Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Spent Coffee Grounds, Soil, and Lettuce
2.2. Hydrochars Production via Hydrothermal Carbonization
2.3. Bio-Chelates
2.4. Experimental Design
- (1)
- Fixed Zn concentration of 10 mg kg−1 soil from the functionalized bio-products (assay Zn-1).
- (2)
- Fixed 0.5% dose of bio-product corresponding to 2 g per pot, regardless of Zn content (assay Zn-2).
- Control A: soil containing NPK alone.
- Control B: a commercial chelate at a concentration of 10 mg kg−1 soil.
2.5. Analytical Procedures
2.6. Efficiency Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Assayed By-Products
3.2. Effects on Soil Fertility Properties
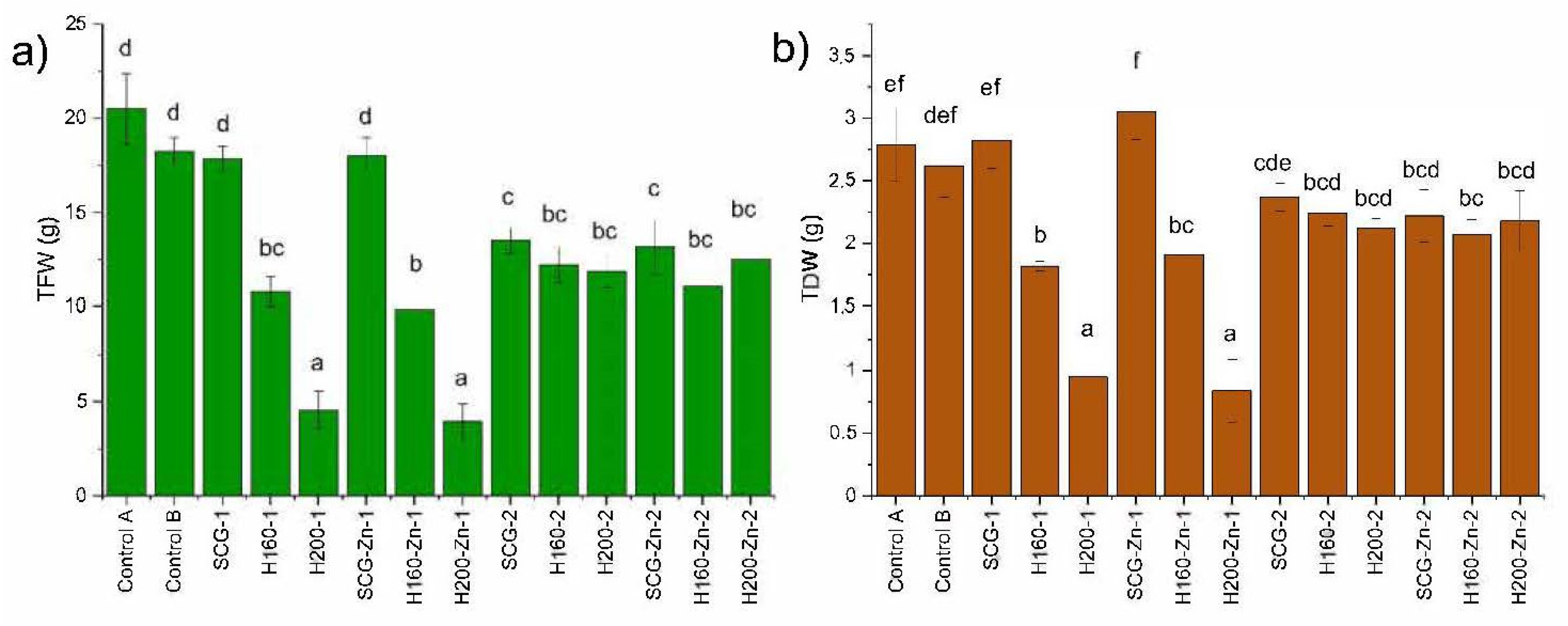
3.3. Effects on Plant Growth
3.4. Agronomic Biofortification of Zn. Evaluation Parameters
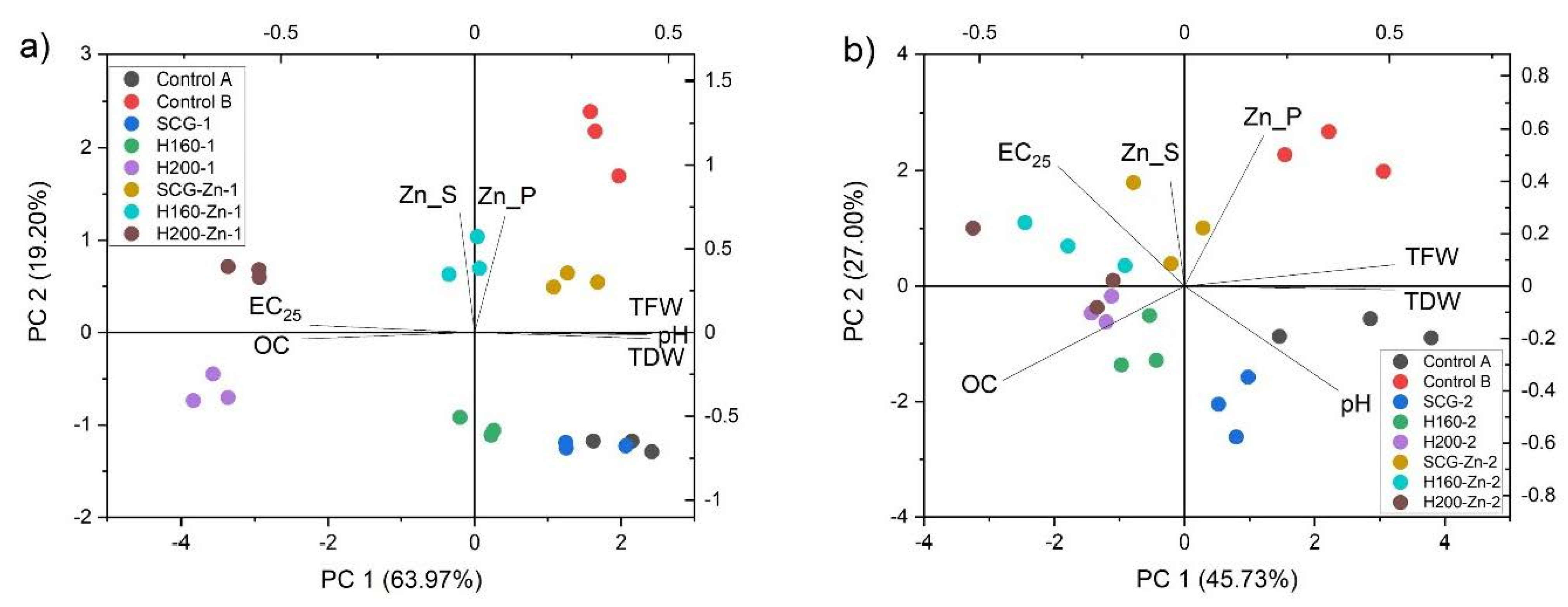
3.5. Relationship between Examined Variables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Hunger Index, The Challenge of Hidden Hunger. Available online: https://www.ifpri.org/sites/default/files/ghi/2014/index.html (accessed on 3 November 2021).
- Levenson, C.W.; Morris, D. Zinc and Neurogenesis: Making new neurons from development to adulthood. Adv. Nutr. 2011, 2, 96–100. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in the Terrestrial Environment, 2nd ed.; Springer: New York, NY, USA, 2001; Volume 1, pp. 61–86. [Google Scholar]
- Kozik, E.; Tyksiñski, W.; Bosiacki, M. A comparison of the efficiency of organic and mineral iron compounds in the greenhouse cultivation of lettuce. J. Elem. 2011, 16, 59–68. [Google Scholar] [CrossRef]
- Mohammadi, P.; Khoshgoftarmanesh, A.H. The effectiveness of synthetic zinc (Zn)-amino chelates in supplying Zn and alleviating salt-induced damages on hydroponically grown lettuce. Sci. Hortic. 2014, 172, 117–123. [Google Scholar] [CrossRef]
- Gregory, P.J.; Wahbi, A.; Adu-Gyamfi, J.; Heiling, M.; Gruber, R.; Joy, E.J.; Broadley, M.R. Approaches to reduce zinc and iron deficits in food systems. J. Glob. Food Sec. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 29, 122223. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Archer, S.A.; Gomes, H.I.; Christgen, B.; Lag-Brotons, A.J.; Purnell, P. Circular economy and the matter of integrated resources. Sci. Total Environ. 2019, 689, 963–969. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.W.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State-of-the-art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Khiari, B.; Jellali, S.; Ghimbeu, C.M.; Jeguirim, M. Hydrochars production, characterization and application for wastewater treatment: A review. Renew. Sustain. Energy. Rev. 2020, 127, 109882. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Ryu, C.; Park, J. Characterization of biochar and byproducts from slow pyrolysis of hinoki cypress. Bioresour. Technol. Rep. 2019, 6, 217–222. [Google Scholar] [CrossRef]
- Afolabi, O.O.D.; Sohail, M.; Cheng, Y.L. Optimisation and characterisation of hydrochar production from spent coffee grounds by hydrothermal carbonisation. Renew. Energy 2020, 147, 1380–1391. [Google Scholar] [CrossRef]
- García-Hernández, P.N.; Baas-López, J.M.; Toledano-Thompson, T.; Valdez-Ojeda, R.; Pacheco-Catalán, D. Revalorization of pleurotus djamor fungus culture: Fungus-derived carbons for supercapacitor application. Sustainability 2021, 13, 10765. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Rufián-Henares, J.Á.; Pastoriza, S.; Montilla-Gómez, J.; Delgado, G. Phytotoxicity and chelating capacity of spent coffee grounds: Two contrasting faces in its use as soil organic amendment. Sci. Total Envi. 2020, 717, 137247. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Mata, A.; Lara, L.; Fernández-Arteaga, A.; Ángel Rufián-Henares, J.; Delgado, G. Washed hydrochar from spent coffee grounds: A second generation of coffee residues. Evaluation as organic amendment. Waste Manag. 2021, 120, 322–329. [Google Scholar] [CrossRef]
- Gemechu, F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020, 104, 235–261. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Comino, F.; Cervera-Mata, A.; Aranda, V.; Martín-García, J.M.; Delgado, G. Short-term impact of spent coffee grounds over soil organic matter composition and stability in two contrasted Mediterranean agricultural soils. J. Soils Sediments 2020, 20, 1182–1198. [Google Scholar] [CrossRef]
- Vela-Cano, M.; Cervera-Mata, A.; Purswani, J.; Pozo, C.; Delgado, G.; González-López, J. Bacterial community structure of two Mediterranean agricultural soils amended with spent coffee grounds. Appl. Soil Ecol. 2019, 137, 12–20. [Google Scholar] [CrossRef]
- Leifa, F.; Pandey, A.; Soccol, C.R. Solid state cultivation-an efficient method to use toxic agro-industrial residues. J. Basic Microbiol. 2000, 40, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Mata, A.; Navarro-Alarcón, M.; Delgado, G.; Pastoriza, S.; Montilla-Gómez, J.; Llopis, J.; Sánchez-González, C.; Rufián-Henares, J.Á. Spent coffee grounds improve the nutritional value in elements of lettuce (Lactuca sativa L.) and are an ecological alternative to inorganic fertilizers. Food Chem. 2019, 282, 1–8. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; de La Cueva, S.P. Antimicrobial activity of coffee melanoidins—A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M. Recycling coffee and tea wastes to increase plant available Fe in alkaline soils. Plant Soil 2008, 304, 249–255. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.Á. Spent coffee grounds as a source of smart biochelates to increase Fe and Zn levels in lettuces. J. Clean Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; de Pascale, S.; Rouphael, Y. Iron biofortification of red and green pigmented lettuce in closed soilless cultivation impacts crop performance and modulates mineral and bioactive composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Unated States Department of Agriculture. Soil Survey Field and Laboratory Methods Manual—Soil Survey Investigations Report No. 51; Unated States Department of Agriculture: Washington, DC, USA, 2014; Volume 2. [Google Scholar]
- Zhao, A.; Yang, S.; Wang, B.; Tian, X. Effects of ZnSO4 and Zn-EDTA applied by broadcasting or by banding on soil Zn fractions and Zn uptake by wheat (Triticum aestivum L.) under greenhouse conditions. J. Plant Nutr. Soil Sci. 2019, 182, 307–317. [Google Scholar] [CrossRef]
- Almendros, P.; Gonzalez, D.; Alvarez, J.M. Long-term bioavailability effects of synthesized zinc chelates fertilizers on the yield and quality of a flax (Linum usitatissimum L.) crop. Plant. Soil. 2013, 368, 251–265. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Bae, D.; Park, K.Y. Characterizations of biochar from hydrothermal carbonization of exhausted coffee residue. J. Mater. Cycles Waste Manag. 2017, 19, 1036–1043. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Li, S.; Zhang, X.; Xia, M.; Xia, T.; Wang, M. Formation mechanisms and characterisation of the typical polymers in melanoidins from vinegar, coffee and model experiments. Food Chem. 2021, 355, 129444. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Delgado, G.; Fernández-Arteaga, A.; Fornasier, F.; Mondini, C. Spent coffee grounds by-products and their influence on soil C–N dynamics. J. Environ. Manage. 2022, 302, 114075. [Google Scholar] [CrossRef]
- Law, X.N.; Cheah, W.Y.; Chew, K.W.; Ibrahim, M.F.; Park, Y.-K.; Ho, S.-H.; Show, P.L. Microalgal-based biochar in wastewater remediation: Its synthesis, characterization and applications. Environ. Res. 2022, 204, 111966. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Sato, N.; Asakawa, H.; Wen, X.; Murata, M.; Homma, S. Characterization of a Metal-Chelating Substance in Coffee. Biosci. Biotechnol. Biochem. 2005, 69, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Han, L.; Yang, Y.; Xia, X.; Yang, Z.; Wu, F.; Li, F.; Feng, Y.; Xing, B. Application of Hydrochar Altered Soil Microbial Community Composition and the Molecular Structure of Native Soil Organic Carbon in a Paddy Soil. Environ. Sci. Technol. 2020, 54, 2715–2725. [Google Scholar] [CrossRef]
- Janissen, B.; Huynh, T. Chemical composition and value-adding applications of coffee industry by-products: A review. Resour. Conserv. Recycl. 2018, 128, 110–117. [Google Scholar] [CrossRef]
- Caliskan, S.; Ozok, N.; Makineci, E. Utilization of Spent Coffee Grounds as Media for Stone Pine (Pinus pinea) Seedlings. J. Soil Sci. Plant Nutr. 2020, 20, 2014–2024. [Google Scholar] [CrossRef]
- Gomes, T.; Pereira, J.A.; Ramalhosa, E.; Casal, S.; Baptista, P. Effect of fresh and composted spent coffee grounds on lettuce growth, photosynthetic pigments and mineral composition. In Proceedings of the Congreso Ibérico de Agroingeniería y Ciencias Hortícolas, Madrid, Spain, 26–29 August 2013; pp. 1–5. [Google Scholar]
- Kasongo, R.K.; Verdoodt, A.; Kanyankogote, P.; Baert, G.; Van Ranst, E. Response of Italian ryegrass (Lolium multiflorum Lam.) to coffee waste application on a humid tropical sandy soil. Soil Use Manag. 2013, 29, 22–29. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.G.; Titirici, M.M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]

| Bio-Product | pH | EC25 (dS m−1) | P av. (ppm) | K av. (ppm) | TP (mg GAE g−1) | Zn Content (ppm) | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-Func. | Func. | ||||||||
| SCG | 5.43 | 9.01 | 666.15 | 3706.07 | 10.23 | 10.16 | 6009 | ||
| H160 | 4.60 | 4.34 | 540.24 | 684.11 | 14.08 | 10.05 | 1748 | ||
| H200 | 3.89 | 4.17 | 324.23 | 506.99 | 36.42 | 11.65 | 332 | ||
| Bio-product | Elemental analysis (%) | C/N | Proximate analysis (%) | ||||||
| C | N | H | O * | Fixed carbon | Volatile matter | Ash content | Moisture | ||
| SCG | 47.96 | 2.29 | 7.58 | 42.17 | 20.94 | 13.97 | 84.55 | 1.45 | 7.53 |
| H160 | 52.01 | 2.21 | 8.08 | 37.70 | 23.53 | 13.45 | 86.10 | 0.41 | 3.58 |
| H200 | 62.57 | 2.47 | 7.62 | 27.34 | 25.33 | 26.02 | 73.42 | 0.54 | 3.46 |
| Bio-Product | Specific Surface Area (m² g−1) | Total Vol in Pores (cm³ g−1) | Total Area in Pores (m² g−1) |
|---|---|---|---|
| SCG | 0.503 | 0.00084 | 0.248 |
| H160 | 1.041 | 0.00162 | 0.512 |
| H200 | 15.942 | 0.06485 | 9.348 |
| Sample | pH | EC25 (dS/m) | OC (%) |
|---|---|---|---|
| Control A | 8.37 ± 0.05 fg | 0.79 ± 0.05 ab | 1.10 ± 0.07 a |
| Control B | 8.31 ± 0.02 efg | 0.88 ± 0.07 ab | 1.02 ± 0.08 a |
| SCG-1 | 8.26 ± 0.12 cdef | 0.82 ± 0.13 ab | 1.25 ± 0.25 a |
| H160-1 | 8.18 ± 0.03 cd | 0.90 ± 0.07 ab | 1.32 ± 0.10 a |
| H200-1 | 7.75 ± 0.03 a | 1.39 ± 0.15 d | 2.96 ± 0.43 b |
| SCG-Zn-1 | 8.19 ± 0.02 cd | 0.90 ± 0.09 ab | 1.14 ± 0.04 a |
| H160-Zn-1 | 8.15 ± 0.02 c | 0.92 ± 0.07 ab | 1.33 ± 0.13 a |
| H200-Zn-1 | 7.85 ± 0.05 b | 1.15 ± 0.06 c | 3.02 ± 0.19 b |
| SCG-2 | 8.41 ± 0.02 g | 0.70 ± 0.03 a | 1.31 ± 0.08 a |
| H160-2 | 8.33 ± 0.02 efg | 0.83 ± 0.02 ab | 1.34 ± 0.13 a |
| H200-2 | 8.28 ± 0.02 def | 0.87 ± 0.03 ab | 1.31 ± 0.04 a |
| SCG-Zn-2 | 8.31 ± 0.03 efg | 0.83 ± 0.10 ab | 1.22 ± 0.03 a |
| H160-Zn-2 | 8.25 ± 0.02 cdef | 0.94 ± 0.06 ab | 1.28 ± 0.06 a |
| H200-Zn-2 | 8.22 ± 0.02 cde | 0.95 ± 0.10 b | 1.38 ± 0.06 a |
| Treatment | Plant Mineral Content (mg/100 g) | UE (%) | Soil Available Content (ppm) | ARE (%) | TF |
|---|---|---|---|---|---|
| Control A | 0.11 ± 0.01 a | - | 1.19 ± 0.12 a | - | - |
| Control B | 0.65 ± 0.10 c | 0.34 | 4.71 ± 0.11 b | 4.92 | 0.06 |
| SCG-1 | 0.11 ± 0.01 a | −0.09 | 1.15 ± 0.02 a | −0.40 | 0.09 |
| H160-1 | 0.13 ± 0.02 a | −0.23 | 1.28 ± 0.08 a | 0.85 | 0.10 |
| H200-1 | 0.19 ± 0.01 ab | −0.36 | 1.52 ± 0.24 a | 3.29 | 0.12 |
| SCG-Zn-1 | 0.18± 0.02 ab | 0.22 | 5.95 ± 0.25 cd | 47.54 | 0.03 |
| H160-Zn-1 | 0.18 ± 0.03 ab | −0.12 | 6.37 ± 0.31 d | 51.82 | 0.03 |
| H200-Zn-1 | 0.20 ± 0.01 ab | −0.38 | 5.70 ± 016 cd | 45.11 | 0.03 |
| SCG-2 | 0.14 ± 0.03 a | −0.04 | 1.21 ± 0.07 a | 0.05 | 0.11 |
| H160-2 | 0.14 ± 0.04 a | −0.18 | 1.16 ± 0.05 a | −0.38 | 0.12 |
| H200-2 | 0.14 ± 0.02 a | −0.88 | 1.20 ± 0.13 a | 0.51 | 0.12 |
| SCG-Zn-2 | 0.26 ± 0.03 b | 0.10 | 14.71 ± 0.76 e | 45.00 | 0.02 |
| H160-Zn-2 | 0.21 ± 0.05 ab | 0.00 | 5.31 ± 0.38 bc | 47.17 | 0.04 |
| H200-Zn-2 | 0.15 ± 0.01 a | −0.52 | 1.89 ± 0.13 a | 42.31 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Ramos, L.; Cervera-Mata, A.; Fernández-Bayo, J.; Navarro-Alarcón, M.; Delgado, G.; Fernández-Arteaga, A. Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification. Sustainability 2023, 15, 10700. https://doi.org/10.3390/su151310700
Lara-Ramos L, Cervera-Mata A, Fernández-Bayo J, Navarro-Alarcón M, Delgado G, Fernández-Arteaga A. Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification. Sustainability. 2023; 15(13):10700. https://doi.org/10.3390/su151310700
Chicago/Turabian StyleLara-Ramos, Leslie, Ana Cervera-Mata, Jesús Fernández-Bayo, Miguel Navarro-Alarcón, Gabriel Delgado, and Alejandro Fernández-Arteaga. 2023. "Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification" Sustainability 15, no. 13: 10700. https://doi.org/10.3390/su151310700
APA StyleLara-Ramos, L., Cervera-Mata, A., Fernández-Bayo, J., Navarro-Alarcón, M., Delgado, G., & Fernández-Arteaga, A. (2023). Hydrochars Derived from Spent Coffee Grounds as Zn Bio-Chelates for Agronomic Biofortification. Sustainability, 15(13), 10700. https://doi.org/10.3390/su151310700









