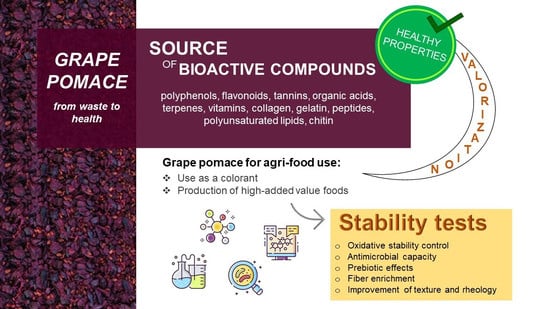
Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects
Abstract
1. Introduction
2. Chemical Composition of Grape Pomace
- -
- The skin or epicarp—the membrane that encloses the pulp and the seeds—is formed by an epidermis of 6–10 layers of flattened cells, covered with a waxy substance called pruine, an ideal substrate for yeasts and other microorganisms’ growth [22].
- -
- The grape seeds, normally two or three per berry, are covered with a tough epidermis, making them passive to the fermentation process (and distillation to obtain grappa). They represent 25–35% by weight of the fresh destemmed grape pomace and are very rich in antioxidant compounds, mainly linoleic acid, an essential fatty acid belonging to the omega-6 family [23].
- -
- The stem is made up mostly of cellulosic substances, small quantities of simple carbohydrates, and organic and mineral salts; it performs important functions in the transport of all the substances that are deposited in the berries and is characterized by a high content of tannins [24].
2.1. Phenolic Compounds
2.2. Minor Components
2.3. Dietary Fiber
2.4. Fatty Acids
2.5. Amino Acids and Biogenic Amines
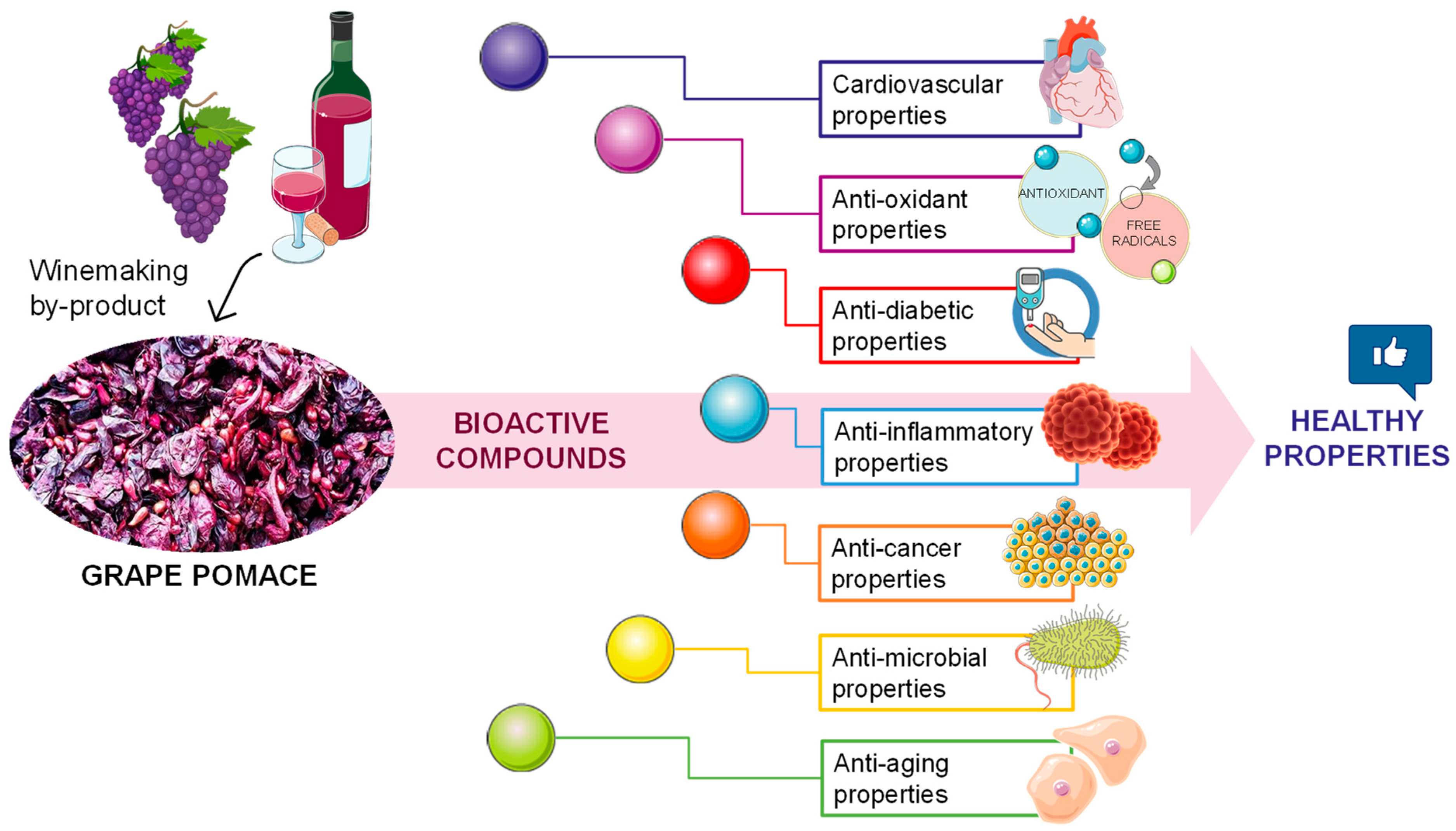
3. Bioactive Compounds from Grape Pomace and Its Healthy/Functional Applications
3.1. Cardiovascular Properties
3.2. Antioxidant and Antidiabetic Properties
3.3. Anti-Inflammatory Properties
3.4. Anti-Cancer Properties
3.5. Anti-Microbial Properties
3.6. Anti-Aging Properties
4. Grape Pomace for Agri-Food Use
4.1. Biogas and Bioethanol
4.2. Bio-Fertilizer
4.3. Tartaric Acid
4.4. Natural Dyes
4.5. Oxidative Stability and Shelf-Life Improvement
4.6. Source of Fiber
4.7. Source of Pectins
4.8. Prebiotic Effects
5. Limitation: Research Gaps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva, A.; Silva, V.; Igrejas, G.; Gaivão, I.; Aires, A.; Klibi, N.; Dapkevicius, M.d.L.; Valentão, P.; Falco, V.; Poeta, P. Valorization of Winemaking By-Products as a Novel Source of Antibacterial Properties: New Strategies to Fight Antibiotic Resistance. Molecules 2021, 26, 2331. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Tikhonova, A.; Ageeva, N.; Globa, E. Grape pomace as a promising source of biologically valuable components. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 34, p. 06002. [Google Scholar]
- Caponio, G.R.; Noviello, M.; Calabrese, F.M.; Gambacorta, G.; Giannelli, G.; De Angelis, M. Effects of grape pomace polyphenols and in vitro gastrointestinal digestion on antimicrobial activity: Recovery of bioactive compounds. Antioxidants 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on anti-inflammatory, anti-cancer, antioxidant properties in gastrointestinal tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Lo, S.; Pilkington, L.I.; Barker, D.; Fedrizzi, B. Attempts to Create Products with Increased Health-Promoting Potential Starting with Pinot Noir Pomace: Investigations on the Process and Its Methods. Foods 2022, 11, 1999. [Google Scholar] [CrossRef]
- Sabetta, W.; Centrone, M.; D’Agostino, M.; Difonzo, G.; Mansi, L.; Tricarico, G.; Venerito, P.; Picardi, E.; Ceci, L.R.; Tamma, G.; et al. “Good Wine Makes Good Blood”: An Integrated Approach to Characterize Autochthonous Apulian Grapevines as Promising Candidates for Healthy Wines. Int. J. Biol. Sci. 2022, 18, 2851. [Google Scholar] [CrossRef]
- Munoz-Bernal, O.A.; Coria-Oliveros, A.J.; de la Rosa, L.A.; Rodrigo-García, J.; del Rocío Martínez-Ruiz, N.; Sayago-Ayerdi, S.G.; Alvarez-Parrilla, E. Cardioprotective effect of red wine and grape pomace. Food Res. Int. 2021, 140, 110069. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Bucić-Kojić, A.; Fernandes, F.; Silva, T.; Planinić, M.; Tišma, M.; Šelo, G.; Šibalić, D.; Pereira, D.M.; Andrade, P.B. Enhancement of the anti-inflammatory properties of grape pomace treated by Trametes versicolor. Food Funct. 2020, 11, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Spinei, M.; Oroian, M. The potential of grape pomace varieties as a dietary source of pectic substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.C.; Minatel, I.O.; Junior, A.P.; Gomez-Gomez, H.A.; de Camargo, J.P.C.; Diamante, M.S.; Basílio, L.S.P.; Tecchio, M.A.; Lima, G.P.P. Bioactive compounds and antioxidant capacity of grape pomace flours. LWT 2021, 135, 110053. [Google Scholar] [CrossRef]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2022, 60, 1388–1416. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the art in grape processing by-products. In Handbook of Grape Processing By-Products: Sustainable Solution; Galanakis, C.M., Ed.; Academic Press Elsevier: London, UK, 2017; pp. 1–27. [Google Scholar]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-pressure recovery of anthocyanins from grape skin pomace (Vitis vinifera cv. Teran) at moderate temperature. J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar] [CrossRef]
- Unusan, N. Proanthocyanidins in grape seeds: An updated review of their health benefits and potential uses in the food industry. J. Funct. Foods 2020, 67, 103861. [Google Scholar] [CrossRef]
- Barros, A.; Gironés-Vilaplana, A.; Texeira, A.; Baenas, N.; Domínguez-Perles, R. Grape stems as a source of bioactive compounds: Application towards added-value commodities and significance for human health. Phytochem. Rev. 2015, 14, 921–931. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Arnous, A.; Meyer, A.S. Quantitative prediction of cell wall polysaccharide composition in grape (Vitis vinifera L.) and apple (Malus domestica) skins from acid hydrolysis monosaccharide profiles. J. Agric. Food Chem. 2009, 57, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape pomace valorization: A systematic review and meta-analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Ahmed, I.A.; Özcan, M.M.; Al Juhaimi, F.; Babiker, E.F.E.; Ghafoor, K.; Banjanin, T.; Osman, M.A.; Gassem, M.A.; Alqah, H.A. Chemical composition, bioactive compounds, mineral contents, and fatty acid composition of pomace powder of different grape varieties. J. Food Process. Preserv. 2020, 44, e14539. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J. Chromatogr. B Biomed. Appl. 2015, 1007, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.C.; Uchôa-Thomaz, A.M.A.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition and bioactive compounds of grape pomace (Vitis vinifera L.), Benitaka variety, grown in the semiarid region of Northeast Brazil. Food Sci. Technol. 2014, 34, 135–142. [Google Scholar] [CrossRef]
- John, W.P.; Li, H.; Liu, J.-R.; Zhou, K.; Zhang, L.; Ren, S. Antioxidant activity, antiproliferation of colon cancer cells, and chemical composition of grape pomace. Food Sci. Nutr. 2011, 2, 6610. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green extraction methods and microencapsulation technologies of phenolic compounds from grape pomace: A review. Food Bioproc. Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Martins, I.M.; Roberto, B.S.; Blumberg, J.B.; Chen, C.Y.O.; Macedo, G.A. Enzymatic biotransformation of polyphenolics increases antioxidant activity of red and white grape pomace. Food Res. Intern. 2016, 89, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Baydar, N.G.; Akkurt, M. Oil content and oil quality properties of some grape seeds. Turk. J. Agric. For. 2001, 25, 163–168. [Google Scholar]
- Rondeau, P.; Gambier, F.; Jolibert, F.; Brosse, N. Compositions and chemical variability of grape pomaces from French vineyard. Ind. Crops Prod. 2013, 43, 251–254. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Singh, A.P.; Kumar, S. Applications of Tannins in Industry. In Tannins-Structural Properties, Biological Properties and Current Knowledge; Aires, A., Ed.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–13. [Google Scholar]
- Bosso, A.; Guaita, M.; Petrozziello, M. Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chem. 2016, 207, 162–169. [Google Scholar] [CrossRef]
- Sallam, I.E.; Abdelwareth, A.; Attia, H.; Aziz, R.K.; Homsi, M.N.; von Bergen, M.; Farag, M.A. Effect of gut microbiota biotransformation on dietary tannins and human health implications. Microorganisms 2021, 9, 965. [Google Scholar] [CrossRef]
- Report, A.A.C.C. The definition of dietary fiber. Cereal Food World 2001, 46, 112–125. [Google Scholar]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Beres, C.; Simas-Tosin, F.F.; Cabezudo, I.; Freitas, S.P.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M. Antioxidant dietary fibre recovery from Brazilian Pinot noir grape pomace. Food Chem. 2016, 201, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green processing and biotechnological potential of grape pomace: Current trends and opportunities for sustainable biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Penner, M.H.; Zhao, Y. Chemical composition of dietary fiber and polyphenols of five different varieties of wine grape pomace skins. Food Res. Int. 2011, 44, 2712–2720. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Pinho, M.; Cabral, L.C. Solid-Liquid Extraction and Concentration with Processes of Membrane Technology of Soluble Fibers from Wine Grape Pomace; Técnico Lisboa: Lisbon, Portugal, 2013. [Google Scholar]
- Difonzo, G.; de Gennaro, G.; Caponio, G.R.; Vacca, M.; Dal Poggetto, G.; Allegretta, I.; Immirzi, B.; Pasqualone, A. Inulin from Globe Artichoke Roots: A Promising Ingredient for the Production of Functional Fresh Pasta. Foods 2022, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- Merenkova, S.P.; Zinina, O.V.; Stuart, M.; Okuskhanova, E.K.; Androsova, N.V. Effects of dietary fiber on human health: A review. Челoвек. Спoрт. Медицина 2020, 20, 106–113. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT-Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Corbin, K.R.; Hsieh, Y.S.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef]
- Anđelković, M.; Radovanović, B.; Anđelković, A.M.; Radovanović, V.; Zarubica, A.; Stojković, N.; Nikolić, V. The determination of bioactive ingredients of grape pomace (Vranac variety) for potential use in food and pharmaceutical industries. Adv. Technol. 2015, 4, 32–36. [Google Scholar] [CrossRef]
- Özcan, M.M.; Al Juhaimi, F. Effect of microwave roasting on yield and fatty acid composition of grape seed oil. Chem. Nat. Compd. 2017, 53, 132–134. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res. Intern. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz, S.; Mostofsky, D.I. Mixture of essential fatty acids lowers test anxiety. Nutr. Neurosci. 2005, 8, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Ngamukote, S.; Mäkynen, K.; Thilawech, T.; Adisakwattana, S. Cholesterol-lowering activity of the major polyphenols in grape seed. Molecules 2011, 16, 5054–5061. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, T.; Litts, C.; Horgan, G.; Zhang, X.; Hoggard, N.; Russell, W.; de Roos, B. Efficacy of bilberry and grape seed extract supplement interventions to improve glucose and cholesterol metabolism and blood pressure in different populations—A systematic review of the literature. Nutrients 2021, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef]
- Purushothaman, A.; Sheeja, A.A.; Janardanan, D. Hydroxyl radical scavenging activity of melatonin and its related indolamines. Free Radic. Res. 2020, 54, 373–383. [Google Scholar] [CrossRef]
- Islam, J.; Shirakawa, H.; Nguyen, T.K.; Aso, H.; Komai, M. Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci. 2016, 13, 56–59. [Google Scholar] [CrossRef]
- Linares, D.M.; del Rio, B.; Redruello, B.; Ladero, V.; Martin, M.C.; Fernandez, M.; Ruas-Madiedo, P.; Alvarez, M.A. Comparative analysis of the in vitro cytotoxicity of the dietary biogenic amines tyramine and histamine. Food Chem. 2016, 197, 658–663. [Google Scholar] [CrossRef]
- Moncalvo, A.; Marinoni, L.; Dordoni, R.; Duserm Garrido, G.; Lavelli, V.; Spigno, G. Waste grape skins: Evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Addit. Contam. Part A 2016, 33, 1116–1126. [Google Scholar] [CrossRef]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas, L.; Moreno-Luna, R.; Mantell, C.; Durán-Ruiz, M.C. Pro-angiogenic effects of natural antioxidants extracted from mango leaf, olive leaf and red grape pomace over endothelial colony-forming cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FOXO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Vita, J.A. Grapes and cardiovascular disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [PubMed]
- Adili, R.; Hawley, M.; Holinstat, M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018, 139, 10–18. [Google Scholar] [CrossRef]
- Lee, L.; Stefanini, W.; Bergmeier, W. Platelet Signal Transduction. In Platelets; Michelson, A.D., Ed.; Academic Press Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 329–348. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Demopoulos, C.A.; Antonopoulou, S. Lipid minor constituents in wines. A biochemical approach in the French paradox. Int. J. Wine Res. 2009, 1, 131–143. [Google Scholar]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Bonechi, C.; Lamponi, S.; Donati, A.; Tamasi, G.; Consumi, M.; Leone, G.; Rossi, C.; Magnani, A. Effect of resveratrol on platelet aggregation by fibrinogen protection. Biophys. Chem. 2017, 222, 41–48. [Google Scholar] [CrossRef]
- Gresele, P.; Pignatelli, P.; Guglielmini, G.; Carnevale, R.; Mezzasoma, A.M.; Ghiselli, A.; Momi, S.; Violi, F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J. Nutr. 2008, 138, 1602–1608. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Justo, M.L.; Claro, C.M.; Vila, E.; Parrado, J.; Herrera, M.D.; De Sotomayor, M.A. Endothelium-dependent vasodilator and antioxidant properties of a novel enzymatic extract of grape pomace from wine industrial waste. Food Chem. 2012, 135, 1044–1051. [Google Scholar] [CrossRef]
- Taladrid, D.; de Celis, M.; Belda, I.; Bartolomé, B.; Moreno-Arribas, M.V. Hypertension-and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome. Food Funct. 2022, 13, 2068–2082. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.; Canning, C.; Sun, S.; Sun, X.; Zhou, K. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J. Agric. Food. Chem. 2010, 58, 11250–11256. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89. [Google Scholar] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Wine pomace product modulates oxidative stress and microbiota in obesity high-fat diet-fed rats. J. Funct. Foods 2020, 68, 103903. [Google Scholar] [CrossRef]
- Goutzourelas, N.; Stagos, D.; Housmekeridou, A.; Karapouliou, C.; Kerasioti, E.; Aligiannis, N.; Skaltsounis, A.L.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Grape pomace extract exerts antioxidant effects through an increase in GCS levels and GST activity in muscle and endothelial cells. Int. J. Mol. Med. 2015, 36, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Raghuram, G.V.; Dsouza, J.; Shinde, S.; Jadhav, V.; Shaikh, A.; Rane, B.; Tandel, H.; Kondhalkar, D.; Chaudhary, S.; et al. A pro-oxidant combination of resveratrol and copper down-regulates multiple biological hallmarks of ageing and neurodegeneration in mice. Sci. Rep. 2022, 12, 17209. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Li, B.; Hou, D.; Guo, H.; Zhou, H.; Zhang, S.; Xu, X.; Liu, Q.; Zhang, X.; Zou, Y.; Gong, Y.; et al. Resveratrol sequentially induces replication and oxidative stresses to drive p53-CXCR2 mediated cellular senescence in cancer cells. Sci. Rep. 2017, 7, 208. [Google Scholar] [CrossRef]
- Demoulin, B.; Hermant, M.; Castrogiovanni, C.; Staudt, C.; Dumont, P. Resveratrol induces DNA damage in colon cancer cells by poisoning topoisomerase II and activates the ATM kinase to trigger p53-dependent apoptosis. Toxicol. In Vitro 2015, 29, 1156–1165. [Google Scholar] [CrossRef]
- Kato-Schwartz, C.G.; Corrêa, R.C.G.; de Souza Lima, D.; de Sá-Nakanishi, A.B.; de Almeida Gonçalves, G.; Seixas, F.A.V.; Haminiuk, C.W.I.; Barros, L.; Ferreira, I.C.F.R.; Bracht, A.; et al. Potential anti-diabetic properties of Merlot grape pomace extract: An in vitro, in silico and in vivo study of α-amylase and α-glucosidase inhibition. Food Res. Int. 2020, 137, 109462. [Google Scholar] [CrossRef]
- Hogan, S.; Zhang, L.; Li, J.; Sun, S.; Canning, C.; Zhou, K. Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr. Metab. 2010, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Howard, L.R.; Rogers, T.J.; Wilkes, S.E.; Dhakal, I.B.; Prior, R.L. Effect of feeding grape pomace on selected metabolic parameters associated with high fructose feeding in growing Sprague–Dawley rats. J. Med. Food 2011, 14, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Renna, N.F.; de Las Heras, N.; Miatello, R.M. Pathophysiology of vascular remodeling in hypertension. Int. J. Hypertens. 2013, 2013, 808353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhou, J.; Jiang, B.; Miao, M. Resveratrol and inflammatory bowel disease. Ann. N. Y. Acad. Sci. 2017, 1403, 38–47. [Google Scholar] [CrossRef]
- Chedea, V.S.; Macovei, Ș.O.; Bocsan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape pomace polyphenols as a source of compounds for management of oxidative stress and inflammation—A possible alternative for non-steroidal anti-inflammatory drugs? Molecules 2022, 27, 6826. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and anti-inflammatory actions of polyphenols from red and white grape pomace in ischemic heart diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. JNB 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Soares, A.A.; Correa, R.C.; Barros, L.; Haminiuk, C.W.; Peralta, R.M.; Ferreira, I.C.F.R.; Bracht, A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods 2017, 33, 408–418. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Gm Naidu, V.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-inflammatory treatments for chronic diseases: A review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Denny, C.; Lazarini, J.G.; Franchin, M.; Melo, P.S.; Pereira, G.E.; Massarioli, A.P.; Moreno, I.A.M.; Pashoal, J.A.R.; Alencar, S.M.; Rosalen, P.L. Bioprospection of Petit Verdot grape pomace as a source of anti-inflammatory compounds. J. Funct. Foods 2014, 8, 292–300. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A.; Roberto, B.S.; Chen, Q.; Blumberg, J.B.; Chen, C.Y.O. Tannase enhances the anti-inflammatory effect of grape pomace in Caco-2 cells treated with IL-1β. J. Funct. Foods 2017, 29, 69–76. [Google Scholar] [CrossRef]
- Martins, I.M.; Macedo, G.A.; Macedo, J.A. Biotransformed grape pomace as a potential source of anti-inflammatory polyphenolics: Effects in Caco-2 cells. Food Biosci. 2020, 35, 100607. [Google Scholar] [CrossRef]
- Rodríguez-Morgado, B.; Candiracci, M.; Santa-María, C.; Revilla, E.; Gordillo, B.; Parrado, J.; Castaño, A. Obtaining from grape pomace an enzymatic extract with anti-inflammatory properties. Plant Foods Hum. Nutr. 2015, 70, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ferri, M.; Rondini, G.; Calabretta, M.M.; Michelini, E.; Vallini, V.; Fava, F.; Roda, A.; Minnucci, G.; Tassoni, A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017, 39, 51–58. [Google Scholar] [CrossRef]
- Caleja, C.; Ribeiro, A.; Filomena Barreiro, M.; CFR Ferreira, I. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Kim, Y.A.; Rhee, S.H.; Park, K.Y.; Choi, Y.H. Antiproliferative effect of resveratrol in human prostate carcinoma cells. J. Med. Food 2003, 6, 273–280. [Google Scholar] [CrossRef]
- Tian, Q.; Xu, Z.; Sun, X.; Deavila, J.; Du, M.; Zhu, M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J. Nutr. Biochem. 2023, 84, 108443. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Q.; Xu, Z.; Du, M.; Zhu, M.J. Metabolomic profiling for the preventive effects of dietary grape pomace against colorectal cancer. J. Nutr. Biochem. 2023, 116, 109308. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and antiproliferative potentials of phenolic-rich extracts from biotransformed grape pomace in colorectal Cancer. BMC Complementary Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-proliferative and pro-apoptotic effects of digested aglianico grape pomace extract in human colorectal cancer cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and cytotoxic effects of grape pomace and grape seed extracts on colorectal cancer cell lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kunova, S.; Felšöciová, S.; Tvrda, E.; Ivanišová, E.; Kántor, A.; Ziarovska, J.; Terentjeva, M.; Kacaniova, M. Antimicrobial activity of resveratrol and grape pomace extract. Potravinarstvo Slovak. J. Food Sci. 2019, 13, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A., Jr.; Smânia, E.F.; Maraschin, M.; Ferreira, S.R. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Kabir, F.; Sultana, M.S.; Kurnianta, H. Antimicrobial activities of grape (Vitis vinifera L.) pomace polyphenols as a source of naturally occurring bioactive components. Afr. J. Biotechnol. 2015, 14, 2157–2161. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food. Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Tsiviki, M.; Goula, A.M. Chapter 16—Valorization of grape seeds. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 331–346. [Google Scholar]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Da Ros, C.; Cavinato, C.; Bolzonella, D.; Pavan, P. Renewable energy from thermophilic anaerobic digestion of winery residue: Preliminary evidence from batch and continuous lab-scale trials. Biomass Bioenergy 2016, 91, 150–159. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef] [PubMed]
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Caramiello, C.; Lancellotti, I.; Righi, F.; Tatàno, F.; Taurino, R.; Barbieri, L. Anaerobic digestion of selected Italian agricultural and industrial residues (grape seeds and leather dust): Combined methane production and digestate characterization. Environ. Technol. 2013, 34, 1225–1237. [Google Scholar] [CrossRef] [PubMed]
- Lempereur, V.; Penavayre, S. Grape marc, wine lees and deposit of the must: How to manage oenological by-products? BIO Web Conf. 2014, 3, 01011. [Google Scholar] [CrossRef]
- Burg, P.; Vítěz, T.; Turan, J.; Burgová, J. Evaluation of grape pomace composting process. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 62, 875–881. [Google Scholar] [CrossRef]
- Paradelo, R.; Moldes, A.B.; Barral, M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 2013, 116, 18–26. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Improving green waste composting by addition of sugarcane bagasse and exhausted grape marc. Bioresour. Technol. 2016, 218, 335–343. [Google Scholar] [CrossRef]
- Hungría, J.; Gutiérrez, M.C.; Siles, J.A.; Martín, M.A. Advantages and drawbacks of OFMSW and winery waste co-composting at pilot scale. J. Clean. Prod. 2017, 164, 1050–1057. [Google Scholar] [CrossRef]
- Achmon, Y.; Harrold, D.R.; Claypool, J.T.; Stapleton, J.J.; VanderGheynst, J.S.; Simmons, C.W. Assessment of tomato and wine processing solid wastes as soil amendments for biosolarization. Waste Manag. 2016, 48, 156–164. [Google Scholar] [CrossRef]
- Domínguez, J.; Martínez-Cordeiro, H.; Álvarez-Casas, M.; Lores, M. Vermicomposting grape marc yields high quality organic biofertiliser and bioactive polyphenols. Waste Manag. Res. 2014, 32, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, T.; Mahmud-Ali, A.; Mussak, R. Anthocyanin dyes extracted from grape pomace for the purpose of textile dyeing. J. Sci. Food Agric. 2007, 87, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Baaka, N.; Haddar, W.; Ben Ticha, M.; Mhenni, M.F. Eco-friendly dyeing of modified cotton fabrics with grape pomace colorant: Optimization using full factorial design approach. J. Nat. Fibers 2019, 16, 652–661. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.M.; Spigno, G. Grape march phenolics: Extraction kinetics, quality and stability of extracts. J. Food Eng. 2010, 97, 384–392. [Google Scholar] [CrossRef]
- Spigno, G.; Donsì, F.; Amendola, D.; Sessa, M.; Ferrari, G.; De Faveri, D.M. Nanoencapsulation systems to improve solubility and antioxidant efficiency of a grape marc extract into hazelnut paste. J. Food Eng. 2013, 114, 207–214. [Google Scholar] [CrossRef]
- Aquilani, C.; Sirtori, F.; Flores, M.; Bozzi, R.; Lebret, B.; Pugliese, C. Effect of natural antioxidants from grape seed and chestnut in combination with hydroxytyrosol, as sodium nitrite substitutes in Cinta Senese dry-fermented sausages. Meat Sci. 2018, 145, 389–398. [Google Scholar] [CrossRef]
- Gaione-Mendes, A.C.; Rettore, D.M.; Ramos, A.L.S.; da Cunha, S.F.V.; de Oliveira, L.C.; Ramos, E.M. Milano type salami elaborated with fibers of red wine by products. Cienc. Rural 2014, 44, 1291–1296. [Google Scholar] [CrossRef]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. Int. J. Food Sci. Technol. 2019, 54, 952–958. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT-Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Cilli, L.P.; Contini, L.R.F.; Sinnecker, P.; Lopes, P.S.; Andreo, M.A.; Neiva, C.R.P.; Nascimento, M.S.; Yoshida, C.M.P.; Venturini, A.C. Effects of grape pomace flour on quality parameters of salmon burger. J. Food Process. Preserv. 2020, 44, e14329. [Google Scholar] [CrossRef]
- Gai, F.; Ortoffi, M.; Giancotti, V.; Medana, C.; Peiretti, P.G. Effect of red grape pomace extract on the shelf life of refrigerated rainbow trout (Oncorhynchus mykiss) minced muscle. J. Aquat. Food Prod. Technol. 2015, 24, 468–480. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Antioxidant protection of white grape pomace on restructured fish products during frozen storage. LWT-Food Sci. Technol. 2008, 41, 42–50. [Google Scholar] [CrossRef]
- Tayengwa, T.; Chikwanha, O.C.; Gouws, P.; Dugan, M.E.; Mutsvangwa, T.; Mapiye, C. Dietary citrus pulp and grape pomace as potential natural preservatives for extending beef shelf life. Meat Sci. 2020, 162, 108029. [Google Scholar] [CrossRef]
- Bianchi, F.; Lomuscio, E.; Rizzi, C.; Simonato, B. Predicted shelf-life, thermodynamic study and antioxidant capacity of breadsticks fortified with grape pomace powders. Foods 2021, 10, 2815. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Allegretta, I.; Pasqualone, A.; Caponio, F. Grape skin and seed flours as functional ingredients of pizza: Potential and drawbacks related to nutritional, physicochemical and sensory attributes. LWT 2023, 175, 114494. [Google Scholar] [CrossRef]
- Troilo, M.; Difonzo, G.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Grape Pomace as Innovative Flour for the Formulation of Functional Muffins: How Particle Size Affects the Nutritional, Textural and Sensory Properties. Foods 2022, 11, 1799. [Google Scholar] [CrossRef]
- Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G.; Simonato, B. Breadstick fortification with red grape pomace: Effect on nutritional, technological and sensory properties. J. Sci. Food Agric. 2022, 102, 2545–2552. [Google Scholar] [CrossRef]
- Smith, I.N.; Yu, J. Nutritional and sensory quality of bread containing different quantities of grape pomace from different grape cultivars. EC Nutr. 2015, 2, 291–301. [Google Scholar]
- Spinei, M.; Oroian, M. The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Q.; Chin, N.L.; Yusof, Y.A. Extraction and characterization of pectin from passion fruit peels. Agric. Agric. Sci. Procedia 2014, 2, 231–236. [Google Scholar] [CrossRef]
- Castillo-Israel, K.A.T.; Baguio, S.F.; Diasanta, M.D.B.; Lizardo, R.C.M.; Dizon, E.I.; Mejico, M.I.F. Extraction and characterization of pectin from Saba banana (Musa’saba’(Musa acuminata x Musa balbisiana)) peel wastes. Int. Food Res. J. 2015, 22, 190–195. [Google Scholar]
- Klen, T.J.; Vodopivec, B.M. Optimisation of olive oil phenol extraction conditions using a high-power probe ultrasonication. Food Chem. 2012, 134, 2481–2488. [Google Scholar] [CrossRef]
- Vásquez, P.; Vega-Gálvez, A.; Bernal, C. Production of antioxidant pectin fractions, drying pretreatment methods and physicochemical properties: Towards pisco grape pomace revalue. J. Food Meas. Charact. 2022, 16, 3722–3734. [Google Scholar] [CrossRef]
- Marchiani, R.; Bertolino, M.; Belviso, S.; Giordano, M.; Ghirardello, D.; Torri, L.; Piochi, M.; Zeppa, G. Yogurt enrichment with grape pomace: Effect of grape cultivar on physicochemical, microbiological and sensory properties. J. Food Qual. 2016, 39, 77–89. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an organic yogurt based on sensorial, nutritional, and functional perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; de Oliveira, I.C.; Lopes, M.A.; Cruz, A.P.G.; Buriti, F.C.; Cabral, L.M. Addition of grape pomace extract to probiotic fermented goat milk: The effect on phenolic content, probiotic viability and sensory acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef]
- de Azevedo, P.O.D.S.; Aliakbarian, B.; Casazza, A.A.; LeBlanc, J.G.; Perego, P.; de Souza Oliveira, R.P. Production of fermented skim milk supplemented with different grape pomace extracts: Effect on viability and acidification performance of probiotic cultures. PharmaNutrition 2018, 6, 64–68. [Google Scholar] [CrossRef]
- Pistol, G.C.; Marin, D.E.; Dragomir, C.; Taranu, I. Synbiotic combination of prebiotic grape pomace extract and probiotic Lactobacillus sp. reduced important intestinal inflammatory markers and in-depth signalling mediators in lipopolysaccharide-treated Caco-2 cells. Br. J. Nutr. 2019, 121, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.R.; Cabezudo, I.; Galetto, C.S.; Romanini, D. Production of grape pomace extracts with enhanced antioxidant and prebiotic activities through solid-state fermentation by Aspergillus niger and Aspergillus oryzae. Food Biosci. 2021, 42, 101168. [Google Scholar] [CrossRef]
- Costa, J.R.; Amorim, M.; Vilas-Boas, A.; Tonon, R.V.; Cabral, L.M.; Pastrana, L.; Pintado, M. Impact of in vitro gastrointestinal digestion on the chemical composition, bioactive properties, and cytotoxicity of Vitis vinifera L. cv. Syrah grape pomace extract. Food Func. 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Impact of biogenic amines on food quality and safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]


| Compounds | Dry Matter Content * | References |
|---|---|---|
| Moisture | 7.8 ± 4.6 g/100 g | [29,30] |
| Ash | 4.0 ± 1.4 g/100 g | [28,29] |
| Protein | 10.8 ± 5.3 g/100 g | [28,29] |
| Fat | 7.5 ± 0.7 g/100 g | [28,29] |
| Carbohydrates | 25.0 ± 4.2 g/100 g | [30] |
| Total dietary fiber | 45.0 ± 7.1 g/100 g | [29,30] |
| Insoluble fiber | 39.0 ± 32.5 g/100 g | [29,30] |
| Soluble fiber | 7.0 ± 7.1 g/100 g | [29,30] |
| ABTS | 101.5 ± 16.3 µmol TE/g | [6,29,32] |
| DPPH | 96.5 ± 14.8 µmol TE/g | [6,29,32] |
| TPC | 59.5 ± 27.6 mg GAE/g | [29,32] |
| Calcium | 9.9 g/kg | [14,31] |
| Phosphorous | 2.7 g/kg | [14,31] |
| Magnesium | 0.8 g/kg | [14,31] |
| Sodium | 0.22 g/kg | [14,31] |
| Sulfur | 1.5 g/kg | [14,31] |
| Copper | 49.0 mg/kg | [14,31] |
| Zinc | 25.0 mg/kg | [14,31] |
| Iron | 361.0 mg/kg | [14,31] |
| Potassium | 140 mg/kg | [31] |
| Manganese | 13.0 mg/kg | [14,31] |
| AG | AG-Q | NT | NT-Q | CS | ME | IRA | IRB | |
|---|---|---|---|---|---|---|---|---|
| Flavonoids | ||||||||
| Anthocyanins | ||||||||
| Delphinidin-3-O-glucoside | 2356.4 ± 335.2 | 231.1 ± 5.1 | 146.2 ± 2.3 | 1427.9 ± 1630.3 | 71.3 ± 0.7 | / | / | / |
| Cyanidin-3-glucoside | 296.9 ± 1.8 | 51.7 ± 0.1 | 16.6 ± 0.4 | 27.6 ± 1.5 | 50.0 ± 0.7 | / | / | / |
| Petunidin-3-O-glucoside | 4667.4 ± 102.5 | 637.9 ± 1.9 | 365.9 ± 0.4 | 484.0 ± 23.5 | 77.5 ± 0.6 | / | / | / |
| Peonidin-3-glucoside | 3717.3 ± 98.5 | 949.4 ± 8.9 | 127.7 ± 0.6 | 224.8 ± 8.5 | 95.0 ± 1.0 | / | / | / |
| Malvidin-3-O-glucoside | 35,813.3 ± 850.5 | 10,775.9 ± 67.0 | 4908.4 ± 42.3 | 5306.2 ± 482.7 | 669.0 ± 3.9 | / | / | / |
| Vitisin A | 288.3 ± 1.9 | 182.9 ± 7.4 | 120.2 ± 0.3 | 106.6 ± 3.5 | / | / | / | / |
| Peonidin-3-O-acetylglucoside | 340.9 ± 8.5 | 120.7 ± 3.7 | 200.8 ± 3.3 | 240.4 ± 41.6 | / | / | / | / |
| Delphinidin-3-O-p-coumarylglucoside | 546.0 ± 42.1 | 273.4 ± 6.9 | 628.7 ± 2.7 | 646.3 ± 76.8 | / | / | / | / |
| Malvidin-3-O-acetylglucoside | 2210.8 ± 43.1 | 1008.9 ± 14.2 | 3126.2 ± 72.1 | 3150.0 ± 343.2 | / | / | / | / |
| Petunidin-3-O-p-coumarylglucoside | 932.5 ± 28.0 | 607.4 ± 12.6 | 761.5 ± 25.7 | 697.8 ± 27.0 | / | / | / | / |
| Peonidin-3-O-p-coumarylglucoside | 1688.1 ± 35.3 | 1680.9 ± 13.0 | 549.9 ± 10.7 | 637.9 ± 54.7 | / | / | / | / |
| Malvidin-3-O-p-coumarylglucoside | 15,221.2 ± 302.6 | 13,992.5 ± 59.6 | 12,156.2 ± 242.7 | 9843.4 ± 548.5 | / | / | / | / |
| Total | 68,079.2 ± 685.0 | 30,512.8 ± 108.5 | 23,108.3 ± 240.1 | 22,792.9 ± 1140.9 | 962.8 ± 11.3 | / | / | / |
| Flavonols | ||||||||
| Rutin hydrate | 118.5 ± 3.7 | 105.0 ± 0.6 | 113.7 ± 28.9 | 115.2 ± 5.3 | 18.2 ± 0.2 | 9.4 ± 0.1 | 40.3 ± 0.5 | 8.4 ± 0.1 |
| Quercetin-3-glucoside | 31.5 ± 44.5 | 41.7 ± 0.8 | 92.5 ± 5.9 | 106.1 ± 0.1 | 123.0 ± 1.6 | 40.3 ± 0.4 | 234.0 ± 2.8 | 81.7 ± 0.8 |
| Myricetin | 19.6 ± 0.2 | 22.4 ± 0.3 | 29.1 ± 1.3 | 94.7 ± 7.1 | 1627.0 ± 9.3 | / | / | / |
| Quercetin | 14.3 ± 0.1 | 27.4 ± 0.6 | 49.7 ± 3.2 | 91.8 ± 5.2 | 759.0 ± 6.6 | 661.0 ± 5.7 | / | / |
| Kaempferol | 2.9 ± 0.4 | 4.9 ± 0.05 | 9.9 ± 0.7 | 6.9 ± 9.8 | 487.0 ± 4.4 | 473.0 ± 3.3 | / | / |
| Isorhamnetin | 13.6 ± 0.1 | 10.1 ± 0.1 | 18.7 ± 1.1 | 19.9 ± 2.4 | 701.0 ± 4.3 | 606.0 ± 3.0 | / | / |
| Total | 200.4 ± 23.1 | 211.5 ± 0.5 | 313.6 ± 28.6 | 434.6 ± 12.3 | 3715.2 ± 13.8 | 1789.7 ± 39.5 | 274.3 ± 3.1 | 90.1+ ± 0.7 |
| Flavanols | ||||||||
| (+)-Catechin | 20.8 ± 2.6 | 24.2 ± 0.1 | 33.9 ± 0.3 | 47.1 ± 0.4 | 841.0 ± 8.2 | / | 992.0 ± 6.1 | 927.0 ± 9.1 |
| Total | 20.8 ± 2.6 | 24.2 ± 0.1 | 33.9 ± 0.3 | 47.1 ± 0.4 | 841.0 ± 8.2 | / | 992.0 ± 6.1 | 927.0 ± 9.1 |
| Phenolic acid | ||||||||
| Gallic acid | 638.0 ± 29.8 | 579.2 ± 19.5 | 1139.6 ± 19.7 | 1093.7 ± 2.5 | 574.0 ± 8.1 | 607.0 ± 13.0 | 193.0 ± 2.1 | 248.0 ± 3.0 |
| Syringic Acid | 23.7 ± 0.1 | 25.5 ± 0.15 | 20.8 ± 0.2 | 25.7 ± 0.1 | 226.0 ± 2.9 | 432.0 ± 5.0 | / | / |
| Total | 661.7 ± 21.1 | 604.7 ± 13.7 | 1160.5 ± 13.8 | 1119.5 ± 1.8 | 800.0 ± 7.5 | 1039.0 ± 4.0 | 193.0 ± 2.05 | 248.0 ± 3.0 |
| Stilbenes | ||||||||
| trans-Resveratrol | 61.2 ± 18.6 | 34.9 ± 0.2 | 33.93 ± 0.34 | 26.37 ± 1.21 | 9.1 ± 0.1 | / | / | / |
| ԑ-Viniferin | 8.3 ± 0.6 | 7.6 ± 0.1 | / | / | / | / | ||
| Total | 69.5 ± 12.8 | 42.4 ± 0.2 | 33.9 ± 0.2 | 26.4 ± 0.9 | 9.1 ± 0.2 |
| Chardonay a | Chardonay b | Vitis vinifera L. c | Cabernet Sauvignon d | Pinot Noir e | Merlot f | |
|---|---|---|---|---|---|---|
| Glucose | 39.1 | 29.8 | 62.7 | 10.7 | 37.0 | 8.4 |
| Arabinose | 29.8 | 6.4 | 5.5 | 21.2 | 20.4 | 0.6 |
| Mannose | 8.5 | 4.8 | 4.8 | 19.9 | 11.8 | 1.1 |
| Galactose | 14.5 | 3.9 | 4.9 | 15.5 | 8.8 | 1.2 |
| Xylose | 3.5 | 14.1 | 20.4 | 7.7 | 3.0 | 2.1 |
| Rhamnose | 4.6 | 0.1 | 1.7 | 3.8 | 2.0 | – |
| Galacturonic acid | – | 40.7 | – | 21.2 | 17 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. https://doi.org/10.3390/su15119075
Caponio GR, Minervini F, Tamma G, Gambacorta G, De Angelis M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability. 2023; 15(11):9075. https://doi.org/10.3390/su15119075
Chicago/Turabian StyleCaponio, Giusy Rita, Fabio Minervini, Grazia Tamma, Giuseppe Gambacorta, and Maria De Angelis. 2023. "Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects" Sustainability 15, no. 11: 9075. https://doi.org/10.3390/su15119075
APA StyleCaponio, G. R., Minervini, F., Tamma, G., Gambacorta, G., & De Angelis, M. (2023). Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability, 15(11), 9075. https://doi.org/10.3390/su15119075