Modelling the Distribution of Three Invasive Freshwater Turtles in Mainland Guadeloupe: Analysis of Their Presence, Abundance and Co-Occurrence
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Count Data and Selection of Variables
2.3. Statistical Analysis and Model Selection
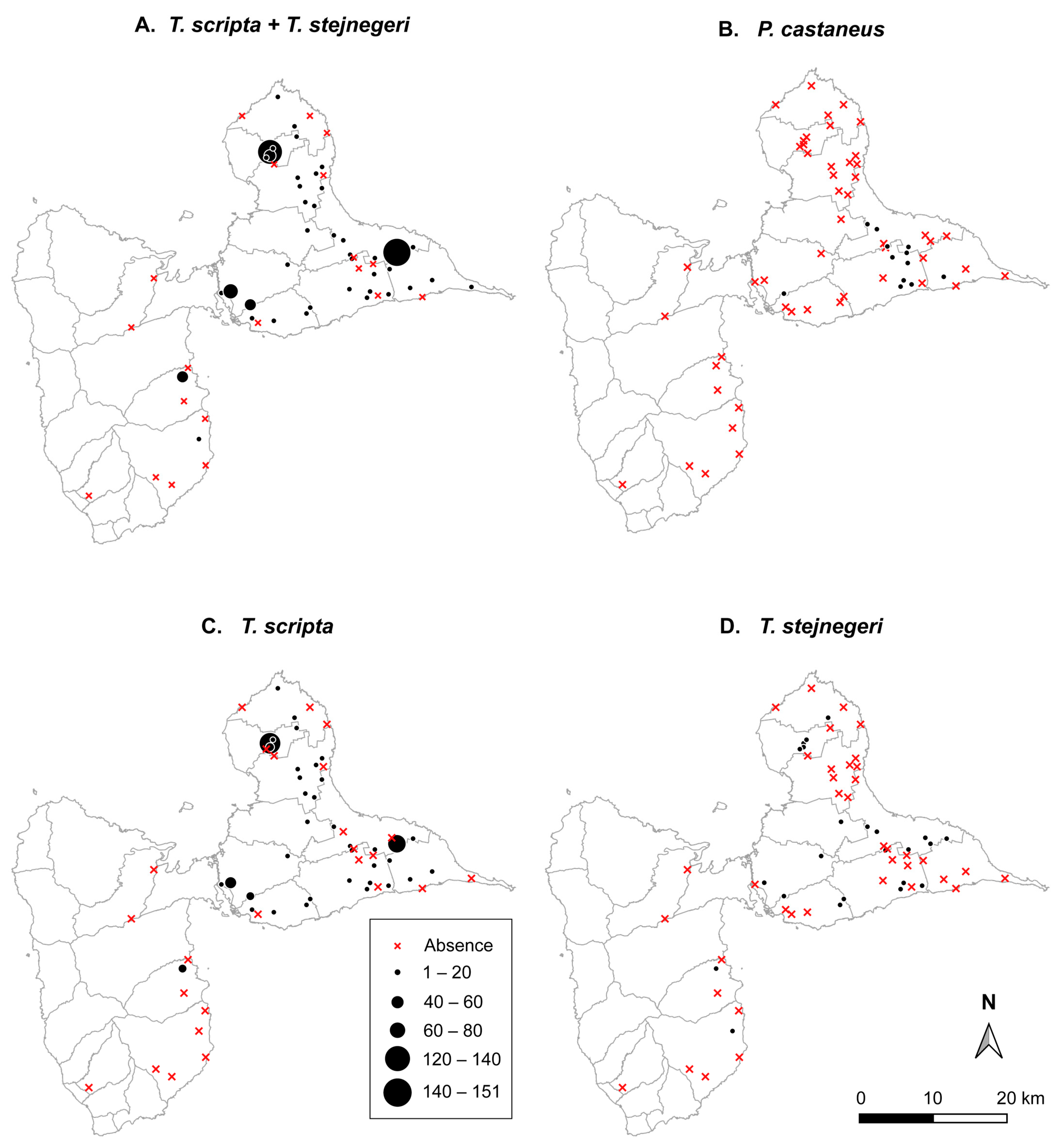
3. Results
3.1. Spatial Variation in Occupancy Rate between Species
3.2. Spatial Variation in Abundance between Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- IPBES. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2019. Available online: https://www.ipbes.net/global-assessment (accessed on 11 May 2023).
- Carlquist, S.J. Island Biology; Columbia University Press: New York, NY, USA, 1974; ISBN 0231035624. [Google Scholar]
- Powell, R.; Henderson, R.W.; Farmer, M.C.; Breuil, M.; Echternacht, A.C.; van Buurt, G.; Romagosa, C.M.; Perry, G. Introduced amphibians and reptiles in the Greater Caribbean: Patterns and conservation implications. In Conservation of Caribbean Island Herpetofaunas Volume 1: Conservation Biology and the Wider Caribbean; Brill: Leiden, The Netherlands, 2011; Volume 1, pp. 63–143. ISBN 9789004183957. [Google Scholar]
- Breuil, M.; Guiougou, F.; Questel, K.; Ibéné, B. Modifications du peuplement herpétologique dans les Antilles françaises: Disparitions et espèces allochtones. 2ème partie: Reptiles. Courr. Nat. 2009, 251, 36–43. [Google Scholar]
- Reaser, J.K.; Meyerson, L.A.; Cronk, Q.; De Poorter, M.; Eldrege, L.G.; Green, E.; Kairo, M.; Latasi, P.; Mack, R.N.; Mauremootoo, J.; et al. Ecological and socioeconomic impacts of invasive alien species in island ecosystems. Environ. Conserv. 2007, 34, 98–111. [Google Scholar] [CrossRef]
- Bridgewater, P.; Kim, R.E.; Bosselmann, K. Ecological integrity: A relevant concept for international environmental law in the Anthropocene? Yearb. Int. Environ. Law 2014, 25, 61–78. [Google Scholar] [CrossRef]
- Arendt, W.J.; Mora, T.A.V. Range expansion of the Shiny Cowbird in the Dominican Republic. J Field Ornithol. 1984, 55, 104–107. [Google Scholar]
- Arendt, W.J. Range expansion of the Cattle Egret (Bubulcus ibis) in the Greater Caribbean Basin. Colon. Waterbirds 1988, 11, 252–262. [Google Scholar] [CrossRef]
- Thorpe, R.S. Reptiles of the Lesser Antilles; Chimaira: Frankfurt am Main, Germany, 2022; ISBN 9783899731231. [Google Scholar]
- Critical Ecosystem Partnership Fund (C.E.P.F.). The Caribbean Islands Biodiversity Hotspot. 2019. Available online: https://www.cepf.net/sites/default/files/cepf-caribbean-islands-ecosystem-profile-december-2020-english.pdf (accessed on 11 May 2023).
- Henderson, R.W.; Powell, R. Natural History of West Indian Reptiles and Amphibians; University Press of Florida: Florida, CA, USA, 2009; ISBN 9780813033945. [Google Scholar]
- Parham, J.F.; Papenfuss, T.J.; Van Dijk, P.P.; Wilson, B.S.; Marte, C.; Schettino, L.R.; Brian Simison, W. Genetic introgression and hybridization in Antillean freshwater turtles (Trachemys) revealed by coalescent analyses of mitochondrial and cloned nuclear markers. Mol. Phylogenet. Evol. 2013, 67, 176–187. [Google Scholar] [CrossRef]
- Héritier, L.; Valdeón, A.; Sadaoui, A.; Gendre, T.; Ficheux, S.; Bouamer, S.; Kechemir-Issad, N.; Du Preez, L.; Palacios, C.; Verneau, O. Introduction and invasion of the red-eared slider and its parasites in freshwater ecosystems of Southern Europe: Risk assessment for the European pond turtle in wild environments. Biodivers. Conserv. 2017, 26, 1817–1843. [Google Scholar] [CrossRef]
- Bour, R.; Luiselli, L.; Petrozzi, F.; Segniagbeto, G.; Chirio, L. Pelusios castaneus (Schweigger 1812)—West African mud turtle, swamp terrapin. Chelonian Res. Monogr. 2016, 5, 095.1–095.11. [Google Scholar] [CrossRef]
- Breuil, M. Histoire Naturelle des Amphibiens et Reptiles Terrestres de l’Archipel Guadeloupéen: Guadeloupe, Saint-Martin, Saint-Barthélemy; Patrimoines naturels; Muséum national d’Histoire Naturelle: Paris, France, 2002; ISBN 9782856535448. [Google Scholar]
- Schwartz, A.; Henderson, R.W. Amphibians and Reptiles of the West Indies: Descriptions, Distributions, and Natural History; University Press of Florida: Florida, CA, USA, 1991; ISBN 9780813010496. [Google Scholar]
- de Massary, J.-C.; Bochaton, C.; Dewynter, M.; Fretey, T.; Ineich, I.; Lorvelec, O.; Vidal, N.; Lescure, J. Taxonomic list of herpetofauna in the French overseas territories: V. Department of Guadeloupe. Bull. Soc. Herpétol. Fr. 2021, 178, 6–23. [Google Scholar] [CrossRef]
- Kraus, F. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2008; ISBN 9781402089459. [Google Scholar]
- Dick, J.T.A.; Gallagher, K.; Avlijas, S.; Clarke, H.C.; Lewis, S.E.; Leung, S.; Minchin, D.; Caffrey, J.; Alexander, M.E.; Maguire, C.; et al. Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol. Invasions 2013, 15, 837–846. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG)—A Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Cuthbert, R.; Coughlan, N.E.; Dickey, J.; Rea, M.; Laverty, C.; South, J.; Crane, K.; McCard, M.; Dick, J.T.A. Shell shocked: High potential impacts on native prey by non-native turtles irrespective of benthic habitat context. Aquat. Invasions 2019, 14, 758–774. [Google Scholar] [CrossRef]
- Vamberger, M.; Ihlow, F.; Asztalos, M.; Dawson, J.E.; Jasinski, S.E.; Praschag, P.; Fritz, U. So different, yet so alike: North American slider turtles (Trachemys scripta). Vertebr. Zool. 2019, 70, 87–96. [Google Scholar] [CrossRef]
- Ministère de la Transition Ecologique. Arrêté du 7 Juillet 2020 Relatif à la Prévention de l’Introduction et de la Propagation des Espèces Animales Exotiques Envahissantes sur le Territoire de la Guadeloupe—Interdiction de Toutes Activités Portant sur des Spécimens Vivants; Ministère de la Transition Ecologique: Paris, France, 2020; pp. 1–6.
- Courchamp, F.; Fournier, A.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Jeschke, J.M.; Russell, J.C. Invasion biology: Specific problems and possible solutions. Trends Ecol. Evol. 2017, 32, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.-F.; David, G. Rapport d’Etudes sur la Répartition à la Martinique de la Tortue de Floride à Tempes Rouges et Eléments de Biologie; Direction de l’Environnement, de l’Aménagement et du Logement: Fort-de-France, France, 2014.
- Elphick, C.S. How you count counts: The importance of methods research in applied ecology. J. Appl. Ecol. 2008, 45, 1313–1320. [Google Scholar] [CrossRef]
- Cunningham, R.B.; Lindenmayer, D.B. Modeling count data of rare species: Some statistical issues. Ecology 2005, 86, 1135–1142. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Ingram, M.; Vukcevic, D.; Golding, N. Multi-output Gaussian processes for species distribution modelling. Methods Ecol. Evol. 2020, 11, 1587–1598. [Google Scholar] [CrossRef]
- Taylor, M.K.; Whelan, C.; Schwarz, C.J.; Hanington, P.C.; Jackson, L.J. Zero-altered modeling of an aquatic parasite host with application to invasive species risk assessments. Manag. Biol. Invasions 2022, 13, 845–862. [Google Scholar] [CrossRef]
- Moshobane, M.C.; Esser, L.F. Ensemble modeling for the potential distribution of invasive weed Verbesina encelioides in South Africa from 2020 to 2090. Manag. Biol. Invasions 2022, 13, 833–844. [Google Scholar] [CrossRef]
- Ménard, I. Etude de Recensement des Zones Humides de Guadeloupe; Office National des Forêts: Basse-Terre, France, 2007.
- Levesque, A. Inventaire de l’Avifaune de 18 Mares de Guadeloupe; Association AMAZONA: Les Abymes, France, 2002. [Google Scholar]
- Meurgey, F.; Daigle, J.J. New status for Orthemis macrostigma (Rambur, 1842) from the Lesser Antilles (Anisoptera: Libellulidae). Odonatologica 2007, 36, 71–78. [Google Scholar]
- Podani, J.; Csontos, P. Quadrat size dependence, spatial autocorrelation and the classification of community data. Community Ecol. 2006, 7, 117–127. [Google Scholar] [CrossRef]
- Zermeño-Díaz, D.M. Diagnostics of observed dry trends in Caribbean precipitation. Int. J. Climatol. 2022, 42, 6927–6943. [Google Scholar] [CrossRef]
- Saint-Louis, L.J.; Paul, J.M.; Célestin, W.; Beaune, D.; Cézilly, F. A baseline survey of waterbirds in five major wetlands of Haiti. Waterbirds 2021, 44, 370–375. [Google Scholar] [CrossRef]
- Grant, P.J.; Sharrock, J.T.R. Binoculars and telescopes survey. Br. Birds 1988, 63, 160. [Google Scholar]
- Strickfaden, K.M.; Fagre, D.A.; Golding, J.D.; Harrington, A.H.; Reintsma, K.M.; Tack, J.D.; Dreitz, V.J. Dependent double-observer method reduces false-positive errors in auditory avian survey data. Ecol. Appl. 2020, 30, e02026. [Google Scholar] [CrossRef]
- Shirley, M.H.; Dorazio, R.M.; Abassery, E.; Elhady, A.A.; Mekki, M.S.; Asran, H.H. A sampling design and model for estimating abundance of Nile crocodiles while accounting for heterogeneity of detectability of multiple observers. J. Wildl. Manag. 2012, 76, 966–975. [Google Scholar] [CrossRef]
- Nichols, J.D.; Hines, J.E.; Sauer, J.R.; Fallon, F.W.; Fallon, J.E.; Heglund, P.J. A double-observer approach for estimating detection probability and abundance from point counts. Auk 2000, 117, 393–408. [Google Scholar] [CrossRef]
- Lambert, M.R.; Nielsen, S.N.; Wright, A.N.; Thomson, R.C.; Shaffer, H.B. Habitat features determine the basking distribution of introduced red-eared sliders and native western pond turtles. Chelonian Conserv. Biol. 2013, 12, 192–199. [Google Scholar] [CrossRef]
- Selman, W. Life in skinny water: Observations of juvenile diamondback terrapins (Malaclemys terrapin) utilizing shallow water habitats. Herpetol. Conserv. Biol. 2018, 13, 399–407. [Google Scholar]
- McKnight, D.T.; Ard, K.; Auguste, R.J.; Barhadiya, G.; Benard, M.F.; Boban, P.; Dillon, M.L.; Downs, C.T.; DeGregorio, B.A.; Glorioso, B.M.; et al. Nocturnal basking in freshwater turtles: A global assessment. Glob. Ecol. Conserv. 2023, 43, e02444. [Google Scholar] [CrossRef]
- Zar, J.H. Spearman Rank Correlation: Overview. In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014; p. stat05964. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence: Probabilistic model. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Griffith, D.; Veech, J.; Marsh, C. Cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Jean-Pierre, A.; Loranger-Merciris, G.; Cézilly, F. Spatial occupancy, local abundance and activity rhythm of three ground dwelling columbid species in the forests of Guadeloupe in relation to environmental factors. Diversity 2022, 14, 480. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Monographs on Statistics and Applied Probability; Chapman & Hall: London, UK, 1989; ISBN 9780412317606. [Google Scholar]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Young, D.S.; Roemmele, E.S.; Yeh, P. Zero-inflated modeling part: Traditional zero-inflated count regression models, their applications, and computational tools. WIREs Comput. Stat. 2022, 14, e1541. [Google Scholar] [CrossRef]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression Models for Count Data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Ritschard, G. Régression robuste et problèmes de colinéarité. Stat. Anal. Données 1990, 15, 77–96. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. 2023. Available online: https://cran.r-project.org/web/packages/MuMIn/MuMIn.pdf (accessed on 20 April 2023).
- Pierce, D.A.; Schafer, D.W. Residuals in generalized linear models. J. Am. Stat. Assoc. 1986, 81, 977–986. [Google Scholar] [CrossRef]
- Lee, Y.; Nelder, J.A. Generalized linear models for the analysis of quality-improvement experiments. Can. J. Stat. 1998, 26, 95–105. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 9780387953649. [Google Scholar]
- Gardner, M.J.; Altman, D.G. Confidence intervals rather than P values: Estimation rather than hypothesis testing. Br. Med. J. 1986, 292, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Cumming, G.; Finch, S. Inference by eye: Confidence intervals and how to read pictures of data. Am. Psychol. 2005, 60, 170–180. [Google Scholar] [CrossRef]
- Mohammed, R.S.; Khan, K.; Ali, S.H. Sightings of Trachemys scripta elegans (Reptilia: Emydidae), a new potential aquatic alien invasive species in Trinidad, West Indies. Living World J. Trinidad Tobago Field Nat. Club 2017, 56–58. [Google Scholar]
- Koo, K.S.; Song, S.; Choi, J.H.; Sung, H.-C. Current distribution and status of non-native freshwater turtles in the wild, Republic of Korea. Sustainability 2020, 12, 4042. [Google Scholar] [CrossRef]
- Rivero, J.A. Los Anfibios y Reptiles de Puerto Rico, 2nd ed.; University of Puerto Rico: San Juan, Puerto Rico, 1998; ISBN 9780847723171. [Google Scholar]
- Jones, M.T.; Sievert, P.R. Effects of stochastic flood disturbance on adult wood turtles, Glyptemys insculpta, in Massachusetts. Can. Field Nat. 2009, 123, 313–322. [Google Scholar] [CrossRef]
- Selman, W.; Qualls, C. The impacts of hurricane Katrina on a population of yellow-blotched sawbacks (Graptemys flavimaculata) in the Lower Pascagoula River. Herpetol. Conserv. Biol. 2008, 3, 224–230. [Google Scholar]
- Moll, E.O.; Legler, J.M. The Life History of a Neotropical Slider Turtle, Pseudemys scripta (Scheopff) in Panama; County Museum of Natural History: Los Angeles, CA, USA, 1971; Volume 11. [Google Scholar]
- Pagney Bénito-Espinal, F. Villes de piémont à risques d’inondations en îles tropicales: Exemple des Antilles françaises. Rev. Geogr. Alp. 1994, 82, 45–57. [Google Scholar] [CrossRef]
- Khouakhi, A.; Villarini, G.; Vecchi, G.A. Contribution of tropical cyclones to rainfall at the global scale. J. Clim. 2017, 30, 359–372. [Google Scholar] [CrossRef]
- Peterson, T.C.; Taylor, M.A.; Demeritte, R.; Duncombe, D.L.; Burton, S.; Thompson, F.; Porter, A.; Mercedes, M.; Villegas, E.; Semexant Fils, R.; et al. Recent changes in climate extremes in the Caribbean region. J. Geophys. Res. Atmos. 2002, 107, ACL 16-1–ACL 16-9. [Google Scholar] [CrossRef]
- Christensen, R.J.; Chow-Fraser, P. Use of GPS loggers to enhance radio-tracking studies of semi-aquatic freshwater turtles. Herpetol. Conserv. Biol. 2014, 9, 18–28. [Google Scholar]
- Reshetnikov, A.N.; Zibrova, M.G.; Ayaz, D.; Bhattarai, S.; Borodin, O.V.; Borzée, A.; Brejcha, J.; Çiçek, K.; Dimaki, M.; Doronin, I.V.; et al. Rarely naturalized, but widespread and even invasive: The paradox of a popular pet terrapin expansion in Eurasia. NeoBiota 2023, 81, 91–127. [Google Scholar] [CrossRef]
- Cadi, A.; Delmas, V.; Prévot-Julliard, A.-C.; Joly, P.; Pieau, C.; Girondot, M. Successful reproduction of the introduced slider turtle (Trachemys scripta elegans) in the South of France. Aquat. Conserv. 2004, 14, 237–246. [Google Scholar] [CrossRef]
- Castro, R.S. Estudio Comparativo Sobre la Estructura Poblacional por Edad y Sexo y la Baundancia Relative de Trachemys scripta Elegans Weld-Neuwied y Trachemys stejnegeri stejnegeri Schmidt en la Reserva de Vida Silvestre en Humacao y el Jardin Botanico de San Juan, Puerto Rico; Universidad Metropolitana: San Juan, Puerto Rico, 2009. [Google Scholar]
- Lantz, S.M.; Gawlik, D.E.; Cook, M.I. The effects of water depth and submerged aquatic vegetation on the selection of foraging habitat and foraging success of wading birds. Condor 2010, 112, 460–469. [Google Scholar] [CrossRef]
- Terral, R.; Sélise, M. Dynamiques urbaines communes et spécificités des villes des Antilles françaises (Guadeloupe, Martinique) des origines de la colonisation (1635) à nos jours. Etudes Caribéennes 2018, 39–40, 12811. [Google Scholar] [CrossRef]
- Unger, S.D.; Santana, A. Turtles and trail cameras: Non-invasive monitoring using artificial platforms. Basic Appl. Herpetol. 2019, 33, 93–100. [Google Scholar] [CrossRef]
- Bluett, R.D.; Schauber, E.M. Estimating abundance of adult Trachemys scripta with camera traps: Accuracy, precision and probabilities of capture for a closed population. Trans. Ill. State Acad. Sci. 2014, 107, 19–24. [Google Scholar]
- Escobar, J.; Rollins, M.A.; Unger, S.D. Telescoping turtles: A comparison of smartphone telephoto magnifiers to non-invasively observe and identify freshwater turtles. Herpetol. J. 2018, 28, 143–147. [Google Scholar]
- Kakuda, A.; Doi, H.; Souma, R.; Nagano, M.; Minamoto, T.; Katano, I. Environmental DNA detection and quantification of invasive red-eared sliders, Trachemy scripta elegans, in ponds and the influence of water quality. PeerJ 2019, 7, e8155. [Google Scholar] [CrossRef]
- Bronnenhuber, J.E.; Wilson, C.C. Combining species-specific COI primers with environmental DNA analysis for targeted detection of rare freshwater species. Conserv. Genet. Resour. 2013, 5, 971–975. [Google Scholar] [CrossRef]
- Davy, C.M.; Kidd, A.G.; Wilson, C.C. Development and validation of environmental DNA (eDNA) markers for detection of freshwater turtles. PLoS ONE 2015, 10, e0130965. [Google Scholar] [CrossRef] [PubMed]
- Luiselli, L.; Demaya, G.S.; Benansio, J.S.; Petrozzi, F.; Akani, G.C.; Eniang, E.A.; Ajong, S.N.; Di Vittorio, M.; Amadi, N.; Dendi, D. A comparative analysis of the diets of a genus of freshwater turtles across Africa. Diversity 2021, 13, 165. [Google Scholar] [CrossRef]
| Variables | Descriptions | Type |
|---|---|---|
| Region | The mainland of Guadeloupe is divided into two large islands that are connected by a bridge (Grande-Terre and Basse-Terre) | Factorial variable |
| Area | Size area of the sites was calculated and then categorized as an ordinal variable according to quantiles of the distribution, with 4 levels for Trachemys (1 < 2 < 3 < 4) and 2 levels (1 < 2) for Pelusios. | Ordinal variable |
| Anthropization | The percentage of anthropized areas was transformed into frequency (ranging from 0 to 1) and then normalized using square root (√×) transformation. (Shapiro–Wilk test; W = 0.981, p > 0.05) | Continuous variable |
| Ardeidae | Species richness of the Ardeidae family included from zero to five different species of herons and egrets: Ardea alba, Egretta thula, Butorides virescens, Nyctanassa violacea and Bubulcus ibis. | Continuous variable |
| Sunshine | Degree of sunlight was categorized and treated as an ordinal variable, with 1 = rainy or cloudy, 2 = cloudy with sunny spells and 3 = sunny. | Ordinal variable |
| Species | Presence | Absence |
|---|---|---|
| T. scripta | 37 (59.68%) | 25 (40.32%) |
| T. stejnegeri | 23 (37.10%) | 39 (62.90%) |
| P. castaneus | 12 (19.35%) | 50 (80.65%) |
| Co-Occurrence | ||||||
|---|---|---|---|---|---|---|
| Pair of Species | Observed | Probability | Expected | P. less | P. greater | |
| T. scripta | T. stejnegeri | 19 | 0.221 | 13.7 | 0.999 | 0.004 |
| T. scripta | P. castaneus | 7 | 0.116 | 7.2 | 0.582 | 0.671 |
| T. stejnegeri | P. castaneus | 6 | 0.072 | 4.5 | 0.912 | 0.240 |
| Model | AICc | ΔAICc | Weight | Loglik | df | |
|---|---|---|---|---|---|---|
| Trachemys spp. | ||||||
| 1 | Anthropization + Region | 64.9 | 0.00 | 0.464 | −29.263 | 3 |
| 2 | Anthropization + Ardeidae + Region | 66.6 | 1.69 | 0.199 | −28.967 | 4 |
| 3 | Anthropization + Sunshine + Region | 67.3 | 2.36 | 0.143 | −28.113 | 5 |
| 4 | Region | 67.8 | 2.88 | 0.110 | −31.807 | 2 |
| 5 | Anthropization + Sunshine + Region + Ardeidae | 68.3 | 3.41 | 0.085 | −27.409 | 6 |
| Pelusios castaneus | ||||||
| 1 | Sunshine + Region | 53.2 | 0.00 | 0.281 | −22.238 | 4 |
| 2 | Ardeidae + Sunshine + Region | 53.3 | 0.08 | 0.270 | −21.094 | 5 |
| 3 | Sunshine + Region + Anthropization + Ardeidae | 54.7 | 1.49 | 0.133 | −20.570 | 6 |
| 4 | Sunshine + Ardeidae + Region + Area | 55.2 | 2.05 | 0.101 | −20.849 | 6 |
| 5 | Sunshine + Region + Area | 55.5 | 2.34 | 0.087 | −22.222 | 5 |
| 6 | Anthropization + Sunshine + Region | 55.5 | 2.35 | 0.087 | −22.227 | 5 |
| 7 | Anthropization + Ardeidae + Area + Region + Sunshine | 57.0 | 3.85 | 0.041 | −20.478 | 7 |
| Model | AICc | ΔAICc | Weight | Loglik | df | |
|---|---|---|---|---|---|---|
| Trachemys spp. | ||||||
| 1 | Anthropization + Ardeidae + Region | 342.4 | 0.00 | 1 | −160.475 | 9 |
| Pelusios castaneus | ||||||
| 1 | Sunshine + Region | 99.8 | 0.00 | 0.237 | −39.195 | 9 |
| 2 | Ardeidae + Sunshine | 100.5 | 0.62 | 0.174 | −39.504 | 9 |
| 3 | Region | 100.8 | 0.99 | 0.144 | −44.885 | 5 |
| 4 | Sunshine + Area + Anthropization | 101.1 | 1.22 | 0.128 | −36.898 | 11 |
| 5 | Null model | 101.3 | 1.48 | 0.113 | −47.459 | 3 |
| 6 | Ardeidae + Anthropization | 101.4 | 1.51 | 0.111 | −42.644 | 7 |
| 7 | Ardeidae + Sunshine + Area | 102.9 | 3.05 | 0.051 | −37.811 | 11 |
| 8 | Sunshine | 103.4 | 3.50 | 0.041 | −43.640 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, J.M.; Cézilly, F.; Bezault, E.; Cambrone, C. Modelling the Distribution of Three Invasive Freshwater Turtles in Mainland Guadeloupe: Analysis of Their Presence, Abundance and Co-Occurrence. Sustainability 2023, 15, 13450. https://doi.org/10.3390/su151813450
Paul JM, Cézilly F, Bezault E, Cambrone C. Modelling the Distribution of Three Invasive Freshwater Turtles in Mainland Guadeloupe: Analysis of Their Presence, Abundance and Co-Occurrence. Sustainability. 2023; 15(18):13450. https://doi.org/10.3390/su151813450
Chicago/Turabian StylePaul, Jeffey Mackenzy, Frank Cézilly, Etienne Bezault, and Christopher Cambrone. 2023. "Modelling the Distribution of Three Invasive Freshwater Turtles in Mainland Guadeloupe: Analysis of Their Presence, Abundance and Co-Occurrence" Sustainability 15, no. 18: 13450. https://doi.org/10.3390/su151813450
APA StylePaul, J. M., Cézilly, F., Bezault, E., & Cambrone, C. (2023). Modelling the Distribution of Three Invasive Freshwater Turtles in Mainland Guadeloupe: Analysis of Their Presence, Abundance and Co-Occurrence. Sustainability, 15(18), 13450. https://doi.org/10.3390/su151813450







