Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts
Abstract
1. Introduction
2. Materials and Methods
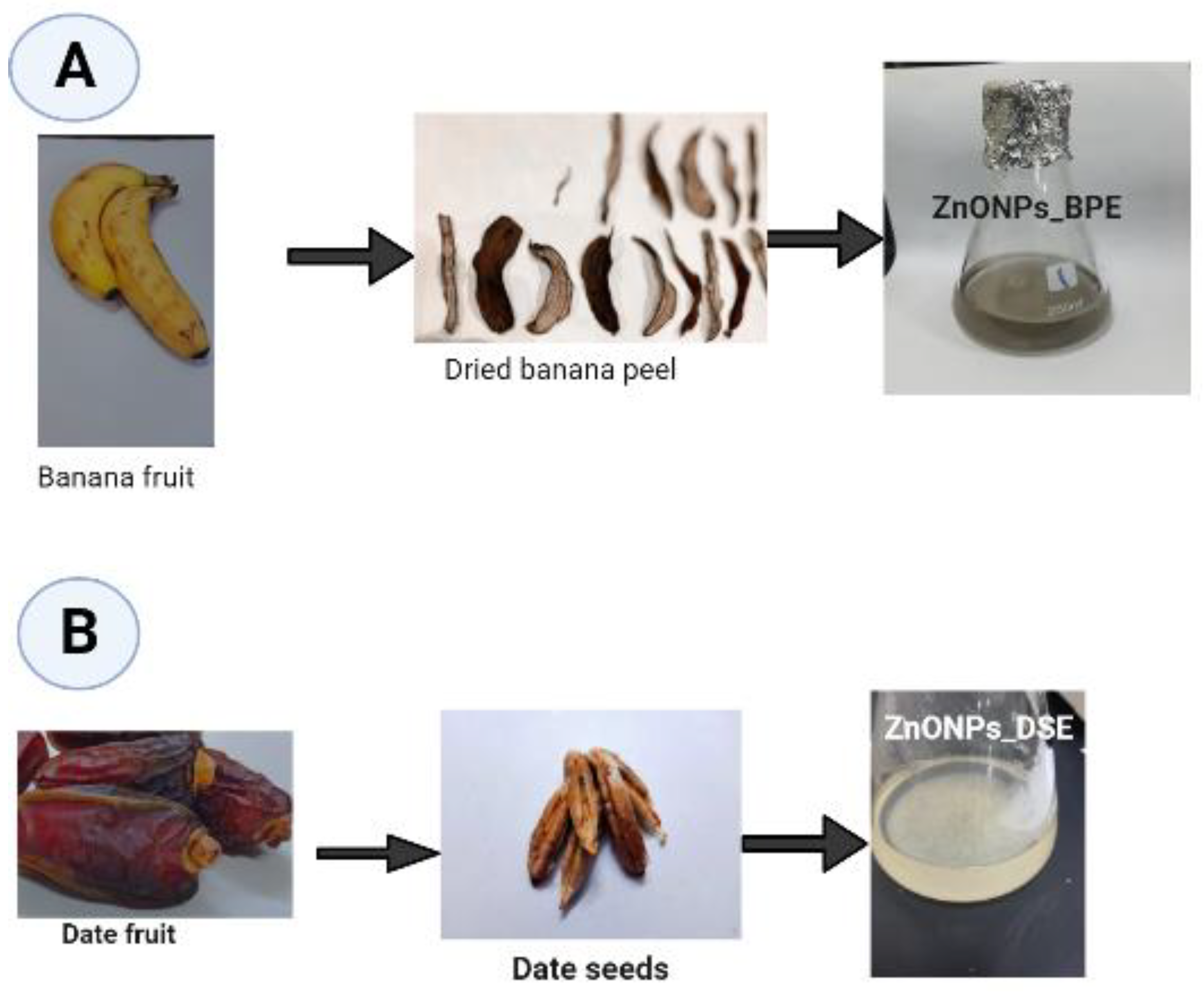
2.1. Banana Peel (BPE) and Date Seed (DSE) Extracts’ Preparation
2.2. Biosynthesis of Zinc Oxide Nanoparticles (ZnONPs)
2.3. Characterization of Prepared ZnONPs
2.3.1. UV–Vis Spectroscopy
2.3.2. Surface Morphology, Charge, and Size Determination
2.3.3. Fourier-Transform Infrared (FTIR) Spectroscopy
2.4. Antimicrobial Assessment
2.4.1. Preparing Inoculum (Colony Suspension Method)
2.4.2. Broth Microdilution Method
2.4.3. Quality Control Results
2.4.4. MIC and MBC Determination
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnONPs
3.1.1. Color Change and UV–Vis Spectroscopy of ZnONPs
3.1.2. Shape, Size, and Surface Charge of Zinc Oxide Nanoparticles
3.1.3. FTIR Analysis
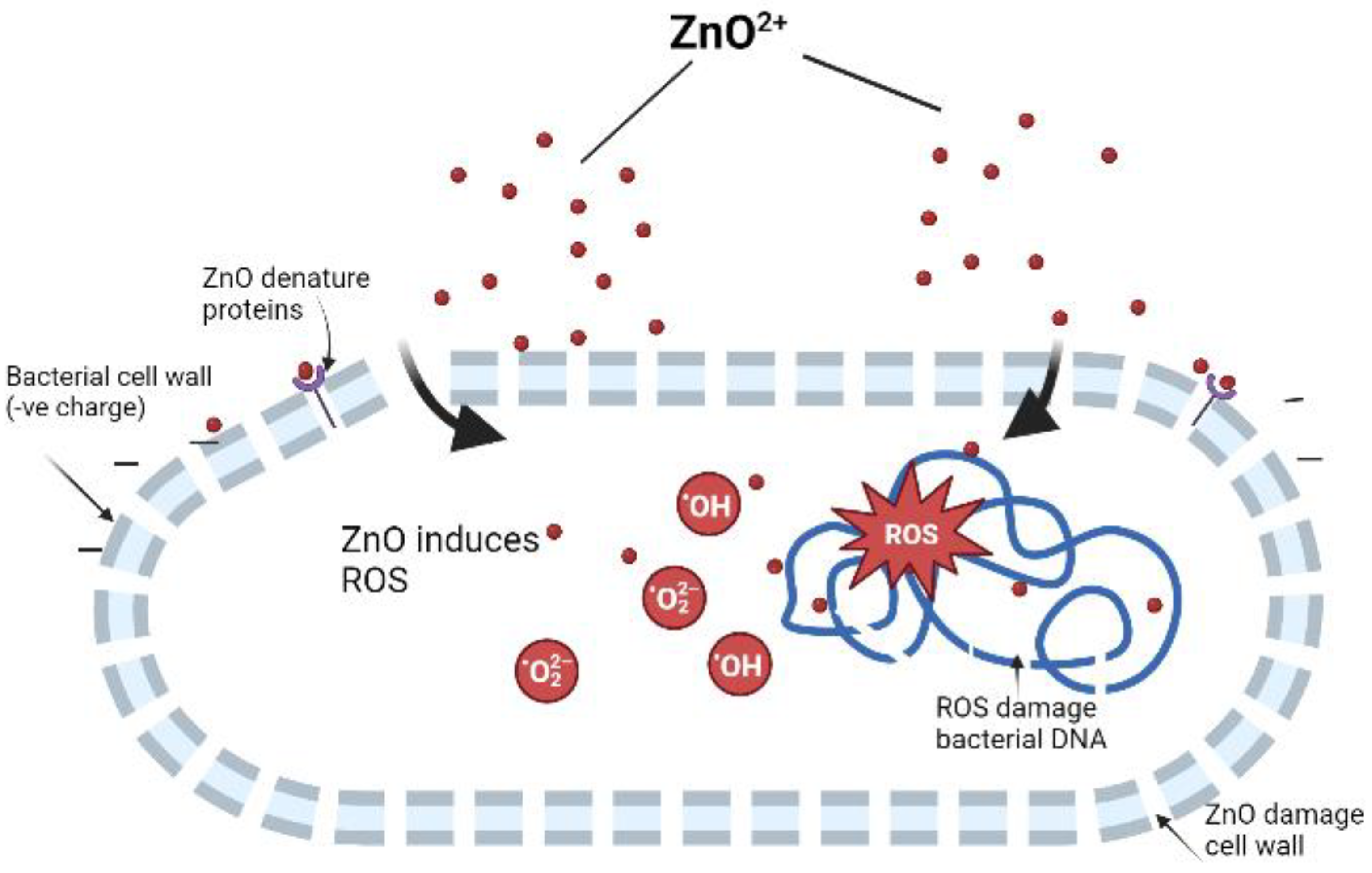
3.2. Antimicrobial Potential of ZnONPs
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharmacol. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Kchaou, M.; Nandee, R.; Shuvho, M.B.A.; Sultana, S. Scope of eco-friendly nanoparticles for anti-microbial activity. Curr. Opin. Green Sustain. Chem. 2021, 4, 100198. [Google Scholar] [CrossRef]
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of nanomaterials insights in green chemistry sustainable route: Review on novel applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Fariq, A.; Khan, T.; Yasmin, A. Microbial synthesis of nanoparticles and their potential applications in biomedicine. J. Appl. Biomed. 2017, 15, 241–248. [Google Scholar] [CrossRef]
- Harshiny, M.; Matheswaran, M.; Arthanareeswaran, G.; Kumaran, S.; Rajasree, S. Enhancement of antibacterial properties of silver nanoparticles-ceftriaxone conjugate through Mukiamaderaspatana leaf extract mediated synthesis. Ecotoxicol. Env. Saf. 2015, 121, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent advances in green synthesis, characterization, and applications of bioactive metallic nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Doble, M.; Kruthiventim, A.K. Green Chemistry and Engineering; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Aguilar, Z. Nanomaterials for Medical Applications; Elsevier: Boston, MA, USA, 2013. [Google Scholar]
- UNEP. Solid Waste Management|UNEP—UN Environment Programme. Available online: https://www.unenvironment.org/explore-topics/resource-efficiency/what-we-do/cities/solid-waste-management (accessed on 10 May 2020).
- Abdelbasir, S.M.; McCourt, K.M.; Lee, C.M.; Vanegas, D.C. Waste-Derived Nanoparticles: Synthesis Approaches, Environmental Applications, and Sustainability Considerations. Front. Chem. 2020, 8, 782. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, N.; Torretta, V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, Q.; Devi, N.; Saini, A.K.; Saini, V.; Pareek, B.; Gaidukovs, S.; Thakur, V.K. Recovery processes of sustainable energy using different biomass and wastes. Renew Sustain. Energy Rev. 2021, 150, 111483. [Google Scholar] [CrossRef]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2022. [Google Scholar] [CrossRef]
- Saffar, R.; Athira, P.V.; Kalita, K.; Manuel, S.G.; Pradeep, N. Nanoparticle synthesis from biowaste and its potential as an antimicrobial agent. Res. Artic. 2021. [Google Scholar] [CrossRef]
- Hassan-Basri, H.; Talib, R.A.; Sukor, R.; Othman, S.H.; Ariffin, H. Effect of synthesis temperature on the size of ZnO nanoparticles derived from pineapple peel extract and antibacterial activity of ZnO–starch nanocomposite films. Nanomaterials 2020, 10, 1061. [Google Scholar] [CrossRef] [PubMed]
- Chankaew, C.; Tapala, W.; Grudpan, K.; Rujiwatra, A. Microwave synthesis of ZnO nanoparticles using longan seeds biowaste and their efficiencies in photocatalytic decolorization of organic dyes. Environ. Sci. Pollut. Res. 2019, 26, 17548–17554. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of nanosilver Particles synthesized by different approaches and their antimicrobial efficacy. J. Nanomater. 2021, 2021, 2204776. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix–xv. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E.D. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Yaseen, T.; Zahra, S.A.; Shahbaz, A.; Shah, S.A.; Uddin, S.; Ma, X.; Raouf, B.; Kanwal, S.; et al. Green synthesis of zinc oxide nanoparticles using Elaeagnus angustifolia L. leaf extracts and their multiple in vitro biological applications. Sci. Rep. 2021, 11, 20988. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, S.; Kaur, G.; Basu, S.; Rawat, M. Biogenic ZnO nanoparticles: A study of blueshift of optical band gap and photocatalytic degradation of reactive yellow 186 dye under direct sunlight. Green Process. Synth. 2019, 8, 272–280. [Google Scholar] [CrossRef]
- Marfu’ah, S.; Rohma, S.M.; Fanani, F.; Hidayati, E.N.; Nitasari, D.W.; Primadi, T.R.; Ciptawati, E.; Sumari, S.; Fajaroh, F. Green Synthesis of ZnO Nanoparticles by Using Banana Peel Extract as Capping agent and Its Bacterial Activity. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012076. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. 2020, 187, 109694. [Google Scholar] [CrossRef] [PubMed]
- Aminuzzaman, M.; Ng, P.S.; Goh, W.-S.; Ogawa, S.; Watanabe, A. Value-adding to dragon fruit (Hylocereus polyrhizus) peel biowaste: Green synthesis of ZnO nanoparticles and their characterization. Inorg. Nano-Met. Chem. 2019, 49, 401–411. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies; Wiley–Interscience Publication: New York, NY, USA, 1980. [Google Scholar]
- Gnanasambandam, P.A.R. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Li, X.F.T.; Yang, H.; Zhao, Y. Novel modified pectin for heavy metal adsorption. China Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Li, Y.; Xia, X.; Hou, W.; Lv, H.; Liu, J.; Li, X. How Effective are Metal Nanotherapeutic Platforms Against Bacterial Infections? A Comprehensive Review of Literature. Int. J. Nanomed. 2023, 18, 1109–1128. [Google Scholar] [CrossRef]
- Mahdavi, B.; Saneei, S.; Qorbani, M.; Zhaleh, M.; Zangeneh, A.; Zangeneh, M.M.; Pirabbasi, E.; Abbasi, N.; Ghaneialvar, H. Ziziphora clinopodioides Lam. leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl. Organomet. Chem. 2019, 33, e5164. [Google Scholar] [CrossRef]
- Devanand Venkatasubbu, G.; Ramakrishnan, V.; Kumar, J. Nanocrystalline hydroxyapatite and zinc-doped hydroxyapatite as carrier material for controlled delivery of ciprofloxacin. 3 Biotech 2011, 1, 173–186. [Google Scholar] [CrossRef]
- Wang, Y.W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- Padam, B.S.; Tin, H.S.; Chye, F.Y.; Abdullah, M.I. Banana by-products: An under utilized renewable food biomass with great potential. J. Food Technol. Res. 2014, 51, 3527–3545. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Encapsulation of natural antioxidant compounds from culinary banana by Cocrystallization. J. Food Process. Preserv. 2017, 41, e13033. [Google Scholar] [CrossRef]
- Colco, R. Gram staining. In Current Protocols in Microbiology; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Norfaradhiah, R.; Rapeah, S. Antibacterial activity of water and methanol extracts of banana pulps against Vibrio cholera. J. Environ. Health 2017, 8, 86–103. [Google Scholar]
- Evbuomwan, L.; Onodje, G.O.; Jacob, I.; Patric, C.E. Evaluating the antibacterial activity of Musa acuminata (banana) fruit peels against multidrug resistant bacterial isolates. Int. J. Nov. Res. Life Sci. 2018, 5, 26–31. [Google Scholar]
- Mostafa, H.S. Banana plant as a source of valuable antimicrobial compounds and its current applications in the food sector. J. Food Sci. 2021, 86, 3778–3797. [Google Scholar] [CrossRef] [PubMed]
- Amira, E.; Behija, S.E.; Beligh, M.; Lamia, L.; Manel, I.; Mohamed, H.; Lotfi, A. Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef]
- Hussain, M.I.; Semreen, M.H.; Shanableh, A.; Khattak, M.N.K.; Saadoun, I.; Ahmady, I.M.; Mousa, M.; Darwish, N.; Radeef, W.; Soliman, S.S.M. Phenolic Composition and Antimicrobial Activity of Different Emirati Date (Phoenix dactylifera L.) Pits: A Comparative Study. Plants 2019, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.A.; Hashim, S.H.; Simarani, K.; Yaacob, J.S. Antibacterial properties and effects of fruit chilling and extract storage on antioxidant activity, total phenolic and anthocyanin content of four date palm (Phoenix dactylifera) cultivars. Molecules 2016, 21, 419. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Marjani, A.; Mousavi, Z. Agricultural waste biomass-assisted nanostructures: Synthesis and application. Green Process. Synth. 2019, 8, 421–429. [Google Scholar] [CrossRef]
- Ghorbani, M.; Biparva, P.; Hosseinzadeh, S. Effect of colloidal silica nanoparticles extracted from agricultural waste on physical, mechanical and antifungal properties of wood polymer composite. Eur. J. Wood Prod. 2018, 76, 749–757. [Google Scholar] [CrossRef]
- Yuan, C.; Lin, H.; Lu, H.; Xing, E.; Zhang, Y.; Xie, B. Synthesis of hierarchically porous MnO2/rice husks derived carbon composite as high-performance electrode material for supercapacitors. Appl. Energy 2016, 178, 260–268. [Google Scholar] [CrossRef]
- Mathur, L.; Hossain, S.K.S.; Majhi, M.R.; Roy, P.K. Synthesis of nano-crystalline forsterite (Mg2SiO4) powder from biomass rice husk silica by solid-state route. Bol. Soc. Esp. Ceram. V 2018, 57, 112–118. [Google Scholar] [CrossRef]
- Lim, J.L.G.; Raman, S.N.; Lai, F.C.; Zain, M.F.M.; Hamid, R. Synthesis of nano cementitious additives from agricultural wastes for the production of sustainable concrete. J. Clean Prod. 2018, 171, 1150–1160. [Google Scholar] [CrossRef]





| Bacterial Strain | ZnONPs_BPE | ZnONPs_DSE | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Escherichia coli ATCC 8739 | 1.5 | >3 | 1.5 | 1.5 |
| Staphylococcus aureus ATCC 6538 | 1.5 | >3 | 0.75 | 3 |
| Bacillus subtilis ATCC 6633 | 1.5 | >3 | 1.5 | 3 |
| Salmonella enteritidis ATCC 13076 | 0.75 | 1.5 | 1.5 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussien, N.A. Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts. Sustainability 2023, 15, 9048. https://doi.org/10.3390/su15119048
Hussien NA. Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts. Sustainability. 2023; 15(11):9048. https://doi.org/10.3390/su15119048
Chicago/Turabian StyleHussien, Nahed Ahmed. 2023. "Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts" Sustainability 15, no. 11: 9048. https://doi.org/10.3390/su15119048
APA StyleHussien, N. A. (2023). Antimicrobial Potential of Biosynthesized Zinc Oxide Nanoparticles Using Banana Peel and Date Seeds Extracts. Sustainability, 15(11), 9048. https://doi.org/10.3390/su15119048






