A Novel Procedure for Comprehensive Recovery of Zinc Fluoride, Manganese Fluorides, Manganese Dioxide, and Carbon Powder from the Electrode Powder of Spent Alkaline Batteries
Abstract
:1. Introduction
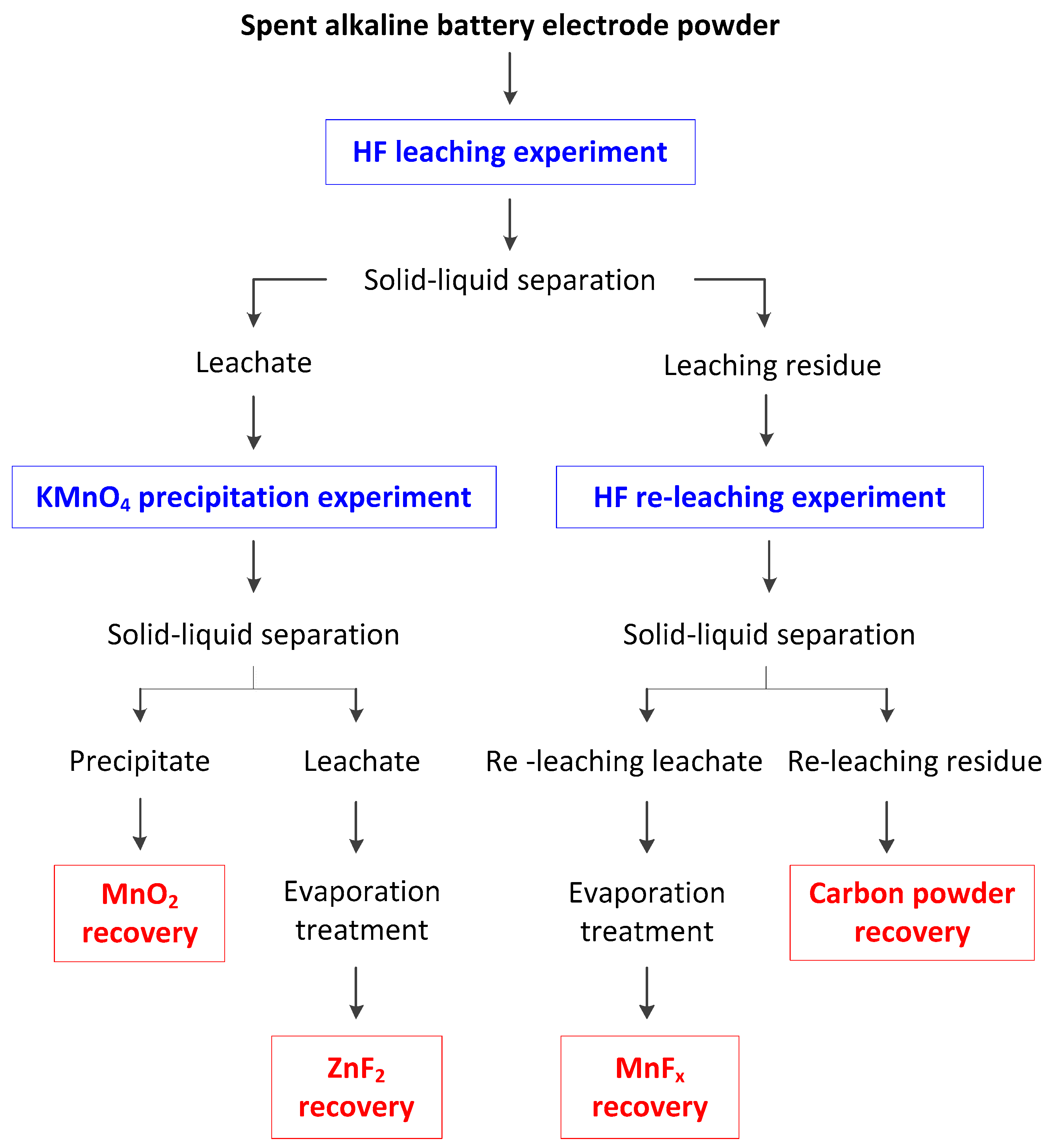
2. Materials and Methods
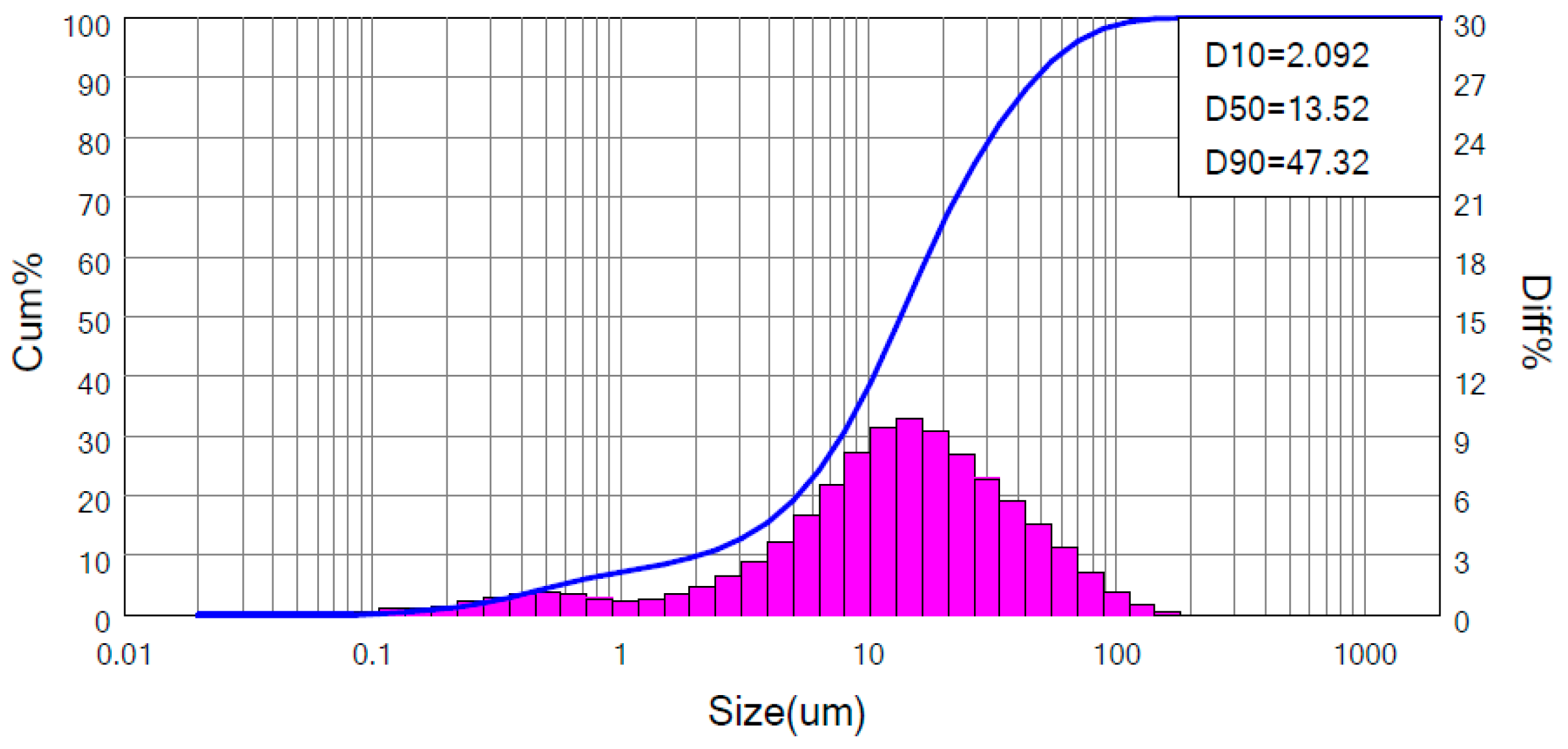
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. HF Leaching Experiments on the Electrode Powder
3.1.1. Effects of the HF Concentration on the Leaching Efficiencies of Zn and Mn
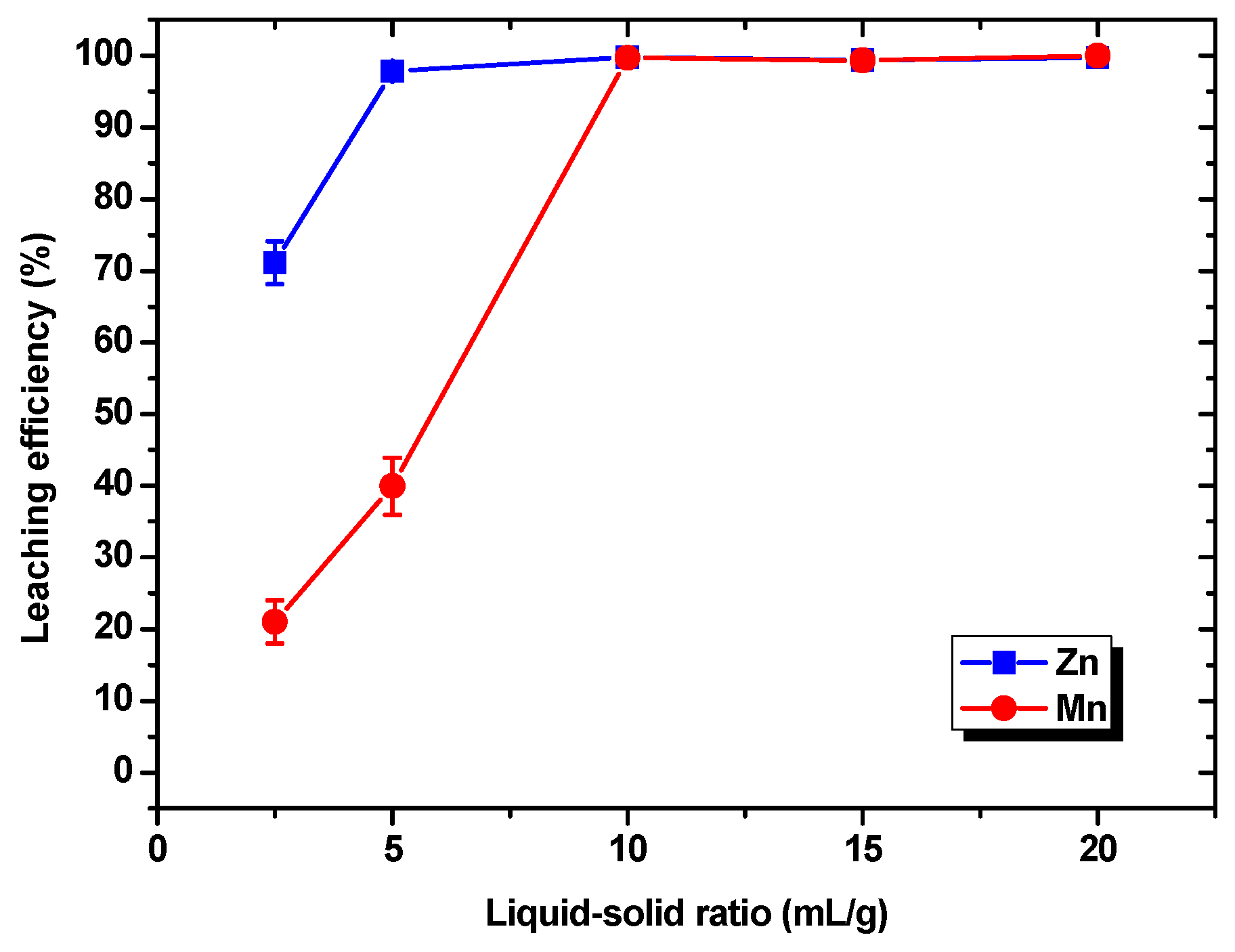
3.1.2. Effects of the Liquid–Solid Ratio on the Leaching Efficiencies of Zn and Mn
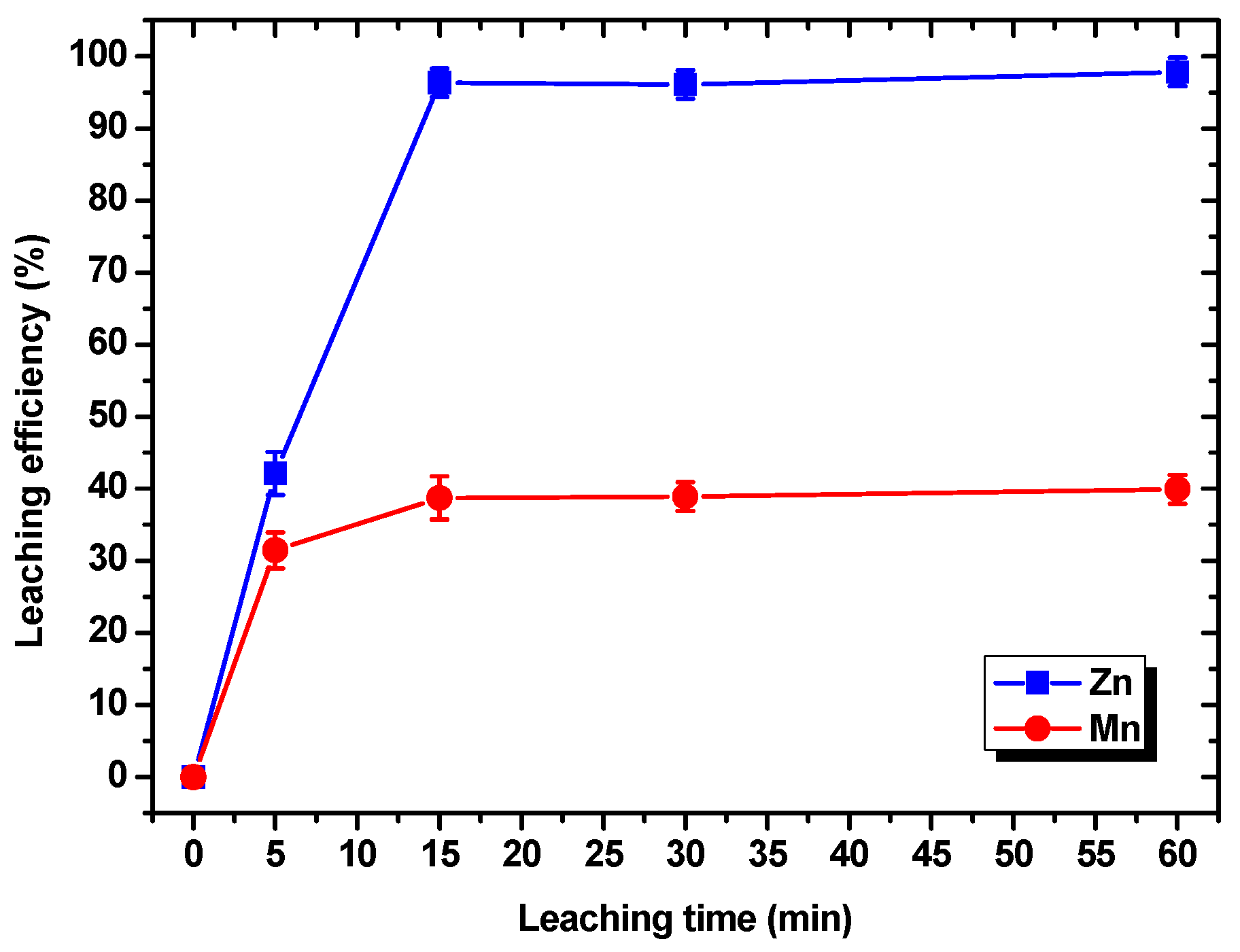
3.1.3. Effects of the Leaching Time on the Leaching Efficiencies of Zn and Mn
3.2. KMnO4 Precipitation Experiments Conducted on the Leachate
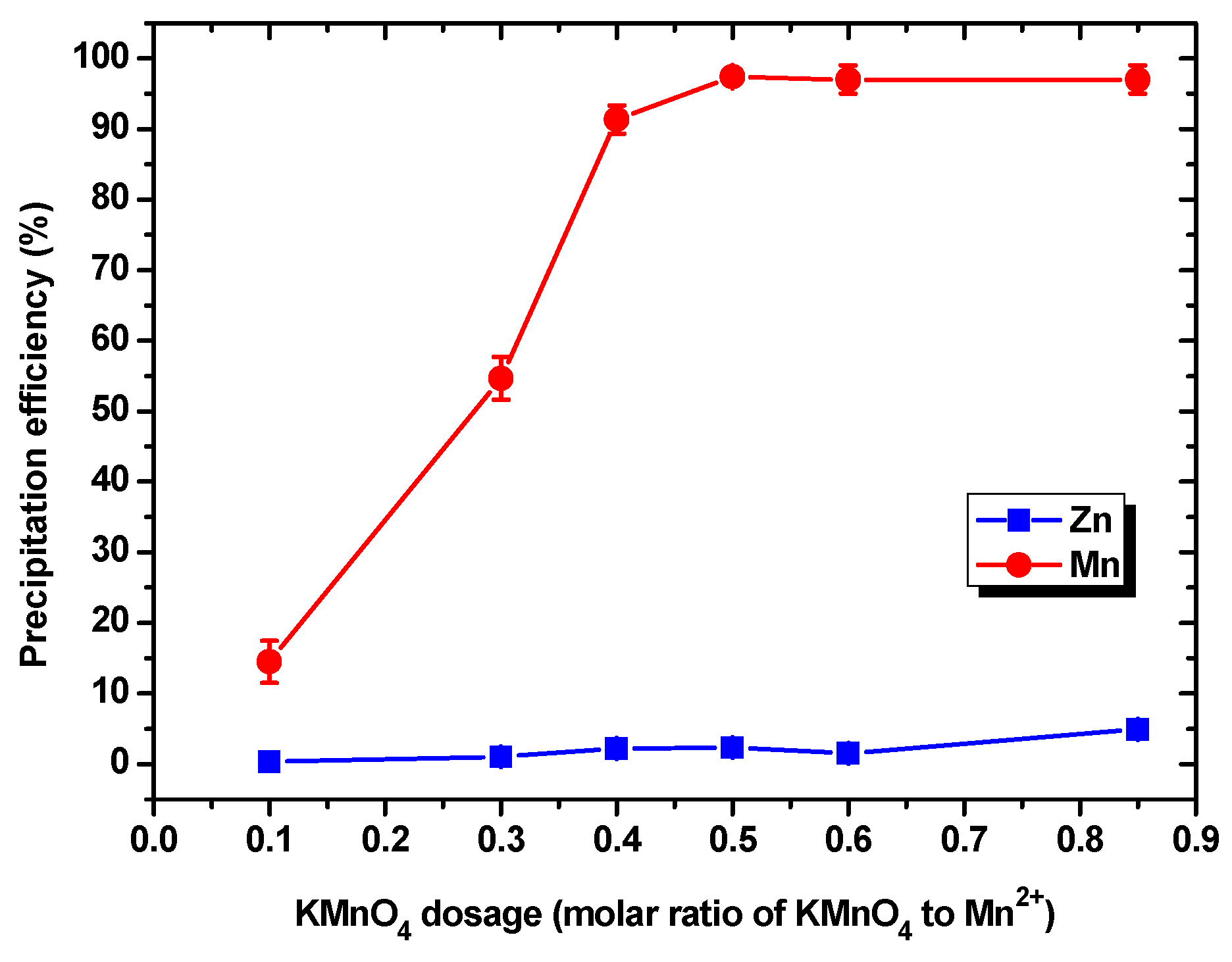
3.2.1. Effect of the KMnO4 Dosage
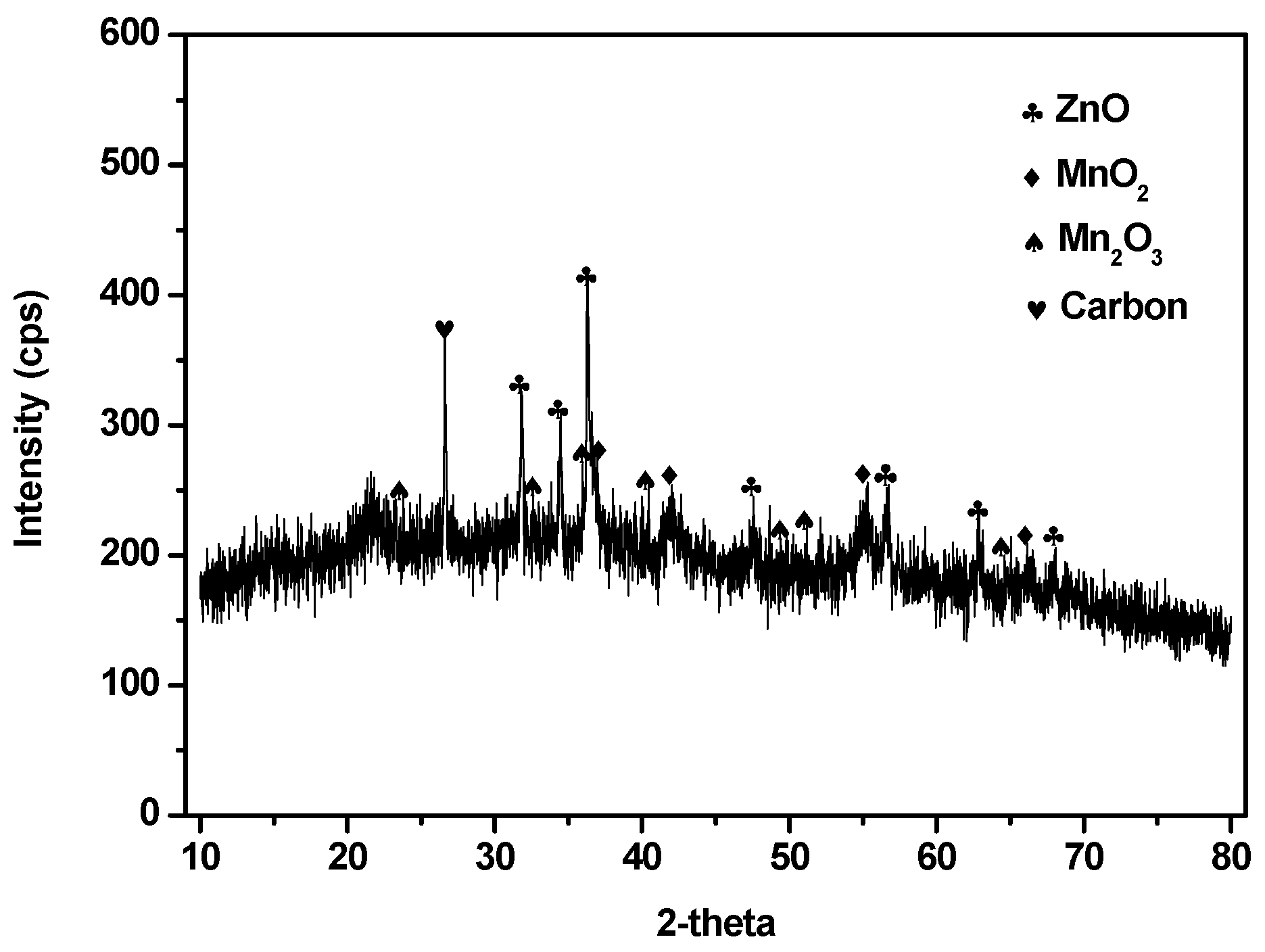
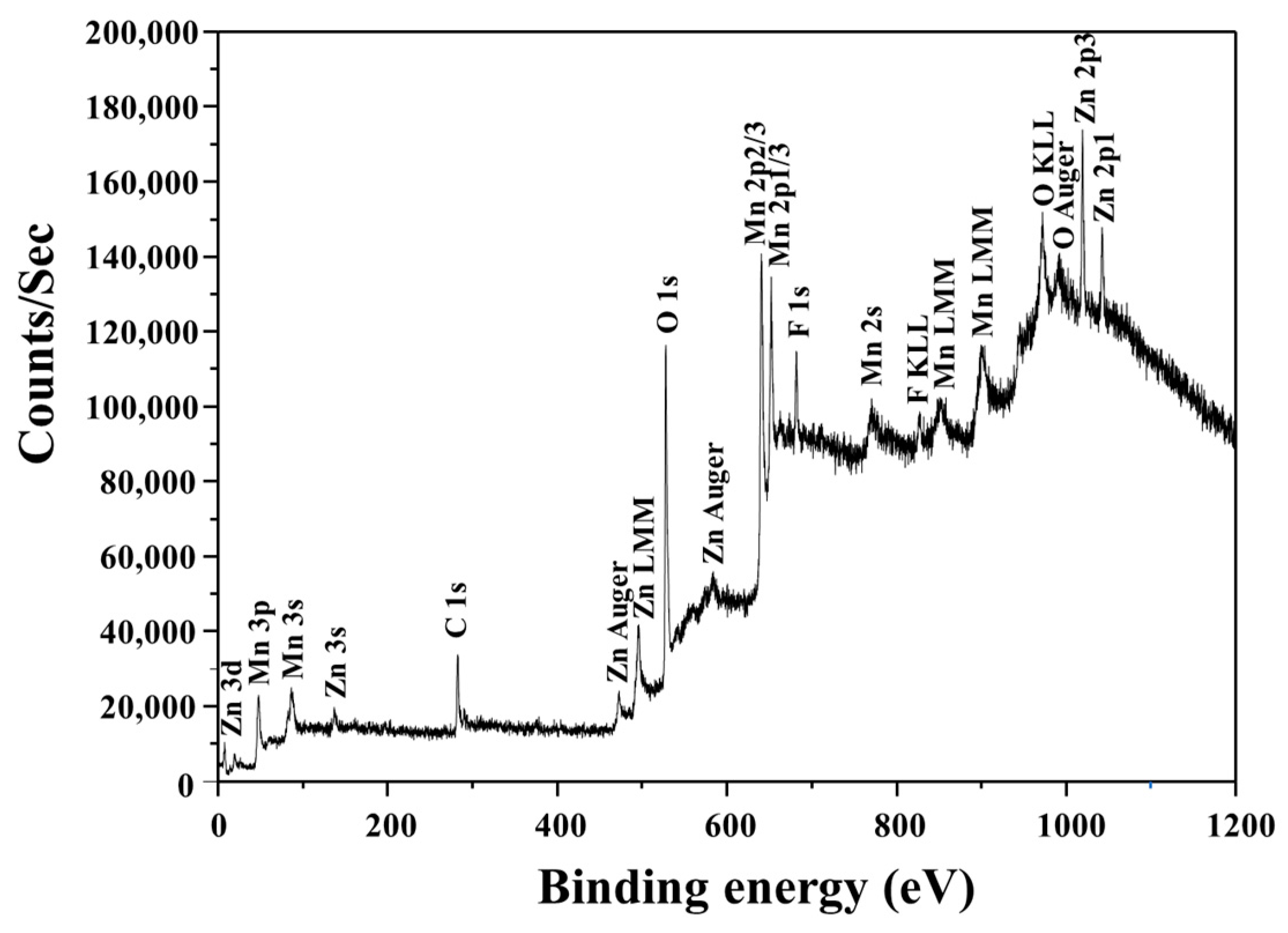
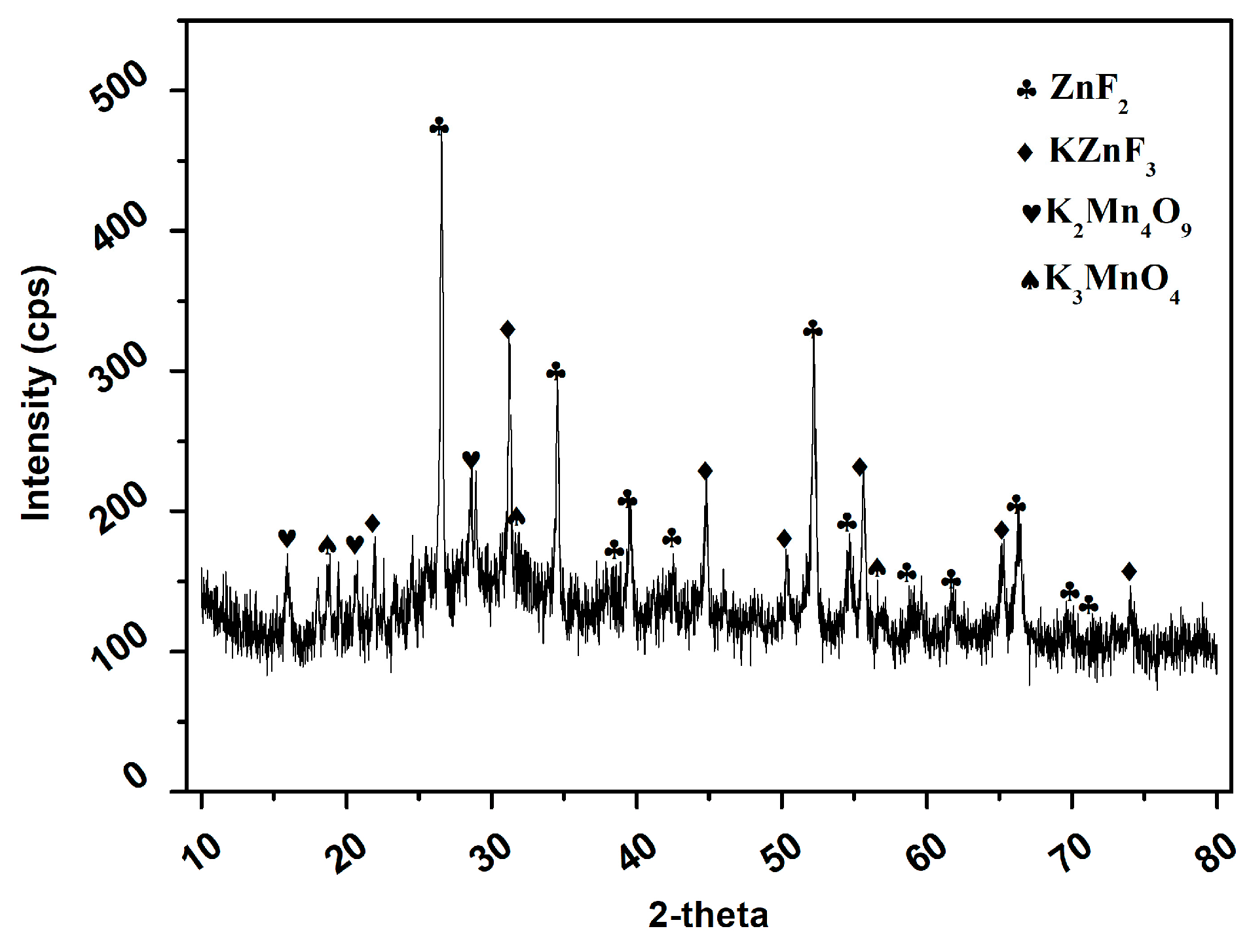
3.2.2. Characterization of the Products Recovered after the Precipitation Experiments
3.3. HF Releaching Experiments for the Leaching Residual
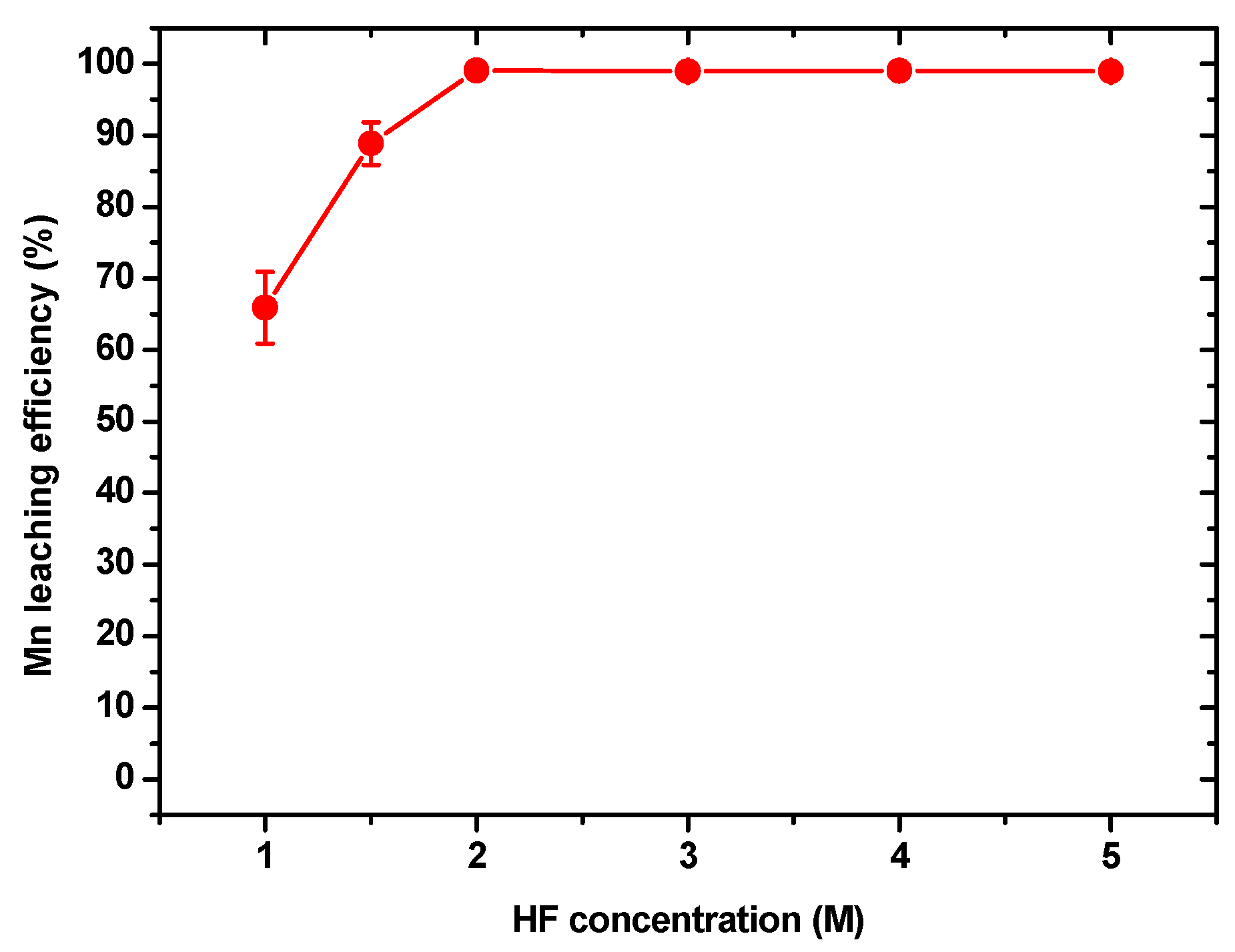
3.3.1. Effect of the HF Concentration on the Leaching Efficiency of Mn in the Leaching Residue
3.3.2. Effect of the Liquid–Solid Ratio on the Leaching Efficiency of Mn in the Leaching Residue
3.3.3. Effect of the Leaching Time on the Leaching Efficiency of Mn in the Leaching Residue
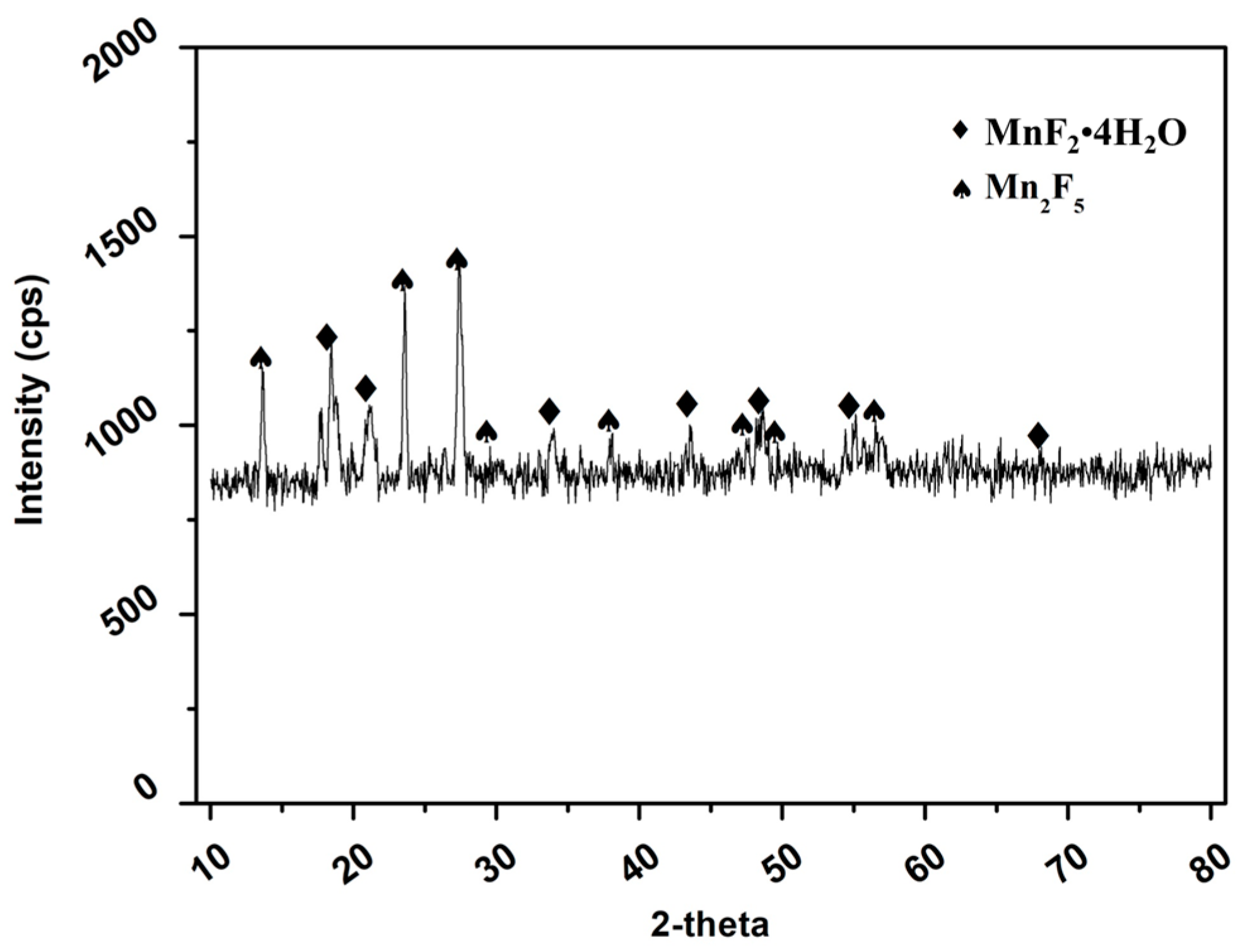
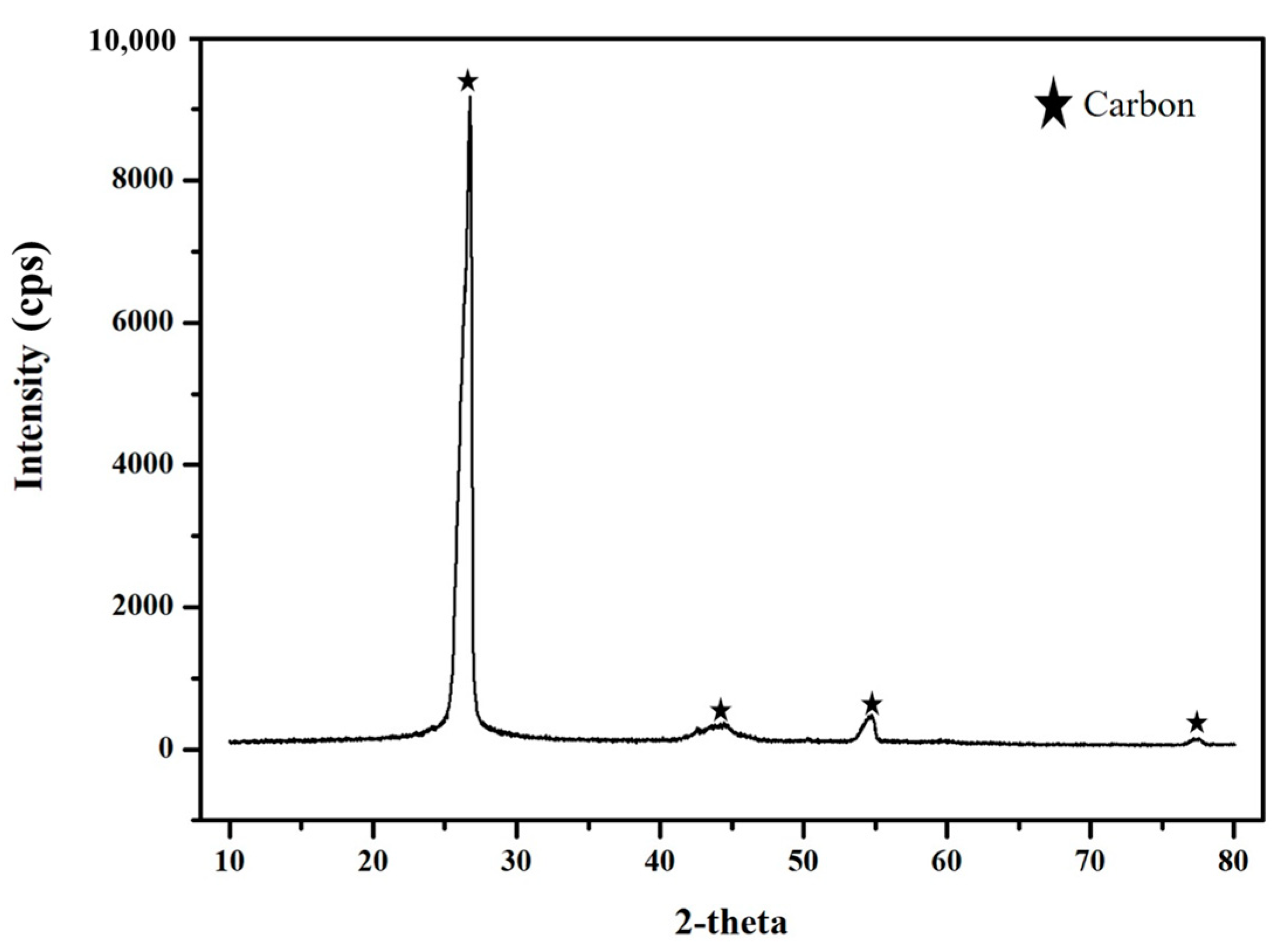
3.3.4. Characterization of the Products Recovered after the Releaching Experiments
3.4. Features and Future Perspective of the Proposed Procedure
4. Conclusions
- (1)
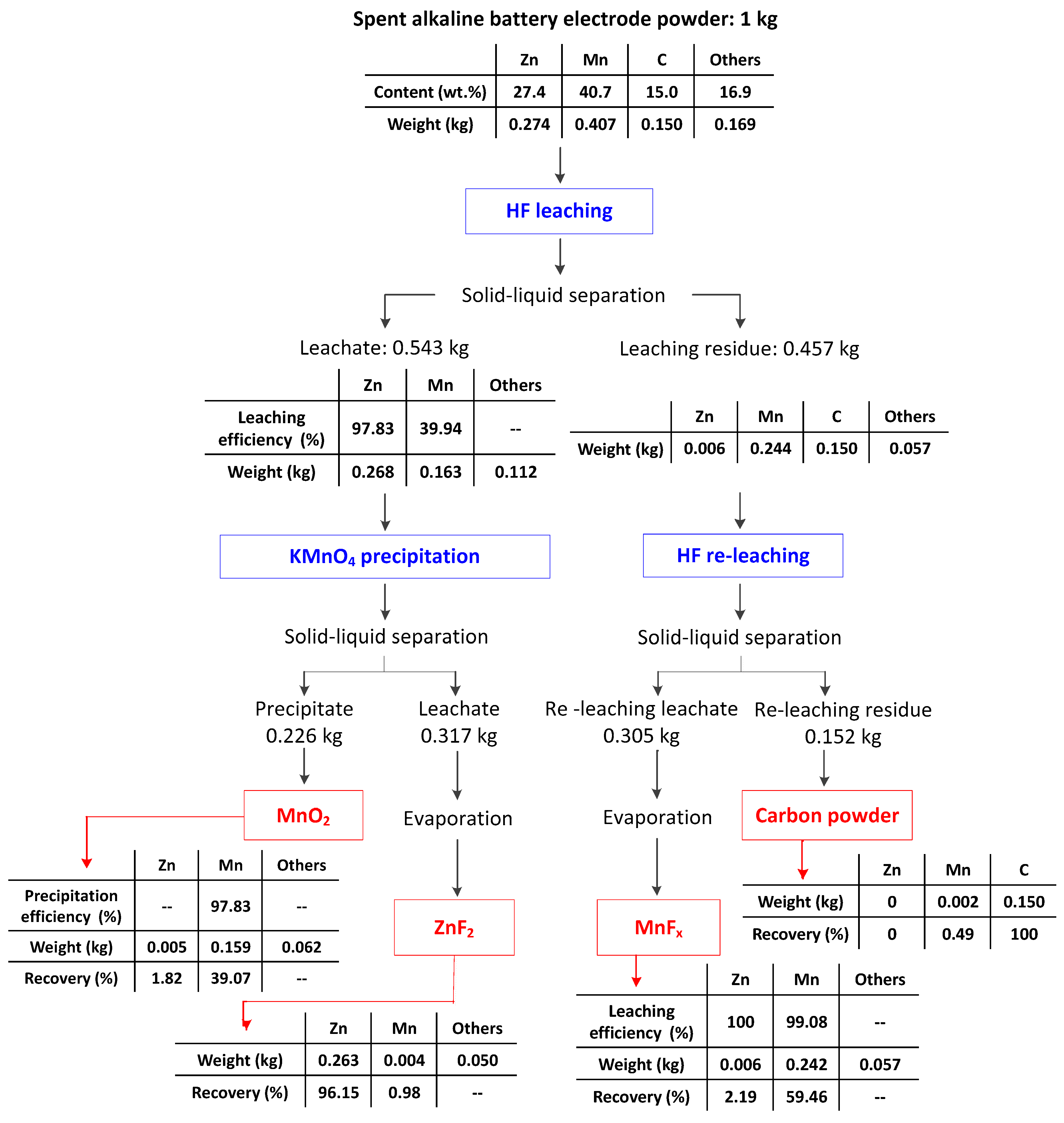
- The HF leaching experiments conducted on the electrode powder indicated that under the optimal conditions of a HF concentration of 4 M, a leaching time of 15 min, and a liquid–solid ratio of 5 mL/g, the leaching efficiencies of Zn and Mn were 97.83% and 39.94%, respectively, and the optimal leaching selectivity for Zn and Mn was achieved.
- (2)
- The KMnO4 precipitation experiments performed in order to precipitate the Mn ions in the leachate indicated that, when KMnO4 with a dosage (KMnO4/Mn ion molar ratio) of 0.5:1 was added to the leachate, the precipitation efficiency of the Mn ions reached 97.43%. The grade and Mn recovery of the recovered α-MnO2 were 91.68% and 39.07%, respectively. After removing the water content from the leachate via evaporation, ZnF2 and KZnF3 with a grade and Zn recovery of 97.98% and 96.15%, respectively, were recovered.
- (3)
- The HF releaching experiments on the leaching residue obtained in the HF leaching experiments indicated that, under the optimal conditions of a HF concentration of 2 M, a leaching time of 15 min, and a liquid–solid ratio of 10 mL/g, the leaching efficiency of Mn was 99.08%. After removing the water content in the leachate via evaporation, MnFx, including MnF2.4H2O and Mn2F5, with a grade and Mn recovery of 94.20% and 59.46%, respectively, was recovered. The residual of the releaching process was carbon powder.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takamura, T. Primary Batteries—Aqueous Systems. Alkaline Manganese–Zinc. Encycl. Electrochem. Power Sources 2009, 28–42. [Google Scholar] [CrossRef]
- Ministry of Environment Executive Yuan, R.O.C. Taiwan. Available online: https://cdx.moenv.gov.tw/CDX/main.aspx (accessed on 6 May 2021).
- Sadeghi, S.M.; Helena, J.J.; Soares, M.V.M. A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries. Waste Manag. 2020, 113, 342–350. [Google Scholar] [CrossRef]
- Sayilgan, E.; Kukrer, T.; Civelekoglu, G.; Ferella, F.; Akcil, A.; Veglio, F.; Kitis, M. A review of technologies for the recovery of metals from spent alkaline and zinc–carbon batteries. Hydrometallurgy 2009, 97, 158–166. [Google Scholar] [CrossRef]
- Belardi, G.; Lavecchia, R.; Medici, F.; Piga, L. Thermal treatment for recovery of manganese and zinc from zinc–carbon and alkaline spent batteries. Waste Manag. 2012, 32, 1945–1951. [Google Scholar] [CrossRef]
- Hu, X.; Robles, A.; Vikström, T.; Väänänen, P.; Zackrisson, M.; Ye, G. A novel process on the recovery of zinc and manganese from spent alkaline and zinc-carbon batteries. J. Hazard. Mater. 2021, 411, 124928. [Google Scholar] [CrossRef]
- Shin, S.-M.; Kang, J.-G.; Yang, D.-H.; Kim, T.-H.; Sohn, J.-S. Comparison of Acid and Alkaline Leaching for Recovery of Valuable Metals from Spent Zinc-carbon Battery. Geosystem Eng. 2008, 10, 21–26. [Google Scholar] [CrossRef]
- Sayilgan, E.; Kukrer, T.; Yigit, N.; Civelekoglu, G.; Kitis, M. Acidic leaching and precipitation of zinc and manganese from spent battery powders using various reductants. J. Hazard. Mater. 2010, 173, 137–143. [Google Scholar] [CrossRef]
- De Michelis, I.; Ferella, F.; Karakaya, E.; Beolchini, F.; Vegliò, F. Recovery of zinc and manganese from alkaline and zinc-carbon spent batteries. J. Power Sources 2007, 172, 975–983. [Google Scholar] [CrossRef]
- Sadeghi, S.M.; Vanpeteghem, G.; Neto, I.F.F.; Soares, H.M.V.M. Selective leaching of Zn from spent alkaline batteries using environmentally friendly approaches. Waste Manag. 2017, 60, 696–705. [Google Scholar] [CrossRef]
- Nogueira, C.; Margarido, F. Selective process of zinc extraction from spent Zn–MnO2 batteries by ammonium chloride leaching. Hydrometallurgy 2015, 157, 13–21. [Google Scholar] [CrossRef]
- Salgado, A.L.; Veloso, A.M.; Pereira, D.D.; Gontijo, G.S.; Salum, A.; Mansur, M.B. Recovery of zinc and manganese from spent alkaline batteries by liquid–liquid extraction with Cyanex 272. J. Power Sources 2003, 115, 367–373. [Google Scholar] [CrossRef]
- Ubaldini, S.; Fornari, P.; Massidda, R.; Michelis, I.D.; Ferella, F.; Vegliò, F. Mn–Zn Recovery From Wastes By Hydrometallurgical Applications. In Proceedings of the 11th International Mineral Processing Symposia, Antalya, Turkey, 21–23 October 2008. [Google Scholar]
- Zhou, L.; Huang, F.; Dou, B.; Li, Y.; Ye, R.; Wang, H.; Xu, S. Sensitization effect between Ln3+ ions in zinc fluoride glasses for MIR applications. Ceram. Int. 2019, 45, 10640–10645. [Google Scholar] [CrossRef]
- Huang, F.; Guob, Y.; Tiana, Y.; Xu, S.; Zhanga, J. Intense 2.7 μm emission in Er3+ doped zinc fluoride glass. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 179, 42–45. [Google Scholar] [CrossRef]
- Sharma, Y.; Surana, S.; Dubedi, R.; Joshi, V. Spectroscopic and radiative properties of Sm3+ doped zinc fluoride borophosphate glasses. Mater. Sci. Eng. B 2005, 119, 131–135. [Google Scholar] [CrossRef]
- Nazabal, V.; Poulain, M.; Olivier, M.; Pirasteh, P.; Camy, P.; Doualan, J.-L.; Guy, S.; Djouama, T.; Boutarfaia, A.; Adam, J. Fluoride and oxyfluoride glasses for optical applications. J. Fluor. Chem. 2012, 134, 18–23. [Google Scholar] [CrossRef]
- Gastev, S.; Hoffman, K.; Kaveev, A.; Reeves, R.; Sokolov, N. Laser spectroscopy of epitaxial manganese and zinc fluoride films on silicon. J. Cryst. Growth 2004, 268, 536–542. [Google Scholar] [CrossRef]
- Jones, L.F.; Raftery, J.; Teat, S.J.; Collison, D.; Brechin, E.K. Manganese (III) fluoride as a new synthon in Mn cluster chemistry. Polyhedron 2005, 24, 2443–2449. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Ma, Q.; Tang, X.; Hao, Q.; Cheng, Y.; Qiu, T. Alloy Engineering in Few-Layer Manganese Phosphorus Trichalcogenides for Surface-Enhanced Raman Scattering. Adv. Funct. Mater. 2020, 30, 1910171. [Google Scholar] [CrossRef]
- Ayres, D.C.; Hellier, D.G. Dictionary of Environmentally Important Chemicals; CRC Press: Boca Raton, FL, USA, 1997; p. 195. ISBN 0-7514-0256-7. [Google Scholar]
- Belardia, G.; Balliranob, P.; Ferrini, M.; Lavecchiac, R.; Medicic, F.; Pigac, L.; Scoppettuoloc, A. Characterization of spent zinc–carbon and alkaline batteries by SEM-EDS, TGA/DTA and XRPD analysis. Thermochim. Acta 2011, 526, 169–177. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Yang, H.; Tang, X.; Luo, X.; Li, G.; Liang, H.; Snyder, S. Oxidants-assisted sand filter to enhance the simultaneous removals of manganese, iron and ammonia from groundwater: Formation of active MnOx and involved mechanisms. J. Hazard. Mater. 2021, 415, 125707. [Google Scholar] [CrossRef] [PubMed]
- Maneechakr, P.; Mongkollertlop, S. Investigation on adsorption behaviors of heavy metal ions (Cd2+, Cr3+, Hg2+ and Pb2+) through low-cost/active manganese dioxide-modified magnetic biochar derived from palm kernel cake residue. J. Environ. Chem. Eng. 2020, 8, 104467. [Google Scholar] [CrossRef]
- Rashid, M.; Ejaz, M. Adsorption studies of ultra-trace concentrations of zinc on manganese dioxide from aqueous solutions. Int. J. Radiat. Appl. Instrumentation. Part A. Appl. Radiat. Isot. 1986, 37, 501–504. [Google Scholar] [CrossRef]
- Guo, Y.; Wuttk, S.; Vimont, A.; Datur, M.; Lavalley, J.C.; Teinz, K.; Kemnitz, E. Novel sol–gel prepared zinc fluoride: Synthesis, characterisation and acid–base sites analysis. J. Mater. Chem. 2012, 22, 14587–14593. [Google Scholar] [CrossRef]
- Huang, B.; Hong, J.-M.; Chen, X.-T.; Yu, Z.; You, X.-Z. Mild solvothermal synthesis of KZnF3 and KCdF3 nanocrystals. Mater. Lett. 2005, 59, 430–433. [Google Scholar] [CrossRef]
- Bandemehr, J.; Zimmerhofer, F.; Ivlev, S.I.; Pietzonka, C.; Eklund, K.; Karttunen, A.J.; Huppertz, H.; Kraus, F. Syntheses and Characterization of the Mixed-Valent Manganese (II/III) Fluorides Mn2F5 and Mn3F8. Inorg. Chem. 2021, 60, 12651–12663. [Google Scholar] [CrossRef]



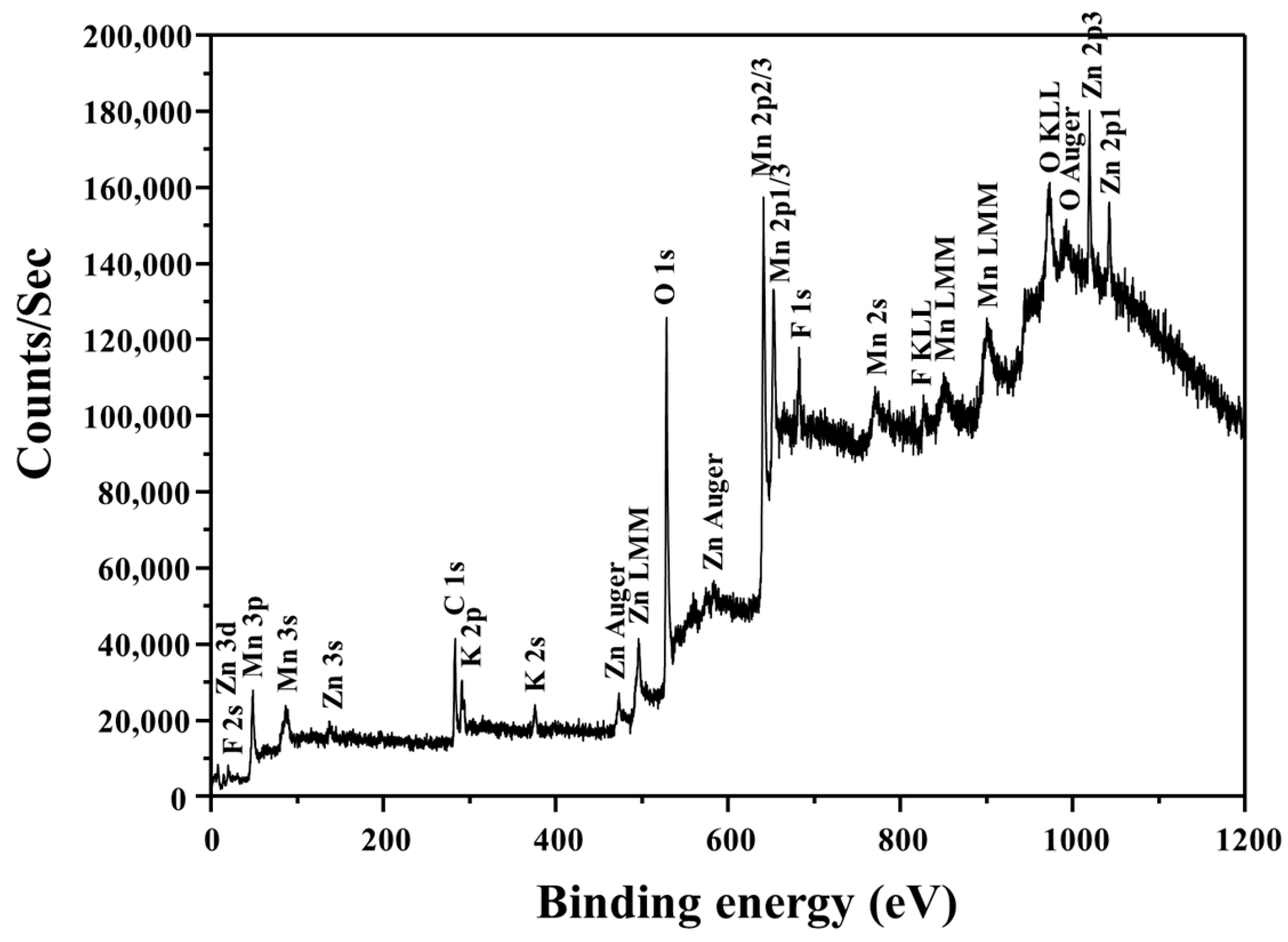


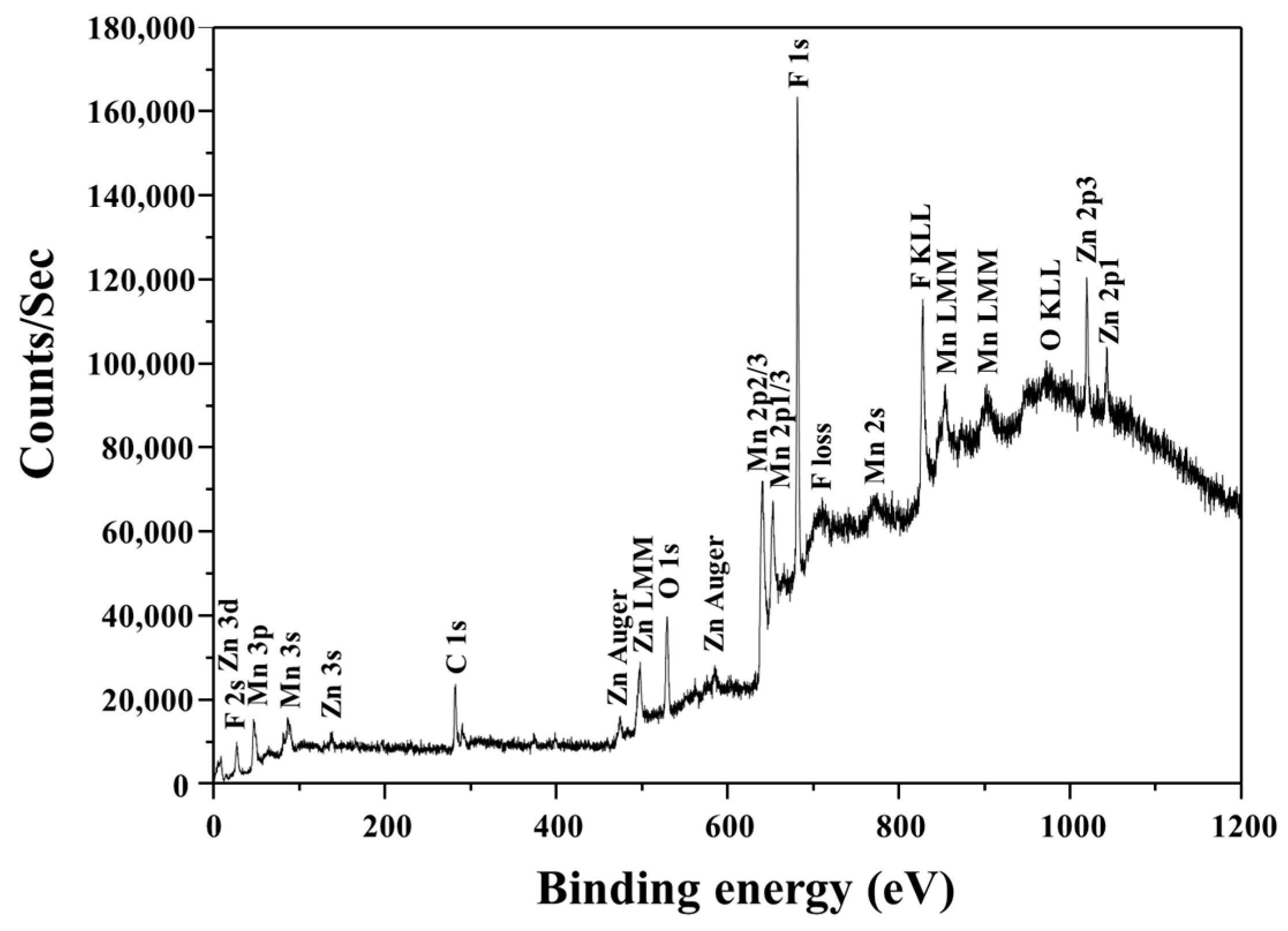
| Element | Zn | Mn | C | Others |
|---|---|---|---|---|
| Content | 27.36 wt.% | 40.71 wt.% | 15.06 wt.% | 16.87 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-P.; Hsu, W.-T.; Chen, Y.-J.; Chen, Y.-F.; Lin, I.-C.; Zhou, H.; Kou, M.; Sreearunothaia, P. A Novel Procedure for Comprehensive Recovery of Zinc Fluoride, Manganese Fluorides, Manganese Dioxide, and Carbon Powder from the Electrode Powder of Spent Alkaline Batteries. Sustainability 2023, 15, 13216. https://doi.org/10.3390/su151713216
Wang L-P, Hsu W-T, Chen Y-J, Chen Y-F, Lin I-C, Zhou H, Kou M, Sreearunothaia P. A Novel Procedure for Comprehensive Recovery of Zinc Fluoride, Manganese Fluorides, Manganese Dioxide, and Carbon Powder from the Electrode Powder of Spent Alkaline Batteries. Sustainability. 2023; 15(17):13216. https://doi.org/10.3390/su151713216
Chicago/Turabian StyleWang, Li-Pang, Wei-Tai Hsu, Yan-Jhang Chen, Yan-Fu Chen, I-Chun Lin, Heng Zhou, Mingyin Kou, and Paiboon Sreearunothaia. 2023. "A Novel Procedure for Comprehensive Recovery of Zinc Fluoride, Manganese Fluorides, Manganese Dioxide, and Carbon Powder from the Electrode Powder of Spent Alkaline Batteries" Sustainability 15, no. 17: 13216. https://doi.org/10.3390/su151713216
APA StyleWang, L.-P., Hsu, W.-T., Chen, Y.-J., Chen, Y.-F., Lin, I.-C., Zhou, H., Kou, M., & Sreearunothaia, P. (2023). A Novel Procedure for Comprehensive Recovery of Zinc Fluoride, Manganese Fluorides, Manganese Dioxide, and Carbon Powder from the Electrode Powder of Spent Alkaline Batteries. Sustainability, 15(17), 13216. https://doi.org/10.3390/su151713216







