A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Algal Biomass
2.2. Physicochemical Characterization of the Collected Algal Biomass
2.3. Extraction of Different Value-Added Products from the Collected Macroalgae
2.4. Analytical Tools
2.5. The Antimicrobial Activity of the Extracted Ulvan
3. Results and Discussion
3.1. Morphological Examination of the Collected Ulva fasciata
3.2. Physicochemical Characterization of the Collected Ulva fasciata
3.3. Value-Added Products from the Collected Ulva faciata
3.3.1. Pigments and Antioxidants
3.3.2. Mineral-Rich Water Extract
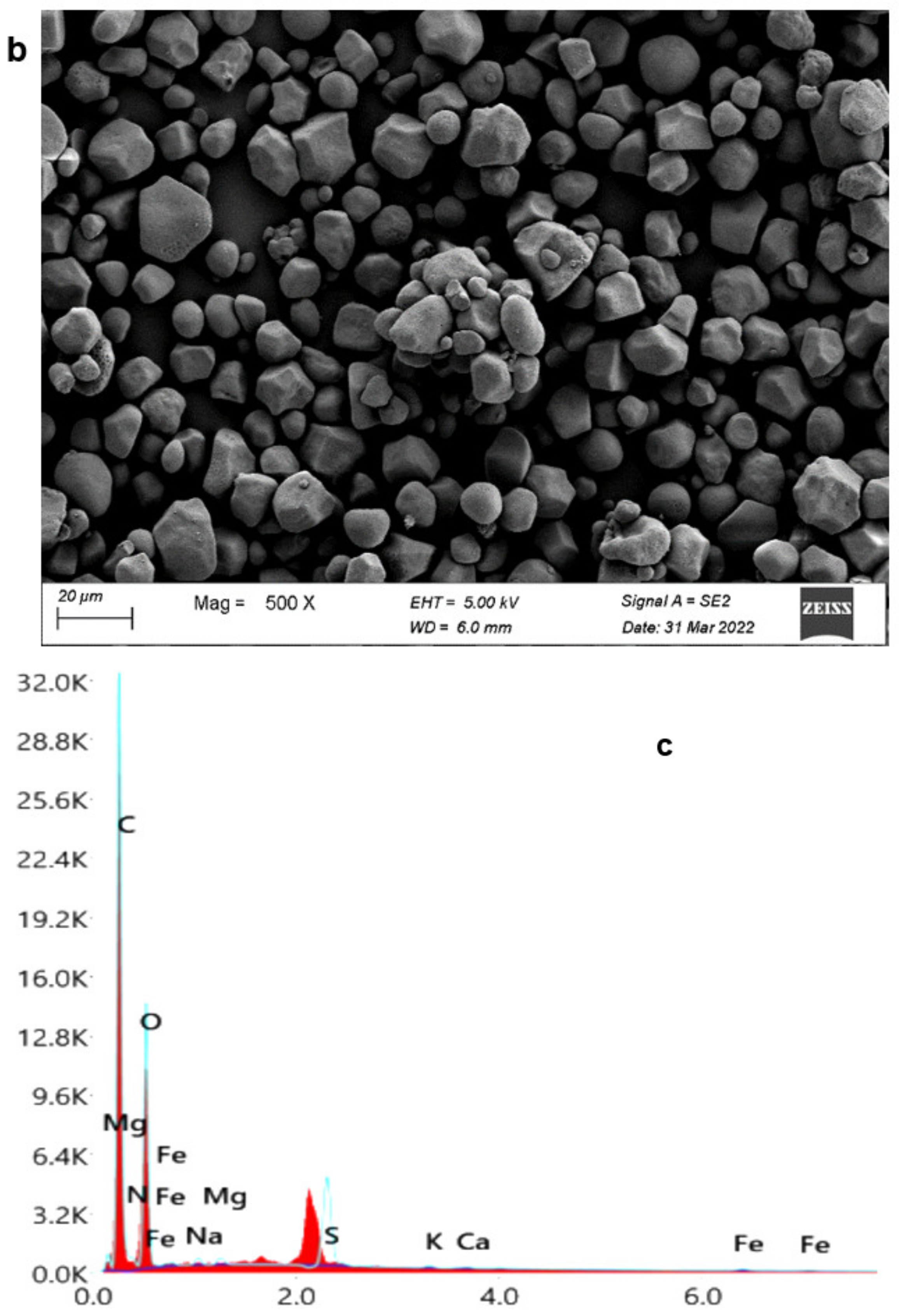
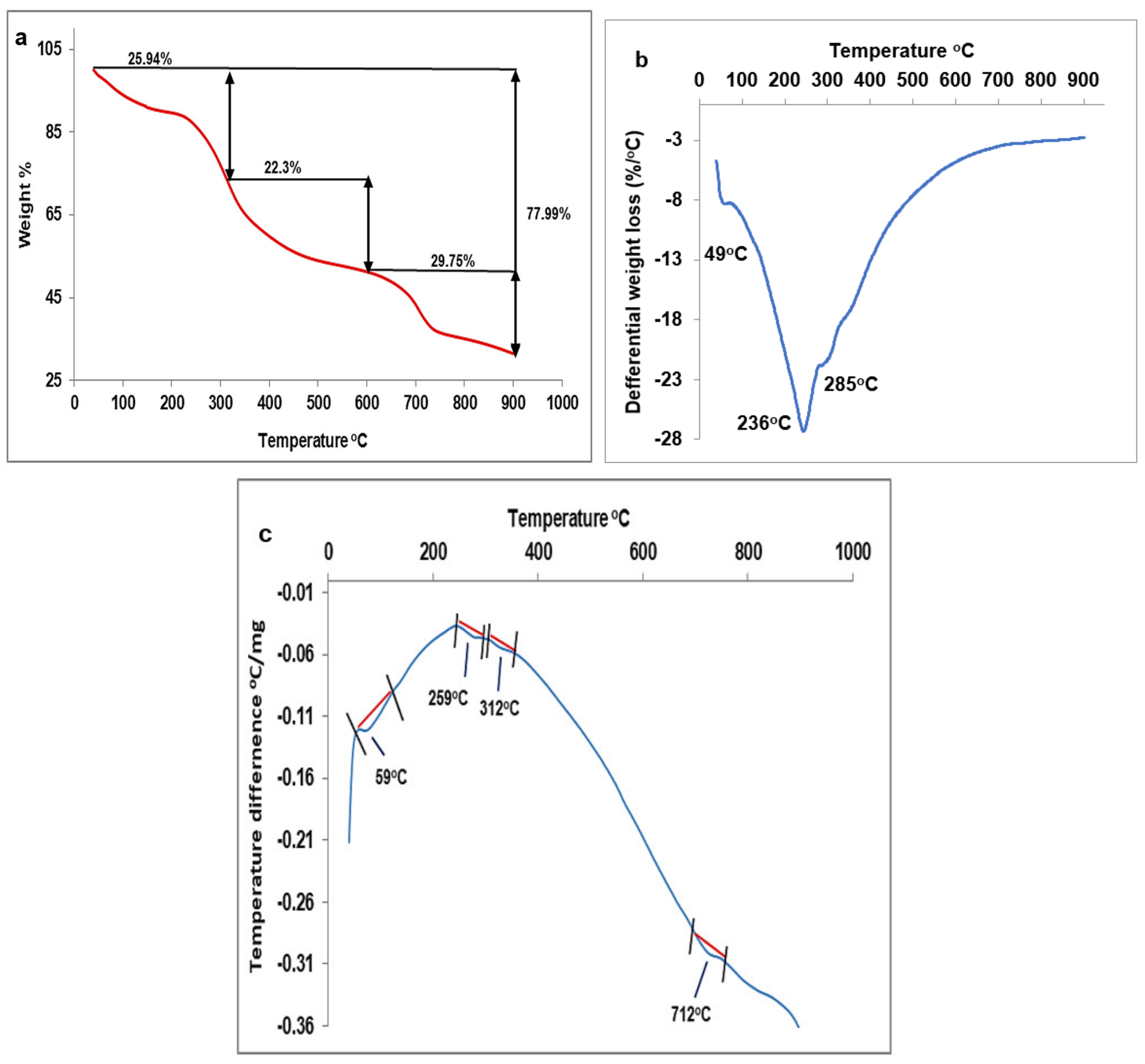
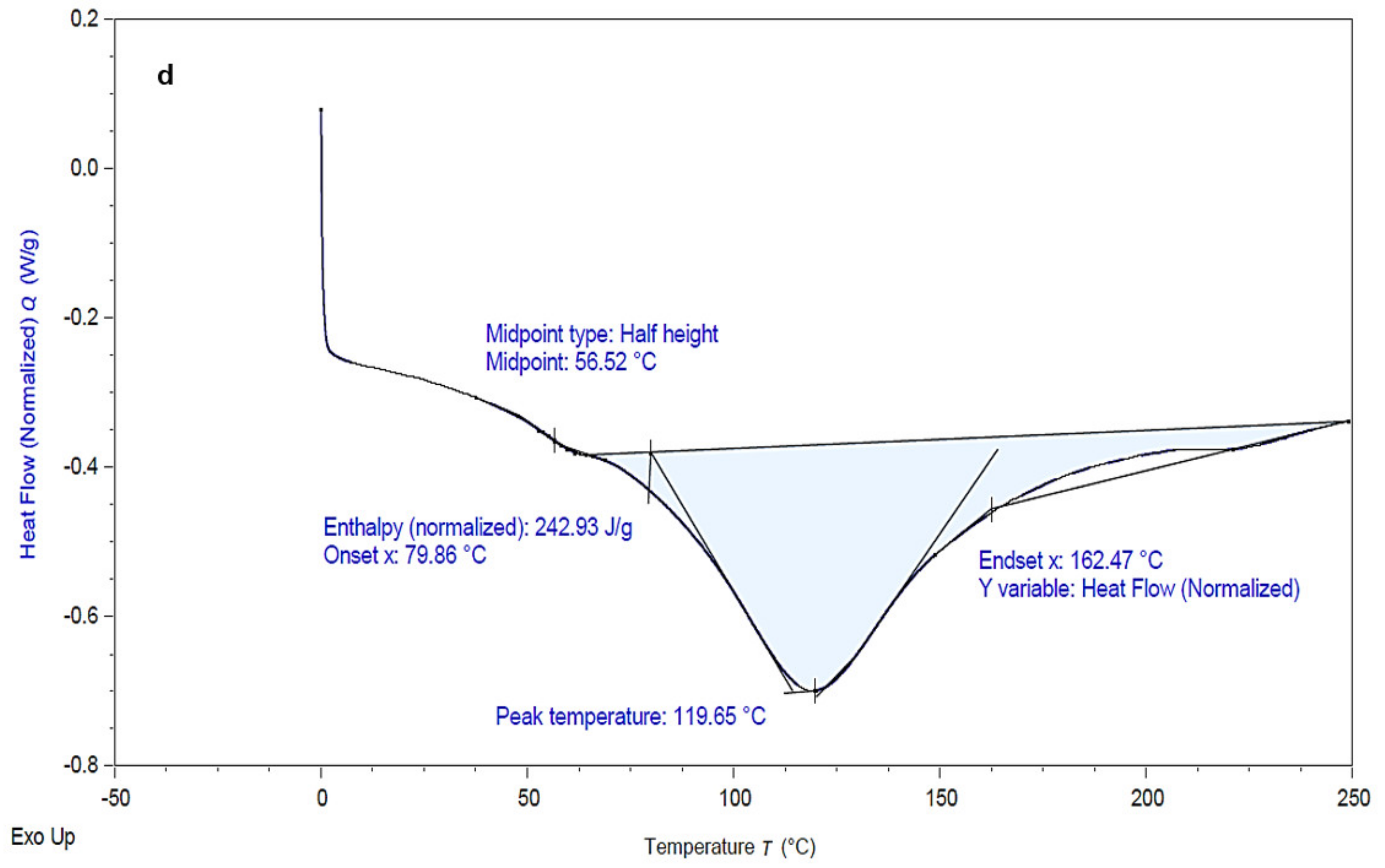
3.3.3. Starch Yield and Characterization
3.3.4. Lipid Yield and Profile
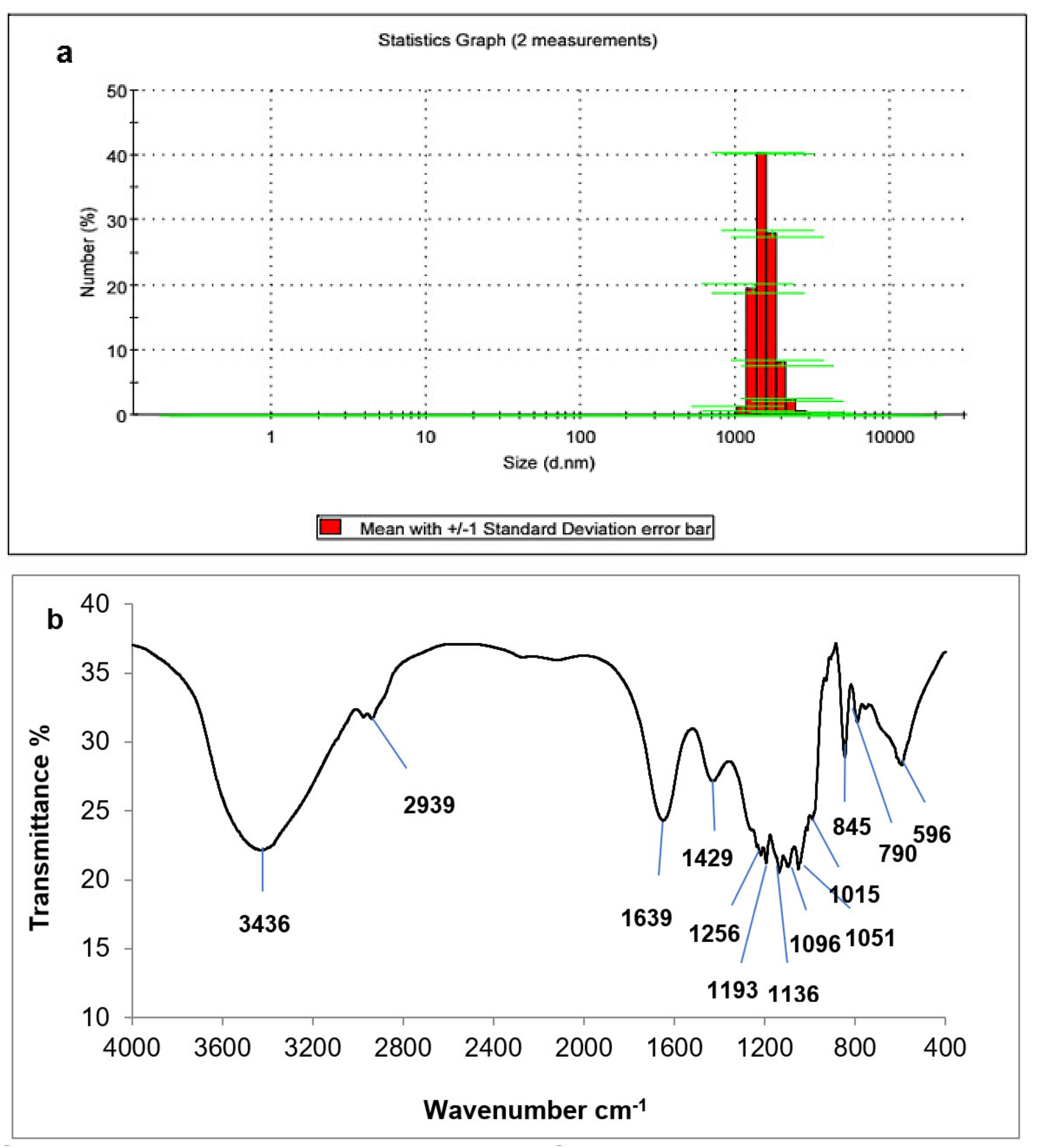
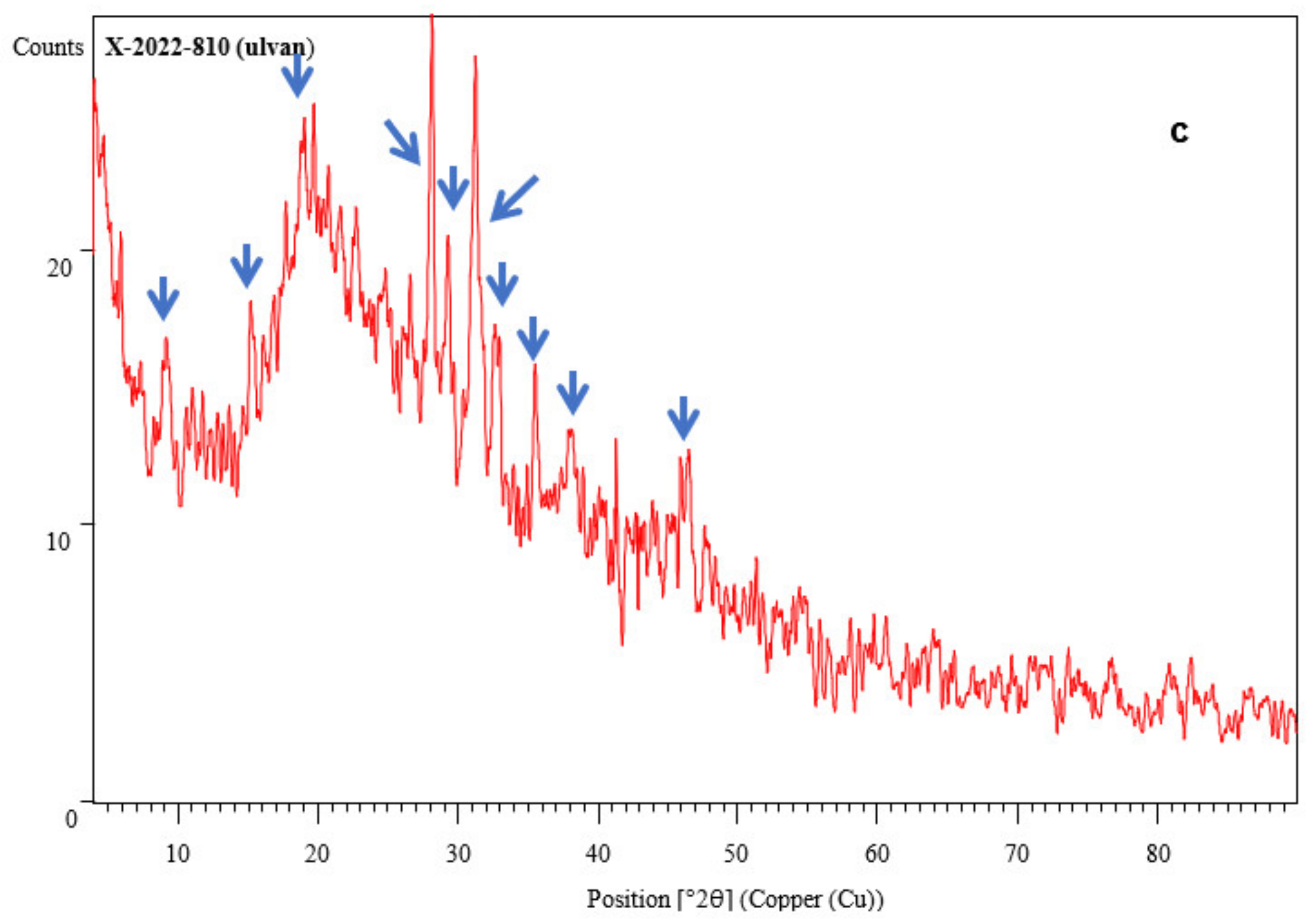
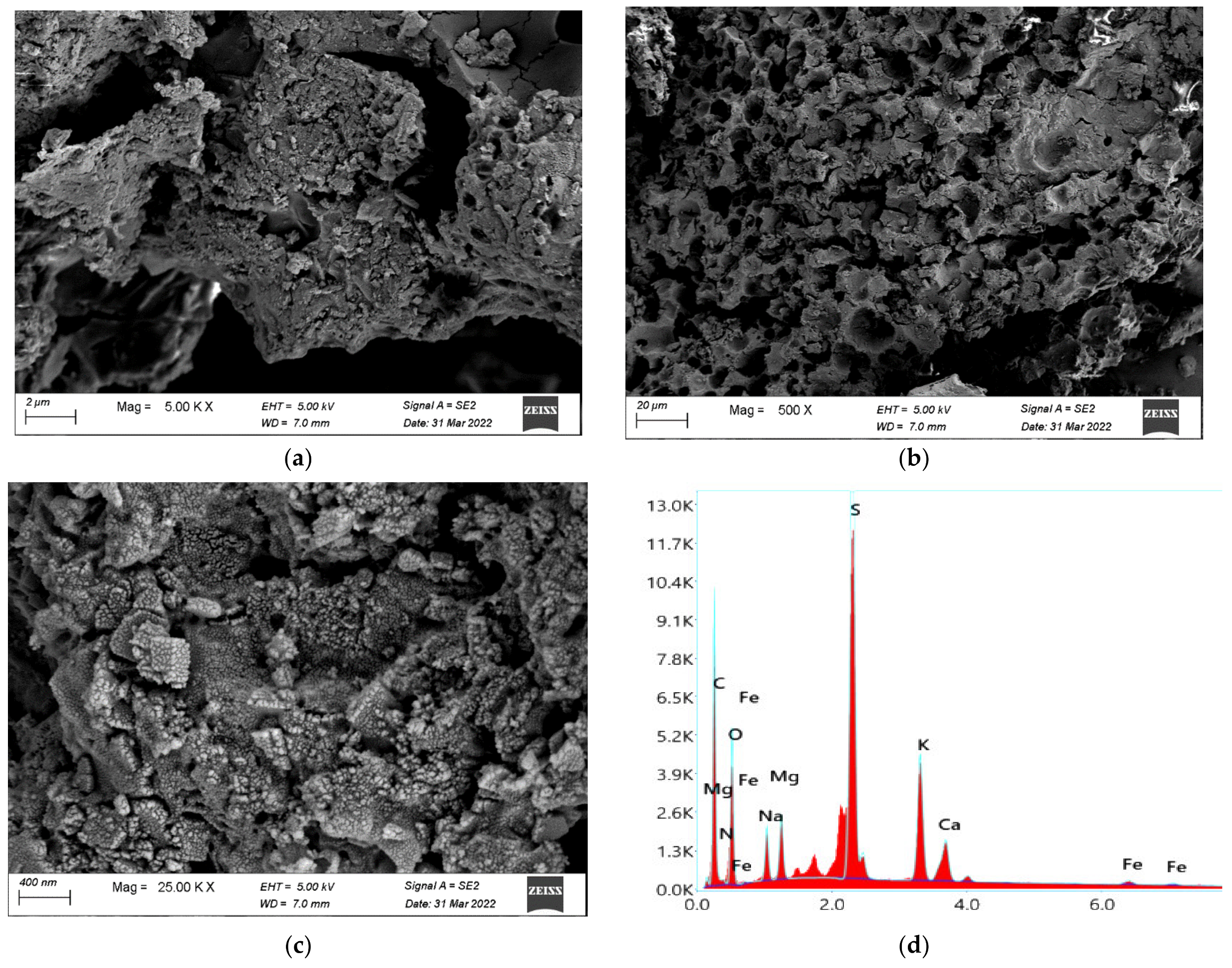
3.3.5. Ulvan Yield and Characterization
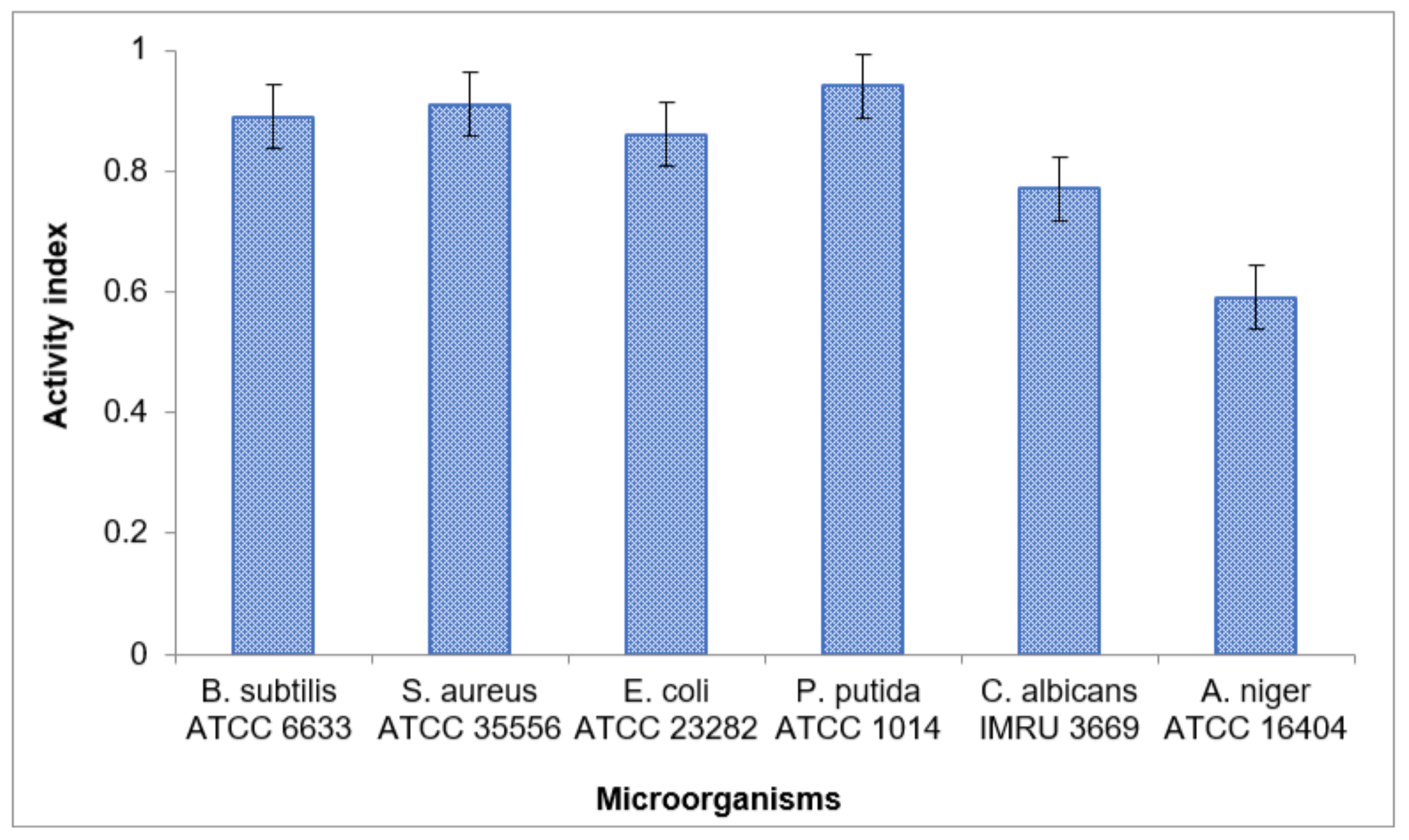
3.3.6. The Antimicrobial Activity of the Extracted Ulva fasciata Ulvan
3.3.7. Protein Yield
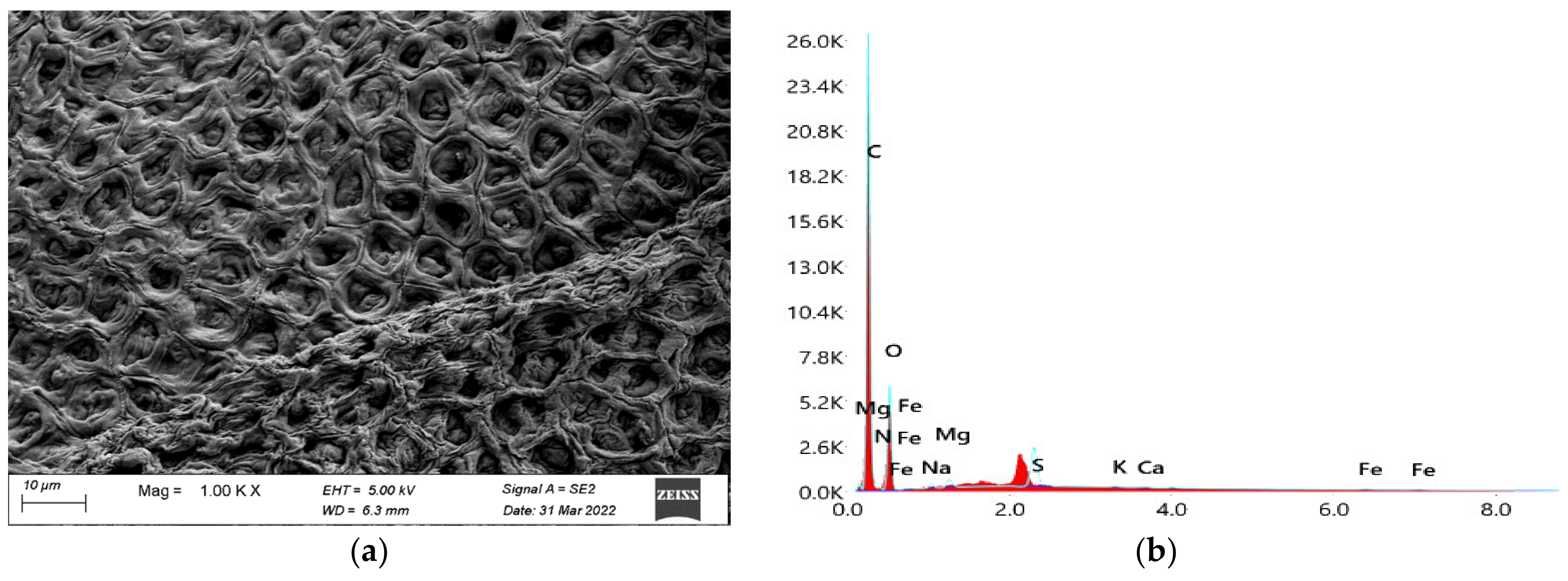
3.3.8. Cellulose Yield and Characterization
4. The Obtained Value-Added Products and Their Suggested Possible Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gajaria, T.K.; Lakshmi, D.S.; Vasu, V.T.; Reddy, C.R.K. Fabrication of ulvan-based ionically cross-linked 3D-biocomposite: Synthesis and characterization. Nanotechnol. Environ. Eng. 2022, 7, 455–459. [Google Scholar] [CrossRef]
- Thygesen, A.; Fernando, D.; Ståhl, K.; Daniel, G.; Mensah, M.; Meyer, A.S. Cell wall configuration and ultrastructure of cellulose crystals in green seaweeds. Cellulose 2021, 28, 2763–2778. [Google Scholar] [CrossRef]
- Egodavitharana, D.I.; Bambaranda, B.V.A.S.M.; Mudannayake, D.C. Phytochemical Composition of Two Green Seaweeds (Ulva lactuca and Ulva fasciata) and their Utilization as a Functional Ingredient in Crackers. J. Aquat. Food Prod. Technol. 2023, 32, 158–174. [Google Scholar] [CrossRef]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Structure and interactions of ulvan in the cell wall of the marine green algae Ulva rotundata (Ulvales, Chlorophyceae). Carbohydr. Polym. 2009, 77, 206–216. [Google Scholar] [CrossRef]
- Hernández-Garibay, E.; Zertuche-González, J.A.; Pacheco-Ruíz, I. Isolation and chemical characterization of algal polysaccharides from the green seaweed Ulva clathrata (Roth) C. Agardh. J. Appl. Phycol. 2011, 23, 537–542. [Google Scholar] [CrossRef]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Effect of extraction conditions on the yield and purity of ulvan extracted from Ulva lactuca. Food Hydrocoll. 2013, 31, 375–382. [Google Scholar] [CrossRef]
- Alves, A.; Caridade, S.G.; Mano, J.F.; Sousa, R.A.; Reis, R.L. Extraction and physico-chemical characterization of a versatile biodegradable polysaccharide obtained from green algae. Carbohydr. Res. 2010, 345, 2194–2200. [Google Scholar] [CrossRef]
- Trivedi, N.; Baghel, R.S.; Bothwell, J.; Gupta, V.; Reddy, C.R.K.; Lali, A.M.; Jha, B. An integrated process for the extraction of fuel and chemicals from marine macroalgal biomass. Sci. Rep. 2016, 6, 30728. [Google Scholar] [CrossRef]
- Lakshmi, D.S.; Trivedi, N.; Reddy, C.R.K. Synthesis and characterization of seaweed cellulose derived carboxymethyl cellulose. Carbohydr. Polym. 2017, 157, 1604–1610. [Google Scholar] [CrossRef]
- Matloub, A.A.; El-Sherbini, M.; Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; Fouad, G.I. Assessment of anti-hyperlipidemic effect and physco-chemical characterization of water soluble polysaccharides from Ulva fasciata Delile. J. Appl. Sci. Res. 2013, 9, 2983–2993. [Google Scholar]
- Madany, M.A.; Abdel-Kareem, M.S.; Al-Oufy, A.K.; Haroun, M.; Sheweita, S.A. The biopolymer ulvan from Ulva fasciata: Extraction towards nanofibers fabrication. Int. J. Biol. Macromol. 2021, 177, 401–412. [Google Scholar] [CrossRef]
- Nunes, N.; Ferraz, S.; Valente, S.; Barreto, M.C.; Pinheiro; de Carvalho, M.A.A. Biochemical composition, nutritional value, and antioxidant properties of seven seaweed species from the Madeira Archipelago. J. Appl. Phycol. 2017, 29, 2427–2437. [Google Scholar] [CrossRef]
- Prabhu, M.; Chemodanov, A.; Gottlieb, R.; Kazir, M.; Nahor, O.; Gozin, M.; Israel, A.; Livney, Y.D.; Golberg, A. Starch from the sea: The green macroalga Ulva ohnoi as a potential source for sustainable starch production in the marine biorefinery. Algal Res. 2019, 37, 215–227. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A Source of Troubles and Potential Riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Gomaa, M.; Al-Badaani, A.A.; Hifney, A.F.; Adam, M.S. Industrial optimization of alkaline and bleaching conditions for cellulose extraction from the marine seaweed Ulva lactuca. J. Appl. Phycol. 2021, 33, 4093–4103. [Google Scholar] [CrossRef]
- Guidara, M.; Yaich, H.; Amor, I.B.; Fakhfakh, J.; Gargouri, J.; Lassoued, S.; Blecker, C.; Richel, A.; Attia, H.; Garna, H. Effect of extraction procedures on the chemical structure, antitumor and anticoagulant properties of ulvan from Ulva lactuca of Tunisia coast. Carbohydr. Polym. 2021, 253, 117283. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Archer, S.D.; Price, N.N. Valorization of waste materials from seaweed industry: An industry survey based biorefinery approach. Rev. Aquac. 2022, 1–8. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.S.; Israel, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Integrated biorefinery process for sustainable fractionation of Ulva ohnoi (Chlorophyta): Process optimization and revenue analysis. J. Appl. Phycol. 2020, 32, 2271–2282. [Google Scholar] [CrossRef]
- Ismail, M.M.; Mohamed, S.E. Differentiation between some Ulva spp. by morphological, genetic and biochemical analyses. Vavilov J. Genet. Breed. 2017, 21, 360–367. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Statistical optimization for cadmium removal using Ulva fasciata biomass: Characterization, immobilization and application for almost-complete cadmium removal from aqueous solutions. Sci. Rep. 2018, 8, 12456. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.; El-Sabagh, S.; Abou; Elsoud, G.; Elbeltagy, A. Efficacy of Immobilized Biomass of the Seaweeds Ulva lactuca and Ulva fasciata for Cadmium Biosorption. Iran. J. Sci. Technol. Trans. A: Sci. 2020, 44, 37–49. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Helrich, K., Arlington, V.A., Eds.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- EN15414-3/2011; Solid Recovered Fuels—Determination of Moisture Content Using the Oven Dry Method—Part 3: Moisture in General Analysis Sample. ISO: London, UK, 2021.
- EN15402:2011; Solid Recovered Fuels—Determination of the Content of Volatile Matter. European Committee for Standardization: Brussels, Belgium, 2011.
- EN15403/2011; Solid Recovered Fuels—Determination of Ash Content. European Committee for Standardization: Brussels, Belgium, 2011.
- ASTM D5865/D5865M-19; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM: West Conshohocken, PA, USA, 2019.
- Moubasher, A.H.; Hafez, S.I.I.; Abdel-Fattah, H.M.; Moharram, A.M. Fungi of wheat and broad-bean straw composts II. Thermophilic fungi. Mycopathologia 1984, 78, 169–176. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis, 1st ed.; Prentice Hall of India Private Limited: New Delhi, India, 1973. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants, and Waters; Division of Agriculture Sciences, University of California: Berkeley, CA, USA, 1961. [Google Scholar]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 29, 677–678. [Google Scholar] [CrossRef]
- Benton, J.J., Jr. Laboratory Guide for Conducting Soil Test and Plant Analysis; CRC Press: Boca Raton, FL, USA; New York, NY, USA; Washington, DC, USA; London, UK, 2001. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.; Pell, A.J.; Jaworski, A.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the green macroalgae Ulva lactuca: Isolation, characterization, optotracing, and production of cellulose nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Mhatre, A.; Gore, S.; Mhatre, A.; Trivedi, N.; Sharma, M.; Pandit, R.; Anil, A.; Lali, A. Effect of multiple product extractions on bio-methane potential of marine macrophytic green alga Ulva lactuca. Renew. Energy 2018, 132, 742–751. [Google Scholar] [CrossRef]
- Ismail, G.A. Biochemical composition of some Egyptian seaweeds with potent nutritive and antioxidant properties. Food Sci. Technol. 2016, 37, 294–302. [Google Scholar] [CrossRef]
- Haryatfrehni, R.H.; Dewi, S.C.; Meilianda, A.; Rahmawati, S.; Sari, I.Z.R. Preliminary Study the Potency of Macroalgae in Yogyakarta: Extraction and Analysis of Algal Pigments from Common Gunungkidul Seaweeds. Procedia Chem. 2015, 14, 373–380. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Pantoa, T.; Roytrakul, S.; Praiboon, J.; Kosawatpat, P.; Tamtin, M.; Thongdang, B. Ultrasonic-Assisted Extraction and Antioxidant Potential of Valuable Protein from Ulva rigida Macroalgae. Life 2023, 13, 86. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; El-Salamony, R.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Novel mycosynthesis of cobalt oxide nanoparticles using Aspergillus brasiliensis ATCC 16404—Optimization, characterization and antimicrobial activity. J. Appl. Microbiol. 2020, 128, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Omran, B.A.; Fatthalah, N.A.; El-Gendy, N.S.; El-Shatoury, E.H.; Abouzeid, A. Green biocides against sulphate reducing bacteria and macrofouling organisms. J. Pure Appl. Microbiol. 2013, 7, 2219–2232. Available online: https://microbiologyjournal.org/green-biocides-against-sulphate-reducing-bacteria-and-macrofouling-organisms/# (accessed on 18 March 2023).
- ASTM-D4412-84; Standard Test Methods for Sulfate Reducing Bacteria in Water and Water Formed Deposits. ASTM: West Conshohocken, PA, USA, 1990.
- AbouGabal, A.A.; Khaled, A.A.; Aboul-Ela, H.M.; Aly, H.M.; Diab, M.H.; Shalaby, O.K. Marine Macroalgal Biodiversity, Spatial Study for the Egyptian Mediterranean Sea, Alexandria Coast. Thalass. Int. J. Mar. Sci. 2022, 38, 639–646. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Samarasinghe, M.B.; van der Heide, M.E.; Weisbjerg, M.R.; Sehested, J.; Sloth, J.J.; Bruhn, A.; Vestergaard, M.; Nørgaard, J.V.; Hernández-Castellano, L.E. A descriptive chemical analysis of seaweeds, Ulva sp., Saccharina latissima and Ascophyllum nodosum harvested from Danish and Icelandic waters. Anim. Feed. Sci. Technol. 2021, 278, 115005. [Google Scholar] [CrossRef]
- Jansen, H.M.; Bernard, M.S.; Nederlof, M.A.J.; van der Meer, I.M.; van der Werf, A. Seasonal variation in productivity, chemical composition and nutrient uptake of Ulva spp. (Chlorophyta) strains. J. Appl. Phycol. 2022, 34, 1649–1660. [Google Scholar] [CrossRef]
- Labib, W.; Hosny, S. Nitrogen and protein contents of Ulva fasciata and Corallina officinalis under environmental variations. Egypt. J. Aquat. Biol. Fish. 2020, 24, 425–438. [Google Scholar] [CrossRef]
- Barakat, K.M.; Ismail, M.M.; El Hassayeb, H.E.A.; El Sersy, N.A.; Elshobary, M.E. Chemical characterization and biological activities of ulvan extracted from Ulva fasciata (Chlorophyta). Rend. Fis. Acc. Lincei. 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Al-Sadek, A.F.; Gad, B.K.; Nassar, H.N.; El-Gendy, N.S. Recruitment of long short-term memory for envisaging the higher heating value of valorized lignocellulosic solid biofuel: A new approach. Energy Sources Part A 2021, 1–17. [Google Scholar] [CrossRef]
- Shin, H.-W.; Smith, C.M. Characterization of light-harvesting pigments in spores of Ulva fasciata Delile. Algae 1996, 11, 309–315. Available online: https://e-algae.org/upload/pdf/algae-1996-11-3-309.pdf (accessed on 18 March 2023).
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of chlorophylls and carotenoids from freshwater algae using different extraction methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2020, 254, 117589. [Google Scholar] [CrossRef]
- Salem, D.M.S.A.; Ismail, M.M. Characterization of cellulose and cellulose nanofibers isolated from various seaweed species. Egypt. J. Aquat. Res. 2022, 48, 307–313. [Google Scholar] [CrossRef]
- Selvam, G.G.; Sivakumar, K. Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental composition using SEM—Energy dispersive spectroscopic analysis. Asian Pac. J. Reprod. 2013, 2, 119–125. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Abu, N.J.; Bujang, J.S.; Zakaria, M.H.; Zulkifly, S. Use of Ulva reticulata as a growth supplement for tomato (Solanum lycopersicum). PLoS ONE 2022, 17, e0270604. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, M.S.; Levkov, K.; Livney, Y.D.; Israel, A.; Golberg, A. High-Voltage Pulsed Electric Field Preprocessing Enhances Extraction of Starch, Proteins, and Ash from Marine Macroalgae Ulva ohnoi. ACS Sustain. Chem. Eng. 2019, 7, 17453–17463. [Google Scholar] [CrossRef]
- Martins, M.M.M.; De Souza, D.C.; Botrel, N.; Resende, L.V.; Pereira, J. Xanthosoma riedelianum starch for use in the food industry. Pesqui. Agropecu. Bras. (Braz. J. Agric. Res.) 2020, 55, e01765. [Google Scholar] [CrossRef]
- Karwasra, B.L.; Gill, B.S.; Kaur, M. Rheological and structural properties of starches from different Indian wheat cultivars and their relationships. Int. J. Food Prop. 2017, 20 (Suppl. S1), S1093–S1106. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Zhang, W.; Li, C.; Yu, J.; Wang, S. Molecular order and functional properties of starches from three waxy wheat varieties grown in China. Food Chem. 2015, 181, 43–50. [Google Scholar] [CrossRef]
- Kazir, M.; Gurevich, D.; Groobman, A.; Prabhu, M.; Israel, A.; Golberg, A.; Livney, Y.D. Physicochemical, rheological and digestibility characterization of starch extracted from the marine green macroalga Ulva ohnoi. Food Hydrocoll. 2021, 120, 106892. [Google Scholar] [CrossRef]
- Todica, M.; Nagy, E.M.; Niculaescu, C.; Stan, O.; Cioica, N.; Pop, C.V. XRD Investigation of Some Thermal Degraded Starch Based Materials. Spectrosc. 2016, 2016, 9605312. [Google Scholar] [CrossRef]
- Kumar, P.; Ganure, A.L.; Subudhi, B.B.; Shukla, S. Synthesis and characterization of pH sensitive ampiphillic new copolymer of methyl methacrylate grafted on modified starch: Influences of reaction variables on grafting parameters. Int. J. Pharm. Pharm. Sci. 2014, 16, 868–880. [Google Scholar]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Toskas, G.; Hund, R.-D.; Laourine, E.; Cherif, C.; Smyrniotopoulos, V.; Roussis, V. Nanofibers based on polysaccharides from the green seaweed Ulva rigida. Carbohydr. Polym. 2011, 84, 1093–1102. [Google Scholar] [CrossRef]
- Yaich, H.; Ben Amira, A.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Ray, B.; Lahaye, M. Cell-wall polysaccharides from the marine green alga Ulva rigida (Ulvales Chlorophyta)-Chemical structure of ulvan. Carbohydr. Res. 1995, 274, 313–318. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Quanbin, Z.; Ning, L.; Zuhong, X.; Yanmei, W.; Zhi’En, L. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.-F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva clathrata and Cladosiphon okamuranus Seaweeds both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Bayro, A.M.; Manlusoc, J.K.; Alonte, R.; Caniel, C.; Conde, P.; Embralino, C. Preliminary Characterization, Antioxidant and Antiproliferative Properties of Polysaccharide from Caulerpa taxifolia. J. Pharm. Sci. Res. 2021, 8, 30–36. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Bhatt, H.; Khandelwal, A.; Vasu, V.T.; Reddy, C.R.K.; Lakshmi, D.S. A facile chemical cross-linking approach toward the fabrication of a sustainable porous ulvan scaffold. J. Bioact. Compat. Polym. 2020, 35, 301–313. [Google Scholar] [CrossRef]
- Ibrahim, M.I.A.; Amer, M.S.; Ibrahim, H.A.H.; Zaghloul, E.H. Considerable Production of Ulvan from Ulva lactuca with Special Emphasis on Its Antimicrobial and Anti-fouling Properties. Appl. Biochem. Biotechnol. 2022, 194, 3097–3118. [Google Scholar] [CrossRef] [PubMed]
- Robic, A.; Rondeau-Mouro, C.; Sassi, J.-F.; Lerat, Y.; Lahaye, M. Seasonal variability of physicochemical and rheological properties of ulvan in two ulva species (Chlorophyta) from the brittany coast1. J. Phycol. 2009, 45, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and Characterization of Ulvan Polysaccharides-Based Hydrogel Films for Potential Wound Dressing Applications. Drug Des. Dev. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef]
- Arul Manikandan, N.A.; Lens, P.N. Green extraction and esterification of marine polysaccharide (ulvan) from green macroalgae Ulva sp. using citric acid for hydrogel preparation. J. Clean. Prod. 2022, 366, 132952. [Google Scholar] [CrossRef]
- Van Tran, T.T.; Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2017, 32, 2291–2296. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Hamdy, A.; Fatthallah, N.A.; Omran, B.A. Recycling of some domestic wastes to produce green corrosion inhibitors and biocides for sulfate reducing bacteria. Energy Sources, Part A 2016, 38, 3722–3732. [Google Scholar] [CrossRef]
- Jmel, M.A.; Anders, N.; Messaoud, G.B.; Marzouki, M.N.; Spiess, A.; Smaali, I. The stranded macroalga Ulva lactuca as a new alternative source of cellulose: Extraction, physicochemical and rheological characterization. J. Clean. Prod. 2019, 234, 1421–1427. [Google Scholar] [CrossRef]
- He, Q.; Wang, Q.; Zhou, H.; Ren, D.; He, Y.; Cong, H.; Wu, L. Highly crystalline cellulose from brown seaweed Saccharina japonica: Isolation, characterization and microcrystallization. Cellulose 2018, 25, 5523–5533. [Google Scholar] [CrossRef]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gaikwad, K.K.; Park, S.-I.; Lee, Y.S. Microwave-assisted step reduced extraction of seaweed (Gelidiella aceroso) cellulose nanocrystals. Int. J. Biol. Macromol. 2017, 99, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cao, Y.; Zhu, Z.; Gao, B.; Zhang, C. Physicochemical Characteristics of Cellulose Nanocrystals Derived from the Residue of Filamentous Microalga Tribonema utriculosum. Appl. Biochem. Biotechnol. 2021, 193, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef]


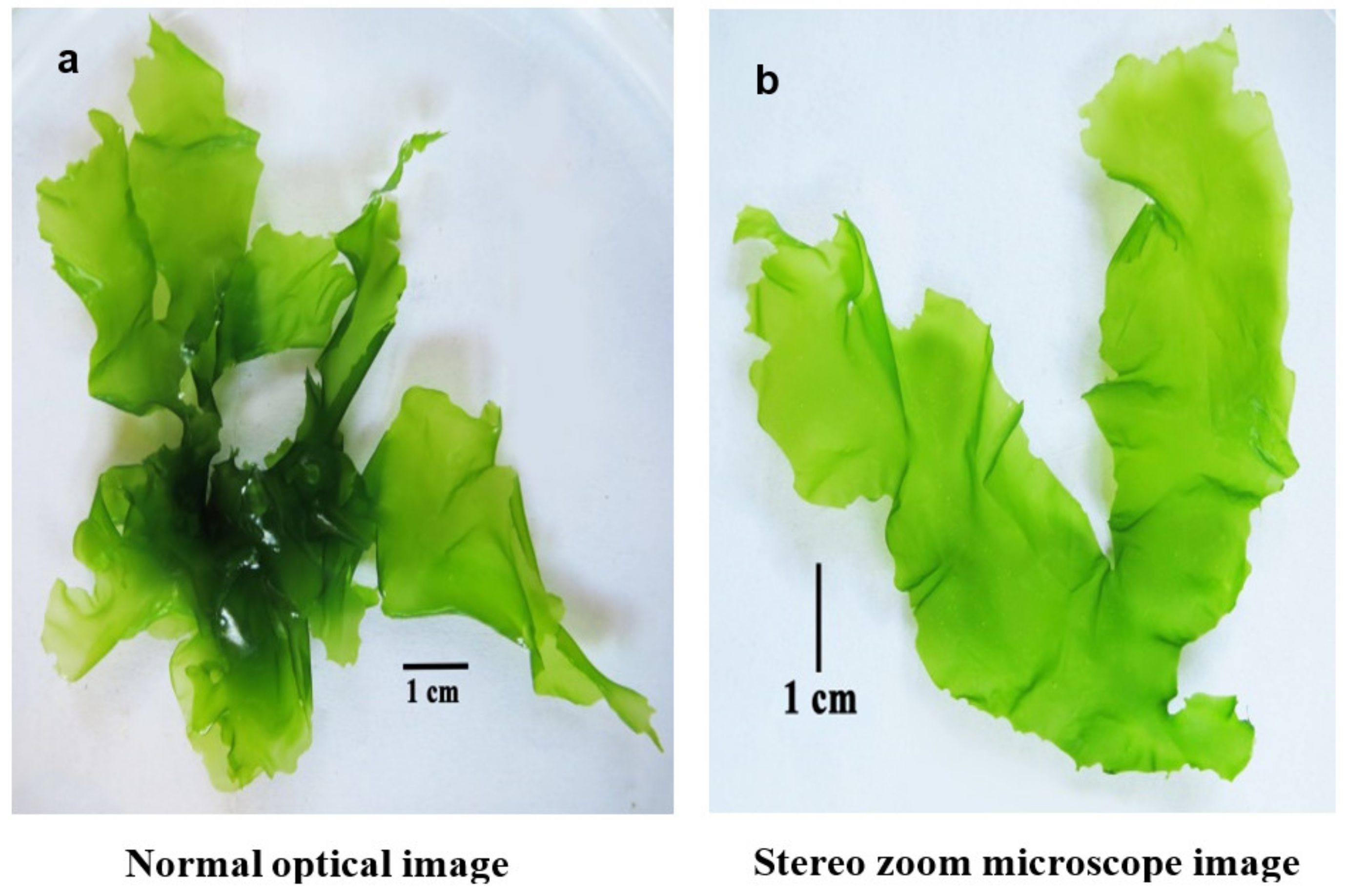
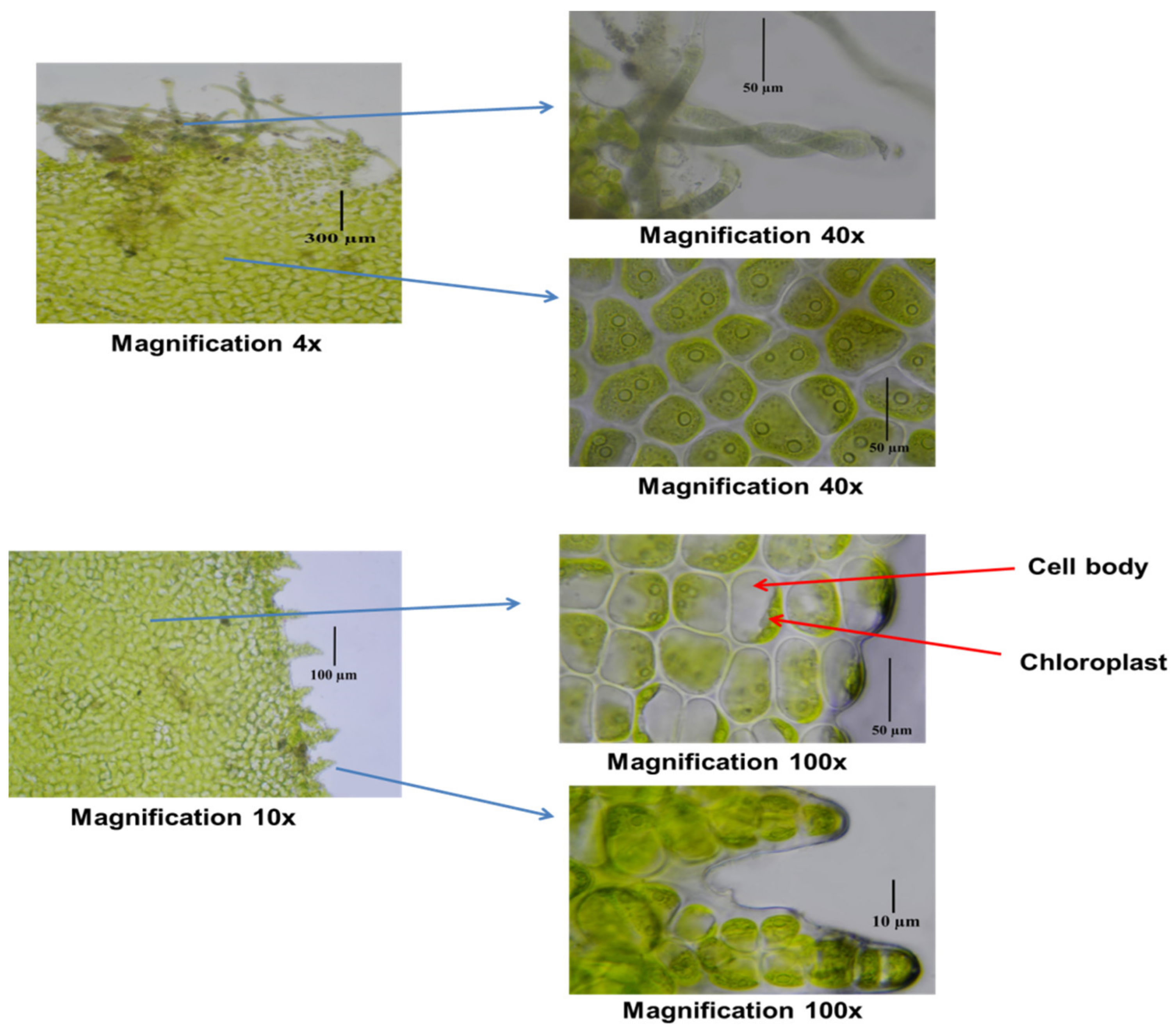
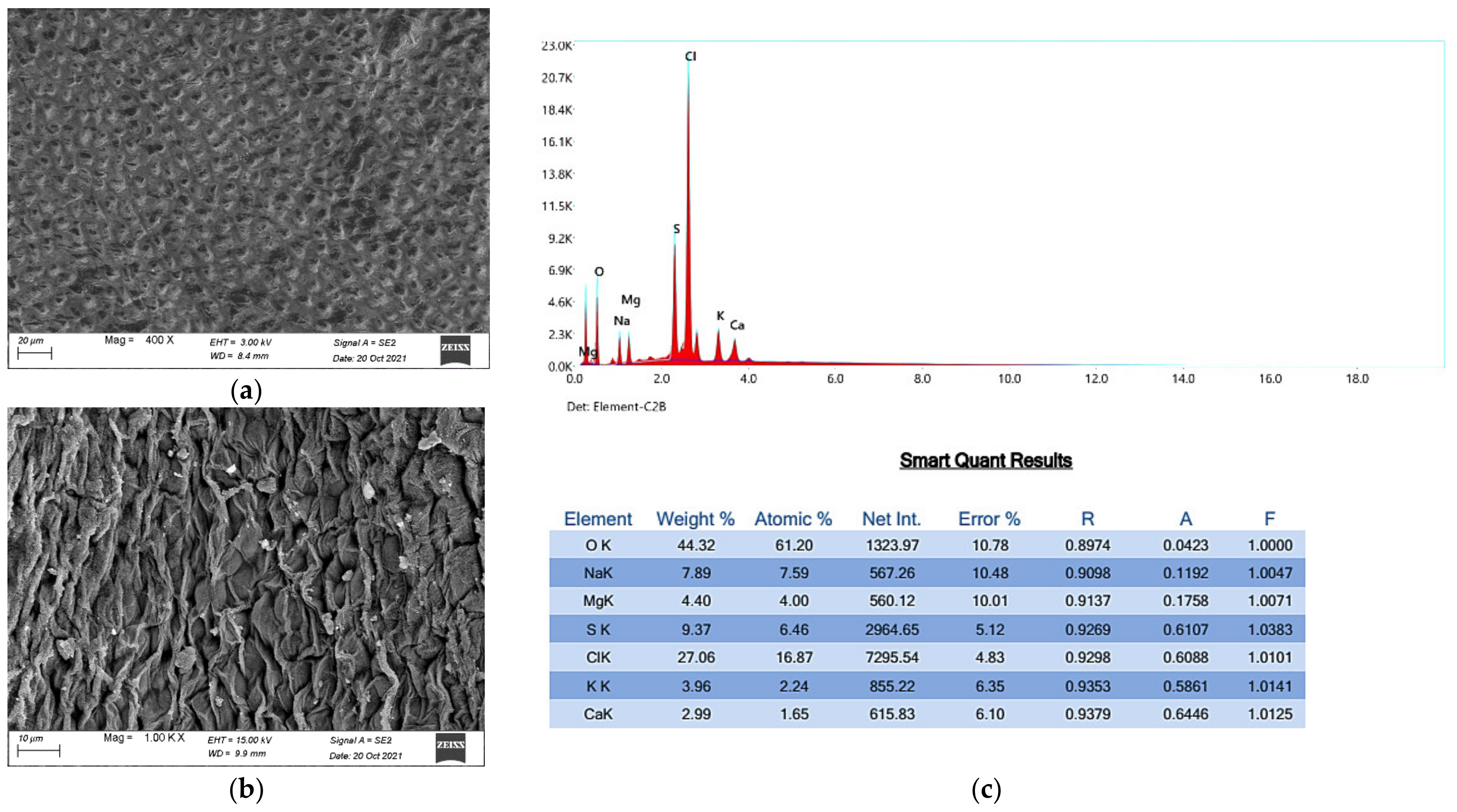

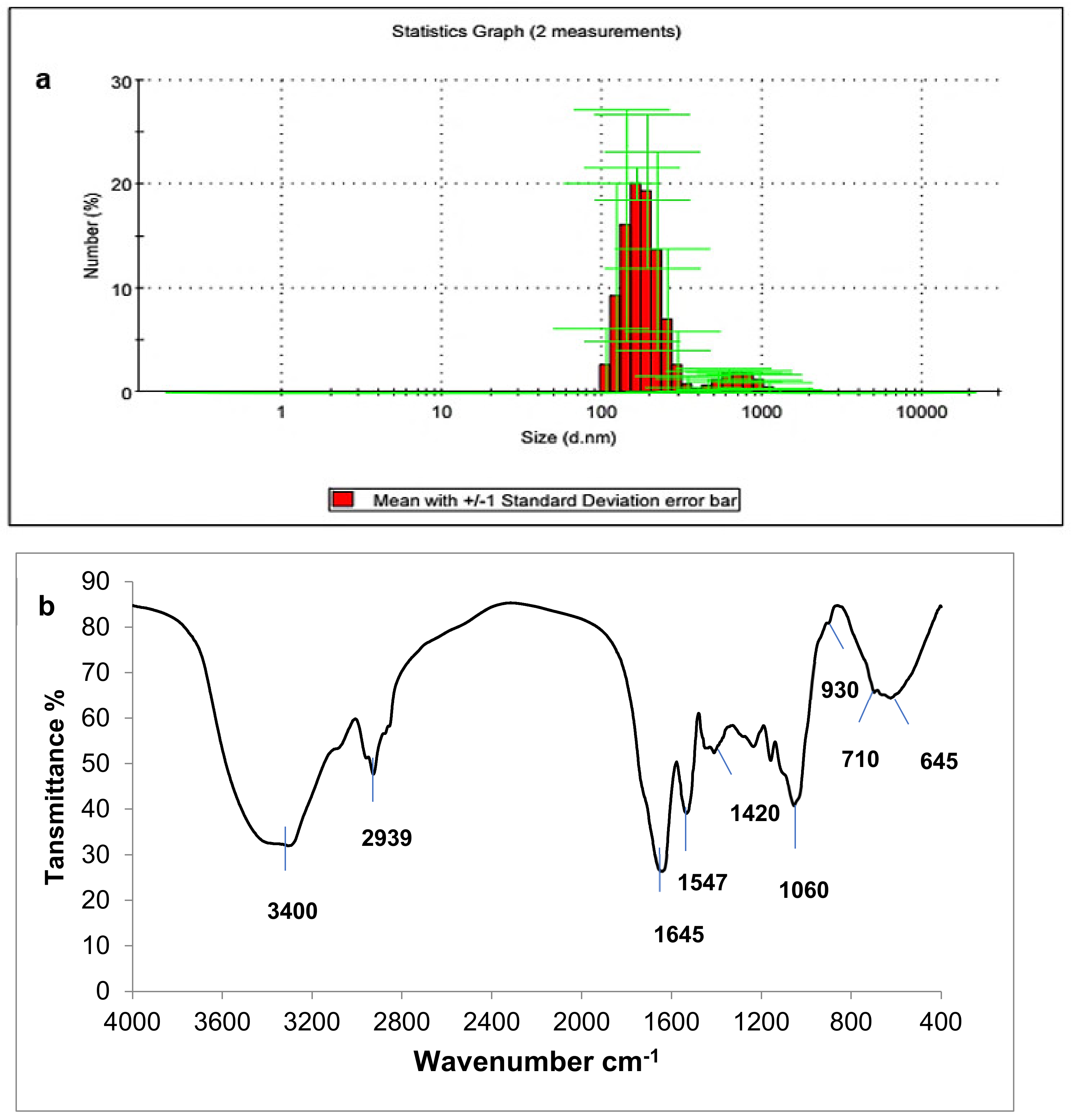








| Dry Matter | Protein Content | Fiber Content | Carbohydrate Content | Lipid Content | Ash Content |
|---|---|---|---|---|---|
| wt% (w:w) | |||||
| 92.15 | 13.13 | 9.9 | 44.85 | 3.27 | 21 |
| Moisture Content | Volatile Content | Fixed Carbon | Ash Content | Calorific Value MJ/kg |
|---|---|---|---|---|
| 8.5% | 70.7% | 0.3% | 20.5% | 15.19 |
| Hemicellulose | Cellulose | Lignin |
|---|---|---|
| 32.91% | 9.80% | 1.50% |
| Parameter | Ulva fasciata | Egyptian Organic Fertilizer Standard (8079/2017) |
|---|---|---|
| Electrical conductivity dS/m | 9.95 | 6–10 |
| Total dissolve solids (mg/L) | 6368 | 3840–6400 |
| Organic C % | 24.59 | Min 15 |
| Organic matter % | 42.30 | Min 18 |
| Moisture content % | 8 | Max 70 |
| Total nitrogen % | 2.1 | Min 0.28 |
| P2O5 % | 0.755 | Min 0.8 |
| K2O % | 0.312 | Min 0.8 |
| N + P2O5 + K2O % | 3.17 | 4 |
| C/N | 11.71 | 18–22:1 |
| pH | 7.1 | 6–8 |
| Macronutrients | Micronutrients | Undesired Heavy Metals | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Fe | Zn | Mn | Cu | Ni | Cd | Cr | Pb | As | Hg | Co | |
| mg/kg | mg/kg | mg/kg | |||||||||||||
| Ulva fasciata | 33 | 260 | 3700 | 82.47 | 180.14 | 66.32 | 15.29 | 3.23 | 0.0519 | 0.591 | 0.31 | 0.0001 | 0.002 | 0.001 | 0.0278 |
| Egyptian organic fertilizer standard 8079/2017 | --- | --- | --- | --- | --- | Max 300 | --- | Max 100 | Max 180 | Max 5 | Max 300 | Max 300 | --- | Max 4 | Max 100 |
| Macronutrients | Micronutrients | Undesired Heavy Metals | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Fe | Zn | Mn | Cu | Ni | Cd | Cr | Pb | As | Hg | Co | |
| mg/L | mg/L | mg/L | |||||||||||||
| MRWE | 3.49 | 23.78 | 1.34 | 0.54 | 12.08 | 29.62 | 30.25 | 0.571 | 0.003 | 0.312 | 0.003 | 0.001 | 0.0001 | 0.0001 | 0.331 |
| Fatty Acid | Trivial Name | Content |
|---|---|---|
| C8:0 | Caprylic acid | 1.17% |
| C10:0 | Capric acid | 0.84% |
| C14:0 | Myristic acid | 4.45% |
| C16:0 | Palimitic acid | 38.93% |
| C17:0 | Margaric acid | 1.63% |
| C18:0 | Stearic acid | 3.72% |
| C20:0 | Arachidic acid | 0.39% |
| C22:0 | Behenic acid | 2.64% |
| C24:0 | Lignoceric acid | 0.92% |
| C16:1 (n-9) | Palmitoleic acid | 3.08% |
| C17:1 | Heptadecenoic acid | 1.84% |
| C18:1 (n-9) | Oleic acid | 1.16% |
| C18:2 (n-6) | Linoleic acid | 1.42% |
| C18:3 (n-3) | α-Linolenic acid | 15.68% |
| C18:3 (n-6) | γ-Linolenic acid | 2.95% |
| C18:4 (n-3) | Stearidonic acid | 16.15% |
| C20:1 (n-9) | Gondoic acid | 1.16% |
| C20:5 (n-3) | Eicosapentaenoic acid | 1.87% |
| Total saturated fatty acids | 54.69% | |
| Total mono-unsaturated fatty acids (MUFAs) | 7.24% | |
| Total poly-unsaturated fatty acids (PUFAs) | 38.07% | |
| Total (n-3) | 33.70% | |
| Total (n-6) | 4.37% | |
| Total (n-9) | 5.4% | |
| n-6/n-3 | 0.13 | |
| Tested Microorganisms | B. subtilis ATCC 6633 | S. aureus ATCC 35556 | E. coli ATCC 23282 | P. putida ATCC 10145 | C. albicans IMRU 3669 | A. niger ATCC 16404 | |
|---|---|---|---|---|---|---|---|
| Compound ID | |||||||
| Ulvan | 31 ± 0.62 | 32 ± 0.64 | 19 ± 0.38 | 31 ± 0.62 | 20 ± 0.4 | 13 ± 0.26 | |
| Reference antibiotic | 35 ± 0.7 | 35 ± 0.7 | 22 ± 0.44 | 33 ± 0.66 | 26 ± 0.52 | 22 ± 0.44 | |
| Tested SRB | Desulfovibrio sapovorans ATCC 33892 (Cell/mL) | Mixed Culture (Cell/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample ID | |||||||||
| +ve control | >3 × 107 | >3 × 107 | |||||||
| −ve control | Nil | Nil | |||||||
| Ulvan concentration mg/L | 500 | 1000 | 200 | 3000 | 500 | 1000 | 2000 | 3000 | |
| 1.5 × 103 | Nil | Nil | Nil | 2.6 × 104 | Nil | Nil | Nil | ||
| Product | Suggested Applications |
|---|---|
| Raw biomass | Animal feeder, biofertilizer, food, and pharmaceutical industries, solid biofuel, bioethanol and biobutanol production |
| Chlorophylla,b Carotenoids | Coloring agent, food, and pharmaceutical industries |
| Mineral rich water extract | Biofertilizer |
| Starch | Starch-based bioplastics, food, and pharmaceutical industries |
| Lipid | Food supplement, animal feeder, and pharmaceutical industries |
| Ulvan | Biocide, water densification, food packaging, biomedical applications, pharmaceutical industries, and biocorrosion mitigation in oil and gas industry. |
| Protein | Animal feeder, food, and pharmaceutical industries |
| Cellulose | Bioethanol production, food, paper, and pharmaceutical industries |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gendy, N.S.; Nassar, H.N.; Ismail, A.R.; Ali, H.R.; Ali, B.A.; Abdelsalam, K.M.; Mubarak, M. A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability 2023, 15, 7319. https://doi.org/10.3390/su15097319
El-Gendy NS, Nassar HN, Ismail AR, Ali HR, Ali BA, Abdelsalam KM, Mubarak M. A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability. 2023; 15(9):7319. https://doi.org/10.3390/su15097319
Chicago/Turabian StyleEl-Gendy, Nour Sh., Hussein N. Nassar, Abdallah R. Ismail, Hager R. Ali, Basma Ahmed Ali, Khaled M. Abdelsalam, and Manal Mubarak. 2023. "A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products" Sustainability 15, no. 9: 7319. https://doi.org/10.3390/su15097319
APA StyleEl-Gendy, N. S., Nassar, H. N., Ismail, A. R., Ali, H. R., Ali, B. A., Abdelsalam, K. M., & Mubarak, M. (2023). A Fully Integrated Biorefinery Process for the Valorization of Ulva fasciata into Different Green and Sustainable Value-Added Products. Sustainability, 15(9), 7319. https://doi.org/10.3390/su15097319







