Effects of Biochar and Apatite on Chemical Forms of Lead and Zinc in Multi-Metal-Contaminated Soil after Incubation: A Comparison of Peanut Shell and Corn Cob Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples and Amendments
- Soil samples
- Biochar
- Apatite
2.2. Design of Experiments
2.3. Analysis Methods of Soil and Materials
2.3.1. Physicochemical Properties Analysis
2.3.2. Heavy Metal Analysis
2.3.3. Surface Characteristics of Biochar and Apatite
2.4. Data Analysis and Statistics
3. Results and Discussion
3.1. Physicochemical Properties of the Studied Soil and Materials
3.2. Characteristics of Amendments
3.2.1. Fourier Transform Infrared Spectroscopy Analysis of Amendments (FT-IR)
- FT-IR of biochar
- FT-IR of apatite
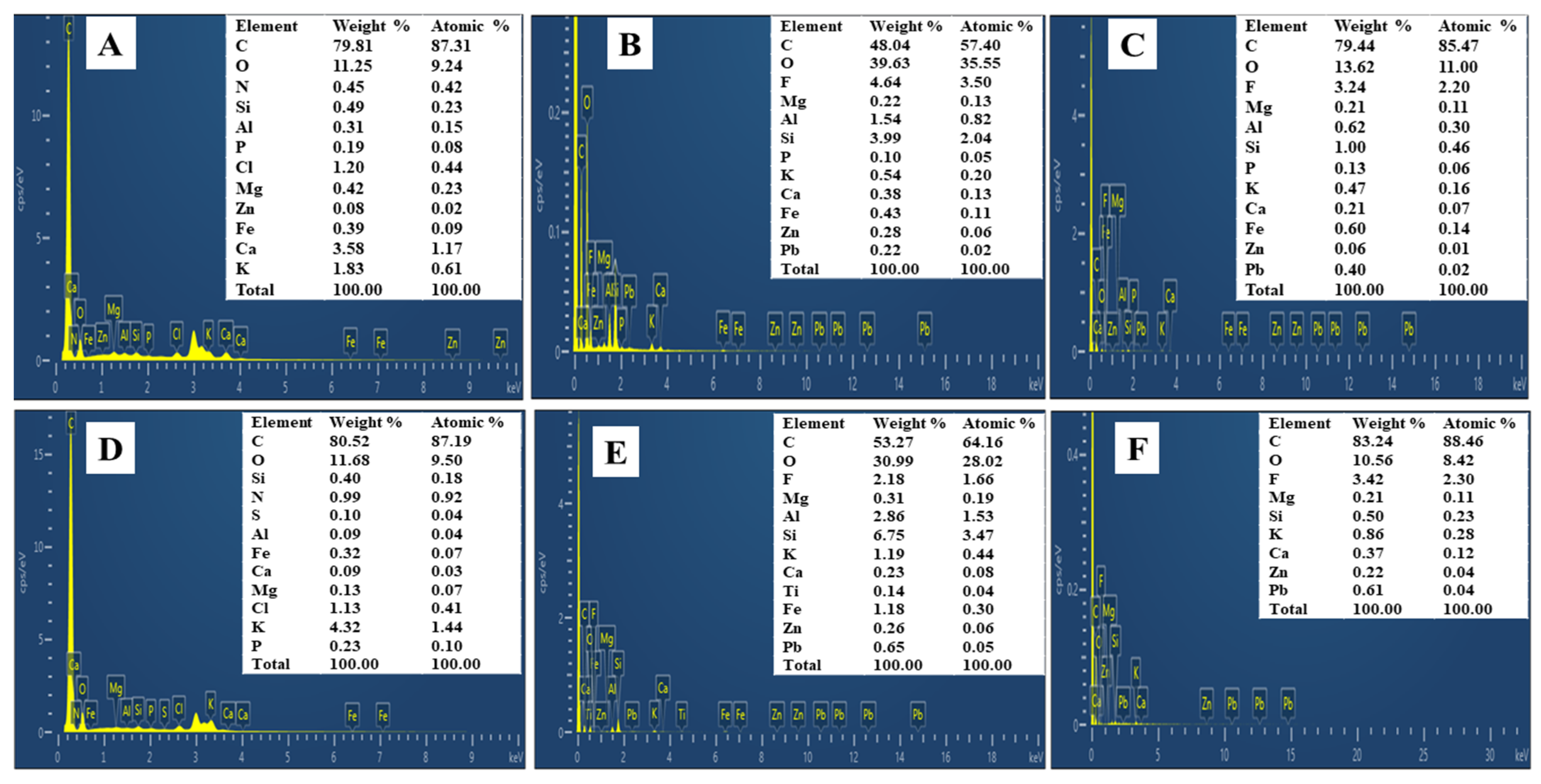
3.2.2. SEM-EDS Analysis of Amendments
- SEM analysis of materials
- EDS analysis of biochar and apatite ore
3.3. Alteration of OC, pH, and EC of the Incubated Soil after a 30-Day Incubation
3.3.1. Soil pH
3.3.2. Organic Carbon (OC)
3.3.3. Electrical Conductivity (EC)
3.4. Chemical Speciation of Lead and Zinc in Control and Incubated Soils after a 30-Day Incubation
3.4.1. Pb Speciation
- Exchangeable fraction (F1-Pb)
- Carbonate fraction (F2-Pb)
- Fe/Mn-Oxide fraction (F3-Pb)
- Organic carbon fraction (F4-Pb)
- Residual fraction (F5-Pb)
3.4.2. Zn Speciation
- Exchangeable fraction of zinc (F1-Zn)
- Carbonate fraction of zinc (F2-Zn)
- Oxide bound fraction of zinc (F3-Zn)
- Organic compound fraction of zinc (F4-Zn)
- Residual fraction of zinc (Zn-F5)
| Biomass Feedstock | pH | Amended Rate | Incubation Time | Heavy Metals | Impacts | Ref |
|---|---|---|---|---|---|---|
| This study | PSB: 9.53, CCB: 9.5 | 3% and 5% | 1 month | Pb, Zn | Reducing 26% and 33% of the exchangeable fraction of Pb and Zn, respectively. | |
| sugarcane bagasse-derived biochar (produced at 450 °C) | SBC: 11.3 | 1.5, 2.25, and 3% | 2 months | Cu, Pb, Cd | Reducing 40.47% labile fraction of Pb. | [51] |
| garden waste biochar produced at 400 °C and 600 °C | BC400: 11 BC600: 11.5 | 2%, 4%, and 6% | 2 months | Cu, Pb, Cd, and Zn | Increasing the soil pH, and decreasing the acid-soluble fraction of Pb 51% and 16% of Zn at a 6% application rate of biochar. | [49] |
| Bamboo and paulownia biochar produced at 700–800 °C | BB: 10.0 PB: 10.5 | 2%, 4%, and 6% | 2 months | Cu, Pb, Cd, and Zn | Increasing the soil pH, and decreasing the acid-soluble fraction of Pb and Zn up to 22.12% and 24.31%, respectively, at a 6% biochar application rate. | [15] |
| Rice straw biochar produced at 350–500 °C | BC: 11.44 | 1%, 3%, and 5% | 30, 60, and 90 days | Pb, Zn, and Cd | Increasing soil pH, and decreasing exchangeable fraction of Pb and Zn up to 57% and 47.5%, respectively, after 30 days at 5% of biochar application rate. | [20] |
| Peanut shell biochar produced at 400 °C and 600 °C | PB400: 10.9 PB400: 11.3 | 3%, 5%, and 10% | 1 month | Zn, Pb | Biochar could increase the pH of the soil and respectively decrease 44.44% and 26.6% of the exchangeable fraction of Pb and Zn at the 10% biochar application ratio. | [23] |
3.5. Mechanism for Immobilizing Heavy Metals in Incubated Soil
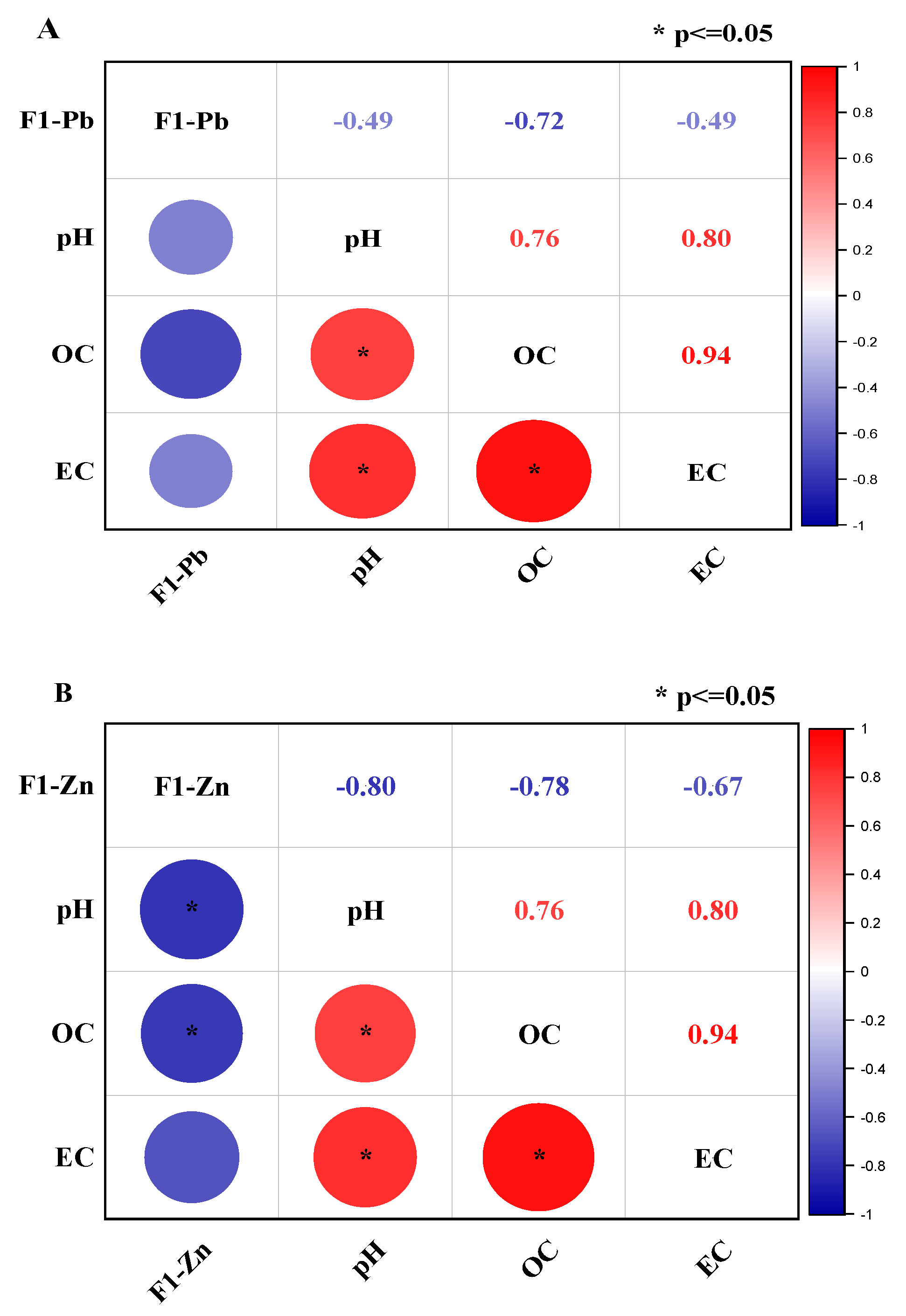
3.6. Correlation of the Exchangeable Fraction of Lead and Zinc with pH, OC, and EC of Incubated Soil after a 30-Day Incubation
- Correlation of F1-Pb with pH, OC, and EC
- Correlation of F1-Zn with pH, OC, and EC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dinu, C.; Vasile, G.-G.; Buleandra, M.; Popa, D.E.; Gheorghe, S.; Ungureanu, E.-M. Translocation and accumulation of heavy metals in Ocimum basilicum L. plants grown in a mining-contaminated soil. J. Soils Sediments 2020, 20, 2141–2154. [Google Scholar] [CrossRef]
- Xu, D.-M.; Fu, R.-B.; Liu, H.-Q.; Guo, X.-P. Current knowledge from heavy metal pollution in Chinese smelter contaminated soils, health risk implications and associated remediation progress in recent decades: A critical review. J. Clean. Prod. 2021, 286, 124989. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks, and best available strategies for remediation. Heavy Met. Contam. Water Soil Anal. Assess. Remediat. Strateg. 2014, 2011, 1–50. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Khan, E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Kiran; Bharti, R.; Sharma, R. Effect of heavy metals: An overview Materials Today: Proceedings Effect of heavy metals: An overview. Mater. Today Proc. 2021, 51, 880–885. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Ju, M.; Liu, L. Preparation and Modification of Biochar Materials and their Application in Soil Remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef] [Green Version]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Chao, X.; Qian, X.; Han-Hua, Z.; Shuai, W.; Qi-Hong, Z.; Dao-You, H.; Yang-Zhu, Z. Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soil. Ecotoxicol. Environ. Saf. 2018, 164, 554–561. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Monisha, R.S.; Mani, R.L.; Sivaprakash, B.; Rajamohan, N.; Vo, D.-V.N. Green remediation of pharmaceutical wastes using biochar: A review. Environ. Chem. Lett. 2021, 20, 681–704. [Google Scholar] [CrossRef]
- Awad, M.; Liu, Z.; Skalicky, M.; Dessoky, E.S.; Brestic, M.; Mbarki, S.; Rastogi, A.; EL Sabagh, A. Fractionation of Heavy Metals in Multi-Contaminated Soil Treated with Biochar Using the Sequential Extraction Procedure. Biomolecules 2021, 11, 448. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, Y.; Cheng, L.; Andserson, B.; Zhao, X.; Wang, D.; Ding, A. Review on utilization of biochar for metal-contaminated soil and sediment remediation. J. Environ. Sci. 2018, 63, 156–173. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Dang, V.M.; Joseph, S.; Van, H.T.; Mai, T.L.A.; Duong, T.M.H.; Weldon, S.; Munroe, P.; Mitchell, D.; Taherymoosavi, S. Immobilization of heavy metals in contaminated soil after mining activity by using biochar and other industrial by-products: The significant role of minerals on the biochar surfaces. Environ. Technol. 2019, 40, 3200–3215. [Google Scholar] [CrossRef]
- Dang, V.M.; Van, H.T.; Duong, H.T.M.; Nguyen, D.H.; Chao, H.-P.; Nguyen, L.H.; Lin, C.-C. Evaluation of fly ash, apatite and rice straw derived-biochar in varying combinations for in situ remediation of soils contaminated with multiple heavy metals. Soil Sci. Plant Nutr. 2020, 66, 379–388. [Google Scholar] [CrossRef]
- Gao, R.; Hu, H.; Fu, Q.; Li, Z.; Xing, Z.; Ali, U.; Zhu, J.; Liu, Y. Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: Speciation transformation, risk evaluation and mechanism inquiry. Sci. Total Environ. 2020, 730, 139119. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.X.; Stephen, J.; Minh, T.B.; Nguyen, T.T.T.; Duong, T.H.; Pham, D.T.N. Chemical Fractionations of Lead and Zinc in the Contaminated Soil Amended with the Blended Biochar/Apatite. Molecules 2022, 27, 8044. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.X.; Stephen, J.; Nguyen, T.T.T.; Cao, V.; Pham, D.T.N. Insight into the Speciation of Heavy Metals in the Contaminated Soil Incubated with Corn Cob-Derived Biochar and Apatite. Molecules 2023, 28, 2225. [Google Scholar] [CrossRef] [PubMed]
- Nejad, Z.D.; Jung, M.C.; Kim, K.-H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, B.; Gao, X.; Cui, S.; Guan, C.-Y.; Zhang, Y.; Zhang, B.; Peng, Y. Recent progresses, challenges, and opportunities of carbon-based materials applied in heavy metal polluted soil remediation. Sci. Total Environ. 2023, 856, 158810. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabbarov, Z.; Arora, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar mitigates effects of pesticides on soil biological activities. Environ. Sustain. 2021, 4, 335–342. [Google Scholar] [CrossRef]
- Vuong, X.T.; Vu, L.D.; Duong, A.T.T.; Duong, H.T.; Hoang, T.H.T.; Luu, M.N.T.; Nguyen, V.D.; Nguyen, T.T.T.; Van, T.H.; Minh, T.B. Speciation and environmental risk assessment of heavy metals in soil from a lead/zinc mining site in Vietnam. Int. J. Environ. Sci. Technol. 2022, 20, 5295–5310. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Miller, W.P.; Miller, D.M. A micro-pipette method for soil mechanical analysis. Commun. Soil Sci. Plant Anal. 1987, 18, 1–15. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, X.; Cai, P.; Huang, Q.; Rong, X.; Liang, W. Preferential adsorption of extracellular polymeric substances from bacteria on clay minerals and iron oxide. Colloids Surfaces B Biointerfaces 2011, 83, 122–127. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. SW-846 method A, 3051. 1994. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-soils-and (accessed on 1 June 2023).
- QCVN03-MT:2005/BTNMT; 2015/BTNMT National Technical Regulations. Vietnamese Ministry of Natural Resources and Environment: Hanoi City, Vietnam, 2015. (In Vietnamese)
- Usman, A.R.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef] [Green Version]
- Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Hadey, C.; Lgaz, H.; Alrashdi, A.A.; Boukhlifi, F. Characterization and adsorption capacity of four low-cost adsorbents based on coconut, almond, walnut, and peanut shells for copper removal. Biomass-Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, P.; Yang, X.; Li, H.; Liu, Y.; Pan, B. An integrated study on the pyrolysis mecanism of peanut shell based on the kinetic analysis and solid/gas characterization. Bioresour. Technol. 2021, 329, 124860. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Srinivasan, P.; Smernik, R.J.; Manley-Harris, M.; Antal, M.J.; Downie, A.; van Zwieten, L. Retention capacity of biochar-amended New Zealand dairy farm soil for an estrogenic steroid hormone and its primary metabolite. Aust. J. Soil Res. 2010, 48, 648–658. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef] [Green Version]
- Tun, Z.M.; Myat, Z.M.; Win, T.T.; Maung, Y.M. Characterization of activated carbons from coconut and peanut shells biomass. J. Myanmar Acad. Arts Sci. 2020, 18, 65–74. [Google Scholar]
- Behazin, E.; Ogunsona, E.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M.; Anyia, A.O. Mechanical, Chemical, and Physical Properties of Wood and Perennial Grass Biochars for Possible Composite Application. Bioresources 2016, 11, 1334–1348. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Jiang, Z.; Zhao, J.; Wang, Z.; Pan, B.; Xing, B. Formation and Physicochemical Characteristics of Nano Biochar: Insight into Chemical and Colloidal Stability. Environ. Sci. Technol. 2018, 52, 10369–10379. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Nikčević, I.; Jokanović, V.; Mitrić, M.; Nedić, Z.; Makovec, D.; Uskoković, D. Mechanochemical synthesis of nanostructured fluorapatite/fluorhydroxyapatite and carbonated fluorapatite/fluorhydroxyapatite. J. Solid State Chem. 2004, 177, 2565–2574. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, X.T.; Van Nguyen, T.; Nguyen, T.T.; Vu, T.Q.; Nguyen, H.T.; Pham, N.T.; Dinh, T.M.T. Treatment of Cd2+ and Cu2+ Ions Using Modified Apatite Ore. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Qian, R.; Bao, M.; Gu, C.; Zhu, P. Raman, FT-IR and XRD study of bovine bone mineral and carbonated apatites with different carbonate levels. Mater. Lett. 2018, 210, 203–206. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; Chaves, A.P.; Ferreira, W.R. Effect of alkyl chain length of amines on fluorapatite and aluminium phosphates floatabilities. J. Mater. Res. Technol. 2019, 8, 3623–3634. [Google Scholar] [CrossRef]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing Heavy Metal Hazards of Contaminated Soil via Biochar Supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Awad, M.; Moustafa-Farag, M.; Wei, L.; Huang, Q.; Liu, Z. Effect of garden waste biochar on the bioavailability of heavy metals and growth of Brassica juncea (L.) in a multi-contaminated soil. Arab. J. Geosci. 2020, 13, 439. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Bolan, N.S.; Naidu, R.; Syers, J.K.; Tillman, R.W. Surface charge and solute interactions in soils. Adv. Agron. 1999, 67, 87–140. [Google Scholar] [CrossRef]
- Namgay, T.; Singh, B.; Singh, B.P. Influence of biochar application to soil on the availability of As, Cd, Cu, Pb, and Zn to maize (Zea mays L.). Soil Res. 2010, 48, 638–647. [Google Scholar] [CrossRef]
- Ndirangu, S.M.; Liu, Y.; Xu, K.; Song, S. Risk Evaluation of Pyrolyzed Biochar from Multiple Wastes. J. Chem. 2019, 2019, 4506314. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Gao, W.; Tang, Y.; Zhang, Q.; Tu, C.; Cheng, J. Remediation via biochar and potential health risk of heavy metal contaminated soils. Environ. Earth Sci. 2022, 81, 482. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [Green Version]
- Sha, H.; Li, J.; Wang, L.; Nong, H.; Wang, G.; Zeng, T. Preparation of phosphorus-modified biochar for the immobilization of heavy metals in typical lead-zinc contaminated mining soil: Performance, mechanism and microbial community. Environ. Res. 2023, 218, 114769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Liu, Y.-X.; Lu, H.-H.; Yang, R.-Q.; Yang, S.-M. Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions. J. Solid State Chem. 2018, 261, 53–61. [Google Scholar] [CrossRef]
- Lei, S.; Shi, Y.; Qiu, Y.; Che, L.; Xue, C. Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Performance and mechanisms of emerging animal-derived biochars for immobilization of heavy metals. Sci. Total Environ. 2018, 646, 1281–1289. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef]
- Zhao, M.; Dai, Y.; Zhang, M.; Feng, C.; Qin, B.; Zhang, W.; Zhao, N.; Li, Y.; Ni, Z.; Xu, Z.; et al. Mechanisms of Pb and/or Zn adsorption by different biochars: Biochar characteristics, stability, and binding energies. Sci. Total Environ. 2020, 717, 136894. [Google Scholar] [CrossRef]
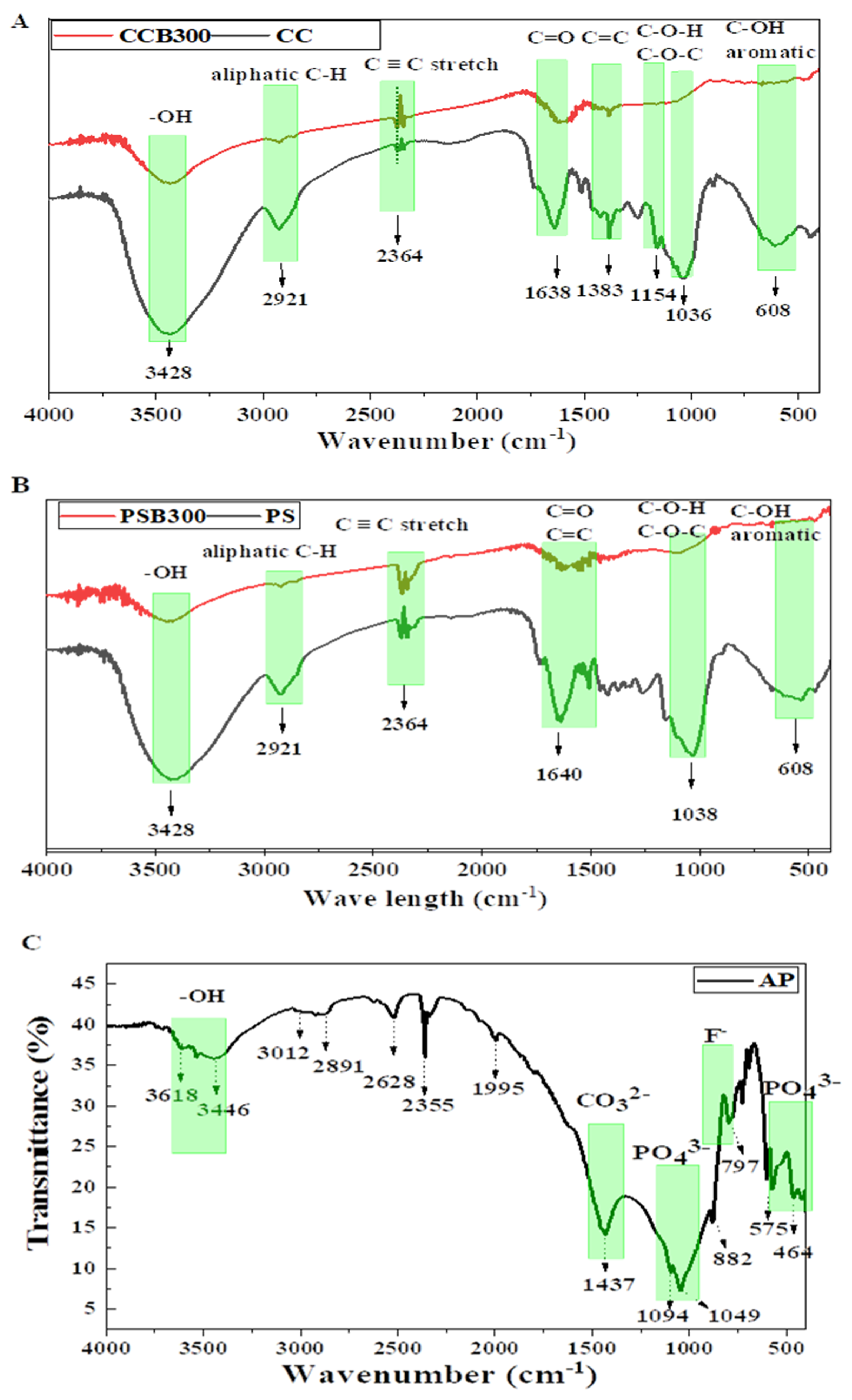
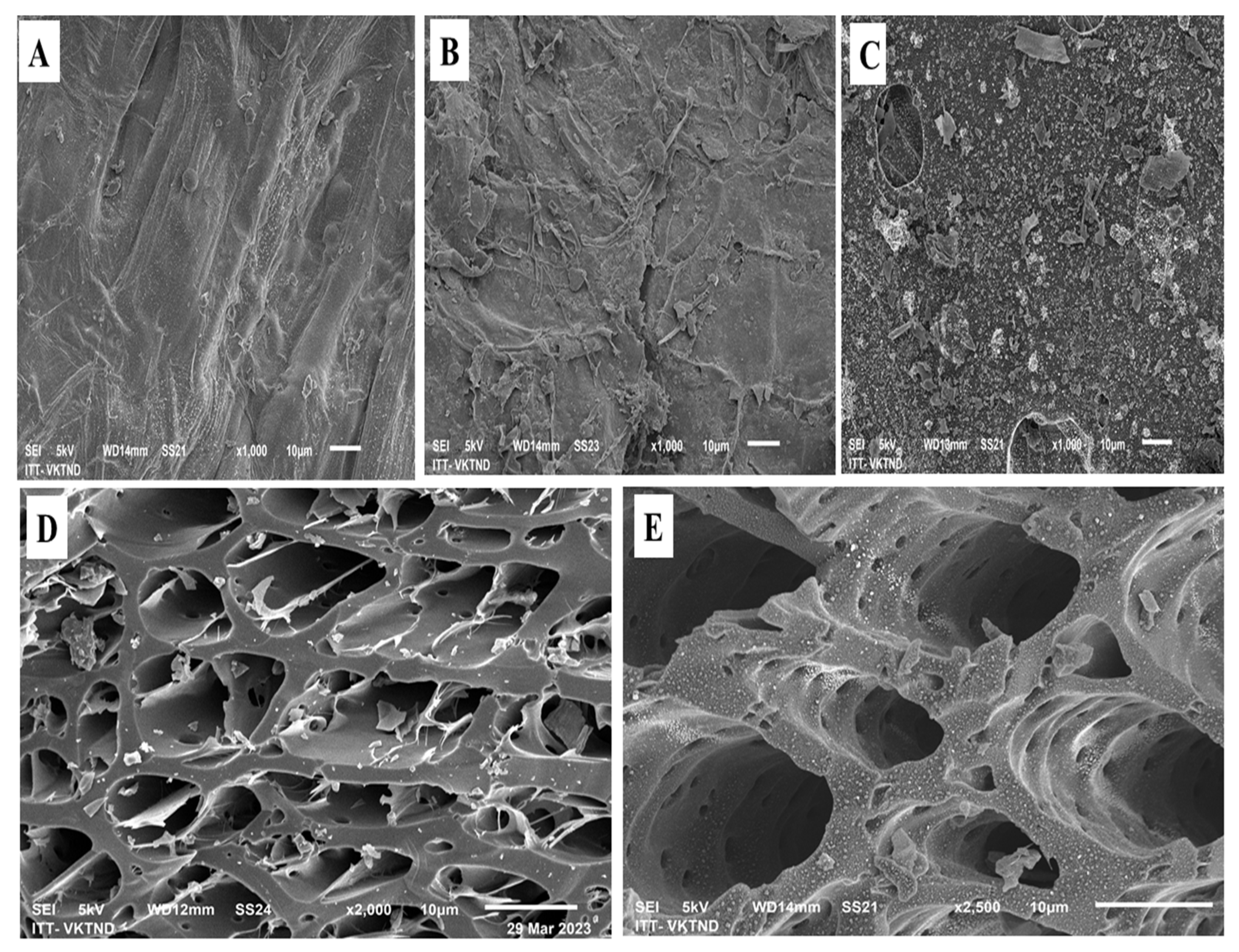
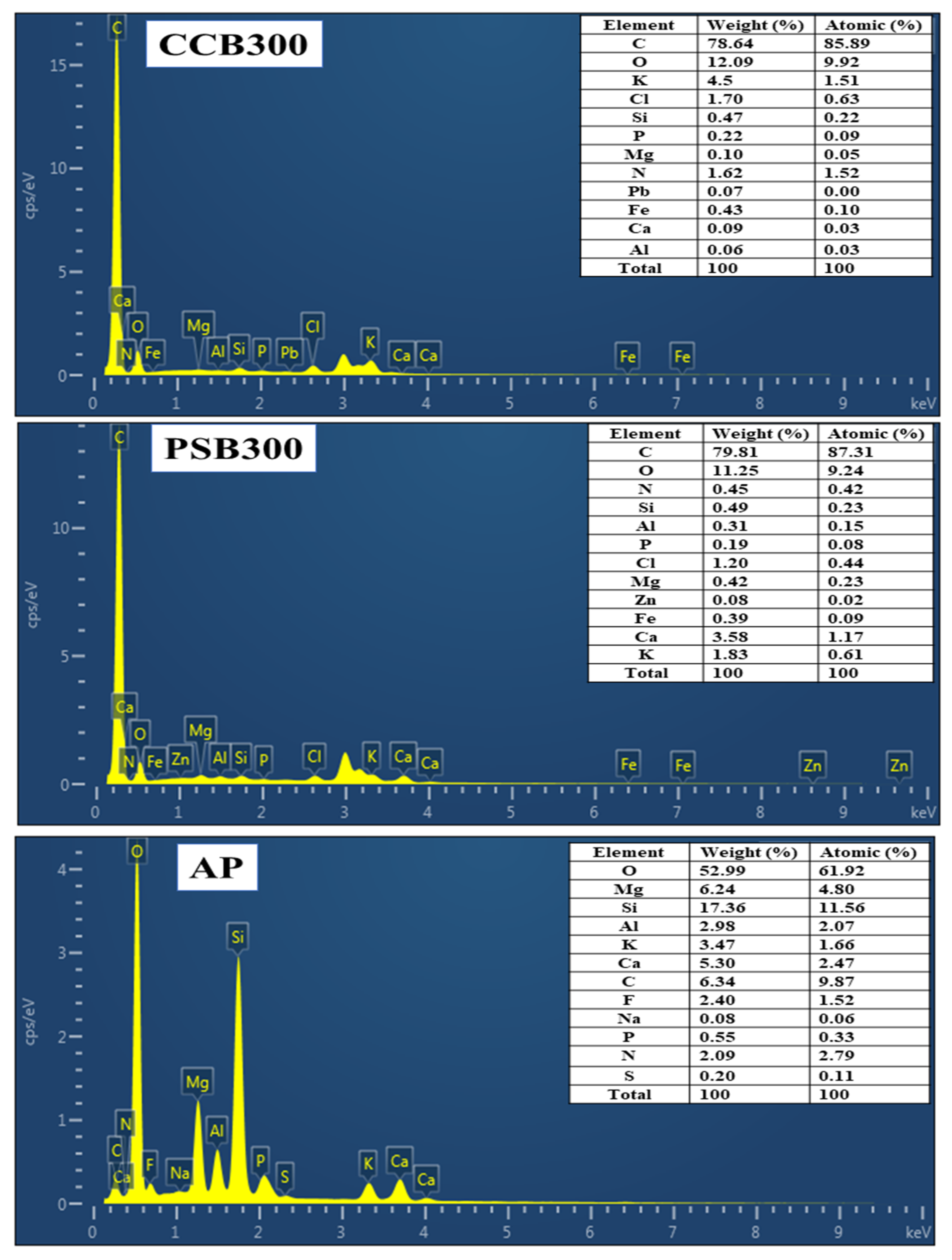
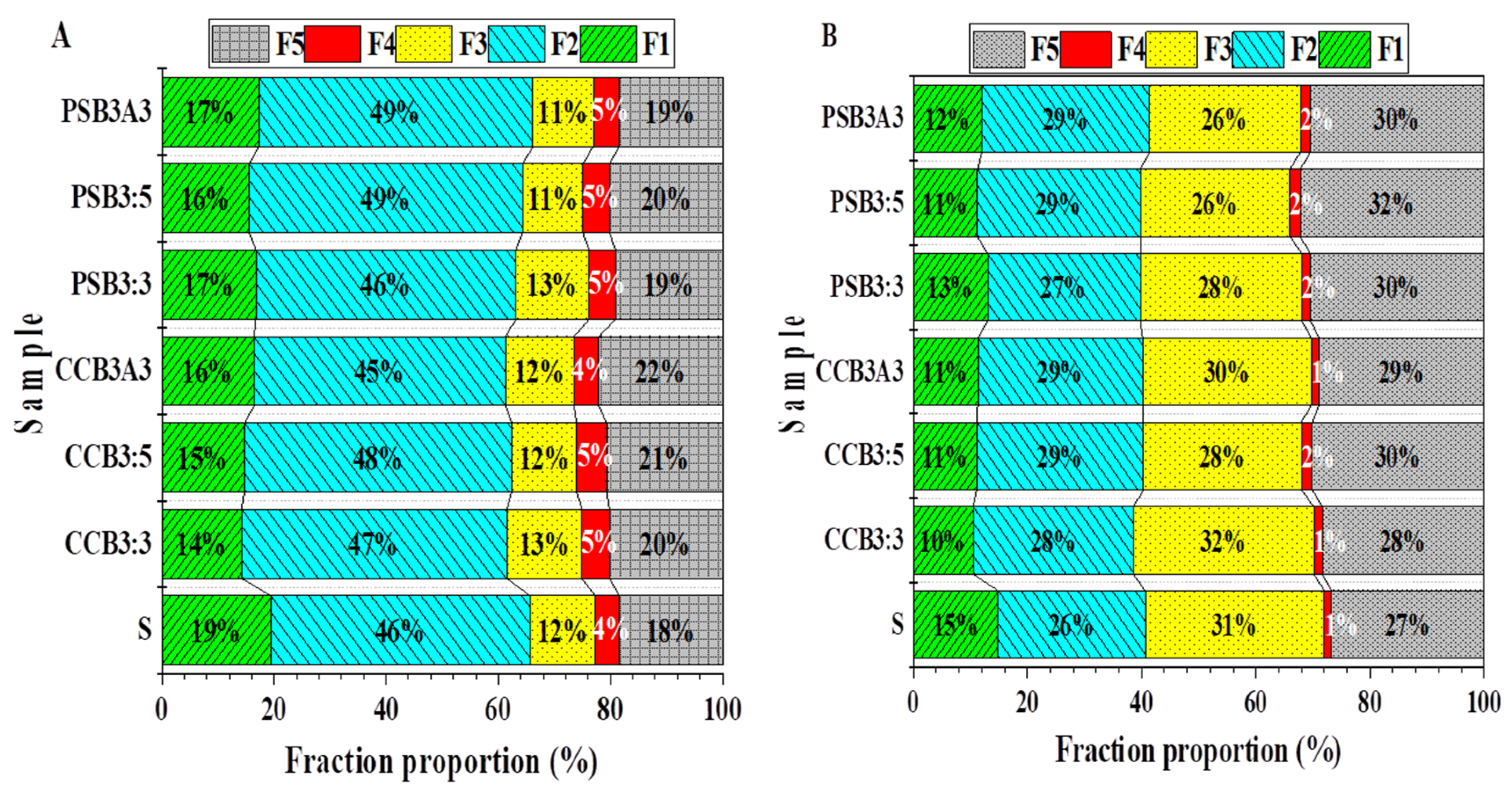

| Sample Plot | Sample Code | Ratio (%) |
|---|---|---|
| CS | CS | 0 |
| CS + 3% PSB300 | PSB3:3 | 3 |
| CS + 5% PSB300 | PSB3:5 | 5 |
| CS + 3% PSB300 + 3% AP | PSB3A3 | 3:3 |
| CS + 3% CCB300 | CCB3:3 | 3 |
| CS + 5% CCB300 | CCB3:5 | 5 |
| CS + 3% CCB300 + 3% AP | CCB3A3 | 3:3 |
| Properties | Unit | Studied Soil | CCB300 | PSB300 | AP |
|---|---|---|---|---|---|
| Sand | % | 69.78 ± 0.72 | - | - | - |
| Silt | % | 5.48 ± 0.32 | - | - | - |
| Clay | % | 24.74 ± 0.43 | - | - | - |
| pH | 6.65 ± 0.01 | 9.53 ± 0.01 | 9.50 ± 0.01 | 8.99 ± 0.01 | |
| OC | % | 1.95 ± 0.31 | 81.29 ± 0.12 | 75.82 ± 0.31 | 2.21 ± 0.10 |
| EC | µS cm−1 | 119.20 ± 0.30 | 1675.40 ± 1.70 | 1115.30 ± 1.41 | 380.71 ± 0.90 |
| Pb | mg kg−1 | 2973.77 ± 33.23 | <LOD | <LOD | <LOD |
| Zn | mg kg−1 | 2462.24 ± 34.29 | 0.20 ± 0.02 | 0.21 ± 0.04 | 7.53 ± 0.02 |
| S(BET) | m2 g−1 | - | 1.73 | 23.41 | 0.43 |
| Sample | F1_Pb | F1_Zn | pH | OC | EC |
|---|---|---|---|---|---|
| mg kg−1 | g kg−1 | µS/cm | |||
| CS | 578.9 ± 10.5 a | 375.0 ± 12.6 a | 6.65 ± 0.01 c | 20.1 ± 0.3 e | 119.1 ± 1.3 g |
| PSB3:3 | 415.6 ± 9.5 e | 259.9 ± 11.8 c | 6.74 ± 0.01 b | 42.1 ± 0.4 d | 150.4 ± 0.3 f |
| PSB3:5 | 429.7 ± 6.8 e | 271.8 ± 13.2 c | 6.79 ± 0.01 a | 56.9 ± 0.1 b | 171.2 ± 0.3 c |
| PSB3A3 | 491.1 ± 8.7 c | 278.7 ± 10.5 c | 6.81 ± 0.01 a | 41.5 ± 0.5 d | 158.2 ± 0.2 e |
| CCB3:3 | 502.7 ± 10.4 bc | 315.3 ± 14.2 b | 6.74 ± 0.01 b | 44.4 ± 0.9 c | 165.7 ± 0.7 d |
| CCB3:5 | 468.2 ± 8.9 d | 277.2 ± 15.2 c | 6.79 ± 0.01 a | 60.7 ± 0.4 a | 196.8 ± 0.8 a |
| CCB3A3 | 519.5 ± 9.3 b | 291.1 ± 9.7 c | 6.81 ± 0.01 a | 43.8 ± 0.9 bc | 173.5 ± 0.4 b |
| Metal | Sample | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| (mg kg−1) | ||||||
| Pb | CS | 578.9 ± 15.5 a | 1376.1 ± 23.1 b | 344.1 ± 9.5 c | 129.4 ± 3.7 c | 549.2 ± 10.1 e |
| PSB3:3 | 415.6 ± 9.5 e | 1384.7 ± 29.3 b | 372.3 ± 6.7 a | 138.9 ± 5.8 b | 592.1 ± 6.4 c | |
| PSB3:5 | 429.7 ± 6.8 e | 1394.7 ± 35.4 b | 338.0 ± 7.5 c | 151.2 ± 2.3 a | 646.4 ± 5.3 a | |
| PSB3A3 | 491.1 ± 8.7 c | 1345.6 ± 26.7 b | 365.4 ± 8.5 b | 130.9 ± 4.4 c | 613.4 ± 6.2 b | |
| CCB3:3 | 502.7 ± 11.4 bc | 1387.8 ± 47.1 b | 372.3 ± 9.3 a | 139.7 ± 3.1 b | 572.1 ± 9.5 d | |
| CCB3:5 | 468.2 ± 8.9 d | 1458.2 ± 19.3 a | 322.8 ± 5.6 d | 154.2 ± 4.2 a | 602.0 ± 10.7 bc | |
| CCB3A3 | 519.5 ± 9.3 b | 1467.4 ± 32.8 a | 319.7 ± 9.1 d | 136.6 ± 5.3 bc | 556.4 ± 12.8 de | |
| Zn | CS | 375.0 ± 12.6 a | 647.0 ± 19.7 b | 788.2 ± 12.4 a | 33.0 ± 2.3 b | 669.8 ± 8.7 d |
| PSB3:3 | 259.9 ± 11.8 c | 698.7 ± 18.4 a | 676.0 ± 8.3 c | 37.3 ± 3.1 b | 705.1 ± 15.8 c | |
| PSB3:5 | 271.8 ± 13.2 c | 716.0 ± 12.8 a | 784.7 ± 9.0 a | 47.2 ± 3.2 a | 737.3 ± 16.3 b | |
| PSB3A3 | 278.7 ± 10.5 c | 715.8 ± 17.2 a | 730.8 ± 11.2 b | 35.2 ± 2.7 b | 715.2 ± 14.2 bc | |
| CCB3:3 | 315.3 ± 14.2 b | 645.7 ± 18.3 b | 684.7 ± 4.5 c | 36.9 ± 3.4 b | 735.1 ± 11.2 b | |
| CCB3:5 | 277.2 ± 15.2 c | 703.5 ± 19.3 a | 645.5 ± 6.1 d | 44.5 ± 1.3 a | 794.8 ± 18.3 a | |
| CCB3A3 | 291.1 ± 9.7 c | 710.8 ± 12.8 a | 639.5 ± 4.3 d | 44.1 ± 4.5 a | 737.3 ± 21.2 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuong, T.X.; Pham, T.T.H.; Nguyen, T.T.T.; Pham, D.T.N. Effects of Biochar and Apatite on Chemical Forms of Lead and Zinc in Multi-Metal-Contaminated Soil after Incubation: A Comparison of Peanut Shell and Corn Cob Biochar. Sustainability 2023, 15, 11992. https://doi.org/10.3390/su151511992
Vuong TX, Pham TTH, Nguyen TTT, Pham DTN. Effects of Biochar and Apatite on Chemical Forms of Lead and Zinc in Multi-Metal-Contaminated Soil after Incubation: A Comparison of Peanut Shell and Corn Cob Biochar. Sustainability. 2023; 15(15):11992. https://doi.org/10.3390/su151511992
Chicago/Turabian StyleVuong, Truong Xuan, Thi Thu Ha Pham, Thi Thu Thuy Nguyen, and Dung Thuy Nguyen Pham. 2023. "Effects of Biochar and Apatite on Chemical Forms of Lead and Zinc in Multi-Metal-Contaminated Soil after Incubation: A Comparison of Peanut Shell and Corn Cob Biochar" Sustainability 15, no. 15: 11992. https://doi.org/10.3390/su151511992
APA StyleVuong, T. X., Pham, T. T. H., Nguyen, T. T. T., & Pham, D. T. N. (2023). Effects of Biochar and Apatite on Chemical Forms of Lead and Zinc in Multi-Metal-Contaminated Soil after Incubation: A Comparison of Peanut Shell and Corn Cob Biochar. Sustainability, 15(15), 11992. https://doi.org/10.3390/su151511992







