A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Near-Infrared Spectral Acquisition
2.3. Water Content Measurement of the T. grandis Kernels
- W—water content, %;
- M—Mass of T. grandis kernels before drying, g;
- m—Mass of T. grandis kernels after drying, g.
2.4. Methods for Removing the Outlier Samples of T. grandis Kernels
2.4.1. Mahalanobis Distance Method
- —The Mahalanobis distance of ;
- —The row vector of the spectrum of the sample;
- —Average spectrum of class ;
- —Centered spectral matrix of Class mean;
- —Transport centered spectral matrix of Class x mean.
- —The average Mahalanobis distance of sample;
- e—Threshold coefficient for adjusting threshold range;
- —Standard deviation of Mahalanobis distance of sample.
2.4.2. Concentration Residual Method
- Res(i)—The absolute concentration residual;
- —The measured value of sample i;
- —Average measured value of samples.
2.5. T. grandis Kernels’ Sample Division
2.6. Preprocessing of the Spectra Data of T. grandis Kernels
2.7. Establishment and Validation of a Near-Infrared Water Content Model of T. grandis Kernels
3. Results and Discussion
3.1. Analysis of the Water Content in T. grandis Kernels
3.2. Near-Infrared Spectra Analysis of the T. grandis Kernels
3.3. Analysis and Removal of the Outlier Samples
3.3.1. Mahalanobis Distance Method—Removal of Spectral Outlier Samples
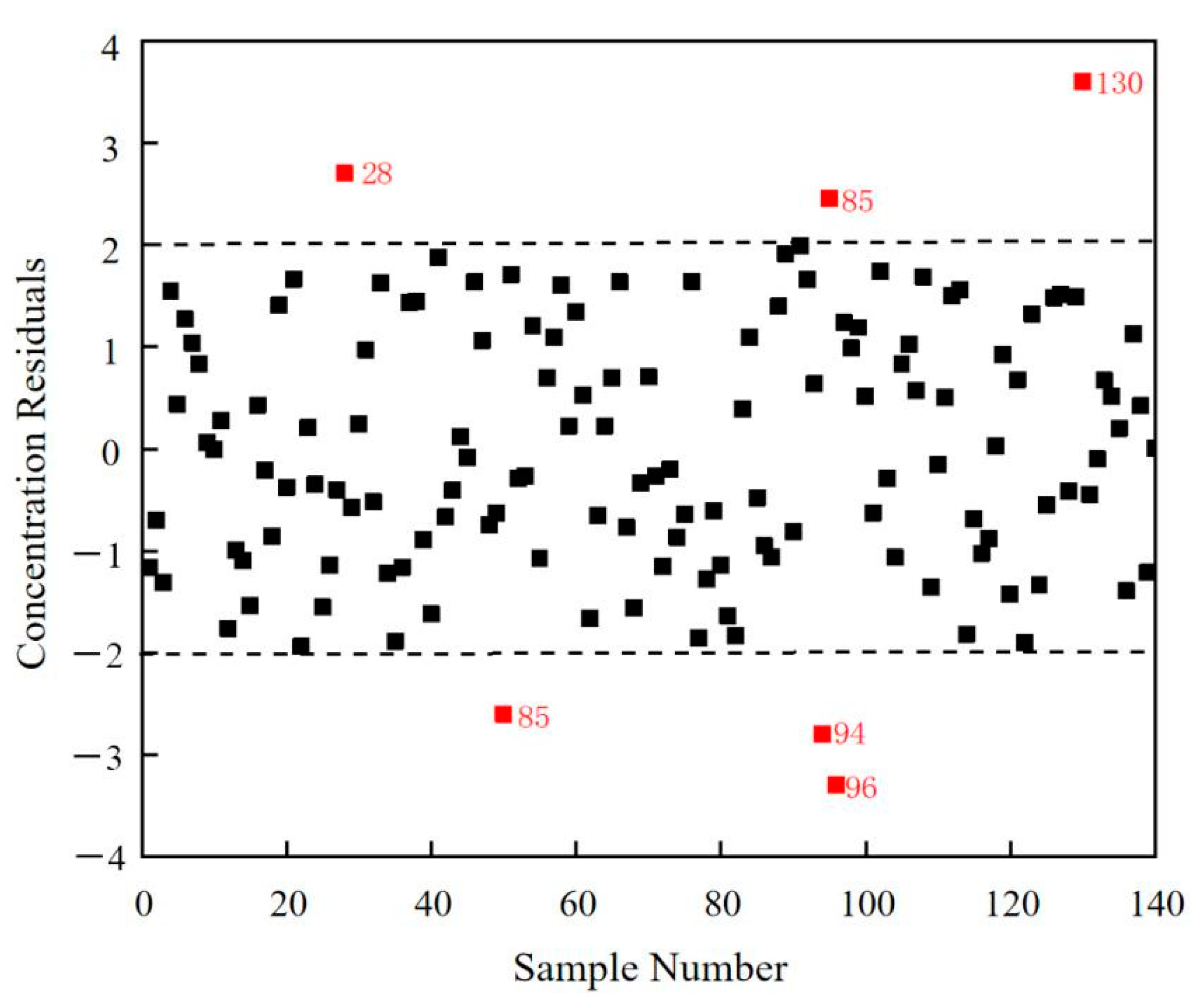
3.3.2. Concentration Residual Method—Removal of the Chemical Outlier Samples
3.4. Sample Division
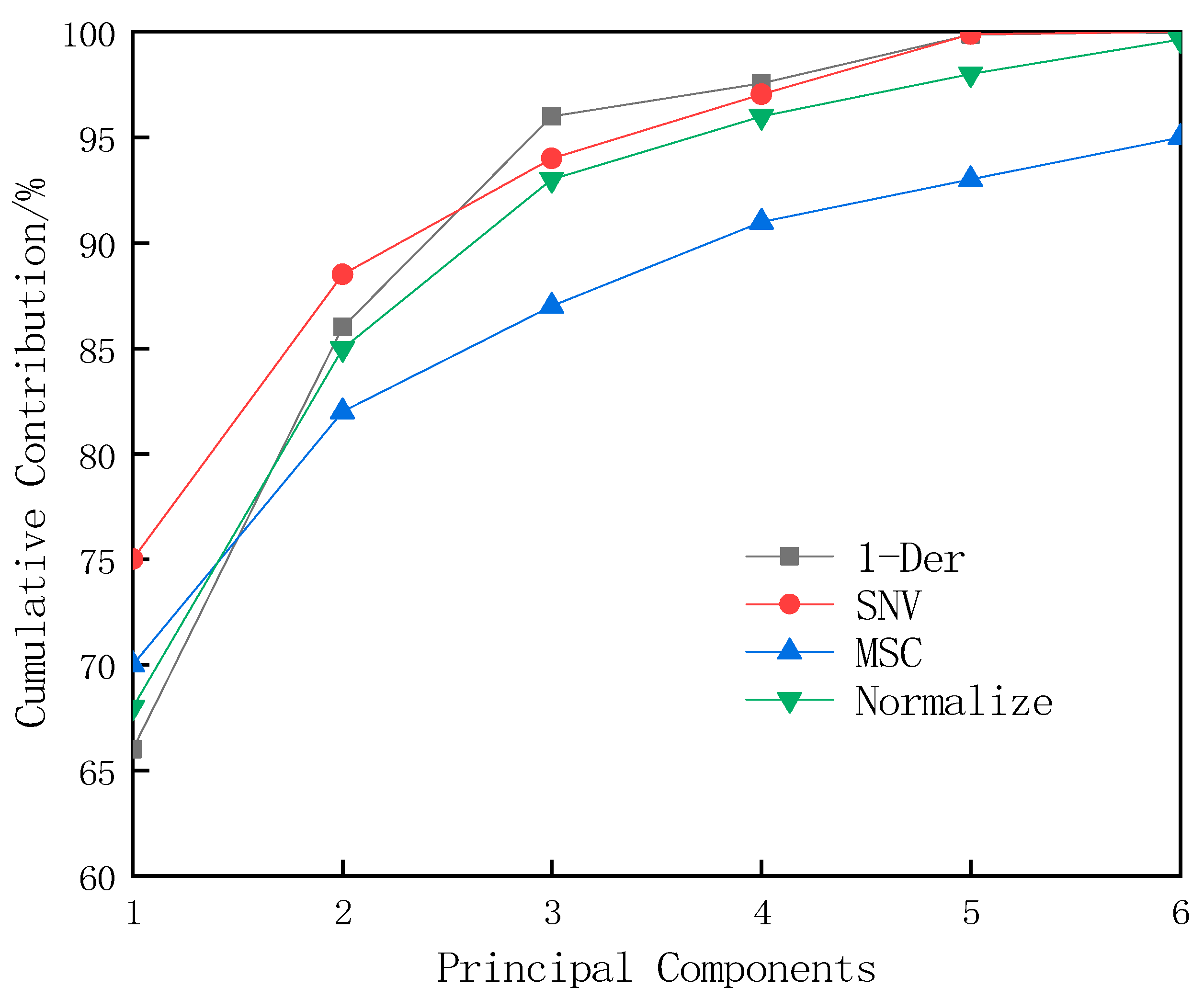
3.5. Establishment and Optimization of the Near-Infrared Calibration Model for the Water Content of T. grandis Kernels
3.6. Comparison of the Near-Infrared Spectroscopy Models for Water Content in Different Foods
4. Conclusions
- (1)
- This study measured the water content of T. grandis kernels samples and obtained their corresponding near-infrared spectral information. The water content of the T. grandis kernels samples ranged from 15.47% to 28.77%. Two obvious absorption peaks of water-content-related groups were located at 1200 nm and 1470 nm, which laid the foundation for the calibration of the near-infrared quantitative model of the water content.
- (2)
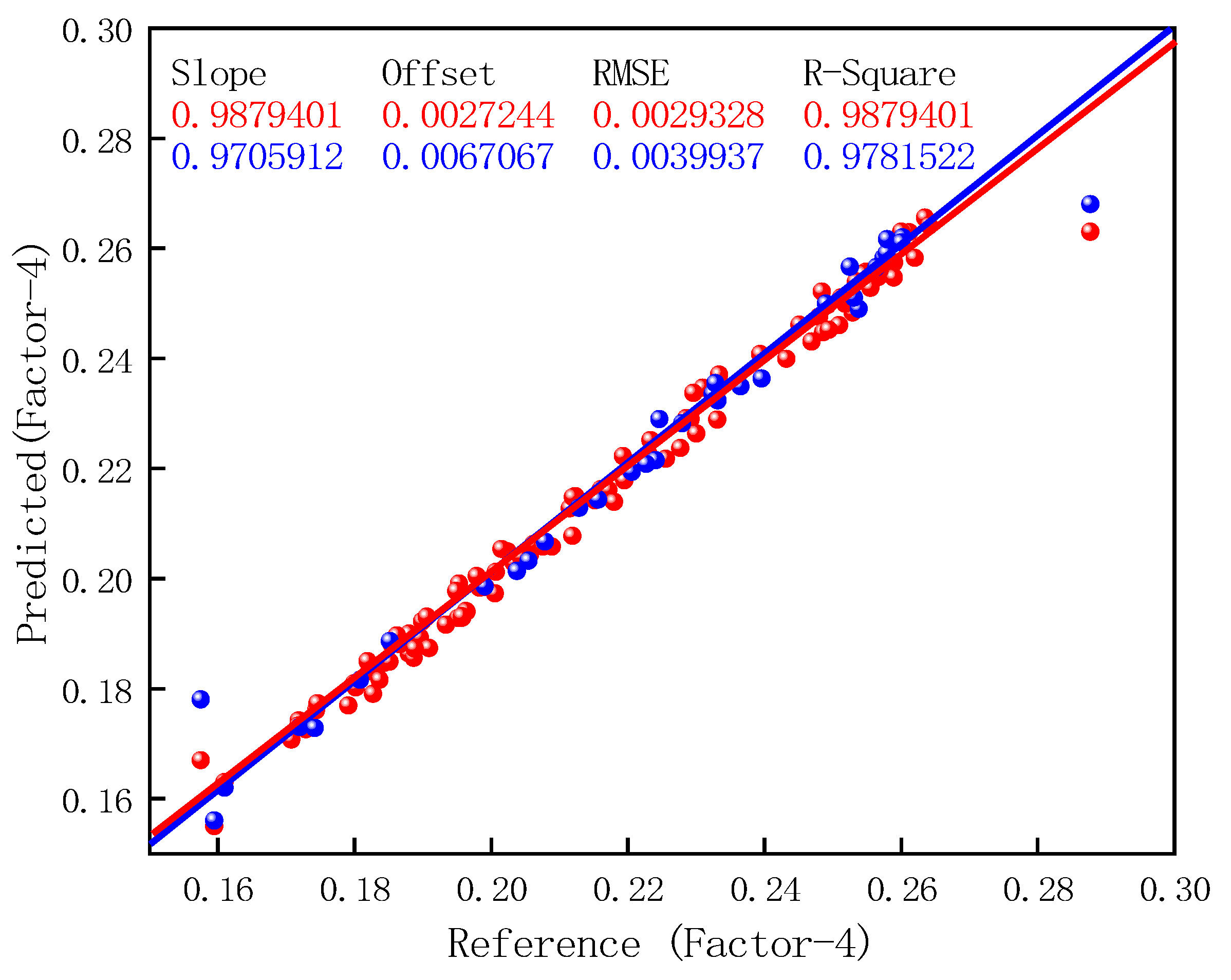
- A quantitative water content model of T. grandis kernels was established using the full spectrum after 10 outlier samples were removed by the Mahalanobis distance method and concentration residual analysis. The optimal calibration model for the water content of T. grandis kernels was the PLS model based on SNV preprocessing. The R2c, RMSEC, R2cv, and RMSECV of this model were 0.9879, 0.0029, 0.9706, and 0.0040, respectively.
- (3)
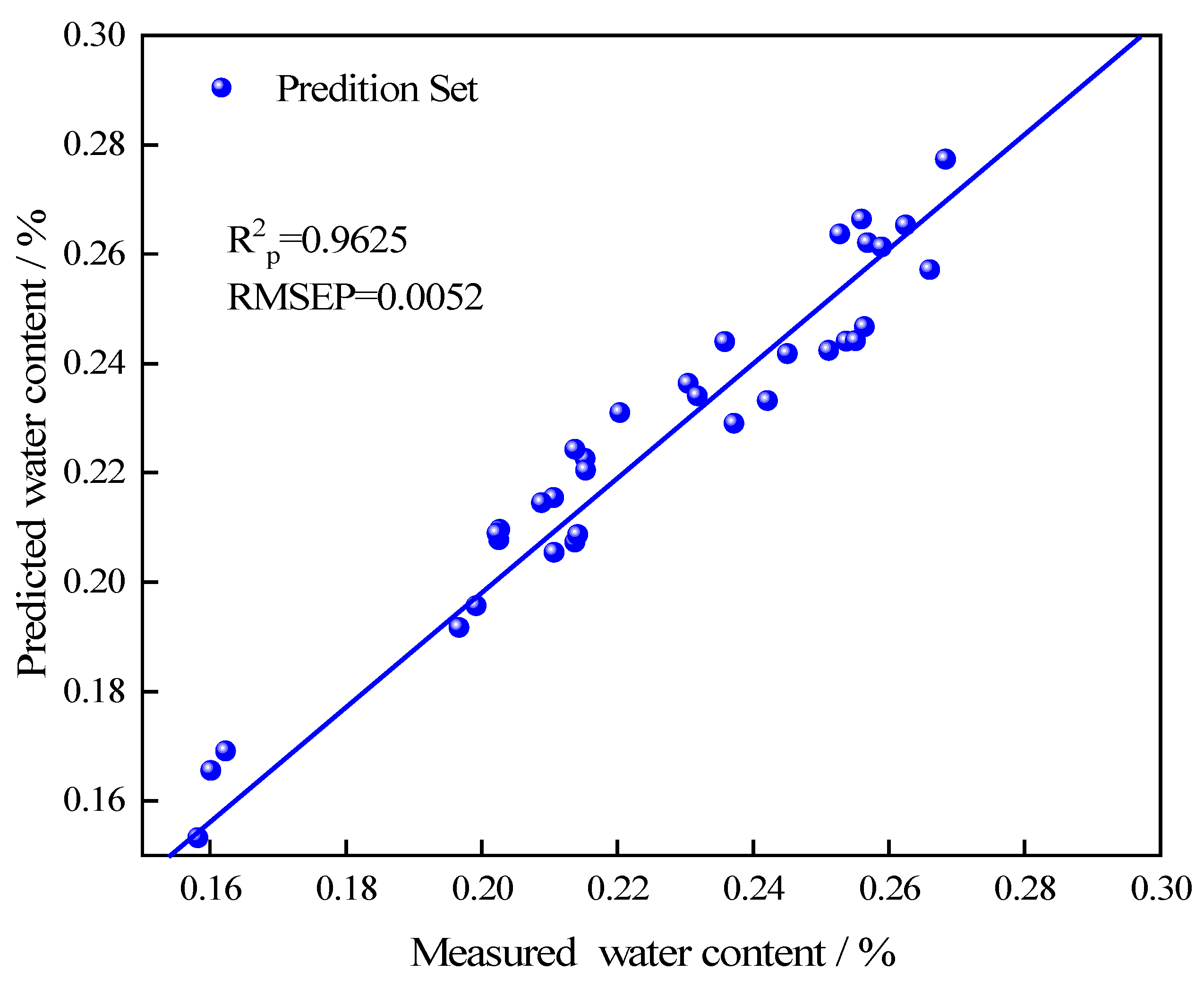
- The samples from the prediction set were used to test the model. The results showed that the prediction correlation coefficient (R2p) of the water content was 0.9625, which indicated that the model had the ability to achieve accurate water content prediction for T. grandis kernels. Near-infrared spectroscopy can be used for rapid nondestructive water determination of nuts. In the future, quantitative determination of other nutrients (such as protein and fat) in T. grandis kernels based on near-infrared spectroscopy could be established, so as to develop a more comprehensive product index evaluation system for T. grandis kernels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Z.; Zhu, H.; Li, W.; Zeng, M.; Wu, S.; Chen, S.; Qin, F.; Chen, J. Chemical components of cold pressed kernel oils from different Torreya grandis cultivars. Food Chem. 2016, 209, 7. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xiao, Q.; Ye, X.; Zhang, S.; Ye, W.; Tu, C.; Zhu, X.; Yu, W.; Li, J. Presentation on Pretreatment Techniques and Moisture Content of Torreya grandis Merrillii Seed. J. Zhejiang For. Sci. Technol. 2022, 42, 81–86. [Google Scholar]
- Guan, S.; Shang, Y.; Zhao, C. Storage Time Detection of Torreya grandis Kernels Using Near Infrared Spectroscopy. Sustainability 2023, 15, 7757. [Google Scholar] [CrossRef]
- Rabanera, J.D.; Guzman, J.D.; Yaptenco, K.F. Rapid and Non-destructive measurement of moisture content of peanut (Arachis hypogaea L.) kernel using a near-infrared hyperspectral imaging technique. J. Food Meas. Charact. 2021, 15, 3069–3078. [Google Scholar] [CrossRef]
- Chala, A.T.; Matula, S.; Batkova, K.; Dolezal, F. Evaluation of methods for water and non-volatile LNAPL contentmeasurement in porous media. Soil Water Res. 2019, 14, 10. [Google Scholar] [CrossRef]
- Gutierrez-Cano, J.D.; Hamilton, I.E.; Catala-Civera, J.M.; Bows, J.; Penaranda-Foix, F.L. Effect of water content on the dynamic measurement of dielectric properties of food snack pellets during microwave. J. Food Eng. 2018, 232, 8. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, C.; Shao, Q.; Yang, Z.; Zhang, X.; Xu, X.; Hassan, M. Determination of water content in corn stover silage using near-infrared spectroscopy. Int. J. Agr. Biol. Eng. 2019, 12, 143–148. [Google Scholar] [CrossRef]
- Hao, Z.; Peng, Y.; Zhang, H.; Zhang, R.; Zhang, R. Nondestructive Testing Research of Wen 185 Walnut Water in South Xinjiang by NIRS. J. Anhui Agric. Sci. 2014, 42, 7191–7193. [Google Scholar]
- Jin, H.; Bian, K. Determinaton of Moisture of Wheat Flour Using near Infrared Spectroscopy. J. Chin. Cereals Oils Assoc. 2010, 25, 109–112. [Google Scholar]
- Ozdemir, I.S.; Orturk, B.; Celik, B.; Santepe, Y.; Aksoy, H. Rapid, simultaneous and non-destructive assessment of the moisture, water activity, firmness and SO2 content ofthe intact sulphured-dried apricot susing FT-NIRS and chemometrics. Talanta 2018, 186, 6. [Google Scholar] [CrossRef]
- Yang, C.; Yu, H.; Guan, S.; Wang, C.; Zhang, J.; Tang, Y.; Wang, X.; Wu, Q. Prediction of Moisture Content in Single Peanut Seed by near Infrared Reflectance Spectroscopy. J. Peanut Sci. 2012, 41, 6–9. [Google Scholar]
- Liu, C.; Hu, Y.; Wu, S.; Sun, X.; Du, S.; Miao, Y.; Du, Y. Outlier sample eliminating methods for building calibration model of near infrared spectroscopy analysis. J. Food Sci. Technol. 2014, 32, 74–79. [Google Scholar]
- Min, S.; Li, N.; Zhang, M. Outlier Diagnosis and Calibration Model Optimization for Near Infrared Spectroscopy Analysis. Spectrosc. Spect. Anal. 2004, 24, 1205–1209. [Google Scholar]
- Chen, B.; Zou, X.; Zhu, W. Eliminating outlier samples in near-infrared model by method of PCA-mahalanobis distance. J. Jiangsu Univ. Nat. Sci. Ed. 2008, 29, 277–292. [Google Scholar]
- Yin, B.; Shi, Y.; Sun, R.; Wang, W. Outlier Samples Detection Method for NIR Multicomponent Analysis. Trans. Chin. Soc. Agric. Mach. 2015, 46, 122–128. [Google Scholar]
- Shi, L.; Zhang, J.; Wang, Y.; Mingdeng, S. Research for eliminating outerlier samples in near-infraredspectroscopy by method of mahalanob distance-concentration residual. J. Chin. Agric. Mech. 2016, 37, 99–103. [Google Scholar]
- Zhu, R.; Duan, H.; Wang, L.; Yao, X.; Xu, C. Comparative research on hyperspectral detection model of beef tenderness with different sample set partitioning methods. Food Ferment. Ind. 2016, 42, 189–192. [Google Scholar]
- Galvão, R.K.H.; Araujo, M.C.U.; José, G.E.; Pontes, M.J.C.; Silva, E.C.; Saldanha, T.C.B. A method for calibration and validation subset partitioning. Talanta 2005, 67, 736–740. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Shi, C.; Chen, M. Determination of oil and moisture content in sunflower seeds based on near infrared diffuse reflectance spectroscopy. Cereals Oils 2022, 35, 142–146. [Google Scholar]
- Bobelyn, E.; Serban, A.; Nicu, M.; Lammertyn, J.; Nicolai, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy: Study of the effect of biological variability on spectra and model performance. Postharvest Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L.; Nieuwoudt, H.; Cronje, P.J.R.; Saeys, W.; Nicolai, B. NIR Spectroscopy Applications for Internal and External Quality Analysis of Citrus Fruit—A Review. Food Bioprocess Technol. 2012, 5, 20. [Google Scholar] [CrossRef]
- Hu, J.; Ma, X.; Liu, L.; Wu, Y.; Ouyang, J. Rapid evaluation of the quality of chestnuts using near-infrared reflectance spectroscopy. Food Chem. 2017, 231, 7. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Sun, Y.; Zhu, Z.; Liu, N.; Lu, J. Near-infrared reflectance spectroscopy for the prediction of chemical composition in walnut kernel. Int. J. Food Prop. 2017, 20, 10. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, S.; Wu, X.; Xing, C.; Yuan, J. Determination of soybean routine quality parameters using near-infrared spectroscopy. Food Sci. Nutr. 2018, 6, 10. [Google Scholar] [CrossRef]
- Macedo, L.L.; Araujo, C.D.S.; Vimercati, W.C.; Hein, P.R.G.; Pimenta, C.J.; Saraiva, S.H. Evaluation of chemical properties of intact green coffee beans usingnear-infrared spectroscopy. J. Sci. Food Agr. 2021, 101, 8. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Roumeliotis, S.; Eglinton, J. Monitoring water uptake in whole barley (Hordeumvulgare L.) grain during steeping using near infrared reflectance spectroscopy. J. Food Eng. 2013, 114, 5. [Google Scholar] [CrossRef]


| Component | Number of Samples | Maximum/% | Minimum/% | Average/% | Standard Deviation |
|---|---|---|---|---|---|
| Water content | 180 | 28.77 | 15.47 | 22.58 | 0.26 |
| Component | Method | Outlier Type | Outlier Sample Number |
|---|---|---|---|
| Water content | Mahalanobis distance | Spectral outlier | 29, 36, 106, 137 |
| Concentration residual | Chemical outlier | 28, 50, 94, 95, 96, 130 |
| Component | Calibration Set (n = 100) | Validation Set (n = 34) | ||||
|---|---|---|---|---|---|---|
| Range/% | Average/% | Standard Deviation | Range/% | Average/% | Standard Deviation | |
| water content | 15.47–28.77 | 22.10 | 0.36 | 15.82–26.60 | 22.79 | 0.23 |
| Preprocessing Methods | R2c | RMSEC | R2cv | RMSECV |
|---|---|---|---|---|
| Origin | 0.8963 | 0.0128 | 0.8773 | 0.0147 |
| 1-Der | 0.8326 | 0.0196 | 0.7948 | 0.0245 |
| 2-Der | 0.8493 | 0.0175 | 0.8034 | 0.024 |
| SG | 0.8826 | 0.014 | 0.829 | 0.0203 |
| Normalize | 0.9021 | 0.0121 | 0.8339 | 0.0199 |
| Baseline | 0.9113 | 0.0109 | 0.8841 | 0.0138 |
| SNV | 0.9879 | 0.0029 | 0.9706 | 0.004 |
| MSC | 0.9767 | 0.0041 | 0.9517 | 0.0065 |
| 1-Der+SNV | 0.9573 | 0.0063 | 0.933 | 0.0075 |
| 2-Der+SNV | 0.9582 | 0.0062 | 0.9213 | 0.0083 |
| Number | Water Range/% | Sample | Preprocessing Method | Calibration Set | Validation Set | Prediction Set | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSEC | R2c | RMSECV | R2cv | RMSEP | R2p | RPD | |||||
| 1 | 9.82~71.09 | Corn stover silage | MSC+1-Der | 4.249 | 0.974 | 4.256 | 0.949 | 4.037 | 0.973 | 4.217 | [7] |
| 2 | 37.13~56.83 | Chestnut | 1-Der+SG | 0.0119 | 0.9650 | 0.547 | 0.904 | N/A | N/A | N/A | [22] |
| 3 | 3.08~4.08 | Walnut | SNV+1-Der | 0.052 | 0.965 | N/A | N/A | 0.058 | 0.952 | 4.14 | [23] |
| 4 | 6.92~13.71 | Soybean | 1-Der+SNV | 0.451 | 0.983 | 0.018 | 0.965 | N/A | 0.966 | 2.00 | [24] |
| 5 | 59.0~82.1 | Peanut | N/A | 0.0168 | 0.935 | 0.0183 | 0.913 | 0.0195 | 0.9445 | N/A | [11] |
| 6 | 28.5~97.9 | Barley | 2-Der | N/A | N/A | 6.36 | 0.94 | 5.36 | 0.9 | 4.1 | [26] |
| 7 | 104.6~134.7 | Raw coffee bean | 2-Der | N/A | N/A | 2.404 | 0.858 | 2.946 | 0.81 | 2.24 | [25] |
| 8 | 15.47~28.77 | T. grandis kernels | SNV | 0.0029 | 0.988 | 0.0039 | 0.978 | 0.0052 | 0.963 | 4.42 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, J.; Huang, Y.; Guan, S.; Shang, Y.; Bao, L.; Yan, X.; Hassan, M.; Xu, L.; Zhao, C. A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy. Sustainability 2023, 15, 12423. https://doi.org/10.3390/su151612423
Xiang J, Huang Y, Guan S, Shang Y, Bao L, Yan X, Hassan M, Xu L, Zhao C. A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy. Sustainability. 2023; 15(16):12423. https://doi.org/10.3390/su151612423
Chicago/Turabian StyleXiang, Jiankai, Yu Huang, Shihao Guan, Yuqian Shang, Liwei Bao, Xiaojie Yan, Muhammad Hassan, Lijun Xu, and Chao Zhao. 2023. "A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy" Sustainability 15, no. 16: 12423. https://doi.org/10.3390/su151612423
APA StyleXiang, J., Huang, Y., Guan, S., Shang, Y., Bao, L., Yan, X., Hassan, M., Xu, L., & Zhao, C. (2023). A Sustainable Way to Determine the Water Content in Torreya grandis Kernels Based on Near-Infrared Spectroscopy. Sustainability, 15(16), 12423. https://doi.org/10.3390/su151612423







