The Influence of Pyrolysis Time and Temperature on the Composition and Properties of Bio-Oil Prepared from Tanjong Leaves (Mimusops elengi)
Abstract
:1. Introduction
2. Materials and Methods
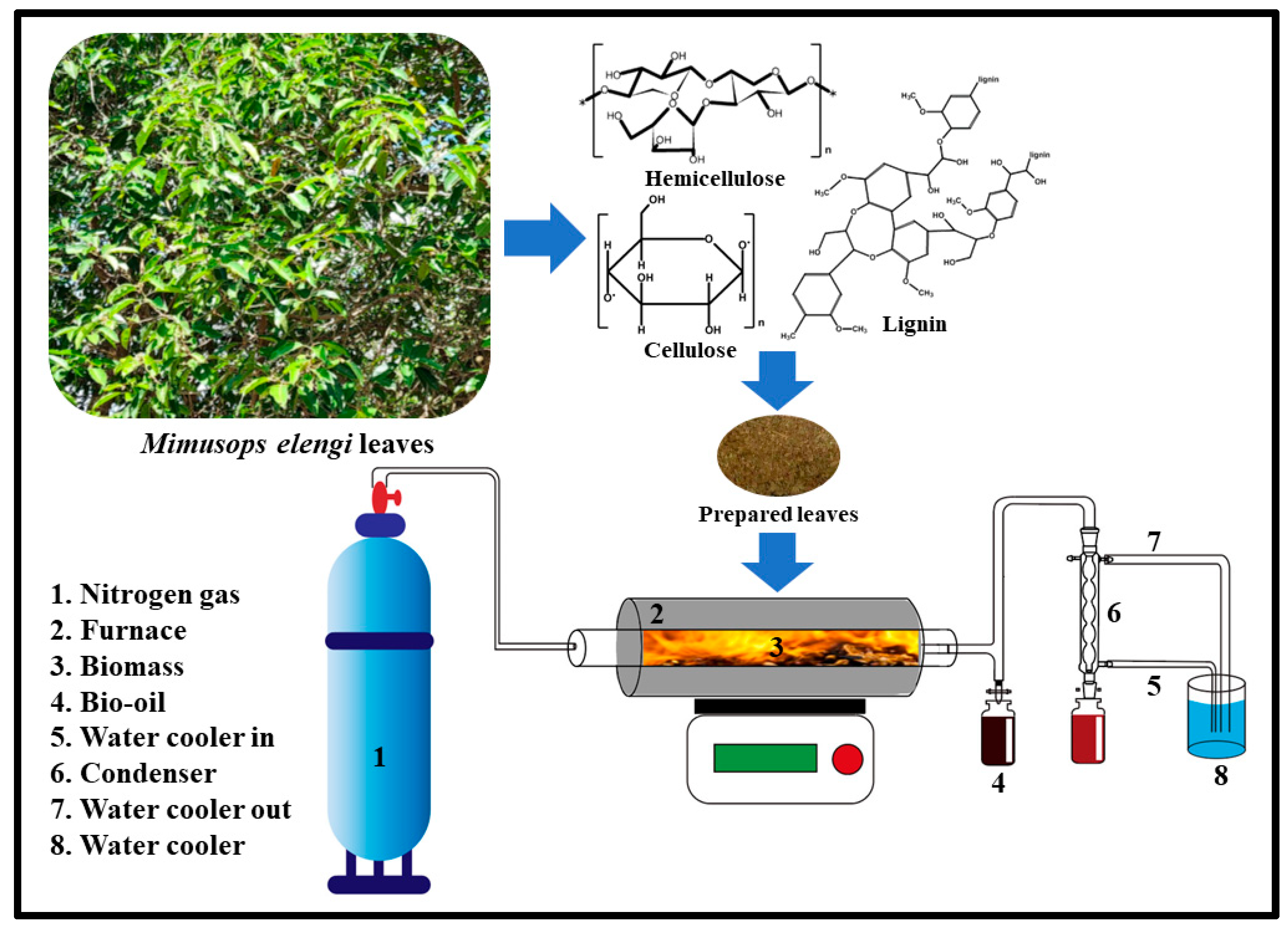
2.1. Material and Sample Preparation
2.2. Pyrolysis Process
2.3. Bio-Oil Characteristics
3. Results
3.1. Characterization of Mimusops elengi
3.1.1. Proximate and Ultimate Analyses
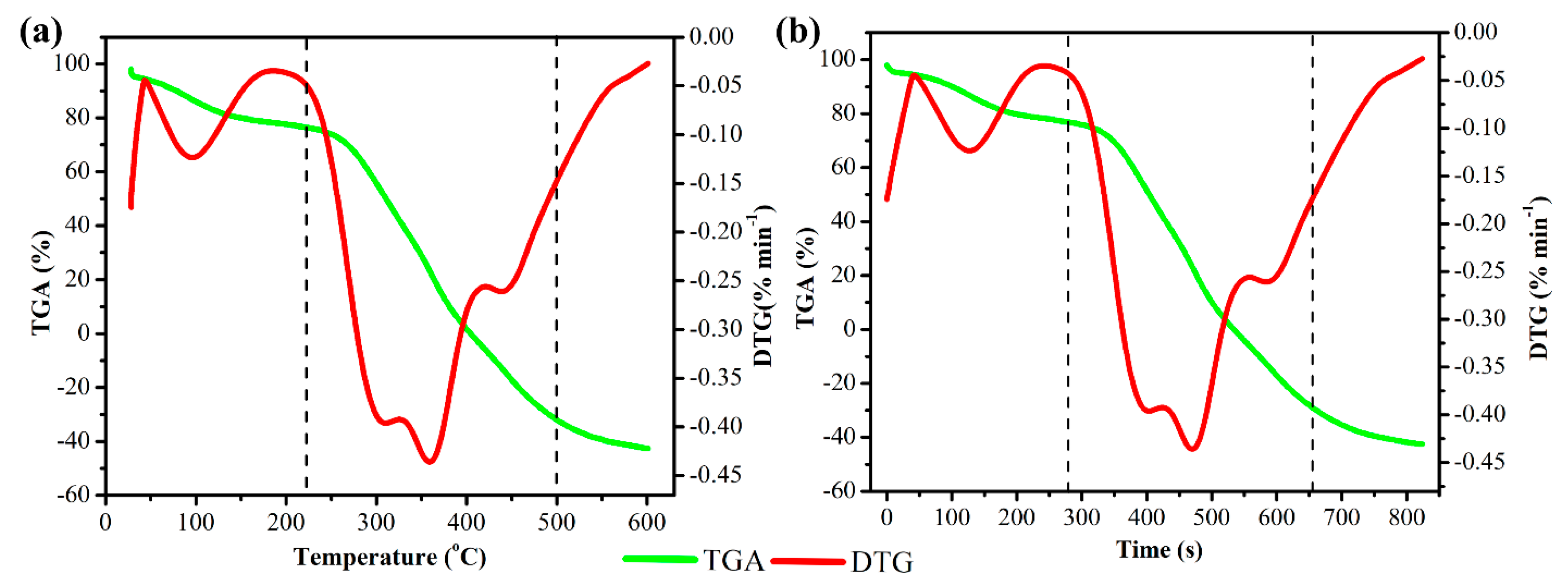
3.1.2. Thermogravimetric Analysis
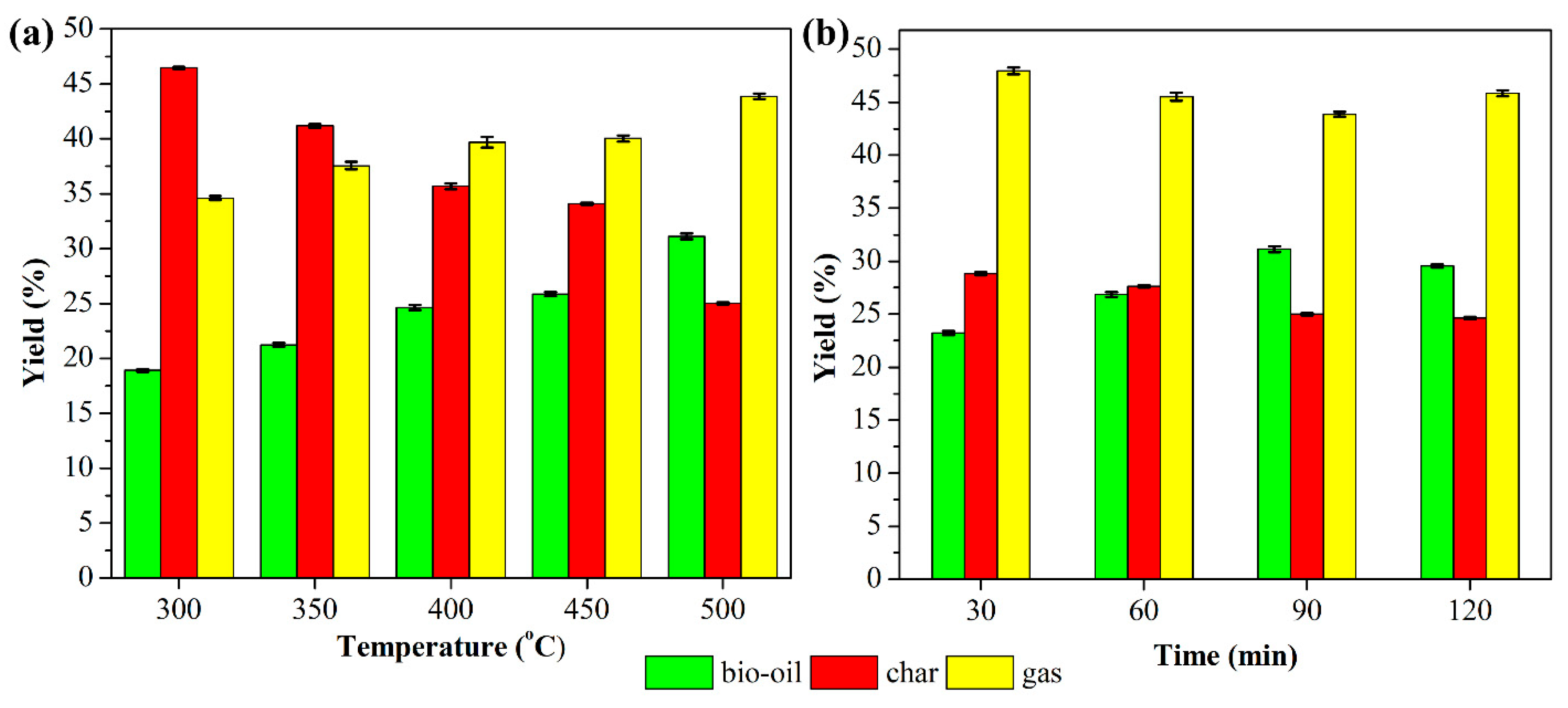
3.1.3. Influence of Pyrolysis Temperature
3.1.4. Influence of Pyrolysis Time
3.2. Characterization of Bio-Oil from Mimusops elengi
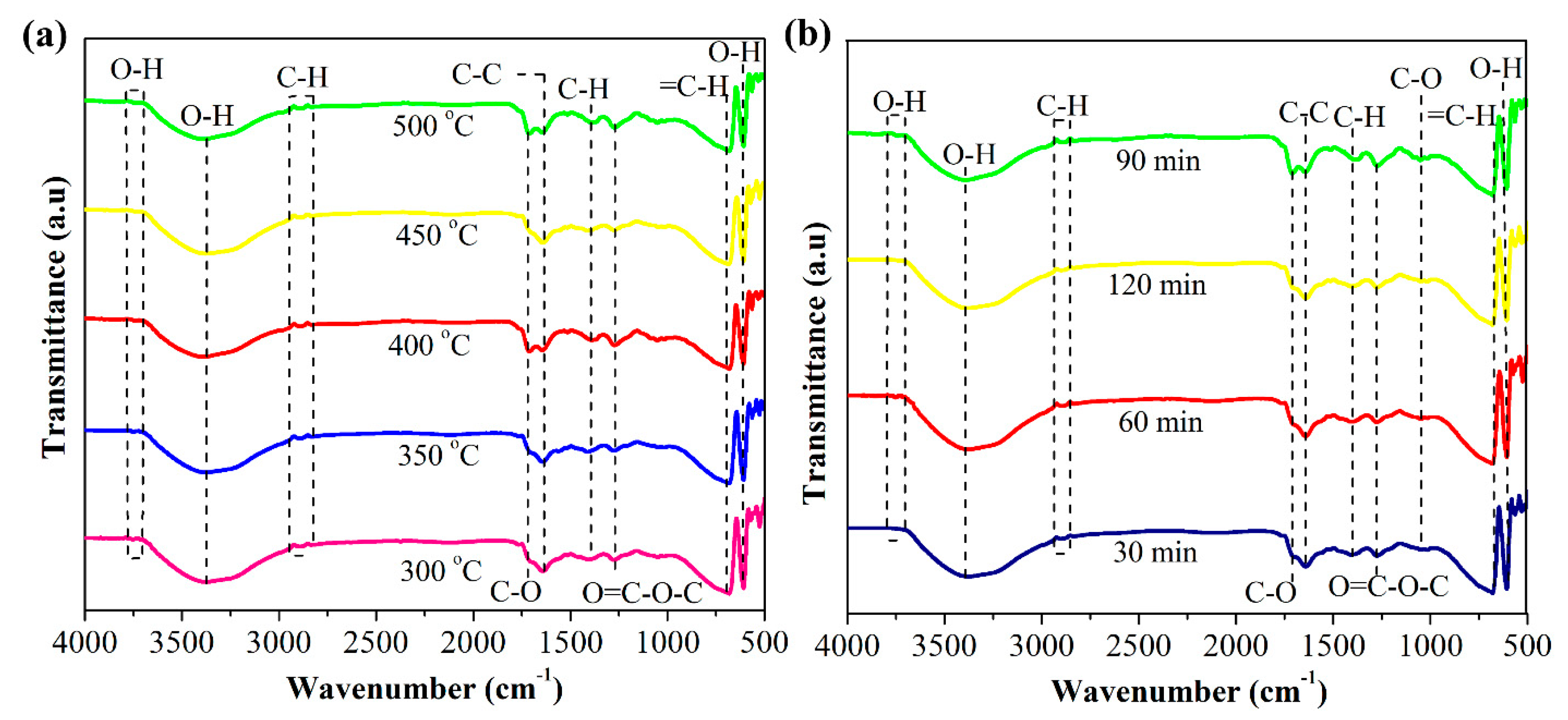
3.3. Chemical Composition Based on FT-IR Analysis
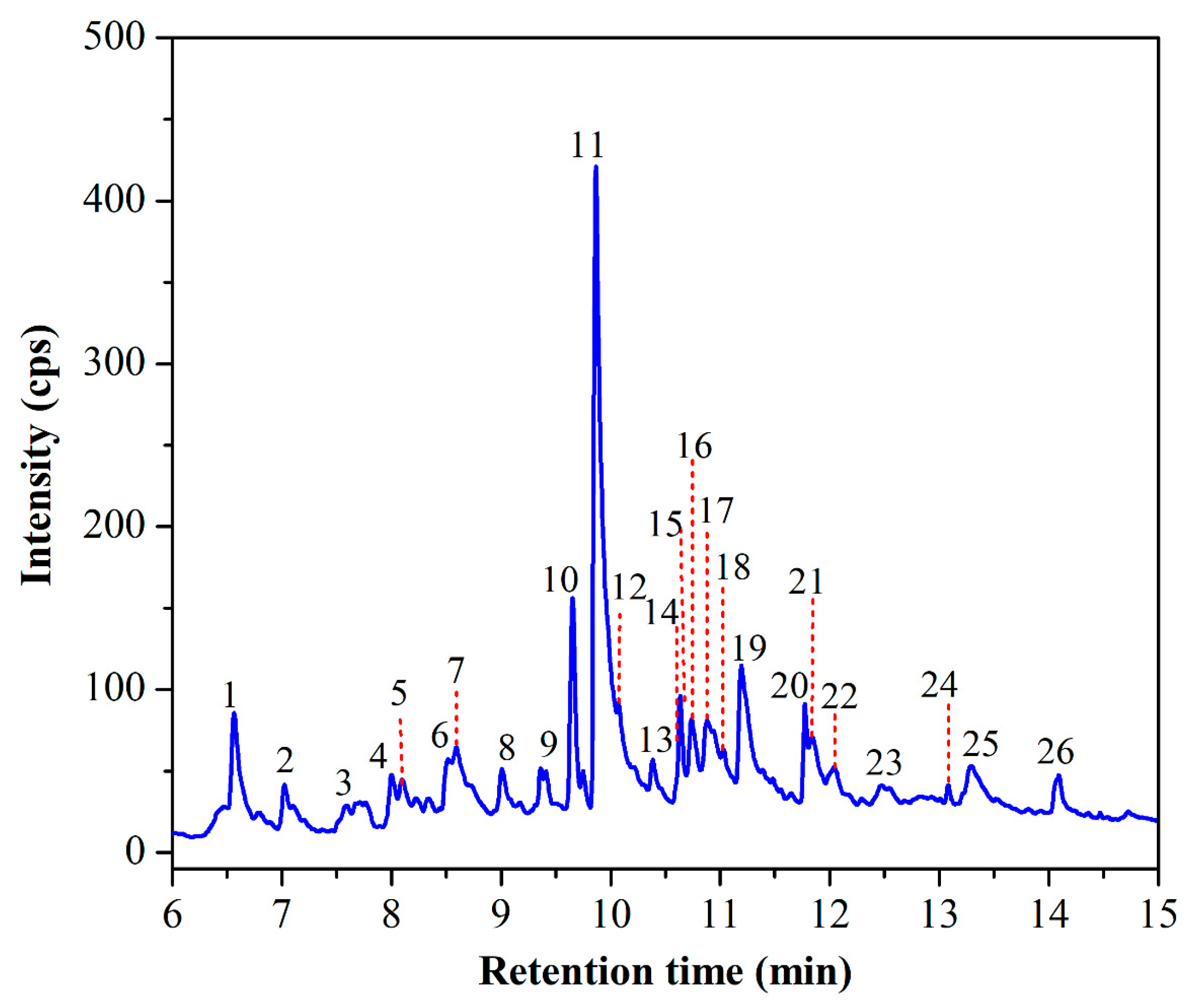
3.4. Chemical Composition Based on GC-MS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yong, J.Y.; Klemeš, J.J.; Varbanov, P.S.; Huisingh, D. Cleaner Energy for Cleaner Production: Modelling, Simulation, Optimisation and Waste Management. J. Clean. Prod. 2016, 111, 1–16. [Google Scholar] [CrossRef]
- Nasution, F.; Husin, H.; Mahidin; Abnisa, F.; Tirta Yani, F.; Maulinda, L.; Ahmadi. Conversion of Pyrolysis Vapors Derived from Non-Biodegradable Waste Plastics (PET) into Valuable Fuels Using Nickel-Impregnated HZSM5-70 Catalysts. Energy Convers. Manag. 2022, 273, 116440. [Google Scholar] [CrossRef]
- Guizani, C.; Valin, S.; Billaud, J.; Peyrot, M.; Salvador, S. Biomass Fast Pyrolysis in a Drop Tube Reactor for Bio Oil Production: Experiments and Modeling. Fuel 2017, 207, 71–84. [Google Scholar] [CrossRef]
- Nižetić, S.; Djilali, N.; Papadopoulos, A.; Rodrigues, J.J.P.C. Smart Technologies for Promotion of Energy Efficiency, Utilization of Sustainable Resources and Waste Management. J. Clean. Prod. 2019, 231, 565–591. [Google Scholar] [CrossRef]
- Rizal, T.A.; Khairil, T.A.; Mahidin, T.A.; Husin, H.; Ahmadi, H.; Nasution, F.; Umar, H. The Experimental Study of Pangium Edule Biodiesel in a High-Speed Diesel Generator for Biopower Electricity. Energies 2022, 15, 5405. [Google Scholar] [CrossRef]
- Husin, H.; Erdiwansyah, E.; Ahmadi, A.; Nasution, F.; Rinaldi, W.; Abnisa, F.; Mamat, R. Efficient Hydrogen Production by Microwave-Assisted Catalysis for Glycerol-Water Solutions via NiO/Zeolite-CaO Catalyst. S. Afr. J. Chem. Eng. 2022, 41, 43–50. [Google Scholar] [CrossRef]
- Balogun, A.O.; Lasode, O.A.; McDonald, A.G. Thermochemical and Pyrolytic Analyses of Musa Spp. Residues from the Rainforest Belt of Nigeria. Environ. Prog. Sustain. Energy 2018, 37, 1932–1941. [Google Scholar] [CrossRef]
- Mudryk, K.; Jewiarz, M.; Wróbel, M.; Niemiec, M.; Dyjakon, A. Evaluation of Urban Tree Leaf Biomass-Potential, Physico-Mechanical and Chemical Parameters of Raw Material and Solid Biofuel. Energies 2021, 14, 818. [Google Scholar] [CrossRef]
- Sayed, D.F.; Afifi, A.H.; Temraz, A.; Ahmed, A.H. Metabolic Profiling of Mimusops Elengi Linn. Leaves Extract and in Silico Anti-Inflammatory Assessment Targeting NLRP3 Inflammasome. Arab. J. Chem. 2023, 16, 104753. [Google Scholar] [CrossRef]
- Shahwar, D.; Raza, M.A. Antioxidant Potential of Phenolic Extracts of Mimusops Elengi. Asian Pac. J. Trop. Biomed. 2012, 2, 547–550. [Google Scholar] [CrossRef]
- Kar, B.; Kumar, R.B.S.; Karmakar, I.; Dola, N.; Bala, A.; Mazumder, U.K.; Hadar, P.K. Antioxidant and in Vitro Anti-Inflammatory Activities of Mimusops Elengi Leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S976–S980. [Google Scholar] [CrossRef]
- Haris, M.; Dedy; Suryatmojo, H.D. Polda Aceh Tanam 6.500 Pohon Penghijauan Di Aceh Besar. Available online: https://aceh.antaranews.com/berita/116183/polda-aceh-tanam-6500-pohon-penghijauan-di-aceh-besar (accessed on 9 September 2023).
- Srinivasan, G.R.; Palani, S.; Munir, M.; Saeed, M.; Jambulingam, R. Biodiesel Production from Mimusops Elengi Seed Oil through Means of Co-Solvent-Based Transesterification Using an Ionic Liquid Catalyst. In Biofuel from Microbes and Plants; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- GR, S.; Palani, S.; Ranjitha, J. Biodiesel Production from the Seeds of Mimusops Elengi Using Potassium Aluminium Silicate as Novel Catalyst. Innov. Energy Res. 2017, 6, 165. [Google Scholar] [CrossRef]
- Meriatna; Husin, H.; Riza, M.; Faisal, M.; Ahmadi; Sulastri. Biodiesel Production Using Waste Banana Peel as Renewable Base Catalyst. Mater. Today Proc. 2023, 87, 214–217. [Google Scholar] [CrossRef]
- Husin, H.; Abubakar, A.; Ramadhani, S.; Sijabat, C.F.B.; Hasfita, F. Coconut Husk Ash as Heterogenous Catalyst for Biodiesel Production from Cerbera Manghas Seed Oil. In MATEC Web of Conferences; EDP Sciences: Castanet-Tolosan, France, 2018. [Google Scholar]
- Kalita, D.; Saikia, C.N. Chemical Constituents and Energy Content of Some Latex Bearing Plants. Bioresour. Technol. 2004, 92, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of Agricultural Biomass Residues: Comparative Study of Corn Cob, Wheat Straw, Rice Straw and Rice Husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research Progress in the Preparation of High-Quality Liquid Fuels and Chemicals by Catalytic Pyrolysis of Biomass: A Review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Yin, M.; Bi, D.; Zhao, W.; Liu, J.; Zhao, A.; Jiang, M. Production of Bio-Oil and Biochar by the Nitrogen-Rich Pyrolysis of Cellulose with Urea: Pathway of Nitrile in Bio-Oil and Evolution of Nitrogen in Biochar. J. Anal. Appl. Pyrolysis 2023, 174, 106137. [Google Scholar] [CrossRef]
- Zhong, D.; Zeng, K.; Li, J.; Qiu, Y.; Flamant, G.; Nzihou, A.; Vladimirovich, V.S.; Yang, H.; Chen, H. Characteristics and Evolution of Heavy Components in Bio-Oil from the Pyrolysis of Cellulose, Hemicellulose and Lignin. Renew. Sustain. Energy Rev. 2022, 157, 111989. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, Y.; Huang, Y.; Yang, X.; Liu, C.; Kang, J.; Zheng, Z. Effect of Temperature on Characteristics of Bio-Oil and Bio-Char during Pyrolysis of Yunnan Pine. J. Biobased Mater. Bioenergy 2016, 10, 81–89. [Google Scholar] [CrossRef]
- Jamilatun, S.; Elisthatiana, Y.; Aini, S.N.; Mufandi, I.; Budiman, A. Effect of Temperature on Yield Product and Characteristics of Bio-Oil From Pyrolysis of Spirulina Platensis Residue. Elkawnie 2020, 6, 96–108. [Google Scholar] [CrossRef]
- Dai, L.; Zeng, Z.; Tian, X.; Jiang, L.; Yu, Z.; Wu, Q.; Wang, Y.; Liu, Y.; Ruan, R. Microwave-Assisted Catalytic Pyrolysis of Torrefied Corn Cob for Phenol-Rich Bio-Oil Production over Fe Modified Bio-Char Catalyst. J. Anal. Appl. Pyrolysis 2019, 143, 104691. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A.; Sahu, J.N. Optimization and Characterization Studies on Bio-Oil Production from Palm Shell by Pyrolysis Using Response Surface Methodology. Biomass Bioenergy 2011, 35, 3604–3616. [Google Scholar] [CrossRef]
- Azzaharo, F.; Mardiyati, Y.; Steven; Rizkiansyah, R.R. Ekstraksi Serat Kulit Jagung Sebagai Bahan Baku Benang Tekstil. Maj. Polim. Indones. 2015, 18, 21–25. [Google Scholar]
- Balagurumurthy, B.; Srivastava, V.; Vinit; Kumar, J.; Biswas, B.; Singh, R.; Gupta, P.; Kumar, K.L.N.S.; Singh, R.; Bhaskar, T. Value Addition to Rice Straw through Pyrolysis in Hydrogen and Nitrogen Environments. Bioresour. Technol. 2015, 188, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, R.; He, Y.; Chai, M.; Cai, J. Bio-Oil Production from Fast Pyrolysis of Rice Husk in a Commercial-Scale Plant with a Downdraft Circulating Fluidized Bed Reactor. Fuel Process. Technol. 2018, 171, 308–317. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The Slow and Fast Pyrolysis of Cherry Seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/Biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–888. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Lup, A.N.K.; Khan, S.; Abnisa, F.; Daud, W.M.A.W. Effect of Temperature and Feed Rate on Pyrolysis Oil Produced via Helical Screw Fluidized Bed Reactor. Korean J. Chem. Eng. 2021, 38, 1797–1809. [Google Scholar] [CrossRef]
- Suman, S.; Gautam, S. Effect of Pyrolysis Time and Temperature on the Characterization of Biochars Derived from Biomass. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 933–940. [Google Scholar] [CrossRef]
- American Standard Technical Material–ASTM D 1102-84; Standard Test Method for Ash in Wood by Celebrating 125 Years. ASTM International: West Conshohocken, PA, USA, 2021.
- American Standard Technical Material–ASTM D 2016-74; Methods of Test for Moisture Content of Wood by Celebrating 125 Years. ASTM International: West Conshohocken, PA, USA, 1983.
- American Standard Technical Material–ASTM D 3178-89; Methods of Test for Carbon and Hydrogen in the Analysis Sample of Coal and Coke by Celebrating 125 Years. ASTM International: West Conshohocken, PA, USA, 2002.
- American Standard Technical Material–ASTM D 3175-07; Standard Specification for Pyrolysis Liquid Biofuel by Celebrating 125 Years. ASTM International: West Conshohocken, PA, USA, 2017.
- Demirbas, A.; Ak, N.; Aslan, A.; Sen, N. Calculation of Higher Heating Values of Hydrocarbon Compounds and Fatty Acids. Pet. Sci. Technol. 2018, 36, 712–717. [Google Scholar] [CrossRef]
- Acar, S.; Ayanoglu, A. Determination of Higher Heating Values (HHVs) of Biomass Fuels. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 28, 749–758. [Google Scholar]
- Noushabadi, A.S.; Dashti, A.; Ahmadijokani, F.; Hu, J.; Mohammadi, A.H. Estimation of Higher Heating Values (HHVs) of Biomass Fuels Based on Ultimate Analysis Using Machine Learning Techniques and Improved Equation. Renew. Energy 2021, 179, 550–562. [Google Scholar] [CrossRef]
- Sukumar, V.; Manieniyan, V.; Senthilkumar, R.; Sivaprakasam, S. Production of Bio Oil from Sweet Lime Empty Fruit Bunch by Pyrolysis. Renew. Energy 2020, 146, 309–315. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of Green Aromatics and Olefins by Catalytic Cracking of Oxygenate Compounds Derived from Biomass Pyrolysis: A Review. Appl. Catal. A Gen. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Younis, M.R.; Farooq, M.; Imran, M.; Kazim, A.H.; Shabbir, A. Characterization of the Viscosity of Bio-Oil Produced by Fast Pyrolysis of the Wheat Straw. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 43, 1853–1868. [Google Scholar] [CrossRef]
- Paenpong, C.; Pattiya, A. Effect of Pyrolysis and Moving-Bed Granular Filter Temperatures on the Yield and Properties of Bio-Oil from Fast Pyrolysis of Biomass. J. Anal. Appl. Pyrolysis 2016, 119, 40–51. [Google Scholar] [CrossRef]
- American Standard Technical Material–ASTM D 240-19; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter by Celebrating 125 Years. ASTM International: West Conshohocken, PA, USA, 2019.
- Sakulkit, P.; Palamanit, A.; Dejchanchaiwong, R.; Reubroycharoen, P. Characteristics of Pyrolysis Products from Pyrolysis and Co-Pyrolysis of Rubber Wood and Oil Palm Trunk Biomass for Biofuel and Value-Added Applications. J. Environ. Chem. Eng. 2020, 8, 104561. [Google Scholar] [CrossRef]
- Geng, A. Upgrading of Oil Palm Biomass to Value-Added Products. In Biomass and Bioenergy: Applications; Springer: Cham, Switerland, 2015. [Google Scholar]
- Pimenidou, P.; Dupont, V. Characterisation of Palm Empty Fruit Bunch (PEFB) and Pinewood Bio-Oils and Kinetics of Their Thermal Degradation. Bioresour. Technol. 2012, 109, 198–205. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Khongphakdi, P.; Palamanit, A.; Phusunti, N.; Tirawanichakul, Y.; Shrivastava, P. Evaluation of Oil Palm Biomass Potential for Bio-Oil Production via Pyrolysis Processes. Int. J. Integr. Eng. 2020, 12, 226–233. [Google Scholar]
- Kumar, R.; Strezov, V. Thermochemical Production of Bio-Oil: A Review of Downstream Processing Technologies for Bio-Oil Upgrading, Production of Hydrogen and High Value-Added Products. Renew. Sustain. Energy Rev. 2021, 135, 110152. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Kay Lup, A.N.; Khan, S.; Abnisa, F.; Wan Daud, W.M.A. Optimization of Palm Shell Pyrolysis Parameters in Helical Screw Fluidized Bed Reactor: Effect of Particle Size, Pyrolysis Time and Vapor Residence Time. Clean. Eng. Technol. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Maulinda, L.; Husin, H.; Rahman, N.A.; Rosnelly, C.M.; Nasution, F.; Abidin, N.Z.; Faisal; Yani, F.T.; Ahmadi. Effects of Temperature and Times on the Product Distribution of Bio-Oils Derived from Typha Latifolia Pyrolysis as Renewable Energy. Results Eng. 2023, 18, 101163. [Google Scholar] [CrossRef]
- Chorazy, T.; Čáslavský, J.; Žvaková, V.; Raček, J.; Hlavínek, P. Characteristics of Pyrolysis Oil as Renewable Source of Chemical Materials and Alternative Fuel from the Sewage Sludge Treatment. Waste Biomass Valorization 2020, 11, 4491–4505. [Google Scholar] [CrossRef]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of Cellulose, Hemicellulose and Lignin of MD2 Pineapple Biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Cai, W.; Dai, L.; Liu, R. Catalytic Fast Pyrolysis of Rice Husk for Bio-Oil Production. Energy 2018, 154, 477–487. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Palamanit, A.; Khongphakdi, P.; Tirawanichakul, Y.; Phusunti, N. Investigation of Yields and Qualities of Pyrolysis Products Obtained from Oil Palm Biomass Using an Agitated Bed Pyrolysis Reactor. Biofuel Res. J. 2019, 6, 1065–1079. [Google Scholar] [CrossRef]
- Chukwuneke, J.L.; Ewulonu, M.C.; Chukwujike, I.C.; Okolie, P.C. Physico-Chemical Analysis of Pyrolyzed Bio-Oil from Swietenia Macrophylla (Mahogany) Wood. Heliyon 2019, 5, e01790. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret Ftir Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Pinto, O.; Romero, R.; Carrier, M.; Appelt, J.; Segura, C. Fast Pyrolysis of Tannins from Pine Bark as a Renewable Source of Catechols. J. Anal. Appl. Pyrolysis 2018, 136, 69–76. [Google Scholar] [CrossRef]
- Adegoke, I.A.; Ogunsanwo, O.Y.; Ige, A.R. Bio-Fuel Properties and Elemental Analysis of Bio-Oil Produced from Pyrolysis of Gmelina Arborea. Acta Chem. Malays. 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Z.; Xu, H.; Yan, K. Selective Production of Phenol-Rich Bio-Oil From Corn Straw Waste by Direct Microwave Pyrolysis Without Extra Catalyst. Front. Chem. 2021, 9, 700887. [Google Scholar] [CrossRef] [PubMed]
- Mullen, C.A.; Boateng, A.A. Chemical Composition of Bio-Oils Produced by Fast Pyrolysis of Two Energy Crops. Energy Fuels 2008, 22, 2104–2109. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-Oil Production from Rice Husk Fast Pyrolysis in a Conical Spouted Bed Reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Lazzari, E.; dos Santos Polidoro, A.; Onorevoli, B.; Schena, T.; Silva, A.N.; Scapin, E.; Jacques, R.A.; Caramão, E.B. Production of Rice Husk Bio-Oil and Comprehensive Characterization (Qualitative and Quantitative) by HPLC/PDA and GC × GC/QMS. Renew. Energy 2019, 135, 554–565. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Proximate Analysis (wt%) | |
| Moisture content | 12.00 |
| Volatile matter | 62.35 |
| Fixed carbon | 21.16 |
| Ash | 5.49 |
| HHV (MJ/kg) | 18.886 |
| LHV (MJ/kg) | 16.41 |
| Ultimate Analysis (wt%) | |
| C | 45.91 |
| H | 6.42 |
| O | 40.28 |
| N | 1.64 |
| S | 0.26 |
| Component Analysis (wt%) [17] | |
| Cellulose | 33.17 |
| Hemicellulose | - |
| Lignin | 10.2 |
| Others | - |
| Property | Test Method | Specification | Units |
|---|---|---|---|
| Density at 20 °C | D4052 | 1.1–1.3 | kg/m3 |
| Kinematic viscosity at 40 °C | D445 | 125 max | cSt |
| pH | E70-07 | report |
| Operating Conditions | Density (kg/m3) @20 °C | Viscosity (cSt) @40 °C | pH | HHV (MJ/Kg) |
|---|---|---|---|---|
| a Temperature (°C) | ||||
| 300 | 1.12 ± 0.026 | 1.31 ± 0.036 | 4.00 ± 0.053 | 16.80 ± 0.062 |
| 350 | 1.13 ± 0.026 | 1.32 ± 0.026 | 4.11 ± 0.061 | 17.44 ± 0.087 |
| 400 | 1.13 ± 0.026 | 1.40 ± 0.036 | 4.12 ± 0.043 | 17.59 ± 0.124 |
| 450 | 1.13 ± 0.043 | 1.42 ± 0.036 | 4.22 ± 0.078 | 18.14 ± 0.213 |
| 500 | 1.15 ± 0.043 | 1.60 ± 0.062 | 4.41 ± 0.046 | 19.91 ± 0.081 |
| b Reaction time (min) | ||||
| 30 | 1.13 ± 0.061 | 1.56 ± 0.088 | 4.06 ± 0.111 | 19.77 ± 0.121 |
| 60 | 1.13 ± 0.070 | 1.37 ± 0.092 | 4.00 ± 0.055 | 16.76 ± 0.079 |
| 90 | 1.15 ± 0.078 | 1.60 ± 0.026 | 4.41 ± 0.053 | 18.14 ± 0.141 |
| 120 | 1.15 ± 0.026 | 1.44 ± 0.070 | 4.33 ± 0.061 | 19.42 ± 0.026 |
| Absorption (cm−1) | Wavenumber (cm−1) | Type of Vibrations | Class of Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction Time (min) | b Temperature (°C) | ||||||||||
| 30 | 60 | 90 | 120 | 300 | 350 | 400 | 450 | 500 | |||
| 3584–3700 | - | - | 3699 | 3699 | 3699 | 3699 | 3699 | 3699 | 3699 | O-H stretch | Alcohols |
| - | - | 3390 | - | - | - | - | - | 3390 | |||
| 3300–3400 | 3379 | - | - | - | 3379 | - | - | - | - | O-H stretch | Alcohols |
| 3377 | - | 3377 | - | 3377 | 3377 | 3377 | - | ||||
| 2850–3000 | 2900 | - | - | - | - | - | - | - | - | C-H stretch | Alkanes |
| - | 2891 | - | - | - | - | - | - | - | |||
| - | - | 2889 | 2889 | 2889 | 2889 | 2889 | 2889 | 2889 | |||
| 2815–2850 | - | 2835 | - | - | - | - | - | - | - | C-H stretch | Esters |
| - | - | - | - | 2833 | 2833 | 2833 | 2833 | - | |||
| - | - | - | - | 2382 | - | - | - | - | |||
| - | - | - | - | - | - | - | 2380 | - | |||
| 2300–2400 | - | 2376 | - | - | - | - | 2376 | - | - | C≡N | Nitrile stretch |
| - | - | 2349 | - | - | - | - | - | 2349 | |||
| - | - | - | 2347 | 2347 | 2347 | - | - | - | |||
| 2100–2140 | - | 2119 | - | - | - | - | - | - | - | C≡C | Alkynes |
| 2112 | - | - | - | - | 2112 | - | 2112 | - | |||
| - | - | - | 2100 | - | - | 2100 | - | - | |||
| 1660–2000 | - | - | 1867 | - | - | - | - | - | 1867 | Aromatic combination bands | Aromatic |
| - | - | - | - | - | - | - | 1865 | - | |||
| C=O stretch | Aldehydes | ||||||||||
| - | 1766 | - | 1766 | 1766 | 1766 | 1766 | - | - | ketones | ||
| 1700–1790 | - | - | - | - | - | - | - | 1764 | - | ||
| 1762 | - | - | - | - | - | - | - | - | |||
| - | - | 1708 | - | - | - | - | - | 1708 | C=C stretch | Alkenes | |
| - | - | - | - | - | - | - | 1647 | - | |||
| 1620–1680 | - | - | 1643 | 1643 | 1643 | 1643 | 1643 | - | 1643 | ||
| 1641 | - | - | - | - | - | - | - | - | C-H blend | Aromatics | |
| - | 1637 | - | - | - | - | - | - | - | |||
| 1450–1600 | - | - | - | - | - | 1566 | - | 1566 | - | ||
| - | - | - | 1560 | 1560 | - | 1560 | - | - | |||
| 1516 | 1516 | - | 1516 | 1516 | 1516 | 1516 | 1516 | - | |||
| - | - | 1514 | - | - | - | - | - | 1514 | |||
| - | - | - | - | 1452 | 1452 | - | - | - | C-H | Alkenes | |
| - | - | - | - | - | - | - | 1450 | - | |||
| 1410–1420 | - | - | - | 1413 | - | 1413 | 1413 | - | - | O-H blend | Alcohols |
| - | - | - | - | - | - | - | 1411 | - | |||
| 1310–1410 | 1409 | - | - | - | - | - | - | - | - | ||
| - | 1404 | - | - | - | - | - | - | - | |||
| - | - | - | 1402 | - | - | - | - | O=C-O-C | Esters | ||
| - | - | 1370 | - | - | - | - | - | 1370 | (aromatics) | ||
| 1250–1310 | - | - | - | 1276 | 1276 | 1276 | 1276 | 1276 | - | ||
| 1274 | 1274 | - | - | - | - | - | - | - | C-O stretch | Alcohols | |
| - | - | 1271 | - | - | - | - | - | 1271 | |||
| 1035–1085 | - | - | 1082 | - | - | - | - | - | 1082 | ||
| - | - | 1051 | - | - | - | - | - | 1051 | |||
| - | 1049 | - | - | - | - | - | - | - | |||
| 1047 | - | - | - | 1047 | - | 1047 | 1047 | - | |||
| 990–1050 | 1016 | - | 1016 | 1016 | - | - | 1016 | 1016 | 1016 | P-O-C stretch | Phosphorus |
| - | - | - | - | 1014 | 1014 | - | - | - | |||
| 610–700 | 680 | 680 | 680 | 680 | 680 | 680 | 680 | 680 | 680 | ≡C-H stretch | Alkynes |
| 607 | 607 | 607 | 607 | 607 | 607 | 607 | 607 | 607 | |||
| No | Compound | Formula | Area (%) | Groups |
|---|---|---|---|---|
| 1 | Phosphonic acid | C6H7O4P | 4.89 | Acid |
| 2 | 2-cyclopentene-1-one, 2-hydroxy-3-methyl | C6H8O2 | 0.97 | Ketone |
| 3 | Oxiranecarboxamide, 2-ethyl-3-propyl | C8H15NO2 | 4.31 | Acid |
| 4 | p-cresol | C7H8O | 1.73 | Phenol |
| 5 | 1,2,3-Propanetriol, 1-acetate | C5H10O4 | 1.37 | Acid |
| 6 | 3-pyridinol | C5H5NO | 2.01 | Aromatic |
| 7 | Glutarimide | C5H7NO2 | 2.22 | Acid |
| 8 | Guanosine | C10H13N5O5 | 1.26 | Acid |
| 9 | 1,4;3,6-Dianhydro-alpha-d-glucopyranose | C6H8O4 | 0.93 | Hydrocarbon |
| 10 | 1,4;3,6-Dianhydro-alpha-d-glucopyranose | C6H8O4 | 7.02 | Hydrocarbon |
| 11 | 3,4-Anhydro-d-galactosan | C6H8O4 | 1.09 | Ester |
| 12 | Catechol | C6H6O2 | 36.63 | Phenol |
| 13 | 5H-Cyclopentapyrazine | C9H12N2 | 2.47 | Pyrazine |
| 14 | 2-Butanone, 4-hydroxy-3,3-dimethyl | C6H12O2 | 0.93 | Ester |
| 15 | 1,2-Benzenediol, 3-methoxy | C7H8O3 | 3.31 | Phenol |
| 16 | 1,2-Benzenediol, 3-methyl | C7H8O2 | 3.85 | Phenol |
| 17 | 1,4-Benzenediol | C6H6O2 | 5.61 | Phenol |
| 18 | Beta-d-Ribopyranoside, methyl, 3-acetate | C8H14O6 | 1.29 | Ester |
| 19 | 1,2-Benzenediol, 4-methyl | C7H8O2 | 6.46 | Phenol |
| 20 | Phenol | C8H10O3 | 2.35 | Phenol |
| 21 | 4,6-Dimethyl (1H) pyrid-2-one | C14H13N3O3 | 2.99 | Ketone |
| 22 | 1,4-Benzenediol, 2,6-dimethyl | C8H10O2 | 1.05 | Phenol |
| 23 | 1,3-Benzenediol, 4-ethyl | C8H10O2 | 1.59 | Phenol |
| 24 | 2,5-Dimethoxybenzyl alcohol | C9H12O3 | 0.38 | Alcohol |
| 25 | D-Allose | C6H12O6 | 1.65 | Hydrocarbon |
| 26 | 4-Oxo-beta-isodamascol | C13H20O2 | 1.66 | Phenol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maulinda, L.; Husin, H.; Arahman, N.; Rosnelly, C.M.; Syukri, M.; Nurhazanah; Nasution, F.; Ahmadi. The Influence of Pyrolysis Time and Temperature on the Composition and Properties of Bio-Oil Prepared from Tanjong Leaves (Mimusops elengi). Sustainability 2023, 15, 13851. https://doi.org/10.3390/su151813851
Maulinda L, Husin H, Arahman N, Rosnelly CM, Syukri M, Nurhazanah, Nasution F, Ahmadi. The Influence of Pyrolysis Time and Temperature on the Composition and Properties of Bio-Oil Prepared from Tanjong Leaves (Mimusops elengi). Sustainability. 2023; 15(18):13851. https://doi.org/10.3390/su151813851
Chicago/Turabian StyleMaulinda, Leni, Husni Husin, Nasrul Arahman, Cut Meurah Rosnelly, Muhammad Syukri, Nurhazanah, Fahrizal Nasution, and Ahmadi. 2023. "The Influence of Pyrolysis Time and Temperature on the Composition and Properties of Bio-Oil Prepared from Tanjong Leaves (Mimusops elengi)" Sustainability 15, no. 18: 13851. https://doi.org/10.3390/su151813851
APA StyleMaulinda, L., Husin, H., Arahman, N., Rosnelly, C. M., Syukri, M., Nurhazanah, Nasution, F., & Ahmadi. (2023). The Influence of Pyrolysis Time and Temperature on the Composition and Properties of Bio-Oil Prepared from Tanjong Leaves (Mimusops elengi). Sustainability, 15(18), 13851. https://doi.org/10.3390/su151813851







