Effects of Basicity Index on Incinerator Fly Ash Melting Process and Stabilization
Abstract
:1. Introduction
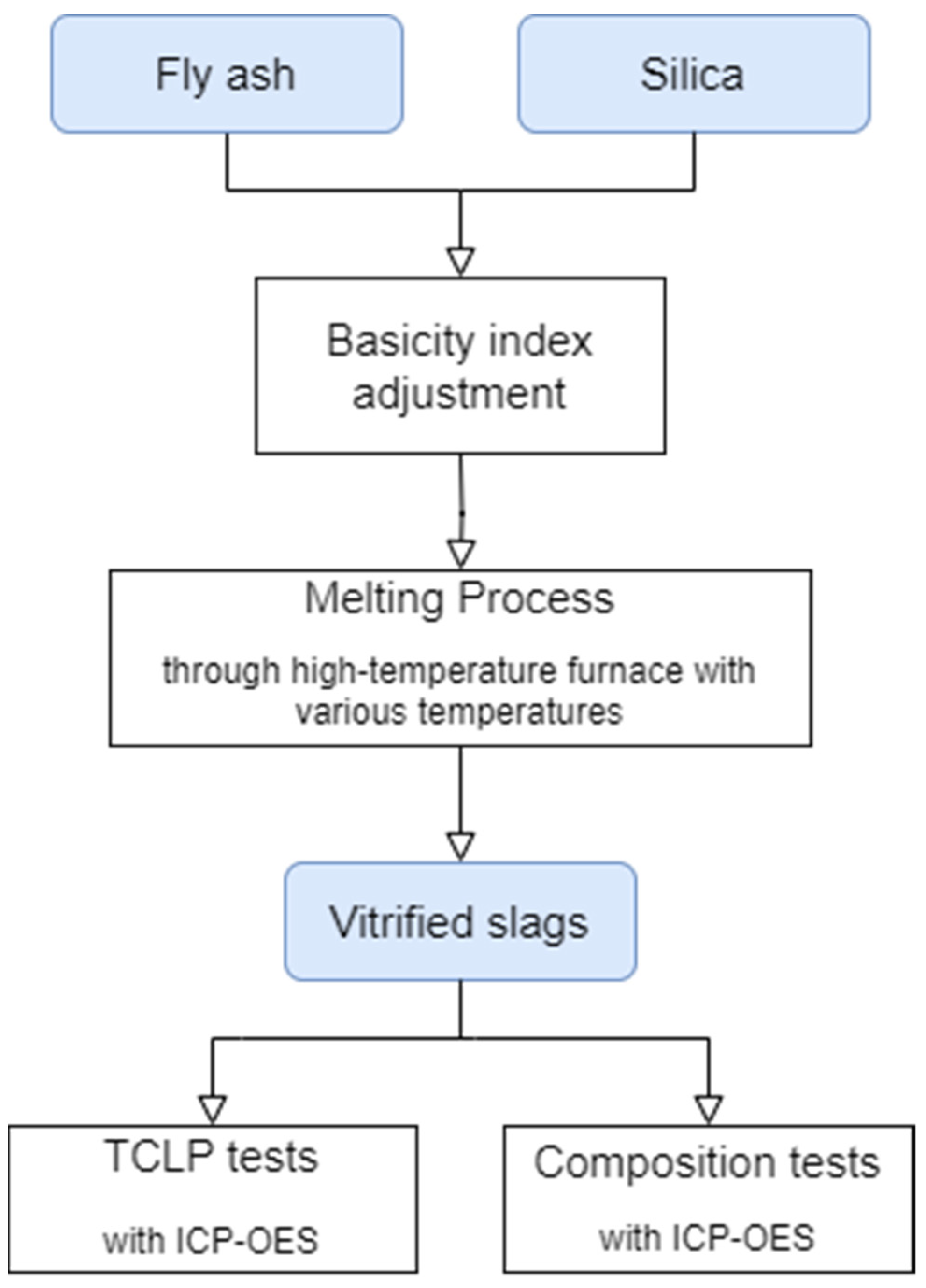
2. Materials and Methods
2.1. Materials, Reagents and Instruments
2.2. Basicity Index Adjustment Experiment
2.2.1. Basicity Index Adjustment with Glass through Melting Process
2.2.2. Basicity Index Adjustment with Silica Sand Powder through Melting Process
2.3. Leaching Property Test
2.4. Vitrified Slag Composition Test
3. Results and Discussion
3.1. Basicity Index Adjustment Experiment
3.1.1. Basicity Index Adjustment with Glass through Melting Process
3.1.2. High-Temperature Melting Process with Silica Sand Powder
3.2. Analysis
3.2.1. TCLP Tests
3.2.2. Composition Tests
4. Conclusions
- By adjusting the basicity index of the fly ash through the addition of silica, it was observed that the melting point of the fly ash could be effectively reduced from 1400 °C to 1200 °C when the basicity index was maintained below 1.28.
- The basicity indices for fly ash’s high temperature melting process were determined optimally by calculating the ratio of CaO/SiO2.
- Both kinds of fly ash, including industrial waste incinerator fly ash (FA1) and laboratory-waste-incinerator fly ash (FA2), could be melted and stabilized by the melting process after the adjustment of basicity indices.
- Even if the basicity index adjustment material was changed from glass to silica sand powder, this study proved that fly ash could be melted with a basicity index under 1.28. It did not make a difference in whether the fly ash could be melted if the SiO2 was in a crystalized phase or not.
- The leaching concentrations for all the vitrified slags were significantly low, including the ones from FA1 mixed with glass, FA1 mixed with silica sand, FA2 mixed with glass, or FA2 mixed with silica sand powder.
- Compared to the leaching concentration regulations in Taiwan, all of the vitrified slags were within the regulations. Thus, they were all considered stabilized.
- Through the ICP tests, the vitrified slags were all mainly composed of SiO2, CaO, Na2O, and a low amount of Al2O3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Environmental Protection Administration Executive Yuan, R.O.C (Taiwan). Available online: https://waste.epa.gov.tw/RWD/Statistics/?page=Year1 (accessed on 10 September 2022).
- Gao, J.; Wang, T.; Zhao, J.; Hu, X.; Dong, C. An experimental study on the melting solidification of municipal solid waste incineration fly ash. Sustainability 2021, 13, 535. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, X. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Fayad, M.A.; Chaichan, M.T.; Dhahad, H.A.; Al-Amiery, A.A.; Wan Isahak, W.N.R. Reducing the Effect of High Sulfur Content in Diesel Fuel on NO x Emissions and PM Characteristics Using a PPCI Mode Engine and Gasoline–Diesel Blends. ACS Omega 2022, 7, 37328–37339. [Google Scholar] [CrossRef]
- Suzuki, K.; Ono, Y. Leaching characteristics of stabilized/solidified fly ash generated from ash-melting plant. Chemosphere 2008, 71, 922–932. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Yan, S.; Li, Y.; Han, D. Application of thermal plasma technology for the treatment of solid wastes in China: An overview. Waste Manag. 2016, 58, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Feng, D.; Bai, C.; Sun, S.; Zhang, Y.; Zhao, Y.; Li, Y.; Zhang, F.; Chang, G.; Qin, Y. Thermal synergistic treatment of municipal solid waste incineration (MSWI) fly ash and fluxing agent in specific situation: Melting characteristics, leaching characteristics of heavy metals. Fuel Process. Technol. 2022, 233, 107311. [Google Scholar] [CrossRef]
- Čarnogurská, M.; Lázár, M.; Puškár, M.; Lengyelová, M.; Václav, J.; Širillová, L. Measurement and evaluation of properties of MSW fly ash treated by plasma. Measurement 2015, 62, 155–161. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Wang, Q.; Huang, Q.; Yang, J. Melting characteristics during the vitrification of MSWI fly ash with a pilot-scale diesel oil furnace. J. Hazard. Mater. 2008, 160, 376–381. [Google Scholar] [CrossRef]
- Szałatkiewicz, J. Construction Materials from Vitrified Lignite Fly Ash in Plasmatron Plasma Reactor. Materials 2019, 12, 905. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Mao, T.; Chen, Z.; Chen, J.; Zhang, S.; Li, X.; Yan, J. Thermal cotreatment of municipal solid waste incineration fly ash with sewage sludge: Phases transformation, kinetics and fusion characteristics, and heavy metals solidification. J. Clean. Prod. 2021, 317, 128429. [Google Scholar] [CrossRef]
- Yang, G.; Ren, Q.; Xu, J.; Lyu, Q. Co-melting properties and mineral transformation behavior of mixtures by MSWI fly ash and coal ash. J. Energy Inst. 2021, 96, 148–157. [Google Scholar] [CrossRef]
- Gao, J.; Dong, C.; Zhao, Y.; Xing, T.; Hu, X.; Wang, X. Effect of B2O3 on the melting characteristics of model municipal solid waste incineration (MSWI) fly ash. Fuel 2021, 283, 119278. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, B.; Boccaccini, A.R. Preparation of low melting temperature glass–ceramics from municipal waste incineration fly ash. Fuel 2009, 88, 1275–1280. [Google Scholar] [CrossRef]
- Károly, Z.; Mohai, I.; Toth, M.; Wéber, F.; Szépvölgyi, J. Production of glass–ceramics from fly ash using arc plasma. J. Eur. Ceram. Soc. 2007, 27, 1721–1725. [Google Scholar] [CrossRef]
- Li, C.; Zhang, P.; Zeng, L.; Yu, L.; Li, D. Study on preparation of glass-ceramics from municipal solid waste incineration (MSWI) fly ash and chromium slag. J. Build. Eng. 2023, 68, 106080. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.S. Glass-ceramic produced from a municipal waste incinerator fly ash with high Cl content. J. Eur. Ceram. Soc. 2004, 24, 2373–2382. [Google Scholar] [CrossRef]
- Mills, K.C.; Hayashi, M.; Wang, L.; Watanabe, T. The structure and properties of silicate slags. Treatise Process Metall. 2014, 1, 149–286. [Google Scholar]
- Ma, W.; Shi, W.; Shi, Y.; Chen, D.; Liu, B.; Chu, C.; Li, D.; Li, Y.; Chen, G. Plasma vitrification and heavy metals solidification of MSW and sewage sludge incineration fly ash. J. Hazard. Mater. 2021, 408, 124809. [Google Scholar] [CrossRef]
- Cheng, T.-W.; Chu, J.P.; Tzeng, C.-C.; Chen, Y.-X. Reutilization of Incinerated Ash Using Plasma Melting Technology. Min. Metall. 2000, 44, 87–91. [Google Scholar]
- Li, C.T.; Huang, Y.J.; Huang, K.L.; Lee, W.J. Characterization of slags and ingots from the vitrification of municipal solid waste incineration ashes. Ind. Eng. Chem. Res. 2003, 42, 2306–2313. [Google Scholar] [CrossRef]
- Lin, C.-Y. Effects of Basicity and Cooling Rate on the Characteristics of Flyash-Molten Slags. Master’s Thesis, National Pingtung University of Science and Technology, Pingtung, Taiwan, 2008. [Google Scholar]
- Yue, Y.; Zhang, J.; Sun, F.; Wu, S.; Pan, Y.; Zhou, J.; Qian, G. Heavy metal leaching and distribution in glass products from the co-melting treatment of electroplating sludge and MSWI fly ash. J. Environ. Manag. 2019, 232, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Hsu, M.H.; Lin, Y.P. Evaluation of heavy metal leachability of incinerating recycled aggregate and solidification/stabilization products for construction reuse using TCLP, multi-final pH and EDTA-mediated TCLP leaching tests. J. Hazard. Mater. 2019, 368, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J. Vitrification of Municipal Solid Waste Incineration Ash. Master’s Thesis, National Cheng Kung University, Tainan, Taiwan, 2002. [Google Scholar]
| Elements | FA1 | FA2 | Glass |
|---|---|---|---|
| Si | 2.71% | 2.60% | 28.73% |
| Na | 4.66% | 9.60% | 2.81% |
| Ca | 22.57% | 20.88% | 0.17% |
| Al | 0.81% | 3.78% | 1.32% |
| Mg | 0.47% | 0.45% | 0.11% |
| Cu | 0.75% | 0.76% | 0.11% |
| Zn | 0.81% | 0.56% | 0.10% |
| Pb | 0.52% | 0.53% | 0.14% |
| K | 1.56% | 1.21% | 0.46% |
| Fe | 0.84% | 1.15% | 0.36% |
| Oxides | FA1 | FA2 | Glass |
|---|---|---|---|
| 5.8% | 7.55% | 88.59% | |
| 6.28% | 14.19% | 5.46% | |
| CaO | 31.59% | 32.23% | 0.34% |
| 1.54% | 7.5% | 3.59% | |
| MgO | 0.78% | 0.84% | 0.27% |
| CuO | 0.94% | 0.94% | 0.19% |
| ZnO | 1.01% | 0.81% | 0.18% |
| Oxides | FA1 | FA2 | Glass |
| 5.8% | 7.55% | 88.59% | |
| 6.28% | 14.19% | 5.46% |
| Ratio | FA1 | FA2 |
|---|---|---|
| 1:0 | 5.45 | 4.27 |
| 6:1 | 1.54 | 1.54 |
| 5:1 | 1.35 | 1.28 |
| 4:1 | 1.13 | 1.09 |
| 3:1 | 0.90 | 0.87 |
| 2:1 | 0.63 | 0.62 |
| 1:1 | 0.34 | 0.34 |
| Ratio | FA1 | FA2 |
|---|---|---|
| 1:0 | 5.45 | 4.27 |
| 6:1 | 1.42 | 1.34 |
| 5:1 | 1.23 | 1.18 |
| 4:1 | 1.03 | 1.00 |
| 1:0 | 6:1 | 5:1 | 4:1 | 3:1 | 2:1 | 1:1 | |
|---|---|---|---|---|---|---|---|
| 1100 °C | X | X | X | X | X | X | X |
| 1200 °C | X | X | X | O | O | O | O |
| 1300 °C | X | X | X | O | O | O | O |
| 1400 °C | O | O | O | O | O | O | O |
| 1:0 | 6:1 | 5:1 | 4:1 | 3:1 | 2:1 | 1:1 | |
|---|---|---|---|---|---|---|---|
| 1100 °C | X | X | X | X | X | X | X |
| 1200 °C | X | X | O | O | O | O | O |
| 1300 °C | X | X | O | O | O | O | O |
| 1400 °C | O | O | O | O | O | O | O |
| 6:1 | 5:1 | 4:1 | |
|---|---|---|---|
| 1100 °C | X | X | X |
| 1200 °C | X | O | O |
| 1300 °C | X | O | O |
| 1400 °C | O | O | O |
| 6:1 | 5:1 | 4:1 | |
|---|---|---|---|
| 1100 °C | X | X | X |
| 1200 °C | X | O | O |
| 1300 °C | X | O | O |
| 1400 °C | O | O | O |
| Regulation | FA1 4:1 | FA1 3:1 | FA1 2:1 | FA1 1:1 | |
|---|---|---|---|---|---|
| Ag | 5.0 | ND | ND | ND | ND |
| As | 5.0 | ND | ND | ND | ND |
| Cd | 1 | ND | ND | ND | ND |
| Cr | 5 | 0.16 | 0.15 | 0.21 | 0.14 |
| Cu | 15 | 0.10 | 0.09 | ND | ND |
| Hg | 0.2 | ND | ND | ND | ND |
| Pb | 5 | ND | 0.05 | ND | ND |
| Se | 1 | 0.04 | 0.05 | 0.03 | 0.02 |
| Ba | 100 | 0.50 | 0.40 | 0.23 | 0.22 |
| Cr(VI) | 2.5 | ND | ND | ND | ND |
| Regulation | FA1 5:1 | FA1 4:1 | |
|---|---|---|---|
| Ag | 5.0 | ND | ND |
| As | 5.0 | ND | ND |
| Cd | 1 | ND | ND |
| Cr | 5 | ND | ND |
| Cu | 15 | ND | ND |
| Hg | 0.2 | ND | ND |
| Pb | 5 | ND | ND |
| Se | 1 | 0.05 | 0.01 |
| Ba | 100 | 0.51 | 0.14 |
| Cr(VI) | 2.5 | ND | ND |
| Regulation | FA2 5:1 | FA2 4:1 | FA2 3:1 | FA2 2:1 | |
|---|---|---|---|---|---|
| Ag | 5.0 | ND | ND | ND | ND |
| As | 5.0 | ND | ND | 0.10 | ND |
| Cd | 1 | ND | ND | ND | ND |
| Cr | 5 | 0.11 | 0.11 | 0.14 | 0.12 |
| Cu | 15 | ND | ND | ND | 0.29 |
| Hg | 0.2 | ND | ND | ND | ND |
| Pb | 5 | ND | ND | ND | ND |
| Se | 1 | 0.18 | 0.13 | 0.12 | 0.19 |
| Ba | 100 | 0.33 | 0.24 | 0.23 | 0.11 |
| Cr(VI) | 2.5 | ND | ND | ND | ND |
| Regulation | FA1 5:1 | FA1 4:1 | |
|---|---|---|---|
| Ag | 5.0 | 0.01 | ND |
| As | 5.0 | ND | ND |
| Cd | 1 | ND | ND |
| Cr | 5 | 0.21 | 0.14 |
| Cu | 15 | ND | ND |
| Hg | 0.2 | ND | ND |
| Pb | 5 | ND | ND |
| Se | 1 | 0.01 | 0.01 |
| Ba | 100 | 0.17 | 0.63 |
| Cr(VI) | 2.5 | ND | ND |
| FA1 4:1 | FA1 3:1 | FA1 2:1 | FA1 1:1 | |
|---|---|---|---|---|
| 37.35% | 37.45% | 47.84% | 51.75% | |
| CaO | 4.58% | 16.55% | 13.63% | 16.44% |
| 4.90% | 3.06% | 3.04% | 4.40% | |
| 0.04% | 0.27% | 0.16% | 0.37% |
| FA1 5:1 | FA1 4:1 | |
|---|---|---|
| 21.94% | 27.38% | |
| CaO | 20.83% | 18.52% |
| 3.11% | 3.18% | |
| 0.50% | 0.85% |
| FA2 5:1 | FA2 4:1 | FA2 3:1 | FA2 2:1 | FA2 1:1 | |
|---|---|---|---|---|---|
| 33.66% | 35.93% | 40.17% | 52.80% | 60.49% | |
| CaO | 4.03% | 3.61% | 4.95% | 6.20% | 7.02% |
| 9.72% | 9.53% | 10.40% | 7.87% | 6.85% | |
| 0.03% | 0.03% | 0.04% | 0.03% | 0.36% |
| FA1 5:1 | FA1 4:1 | |
|---|---|---|
| 31.84% | 25.54% | |
| CaO | 17.69% | 21.42% |
| 6.41% | 6.79% | |
| 0.63% | 0.71% |
| Method | Narrative |
|---|---|
| Thermal treatment | The thermal treatment could stabilize the heavy metals in fly ash by melting it into vitrified slags and the slags could be further used as other materials. Yet, the high energy consumption of the method still needs to be solved. |
| Solidification/stabilization | This method is the most commonly used method in all fly ash treatments due to its simple operation and low processing costs. However, it does lead to the problems of low heavy-metal stability and increase in waste volume. |
| Leaching process | This method is the only method that is able to effectively recycle the Zn, Pb, Cu, Cd and other metals from the fly ash. Yet, the residues of this method would still require further treatments before landfilling. |
| Pyrolysis process | This method shows good results in its dioxins degradation and PCDD/Fs decomposition. Nonetheless, it still presents the problems of high energy consumption and high TEQ residues. |
| Hydrothermal treatment | The hydrothermal method can significantly dichlorination and degradation the POPs in fly ash and is a relatively mature method. Nevertheless, the leaching of some heavy metals such as Pb and Cd could then become even more severe, causing further problems. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-S.; Chen, G.; Lee, C.-H. Effects of Basicity Index on Incinerator Fly Ash Melting Process and Stabilization. Sustainability 2023, 15, 11610. https://doi.org/10.3390/su151511610
Chen W-S, Chen G, Lee C-H. Effects of Basicity Index on Incinerator Fly Ash Melting Process and Stabilization. Sustainability. 2023; 15(15):11610. https://doi.org/10.3390/su151511610
Chicago/Turabian StyleChen, Wei-Sheng, Gregory Chen, and Cheng-Han Lee. 2023. "Effects of Basicity Index on Incinerator Fly Ash Melting Process and Stabilization" Sustainability 15, no. 15: 11610. https://doi.org/10.3390/su151511610
APA StyleChen, W.-S., Chen, G., & Lee, C.-H. (2023). Effects of Basicity Index on Incinerator Fly Ash Melting Process and Stabilization. Sustainability, 15(15), 11610. https://doi.org/10.3390/su151511610








