications Office of the European Union: Luxembourg, 2011; p. 258. [Google Scholar] [CrossRef]
- Johnson, K.M.; Sieburth, J.M.; Williams, P.J.L.; Brandstrom, L. Coulometric total carbon dioxide analysis for marine studies: Automation and calibration. Mar. Chem. 1987, 21, 117–133. [Google Scholar] [CrossRef]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. (Eds.) Guide to Best Practices for Ocean CO2 Measurement; North Pacific Marine Science Organization: Victoria, BC, Canada, 2007; p. 191. [Google Scholar]
- Lewis, E.R.; Wallace, D.W.R.; Allison, L.J. Program Developed for CO2 System Calculations; Environmental System Science Data Infrastructure for a Virtual Ecosystem (ESS-DIVE): Oak Ridge, TN, USA, 1998. [Google Scholar] [CrossRef]
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicx, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard potential of the reaction: AgCl(s) + 12H2(g) = Ag(s) + HCl(aq), and the standard acidity constant of the ion HSO-4 in synthetic sea water from 273.15 to 318.15 K. J. Chem. Thermodyn. 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Uppström, L.R. Boron/chlorinity ratio of deep-sea water from the Pacific Ocean. Deep-Sea Res. 1974, 21, 161–162. [Google Scholar] [CrossRef]
- Leighton, D.L. The Influence of Temperature on Larval and Juvenile Growth in Three Species of Southern California Abalones. Fish. Bull. 1972, 72, 1137–1145. [Google Scholar]
- Noisette, F.; Comtet, T.; Legrand, E.; Bordeyne, F.; Davoult, D.; Martin, S. Does Encapsulation Protect Embryos from the Effects of Ocean Acidification? The Example of Crepidula fornicata. PLoS ONE 2014, 9, e93021. [Google Scholar] [CrossRef]
- Shepard, S.A.; Tegner, M.J.; Guzman del Proo, S.A. Abalone of the World: Biology, Fisheries and Culture, 1st ed.; Wiley-Blackwell: Cambridge, MA, USA, 1972. [Google Scholar]
- Weiss, I.M.; Tuross, N.; Addadi, L.; Weiner, S. Mollusc larval shell formation: Amorphous calcium carbonate is a precursor phase for aragonite. J. Exp. Zool. 2002, 293, 478–491. [Google Scholar] [CrossRef]
- Kurihara, H.; Kato, S.; Ishimatsu, A. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat. Biol. 2007, 1, 91–98. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Leighton, D.L. The Biology and Culture of the California abalones, 1st ed.; Dorrance Pub Co. Inc.: Pittsburg, PA, USA, 2000. [Google Scholar]
- Keough, M.J.; Downes, B.J. Recruitment of marine invertebrates: The role of active larval choices and early mortality. Oecologia 1982, 54, 348–352. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 1 February 2023).
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Friendly, M.; Fox, J. candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. R Package Version 0.8-6. 2021. Available online: https://CRAN.R-project.org/package=candisc (accessed on 1 February 2023).
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F.; et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Li, J.; Jiang, Z.; Zhang, J.; Qiu, J.-W.; Du, M.; Bian, D.; Fang, J. Detrimental effects of reduced seawater pH on the early development of the Pacific abalone. Mar. Pollut. Bull. 2013, 74, 320–324. [Google Scholar] [CrossRef]
- Tahil, A.S.; Dy, D.T. Effects of reduced pH on the early larval development of hatchery-reared Donkey's ear abalone, Haliotis asinina (Linnaeus 1758). Aquaculture 2016, 459, 137–142. [Google Scholar] [CrossRef]
- Hurd, C.L.; Beardall, J.; Comeau, S.; Cornwall, C.E.; Havenhand, J.N.; Munday, P.L.; Parker, L.M.; Raven, J.A.; McGraw, C.M. Ocean acidification as a multiple driver: How interactions between changing seawater carbonate parameters affect marine life. Mar. Freshw. Res. 2020, 71, 263. [Google Scholar] [CrossRef]
- Bednaršek, N.; Calosi, P.; Feely, R.A.; Ambrose, R.; Byrne, M.; Chan, K.Y.K.; Weisberg, S.B. Synthesis of thresholds of ocean acidification impacts on echinoderms. Front. Mar. Sci. 2021, 8, 602601. [Google Scholar] [CrossRef]
- Dupont, S.; Thorndyke, M.C. Impact of CO2-Driven Ocean Acidification on Invertebrates Early Life-History—What We Know, What We Need to Know and What We Can Do. Biogeosciences Discuss. 2009, 6, 3109–3131. [Google Scholar] [CrossRef]
- Morash, A.J.; Alter, K. Effects of environmental and farm stress on abalone physiology: Perspectives for abalone aquaculture in the face of global climate change. Rev. Aquac. 2016, 8, 342–368. [Google Scholar] [CrossRef]
- Eyster, L.S.; Morse, M.P. Early shell formation during molluscan embryogenesis, with new studies on the surf clam, Spisula solidissima. Am. Zool. 1984, 24, 871–882. [Google Scholar] [CrossRef]
- Eyster, L.S. Shell inorganic composition and onset of shell mineralization during bivalve and gastropod embryogenesis. Biol. Bull. 1986, 170, 211–231. [Google Scholar] [CrossRef]
- Huang, Z.-X.; Chen, Z.-S.; Ke, C.-H.; Zhao, J.; You, W.-W.; Zhang, J.; Dong, W.-T.; Chen, J. Pyrosequencing of Haliotis diversicolor Transcriptomes: Insights into Early Developmental Molluscan Gene Expression. PLoS ONE 2012, 7, e51279. [Google Scholar] [CrossRef]
- Auzoux-Bordenave, S.; Badou, A.; Gaume, B.; Berland, S.; Helléouet, M.N.; Milet, C.; Huchette, S. Ultrastructure, chemistry and mineralogy of the growing shell of the European abalone Haliotis tuberculata. J. Struct. Biol. 2010, 171, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Hu, M.Y.; Thomsen, J.; Bleich, M.; Melzner, F. Mussel larvae modify calcifying fluid carbonate chemistry to promote calcification. Nat. Commun. 2017, 8, 1709. [Google Scholar] [CrossRef] [PubMed]
- Zippay, M.L.; Hofmann, G.E. Effect of pH on gene expression and thermal tolerance of early life history stages of red abalone (Haliotis rufescens). J. Shellfish Res. 2010, 29, 429–439. [Google Scholar] [CrossRef]
- Pernet, B. Larval Feeding: Mechanisms, Rates, and Performance in Nature; Oxford University Press: Oxford, UK, 2018; pp. 87–102. [Google Scholar] [CrossRef]
- Jaeckle, W.B.; Manahan, D.T. Feeding by a “nonfeeding” larva: Uptake of dissolved amino acids from seawater by lecithotrophic larvae of the gastropod Haliotis rufescens. Mar. Biol. 1989, 103, 87–94. [Google Scholar] [CrossRef]
- Stumpp, M.; Hu, M.; Casties, I.; Saborowski, R.; Bleich, M.; Melzner, F.; Dupont, S. Digestion in sea urchin larvae impaired under ocean acidification. Nat. Clim. Chang. 2013, 3, 1044–1049. [Google Scholar] [CrossRef]
- Jaeckle, W.B.; Manahan, D.T. Growth and energy imbalance during the development of a lecithotrophic molluscan larva (Haliotis rufescens). Biol. Bull. 1989, 177, 237–246. [Google Scholar] [CrossRef]
- Jardillier, E.; Rousseau, M.; Gendron-Badou, A.; Fröhlich, F.; Smith, D.C.; Martin, M.; Helléouet, M.-N.; Huchette, S.; Doumenc, D.; Auzoux-Bordenave, S. A morphological and structural study of the larval shell from the abalone Haliotis tuberculata. Mar. Biol. 2008, 154, 735–744. [Google Scholar] [CrossRef]
- Cigliano, M.; Gambi, M.C.; Rodolfo-Metalpa, R.; Patti, F.P.; Hall-Spencer, J.M. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar. Biol. 2010, 157, 2489–2502. [Google Scholar] [CrossRef]
- Tahil, A.S.; Dy, D.T. Effects of Reduced pH on Larval Settlement and Survival of the Donkey’s Ear Abalone, Haliotis asinina (Linnaeus 1758). Philipp. J. Sci. 2015, 144, 21–29. [Google Scholar]
- Pörtner, H. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef]
- Cannon, W.B. Bodily Changes in Pain, Hunger, Fear, and Rage; Appleton-Century-Crofts: New York, NY, USA, 1915. [Google Scholar]
- Costantini, D. Does hormesis foster organism resistance to extreme events? Front. Ecol. Environ. 2014, 12, 209–210. [Google Scholar] [CrossRef]
- Parker, L.M.; Ross, P.M.; O’Connor, W.A.; Borysko, L.; Raftos, D.A.; Pörtner, H.O. Adult exposure influences offspring response to ocean acidification in oysters. Glob. Chang. Biol. 2012, 18, 82–92. [Google Scholar] [CrossRef]
- Espinel-Velasco, N.; Lamare, M.; Kluibenschedl, A.; Moss, G.; Cummings, V. Ocean acidification induces carry-over effects on the larval settlement of the New Zealand abalone, Haliotis iris. ICES J. Mar. Sci. 2020, 78, 340–348. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef]
- Phillips, N.E.; Gaines, S.D. Spatial and temporal variability in size at settlement of intertidal mytilid mussels from around Pt. Conception, California. Invertebr. Reprod. Dev. 2002, 41, 171–177. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermúdez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 2012, 7, e34737. [Google Scholar] [CrossRef]
- O’Leary, J.K.; Barry, J.P.; Gabrielson, P.W.; Rogers-Bennett, L.; Potts, D.C.; Palumbi, S.R.; Micheli, F. Calcifying algae maintain settlement cues to larval abalone following algal exposure to extreme ocean acidification. Sci. Rep. 2017, 7, 5774. [Google Scholar] [CrossRef]
- Vargas, C.A.; de la Hoz, M.; Aguilera, V.; Martín, V.S.; Manríquez, P.H.; Navarro, J.M.; Torres, R.; Lardies, M.A.; Lagos, N.A. CO2-driven ocean acidification reduces larval feeding efficiency and changes food selectivity in the mollusk Concholepas concholepas. J. Plankton Res. 2013, 35, 1059–1068. [Google Scholar] [CrossRef]
- Strader, M.E.; Wong, J.M.; Hofmann, G.E. Ocean acidification promotes broad transcriptomic responses in marine metazoans: A literature survey. Front. Zool. 2020, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- López-Landavery, E.A.; Carpizo-Ituarte, E.J.; Pérez-Carrasco, L.; Díaz, F.; la Cruz, F.L.-D.; García-Esquivel, Z.; Hernández-Ayón, J.M.; Galindo-Sánchez, C.E. Acidification stress effect on umbonate veliger larval development in Panopea globosa. Mar. Pollut. Bull. 2021, 163, 111945. [Google Scholar] [CrossRef] [PubMed]
- Kapsenberg, L.; Bitter, M.C.; Miglioli, A.; Aparicio-Estalella, C.; Pelejero, C.; Gattuso, J.-P.; Dumollard, R. Molecular basis of ocean acidification sensitivity and adaptation in Mytilus galloprovincialis. IScience 2022, 25, 104677. [Google Scholar] [CrossRef]
- Gurr, S.J.; Trigg, S.A.; Vadopalas, B.; Roberts, S.B.; Putnam, H.M. Acclimatory gene expression of primed clams enhances robustness to elevated p CO2. Mol. Ecol. 2022, 31, 5005–5023. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
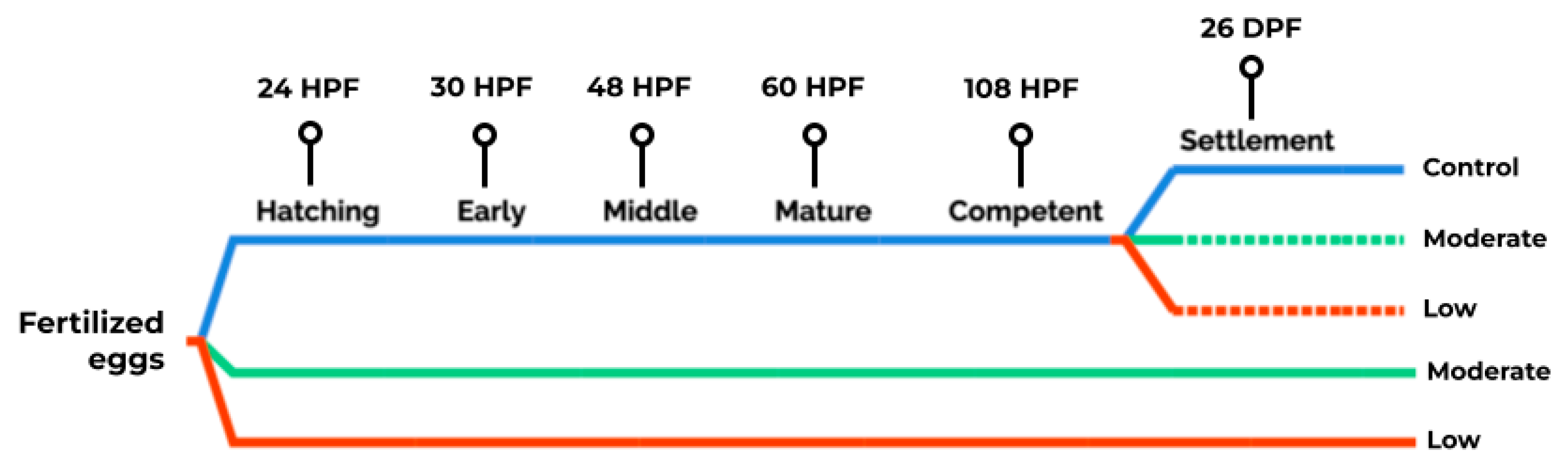
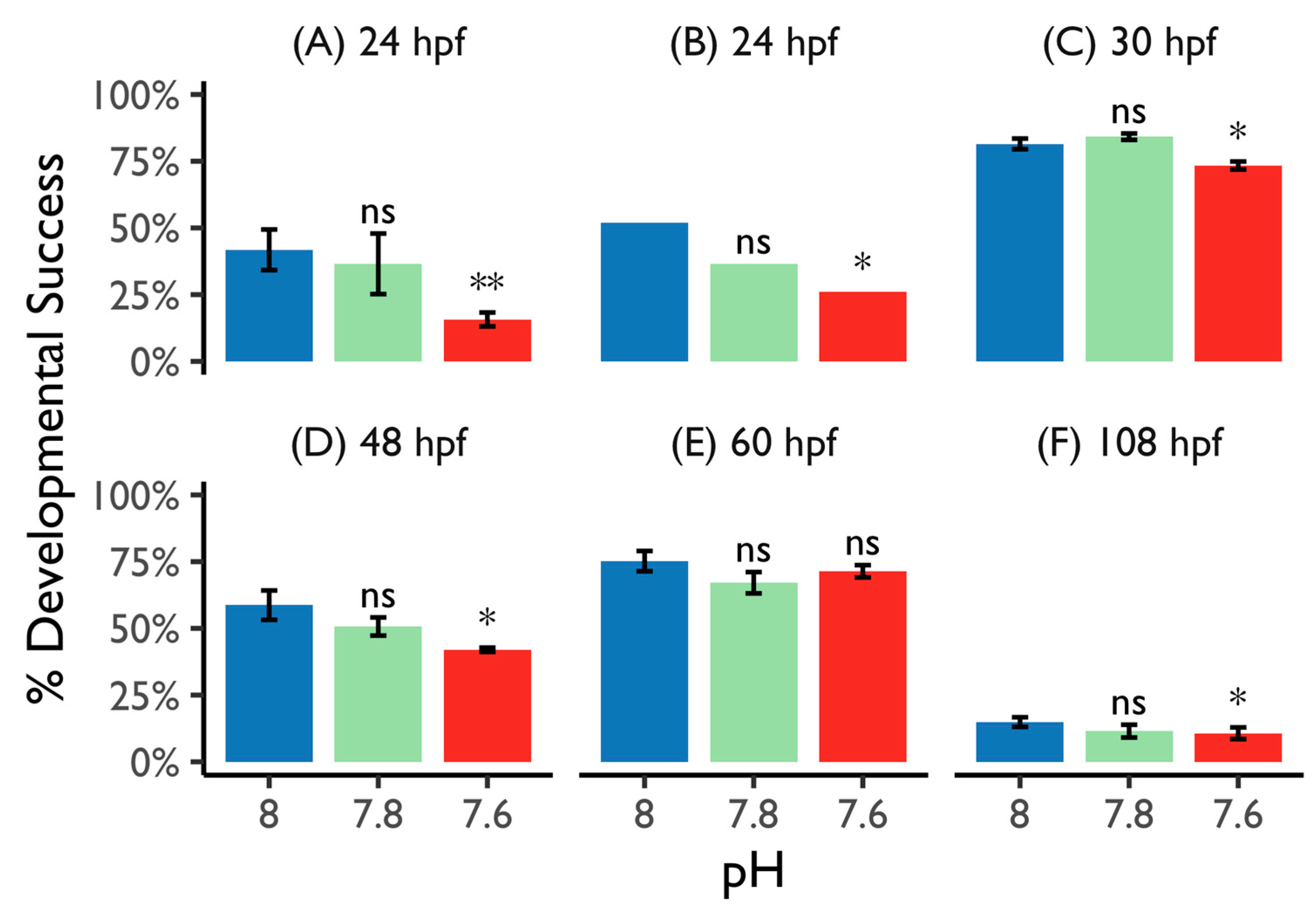
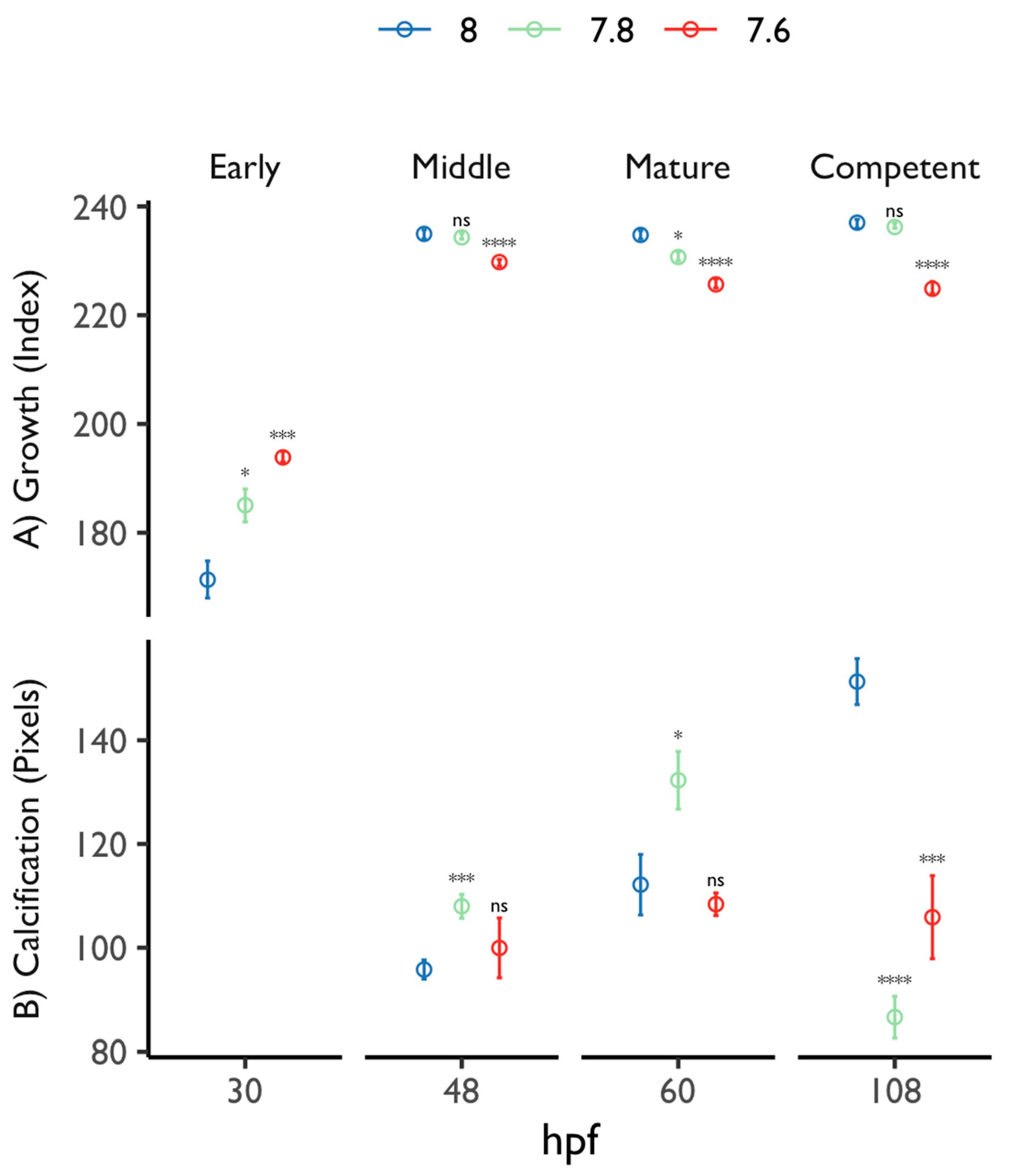
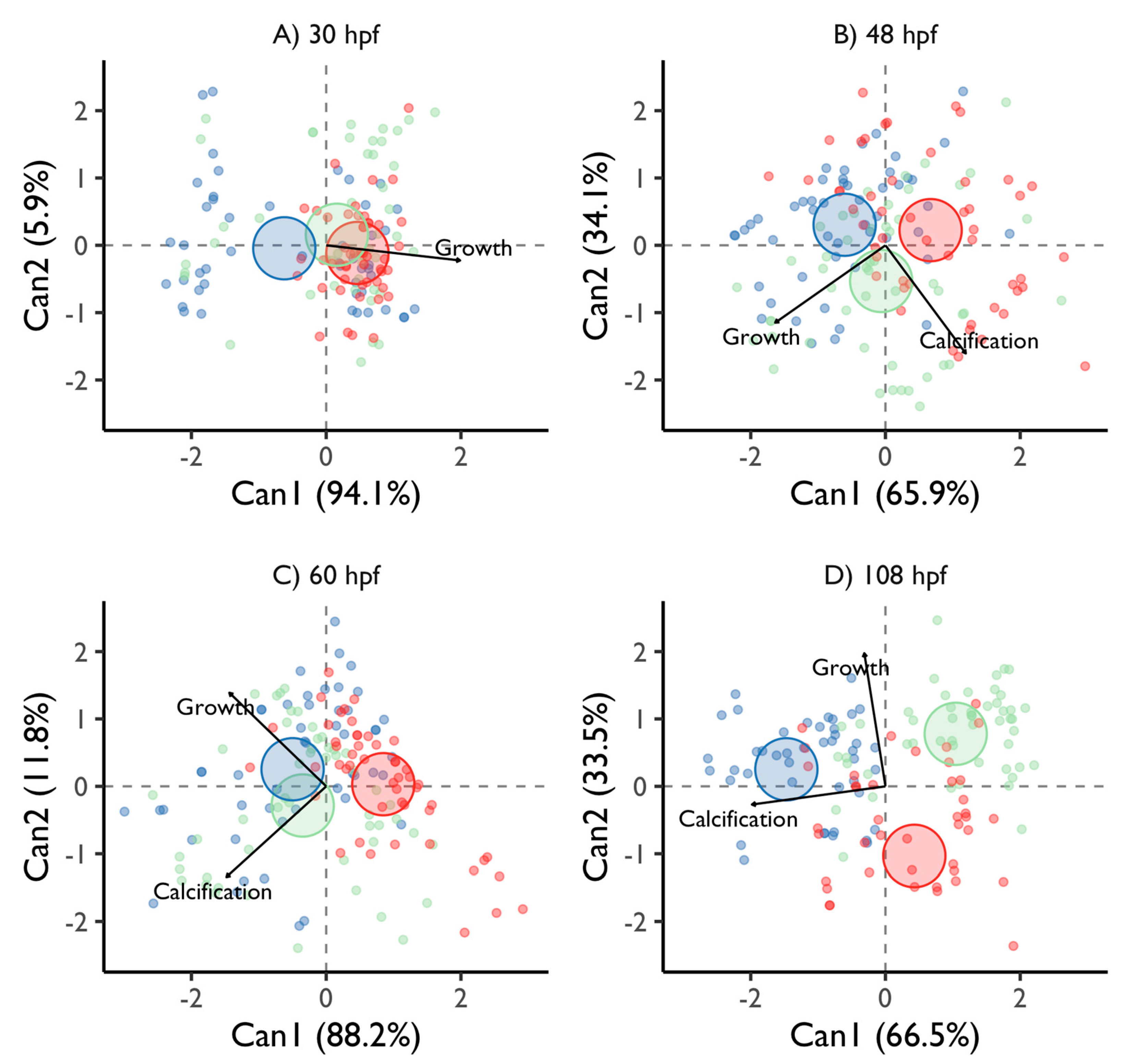
| Time 1 | Stage 2 | Feature | Assessment |
|---|---|---|---|
| 10 HPF | Embryonic Trochophore | Larvae show ciliated prototrochal girdle | Starting bioassay |
| 24 HPF | Hatched Trochophore | Larvae swim upward, congregating at the water surface | Developmental success and normal shelled larvae |
| 30 HPF | Early Veliger | The larval velum appears twisted, and the shell is imbalanced | Developmental success and growth |
| 48 HPF | Middle Veliger | Visceral mass torsion occurs | Developmental success, growth and calcification |
| 60 HPF | Mature Veliger | Larval shell is fully formed; retractor muscle is attached to the shell posterior and operculum distinct | Developmental success, growth and calcification |
| 108 HPF | Competent Veliger | Cilia are retained from hours to days, and swimming may occur intermittently; larvae start to crawl to the vessel bottom | Developmental success, growth and calcification |
| 26 DPF | Settle Larvae | Larvae are settled at the vessel bottom; advanced post-larvae are formed days after | Developmental success and SEM 3 |
| Measured | Calculated (CO2Sys) | |||||
|---|---|---|---|---|---|---|
| Assay | pH ± Sd | DIC ± Sd | TA ± Sd | CO3 ± Sd | Ωara ± Sd | pCO2 ± Sd |
| Control | 8.07 ± 0.029 | 2043 ± 33 | 2187 ± 60 | 111 ± 24.8 | 1.69 ± 0.376 | 591 ± 131 |
| Moderate | 7.74 ± 0.004 | 2057 ± 98 | 2175 ± 66 | 101 ± 65 | 1.3 ± 1 | 897 ± 563 |
| Low | 7.62 ± 0.122 | 1972 ± 202 | 2051 ± 158 | 75.2 ± 32.2 | 1.14 ± 0.489 | 987 ± 510 |
| Time 1 | Assessment | Moderate | Low |
|---|---|---|---|
| 24 HPF | Hatching success | −0.13 | −0.98 (**) |
| Normal shelled larvae | −0.35 | −0.69 (*) | |
| 30 HPF | Growth index | 0.07 (*) | 0.12 (***) |
| Veliger maturation | 0.03 | −0.1 (*) | |
| 48 HPF | Growth index | 0 | −0.02 (***) |
| Birefringence | 0.12 (***) | 0.04 | |
| Veliger maturation | −0.15 | −0.33 (*) | |
| 60 HPF | Growth index | −0.02 (*) | −0.04 (****) |
| Birefringence | 0.16 (*) | −0.03 | |
| Veliger maturation | −0.11 | −0.05 | |
| 108 HPF | Growth index | 0 | −0.05 (****) |
| Birefringence | −0.56 (****) | −0.36 (***) | |
| Competence success | −0.26 | −0.33 (*) | |
| 26 DPF | Settlement (short term) | −0.52 (**) | −0.78 (**) |
| Settlement (long term) | −0.13 | −0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Reyes, R.; Galindo-Sánchez, C.E.; Lafarga-De la Cruz, F.; Hernández-Ayón, J.M.; Valenzuela-Wood, E.; López-Galindo, L. Individual Pattern Response to CO2-Induced Acidification Stress in Haliotis rufescens Suggests Stage-Specific Acclimatization during Its Early Life History. Sustainability 2023, 15, 14010. https://doi.org/10.3390/su151814010
Gómez-Reyes R, Galindo-Sánchez CE, Lafarga-De la Cruz F, Hernández-Ayón JM, Valenzuela-Wood E, López-Galindo L. Individual Pattern Response to CO2-Induced Acidification Stress in Haliotis rufescens Suggests Stage-Specific Acclimatization during Its Early Life History. Sustainability. 2023; 15(18):14010. https://doi.org/10.3390/su151814010
Chicago/Turabian StyleGómez-Reyes, Ricardo, Clara E. Galindo-Sánchez, Fabiola Lafarga-De la Cruz, José M. Hernández-Ayón, Enrique Valenzuela-Wood, and Laura López-Galindo. 2023. "Individual Pattern Response to CO2-Induced Acidification Stress in Haliotis rufescens Suggests Stage-Specific Acclimatization during Its Early Life History" Sustainability 15, no. 18: 14010. https://doi.org/10.3390/su151814010
APA StyleGómez-Reyes, R., Galindo-Sánchez, C. E., Lafarga-De la Cruz, F., Hernández-Ayón, J. M., Valenzuela-Wood, E., & López-Galindo, L. (2023). Individual Pattern Response to CO2-Induced Acidification Stress in Haliotis rufescens Suggests Stage-Specific Acclimatization during Its Early Life History. Sustainability, 15(18), 14010. https://doi.org/10.3390/su151814010








