Particle-Size Effect of Nanoparticles on the Thermal Performance of Solar Flat Plate Technology
Abstract
1. Introduction
2. Effect of Nanoparticle Size (NPS) on Thermophysical Properties
3. Nanofluid Used for Analysis
4. Solar Flat Plate Collector Configuration and Mathematical Modelling
- A steady-state operation of the FPC is assumed in the analysis and discussion;
- There is uniform temperature across the tubes;
- Temperature gradients are treated discretely in the flow direction and along the Y-axis in the absorber plate (along the X-axis);
- There is the assumption of thorough and long-term stability of the nanofluid;
- The heat losses are considered to be occurring from the absorber plate, concerning the bottom, top, and edge of the FPC. In addition, heat loss is occurring at the average temperature of the ambient temperature;
- There is a uniform and unidimensional flow of the heat transfer fluid and heat, respectively.
| Parameters of Flat Plate Collector | Specifications |
|---|---|
| Collector area | 2 m2 |
| Length of plate | 2 m |
| Width of collector plate | 1 m |
| Height of collector plate | 0.15 m |
| Collector tilt angle | 15 |
| Back insulation thickness | 0.05 m |
| Edge insulation thickness | 0.025 m |
| Absorber plate thickness | 0.00045 m |
| Thermal conductivity of absorber plate | 386 W/m k |
| Emissivity of absorber plate | 0.95 |
| Effective transmittance–absorptance product | 0.82 |
| Thickness of glass cover | 0.004 m |
| Absorptivity of glass cover | 0.05 |
| Emissivity of glass cover | 0.88 |
| Tube spacing between risers | 0.095 m |
| Riser pipe’s inner diameter | 0.0095 m |
| Riser pipe’s outer diameter | 0.01 m |
| Diameter of header pipe | 0.0254 m |
4.1. Mathematical Computation of the FPC
4.2. Economic and Environmental Analysis
5. Model Validation
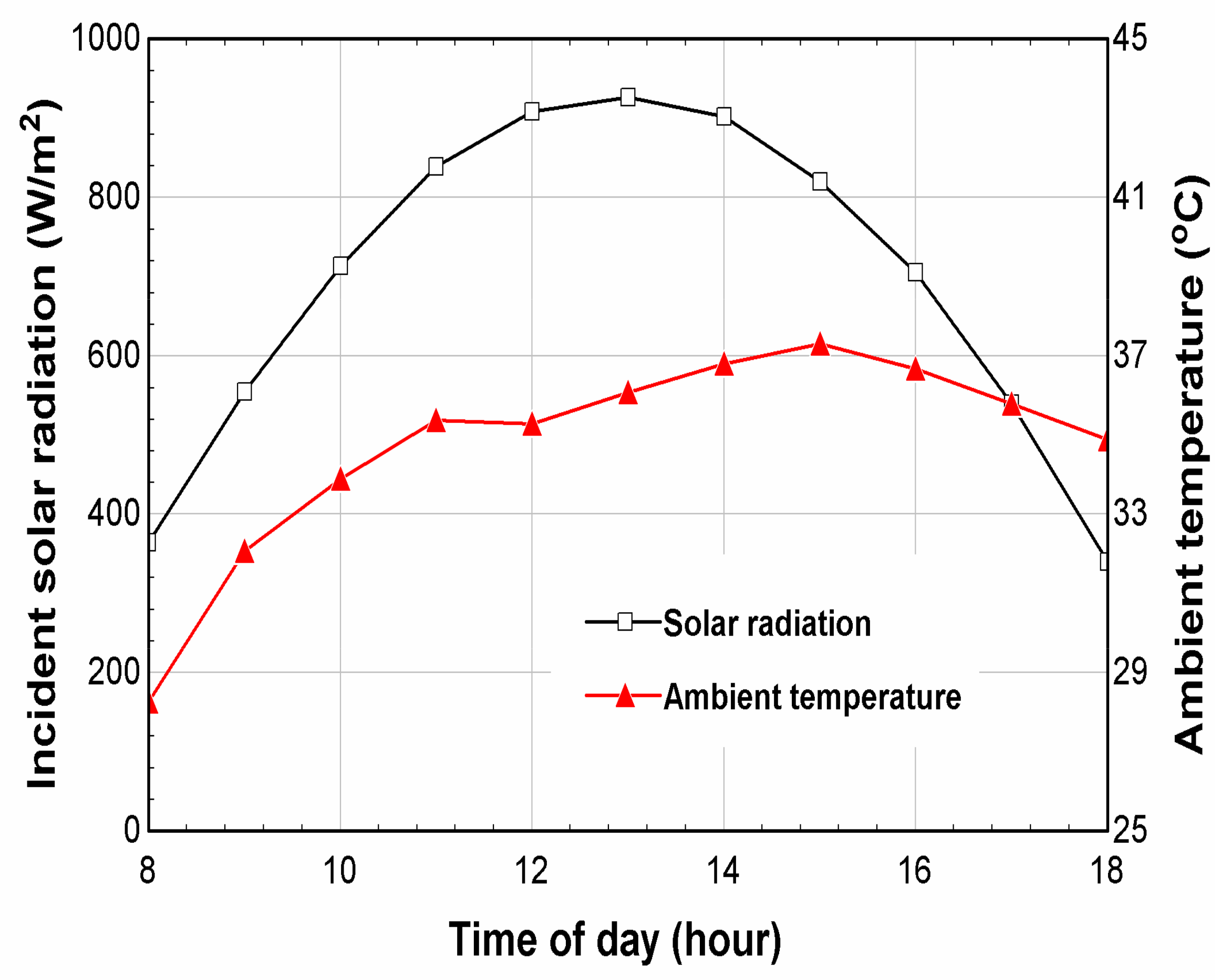
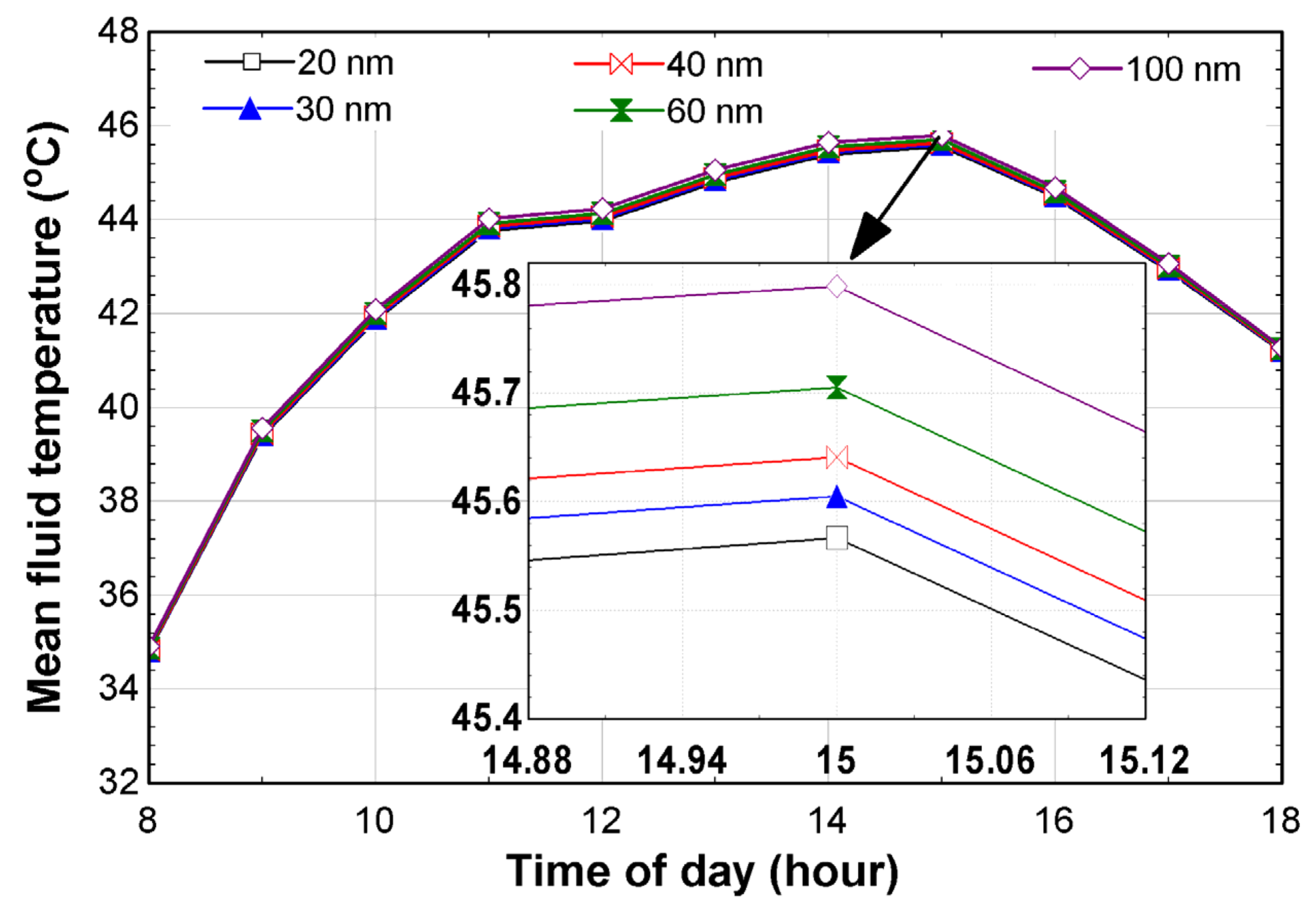
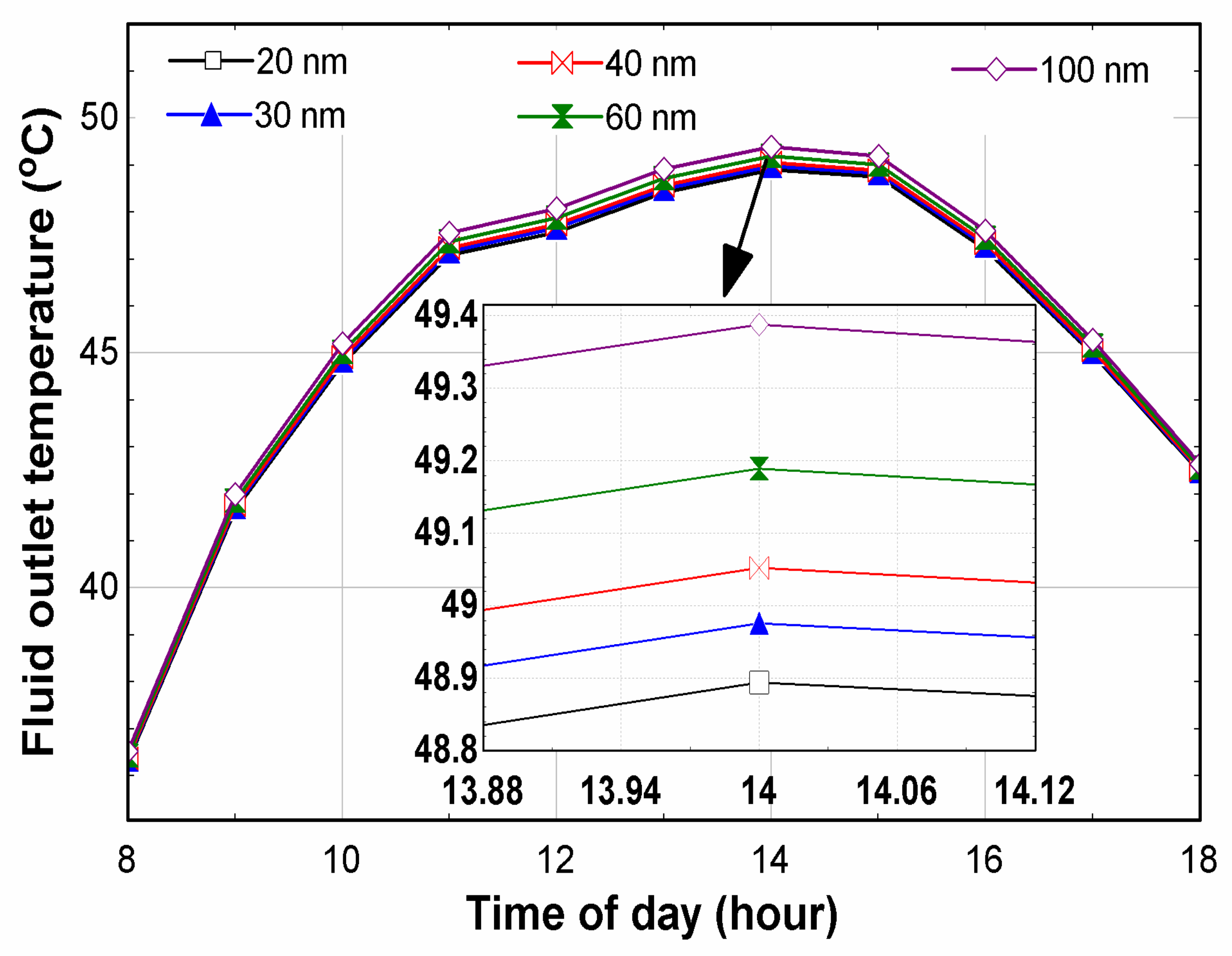
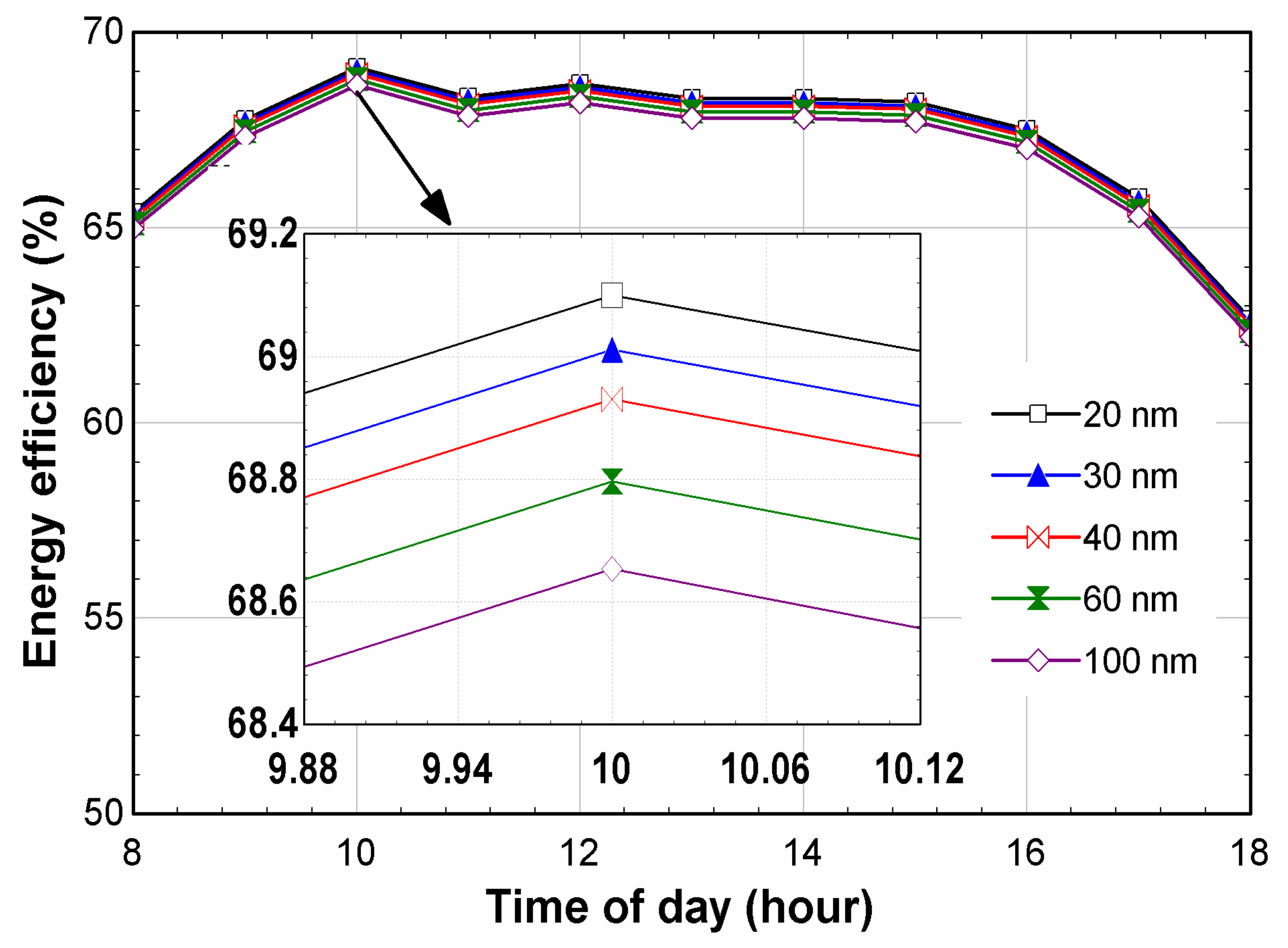
6. Results and Discussion
7. Summary of Findings and Conclusions
- The effect of nanoparticle size on the mean fluid temperature was shown by a maximum temperature of 45.8 °C which was measured for the Ag-100 nm. An increase of 0.25 °C between the Ag-20 nm and Ag-100 nm was calculated;
- The useful heat at 8 am for the Ag-20 nm, Ag-30 nm, Ag-40 nm, Ag-60 nm, and Ag-100 nm was 475.8 kW, 476,2 kW, 475.6 kW, 474.7 kW, and 473.7 kW, respectively. The maximum useful heat (1265 kW) was measured at 1 pm for the Ag-20 nm;
- The maximum energy efficiency, 69.1%, was measured for the Ag-20 nm at 10 am;
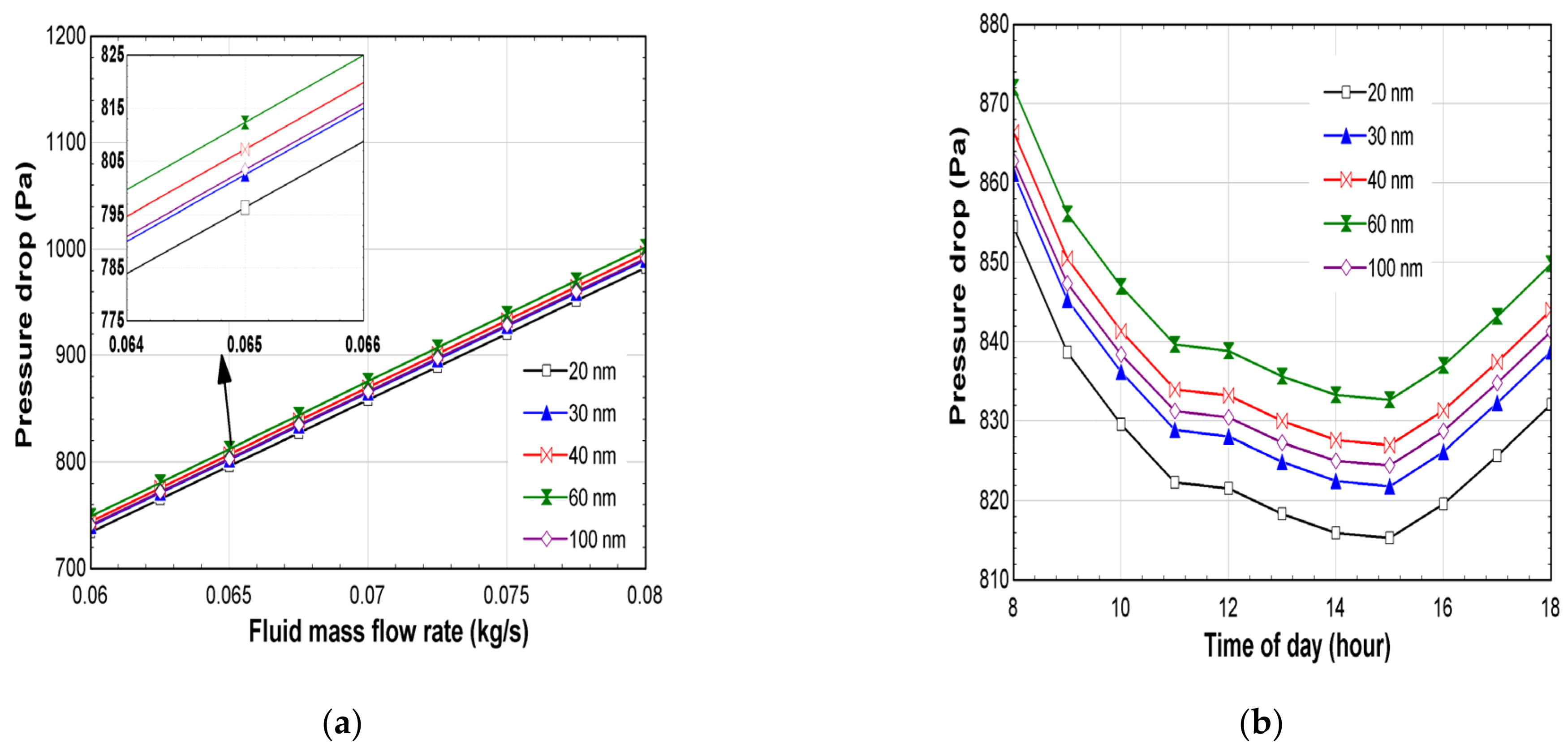
- The lowest pressure drop, 734.3 Pa, which is a net positive for the FPC system, was measured for the Ag-20 nm, while the maximum pressure drop, 1002 Pa, was measured for the Ag-60 nm at 0.08 kg/s;
- Relative to water, the Ag-100 nm resulted in the lowest damage costs: 7.428837, 1.579411, and 4.096075 USD/kg for the CO2, SOx, and NOx, respectively;
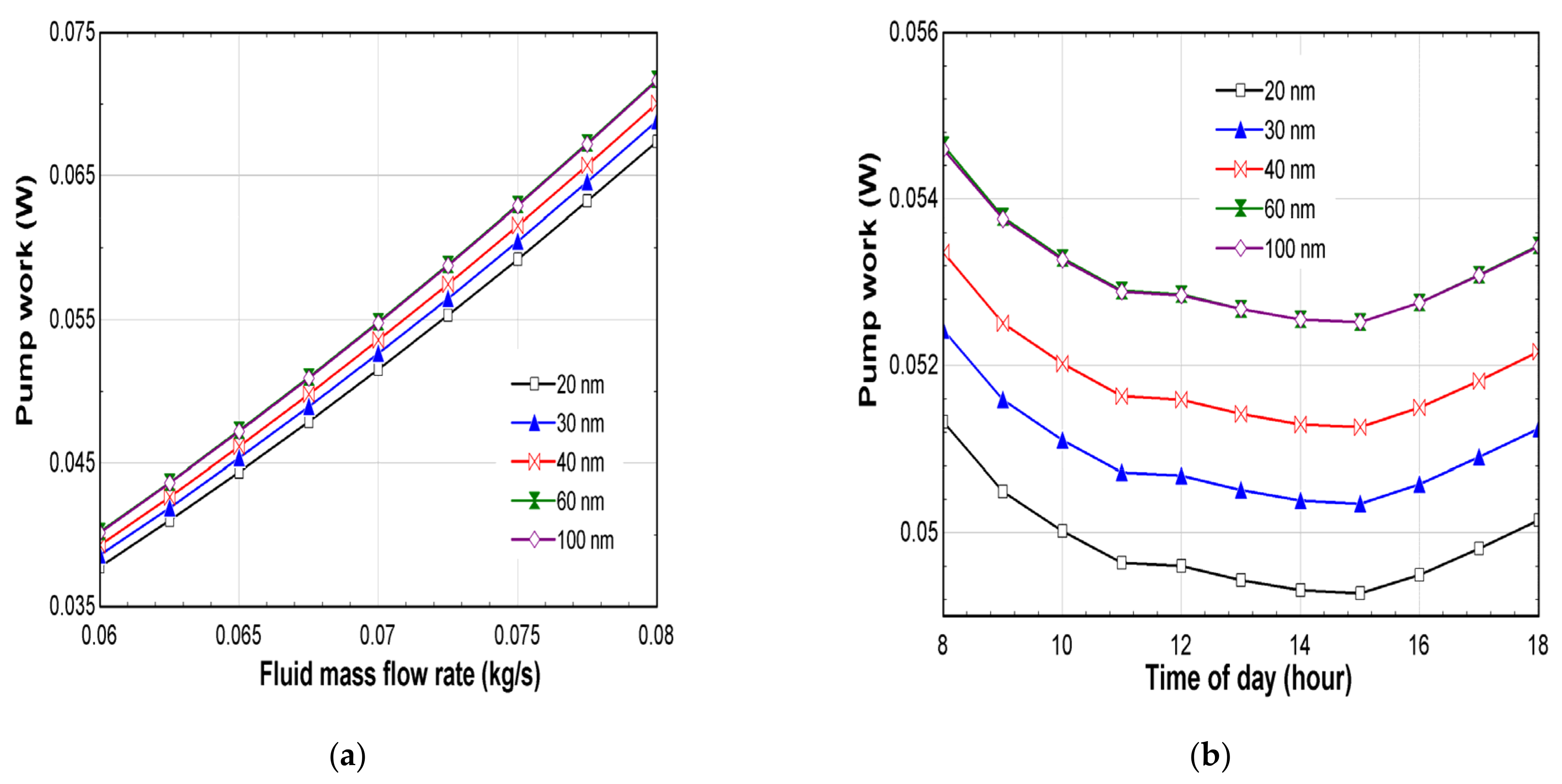
- The maximum pump work was measured for the Ag-60 nm, with 0.072 W.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ΔP | Pressure drop |
| η | Efficiency |
| Ap | Area of aperture |
| Cp | Specific heat capacity |
| EP | Emission production |
| F′ | Coefficient of collector efficiency |
| FPC | Flat plate collector |
| FR | Removal factor |
| hb | Heat transfer coefficient at back of collector |
| Le | Edge thickness |
| m | Mass flow rate |
| Qu | Useful energy |
| S | Solar irradiance |
| Ta | Ambient temperature |
| Tpm | Mean plate temperature |
References
- Wole-Osho, I.; Bamisile, O.; Adun, H.; Yusuf, I. Comparison of renewable energy potential in relation to renewable energy policy in ECOWAS countries. In Proceedings of the IEEE HONET-ICT, Nicosia, Cyprus, 13–14 October 2016; pp. 24–28. [Google Scholar]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Ellabban, O.; Abu-rub, H.; Blaabjerg, F. Renewable energy resources : Current status, future prospects and their enabling technology. Renew. Sustain. Energy Rev. 2014, 39, 748–764. [Google Scholar] [CrossRef]
- Bilen, K.; Ozyurt, O.; Bakırcı, K.; Karslı, S.; Erdogan, S.; Yılmaz, M.; Comaklı, O.K. Energy production, consumption, and environmental pollution for sustainable development : A case study in Turkey. Sustain. Energy Rev. 2008, 12, 1529–1561. [Google Scholar] [CrossRef]
- Heinberg, R. Ur Enewable Future; Island Press: Washington, DC, USA, 2015. [Google Scholar]
- Kohl, W.L. Consumer country energy cooperation: The International Energy Agency and the global energy order. In Global Energy Governance: The New Rules of the Game; Global Public Policy Institute: Berlin, Germany; Brookings Institution Press: Washington, DC, USA, 2010; pp. 195–220. [Google Scholar]
- Gorjian, S.; Ebadi, H.; Calise, F.; Shukla, A.; Ingrao, C. A review on recent advancements in performance enhancement techniques for low-temperature solar collectors. Energy Convers. Manag. 2020, 222, 113246. [Google Scholar] [CrossRef]
- Hottel, B.B.; Woertz, H.C. The performance of flat-plate solar heat collectors. Trans. Am. Soc. Mech. Eng. 2002, 64, 91–103. [Google Scholar]
- Hottel, A.; Whillier, H. Evaluation of flat-plate solar collector performance. Trans. Conf. Use Sol. Energy 1955, 3, 19–41. [Google Scholar]
- Tabarhoseini, S.M.; Sheikholeslami, M.; Said, Z. Solar Energy Materials and Solar Cells Recent advances on the evacuated tube solar collector scrutinizing latest innovations in thermal performance improvement involving economic and environmental analysis. Sol. Energy Mater. Sol. Cells 2022, 241, 111733. [Google Scholar] [CrossRef]
- Murari, K.; Chaurasiya, R. A review on analysis and development of solar fl at plate collector. Renew. Sustain. Energy Rev. 2017, 67, 641–650. [Google Scholar] [CrossRef]
- Al Faris, H.; Khonkar, H. A simulation modeling for optimization of flat plate collector design in Riyadh, Saudi Arabia. Renew. Energy 2003, 28, 1325–1339. [Google Scholar] [CrossRef]
- Beikircher, T.; Möckl, M.; Osgyan, P.; Streib, G. Solar Energy Materials & Solar Cells Advanced solar fl at plate collectors with full area absorber, front side fi lm and rear side vacuum super insulation. Sol. Energy Mater. Sol. Cells 2015, 141, 398–406. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 435873. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal conductivity of nanoparticle-fluid mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Babar, H.; Ali, H.M. Towards hybrid nano fluids: Preparation, thermophysical properties, applications, and challenges. J. Mol. Liq. 2019, 281, 598–633. [Google Scholar] [CrossRef]
- Kumar, N.; Sonawane, S.S.; Sonawane, S.H. Experimental study of thermal conductivity, heat transfer and friction factor of Al2O3 based nanofluid. Int. Commun. Heat Mass Transf. 2017, 90, 1–10. [Google Scholar] [CrossRef]
- Akilu, S.; Baheta, A.T.; Sharma, K.V. Experimental measurements of thermal conductivity and viscosity of ethylene glycol-based hybrid nanofluid with TiO2-CuO/C inclusions. J. Mol. Liq. 2017, 246, 396–405. [Google Scholar] [CrossRef]
- Babu, J.A.R.; Kumar, K.K.; Rao, S.S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Adun, H.; Kavaz, D.; Dagbasi, M.; Umar, H.; Wole-Osho, I. An experimental investigation of thermal conductivity and dynamic viscosity of Al2O3-ZnO-Fe3O4 ternary hybrid nanofluid and development of machine learning model. Powder Technol. 2021, 394, 1121–1140. [Google Scholar] [CrossRef]
- Sahoo, R.R. Thermo-hydraulic characteristics of radiator with various shape nanoparticle-based ternary hybrid nanofluid. Powder Technol. 2020, 370, 19–28. [Google Scholar] [CrossRef]
- Wole-Osho, I.; Okonkwo, E.C.; Kavaz, D.; Abbasoglu, S. An experimental investigation into the effect of particle mixture ratio on specific heat capacity and dynamic viscosity of Al2O3-ZnO hybrid nanofluids. Powder Technol. 2020, 363, 699–716. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Esmaeilzadeh, F.; Wang, X.P. Effects of temperature and particles volume concentration on the thermophysical properties and the rheological behavior of CuO/MgO/TiO2 aqueous ternary hybrid nanofluid: Experimental investigation. J. Therm. Anal. Calorim. 2019, 137, 879–901. [Google Scholar] [CrossRef]
- Moravej, M.; Vahabzadeh, M.; Guan, Y.; Li, L.K.B. Enhancing the e ffi ciency of a symmetric flat-plate solar collector via the use of rutile TiO2-water nano fluids. Sustain. Energy Technol. Assess. 2020, 40, 100783. [Google Scholar] [CrossRef]
- Khetib, Y.; Abo-dief, H.M.; Alanazi, A.K.; Sajadi, S.M. Effect of nanoparticles shape on turbulent nanofluids flow within a solar collector by using hexagonal cross-section tubes. Sustain. Energy Technol. Assess. 2022, 51, 101843. [Google Scholar] [CrossRef]
- Jamal-abad, M.T.; Zamzamian, A.; Imani, E.; Mansouri, M. Experimental Study of the Performance of a Flat-Plate Collector Using Cu—Water Nanofluid. J. Thermophys. Heat Transf. 2013, 27, 756–760. [Google Scholar] [CrossRef]
- Ahmed, M.; Gr, G. Experimental study on the performance of a fl at-plate collector using WO3/Water nano fluids. Energy 2017, 141, 2436–2444. [Google Scholar] [CrossRef]
- Science, E. Potential of size reduction of flat-plate solar collectors when applying MWCNT nanofluid. IOP Conf. Ser. Earth Environ. Sci. 2013, 16, 012004. [Google Scholar] [CrossRef]
- Khetib, Y.; Alzaed, A.; Tahmasebi, A.; Sharifpur, M.; Cheraghian, G. Influence of using innovative turbulators on the exergy and energy efficacy of flat plate solar collector with DWCNTs-TiO2/water nanofluid. Sustain. Energy Technol. Assess. 2022, 51, 101855. [Google Scholar] [CrossRef]
- Abu-hamdeh, N.H.; Alazwari, M.A.; Salilih, E.M.; Sajadi, S.M.; Karimipour, A. Improve the efficiency and heat transfer rate’ trend prediction of a flat-plate solar collector via a solar energy installation by examine the Titanium Dioxide/Silicon Dioxide-water nanofluid. Sustain. Energy Technol. Assess. 2021, 48, 101623. [Google Scholar] [CrossRef]
- Ali, S.A.; Naser, K.A. Improving performance of flat plate solar collector using nanofluid water/zinc oxide. J. Cent. South Univ. 2021, 28, 3391–3403. [Google Scholar]
- Alawi, O.A.; Kamar, H.M.; Mallah, A.R.; Mohammed, H.A.; Sabrudin, M.A.S.; Newaz, K.M.S.; Najafi, G.; Yaseen, Z.M. Experimental and Theoretical Analysis of Energy Efficiency in a Flat Plate Solar Collector Using Monolayer Graphene Nanofluids. Sustainability 2021, 13, 5416. [Google Scholar] [CrossRef]
- Alawi, O.A.; Mohamed, H.; Mallah, A.R.; Kazi, S.N.; Azwadi, N.; Sidik, C. Thermal efficiency of a flat-plate solar collector filled with Pentaethylene Glycol-Treated Graphene Nanoplatelets : An experimental analysis. Sol. Energy 2019, 191, 360–370. [Google Scholar] [CrossRef]
- Alawi, O.A.; Kamar, H.M.; Mallah, A.R.; Mohammed, H.A.; Sabrudin, M.A.S.; Hussein, O.A.; Kazi, S.N.; Najafi, G. Graphene Nanoplatelets Suspended in Different Basefluids Based Solar Collector: An Experimental and Analytical Study. Processes 2021, 9, 302. [Google Scholar] [CrossRef]
- Stalin, P.M.J.; Arjunan, T.V.; Matheswaran, M.M.; Sadanandam, N. Experimental and theoretical investigation on the effects of lower concentration CeO2/water nanofluid in flat-plate solar collector. J. Therm. Anal. Calorim. 2019, 135, 29–44. [Google Scholar] [CrossRef]
- Yousefi, T.; Shojaeizadeh, E.; Veysi, F.; Zinadini, S. An experimental investigation on the effect of pH variation of MWCNT-H 2O nanofluid on the efficiency of a flat-plate solar collector. Sol. Energy 2012, 86, 771–779. [Google Scholar] [CrossRef]
- Youse, T.; Veysi, F.; Shojaeizadeh, E.; Zinadini, S. An experimental investigation on the effect of Al2O3-H2O nanofluid on the efficiency of flat-plate solar collectors. Renew. Energy 2012, 39, 293–298. [Google Scholar] [CrossRef]
- Akram, N.; Montazer, E.; Kazi, S.N.; Soudagar, M.E.M.; Ahmed, W.; Zubir, M.N.M.; Afzal, A.; Muhammad, M.R.; Ali, H.M.; Márquez, F.P.G.; et al. Experimental investigations of the performance of a flat-plate solar collector using carbon and metal oxides based nano fluids. Energy 2021, 227, 120452. [Google Scholar] [CrossRef]
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U.; Chon, C.H.; Kihm, K.D. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107. [Google Scholar] [CrossRef]
- Teng, T.; Hung, Y.; Teng, T.; Mo, H.; Hsu, H. The effect of alumina/water nano fl uid particle size on thermal conductivity. Appl. Therm. Eng. 2010, 30, 2213–2218. [Google Scholar] [CrossRef]
- Chopkar, I.; Sudarshan, S.; Das, P.K.; Manna, I. Effect of particle size on thermal conductivity of nanofluid. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2008, 39, 1535–1542. [Google Scholar] [CrossRef]
- Beck, M.P.; Yuan, Æ.Y.; Warrier, P.; Teja, Æ.A.S. The effect of particle size on the thermal conductivity of alumina nanofluids. 2009, 2009, 1129–1136. J. Nanoparticle Res. 2009, 2009, 1129–1136. [Google Scholar] [CrossRef]
- Warrier, P.; Teja, A. Effect of particle size on the thermal conductivity of nanofluids containing metallic nanoparticles. Nanoscale Res. Lett. 2011, 6, 247. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Maré, T.; Boucher, E.; Mintsa, H.A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Lu, W.-Q.; Fan, Q.-M. Fan Study for the particle’s scale effect on some thermophysical properties of nanofluids by a simplified molecular dynamics method. Eng. Anal. Bound. Elem. 2008, 32, 282–289. [Google Scholar] [CrossRef]
- Dhairiyasamy, R.; Saleh, B.; Govindasamy, M.; Ayman, A.; Afzal, A.; Abdelrhman, Y. Effect of particle size on thermophysical and heat transfer properties of Ag nanofluid in a radiator—An experimental investigation. Inorg. Nano-Metal Chem. 2021, 53, 78–92. [Google Scholar] [CrossRef]
- Mustafa, J.; Alqaed, S.; Sharifpur, M. Evaluation of energy efficiency, visualized energy, and production of environmental pollutants of a solar flat plate collector containing hybrid nanofluid. Sustain. Energy Technol. Assess. 2022, 53, 102399. [Google Scholar] [CrossRef]
- Joseph, P.M.; Arjunan, S.T.V.; Dolli, M.M.M.H. Energy, economic and environmental investigation of a flat plate solar collector with CeO2/water nanofluid. J. Therm. Anal. Calorim. 2020, 139, 3219–3233. [Google Scholar] [CrossRef]
- Fudholi, A.; Sopian, K. A review of solar air flat plate collector for drying application. Renew. Sustain. Energy Rev. 2019, 102, 333–345. [Google Scholar] [CrossRef]
- Elminir, H.K.; Ghitas, A.E.; El-hussainy, F.; Hamid, R. Optimum solar flat-plate collector slope: Case study for Helwan, Egypt. Energy Convers. Manag. 2006, 47, 624–637. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K.; Chauhan, D.S. Experimental evaluation of flat plate solar collector using nanofluids. Energy Convers. Manag. 2017, 134, 103–115. [Google Scholar] [CrossRef]
- Duffie, N.; Beckman, J.A.; Blair, W.A. Solar Engineering of Thermal Processes, Photovoltaics and Wind; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Dominguez, J. Solar Energy Engineering Processes and Systems, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sarsam, W.S.; Kazi, S.N.; Badarudin, A. Thermal performance of a flat-plate solar collector using aqueous colloidal dispersions of multi-walled carbon nanotubes with different outside diameters. Exp. Heat Transf. 2022, 35, 258–281. [Google Scholar] [CrossRef]
- Amin, T.E.; Roghayeh, G.; Fatemeh, R.; Fatollah, P. Evaluation of nanoparticle shape effect on a nanofluid based flat-plate solar collector efficiency. Energy Explor. Exploit. 2015, 33, 659–676. [Google Scholar] [CrossRef]
- Dobriyal, R.; Negi, P.; Sengar, N.; Singh, D.B. Materials Today : Proceedings A brief review on solar flat plate collector by incorporating the effect of nanofluid. Mater. Today Proc. 2020, 21, 1653–1658. [Google Scholar] [CrossRef]
- Said, Z.; Sabiha, M.A.; Saidur, R.; Hepbasli, A.; Rahim, N.A.; Mekhilef, S.; Ward, T.A. Performance enhancement of a Flat Plate Solar collector using Titanium dioxide nanofluid and Polyethylene Glycol dispersant. J. Clean. Prod. 2015, 92, 343–353. [Google Scholar] [CrossRef]
- ADUN, H.; Mukhtar, M.; Adedeji, M.; Agwa, T.; Ibrahim, K.H.; Bamisile, O.; Dagbasi, M. Synthesis and Application of Ternary Nanofluid for Photovoltaic-Thermal System: Comparative Analysis of Energy and Exergy Performance with Single and Hybrid Nanofluids. Energies 2021, 14, 4434. [Google Scholar] [CrossRef]
- Suzuki, A. General theory of exergy-balance analysis and application to solar collectors. Energy 1988, 13, 153–160. [Google Scholar] [CrossRef]
- Farahat, S.; Sarhaddi, F.; Ajam, H. Exergetic optimization of flat plate solar collectors. Renew. Energy 2009, 34, 1169–1174. [Google Scholar] [CrossRef]
- Kotas, T.J. The Exergy Analysis Method of Thermal Plant Analysis; Krieger: Melbourne, Australia, 1995. [Google Scholar]
- Ardente, F.; Beccali, G.; Cellura, M.; Lo Brano, V. Life cycle assessment of a solar thermal collector. Renew. Energy 2005, 30, 1031–1054. [Google Scholar] [CrossRef]
- Adun, H.; Adedeji, M.; Ruwa, T.; Senol, M.; Kavaz, D. Energy, exergy, economic, environmental (4E) approach to assessing the performance of a photovoltaic-thermal system using a novel ternary nanofluid. Sustain. Energy Technol. Assess. 2022, 50, 101804. [Google Scholar] [CrossRef]
- Steen, M. Greenhouse Gas Emissions from Fossil Fuel Fired Power Generation Systems; Institute for Advanced Materials, Joint Research Centre, European Commission: Ispra, VA, Italy, 2000. [Google Scholar]
- Akhtar, N.; Mullick, S.C. International Journal of Heat and Mass Transfer Effect of absorption of solar radiation in glass-cover (s) on heat transfer coefficients in upward heat flow in single and double glazed flat-plate collectors. Int. J. Heat Mass Transf. 2012, 55, 125–132. [Google Scholar] [CrossRef]
- Zayed, M.E.; Zhao, J.; Du, Y.; Kabeel, A.E.; Shalaby, S.M. Factors a ff ecting the thermal performance of the fl at plate solar collector using nano fluids : A review. Sol. Energy 2019, 182, 382–396. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based α-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles transfer study of dispersed fluids with submicron metallic oxide. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Torii, W.J.; Yang, S. Heat transfer augmentation of aqueous suspensions of nanodiamonds in turbulent pipe flow. J. Heat Transfer 2009, 131, 043203. [Google Scholar] [CrossRef]
- Naveen, N.S.; Kishore, P.S.; Bargar, H.E.; Das, D.K. Convective Heat Transfer and Fluid Dynamic Characteristics of SiO2 Ethylene Glycol/Water Nanofluid Convective Heat Transfer and Fluid Dynamic Characteristics of SiO2—Ethylene Glycol/Water Nanofluid. Heat Transf. Eng. 2010, 29, 1027–1035. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Investigation on convective heat transfer and flow features of nanofluids. J. Heat Transfer 2003, 125, 151–155. [Google Scholar] [CrossRef]
- He, Y.; Jin, Y.; Chen, H.; Ding, Y.; Cang, D.; Lu, H. Heat transfer and flow behaviour of aqueous suspensions of TiO2 nanoparticles (nanofluids) flowing upward through a vertical pipe. Int. J. Heat Mass Transf. 2007, 50, 2272–2281. [Google Scholar] [CrossRef]
- Kanti, P.; Sharma, K.V.; Raja Sekhar, Y. Influence of particle size on thermal conductivity and dynamic viscosity of water-based Indian coal fly ash nanofluid. Heat Transfer 2022, 51, 413–433. [Google Scholar] [CrossRef]
- Alim, M.A.; Abdin, Z.; Saidur, R.; Hepbasli, A.; Khairul, M.A.; Rahim, N.A. Analyses of entropy generation and pressure drop for a conventional flat plate solar collector using different types of metal oxide nanofluids. Energy Build. 2013, 66, 289–296. [Google Scholar] [CrossRef]
- Majdi, H.; Abed, A.M. Effect of nanofluids on the performance of corrugated channel within out-of-phase arrangement. Int. Int. J. Sci. Technol. Res. 2014, 3, 220–229. [Google Scholar]
- Asif, M.; Dhiman, A.K. Effect of alumina nanoparticle shape in a triangular porous array of heated periodic pin-fins. Korean J. Chem. Eng. 2021, 38, 704–715. [Google Scholar] [CrossRef]




| Reference | Nanofluid Used | Nanoparticle Size | Volume Concentration | Result Obtained |
|---|---|---|---|---|
| Omer et al. [32] | Covalently functionalized graphene (Gr) | 289.6 nm | 0.025%-wt., 0.05%-wt., 0.075%-wt. | The optimum removal factor determined was 11.67%, using a volume concentration of 0.1 wt%. |
| Omer et al. [33] | Glycol-treated graphene nanoplatelets | - | 0.025, 0.05, 0.075, and 0.1 wt% | When the flow rate was held constant at 0.025 kg/s and the weight concentration was 0.1 wt%, the largest improvement in solar collector thermal performance was 13.3% |
| Omer et al. [34] | Graphene/EG; Graphene/water; Graphene/DW: EG (70:30), DW: EG (50:50) | - | 0.025, 0.05, 0.075, and 0.1 wt% | The maximum heat gain was measured for the nanofluid with a DW:EG ratio of (70:30) for all volume concentrations |
| Stalin et al. [35] | CeO2/water | 25 nm | 0.01% | 78.2% maximum efficiency and 21.5% enhancement against water |
| Yousefi et al. [36] | MWCNT–H2O | - | 0.2 wt.% | According to the experimental findings, the greater the discrepancies between the pH of the nanofluid and the pH of the isoelectric point, the greater the increase in collector efficiency. |
| Tooraj et al. [37] | Al2O3/water | 15 nm | 0.2% and 0.4% | The maximum efficiency enhancement was 28.3% against water, with volume concentration of 0.2%. |
| Naveed et al. [38] | f-GNPs, SiO2, and ZnO | ZnO-50 nm SiO2-10–20 nm | 0.025 wt.%, 0.05 wt.%, 0.075 wt.% and 0.1 wt.% | The results show that compared to DI water, all nanofluids enhanced the thermal performance of FPSCs. |
| Pollutants | Values |
|---|---|
| Sulphur oxide | 9 g per kWh |
| Nitrogen oxide | 2 g per kWh |
| Carbon dioxide | 644 g per kWh |
| Particles released to the air | 0.7 g per kWh |
| Reference | Volume Fraction % | Mass Flux Rate (kg/s.m2) | Error Percentage | ||
|---|---|---|---|---|---|
| Mahmoud et al. [27] | 0.0167 | 0.0156 | 0.6387 | 0.6377 | 0.1568 |
| 0.0183 | 0.6539 | 0.6535 | 0.0612 | ||
| 0.0195 | 0.6602 | 0.6599 | 0.0454 | ||
| 0.0333 | 0.0156 | 0.6585 | 0.6582 | 0.0455 | |
| 0.0183 | 0.6835 | 0.6821 | 0.2052 | ||
| 0.0195 | 0.6966 | 0.6960 | 0.0862 |
| Reference | Mass Flow Rate (kg/min) | Volume Concentration | Error Percentage | ||
|---|---|---|---|---|---|
| Alawi et al. [33] | 0.5 | 0.025 | 0.706 | 0.705 | 0.1418 |
| 0.05 | 0.721 | 0.721 | 0 | ||
| 0.075 | 0.728 | 0.725 | 0.4138 | ||
| 0.1 | 0.735 | 0.730 | 0.6849 | ||
| 1 | 0.025 | 0.722 | 0.719 | 0.4172 | |
| 0.05 | 0.737 | 0.735 | 0.2721 | ||
| 0.075 | 0.745 | 0.742 | 0.4043 | ||
| 0.1 | 0.752 | 0.750 | 0.2667 |
| Reference | Mass Flow Rate (kg/min) | Volume Concentration | Experimental Result (Outlet Temp) | Modelled Result (Outlet Temperature) | Error Percentage |
|---|---|---|---|---|---|
| [4] | 0.5 | 1 | 49.6 | 49.7 | 0.2 |
| 3 | 50.4 | 51.2 | 0.39 | ||
| 5 | 51.6 | 51.5 | 0.19 |
| Water | 20 nm | 30 nm | 40 nm | 60 nm | 100 nm | |
|---|---|---|---|---|---|---|
| Size reduction (%) | 16.81 | 17.07 | 17.35 | 17.75 | 18.30 | |
| Embodied energy (MJ) | 1183 | 984.14 | 816.15 | 674.54 | 554.81 | 453.28 |
| Emission (kg) | ||||||
| Carbon dioxide (CO2) | 718.081 | 597.372 | 495.400 | 409.448 | 336.771 | 275.142 |
| Sulphur oxides (SOx) | 0.3667 | 0.3051 | 0.2530 | 0.2091 | 0.1720 | 0.1405 |
| Nitrogen oxides (NOx) | 0.6270 | 0.5216 | 0.4326 | 0.3575 | 0.2941 | 0.2402 |
| Cost (USD/kg) | Water | 20 nm | 30 nm | 40 nm | 60 nm | 100 nm | |
|---|---|---|---|---|---|---|---|
| Carbon dioxide (CO2) | 0.027 | 19.38819 | 16.12903 | 13.37581 | 11.0551 | 9.092822 | 7.428837 |
| Sulphur oxides (SOx) | 11.24 | 4.122045 | 3.429133 | 2.843776 | 2.350385 | 1.93319 | 1.579411 |
| Nitrogen oxides (NOx) | 17.05 | 10.69018 | 8.893161 | 7.375097 | 6.095511 | 5.01357 | 4.096075 |
| Total (USD) | 34.20041 | 28.45133 | 23.59468 | 19.501 | 16.03958 | 13.10432 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adun, H.; Adedeji, M.; Titus, A.; Mangai, J.J.; Ruwa, T. Particle-Size Effect of Nanoparticles on the Thermal Performance of Solar Flat Plate Technology. Sustainability 2023, 15, 5271. https://doi.org/10.3390/su15065271
Adun H, Adedeji M, Titus A, Mangai JJ, Ruwa T. Particle-Size Effect of Nanoparticles on the Thermal Performance of Solar Flat Plate Technology. Sustainability. 2023; 15(6):5271. https://doi.org/10.3390/su15065271
Chicago/Turabian StyleAdun, Humphrey, Michael Adedeji, Ayomide Titus, Joakim James Mangai, and Tonderai Ruwa. 2023. "Particle-Size Effect of Nanoparticles on the Thermal Performance of Solar Flat Plate Technology" Sustainability 15, no. 6: 5271. https://doi.org/10.3390/su15065271
APA StyleAdun, H., Adedeji, M., Titus, A., Mangai, J. J., & Ruwa, T. (2023). Particle-Size Effect of Nanoparticles on the Thermal Performance of Solar Flat Plate Technology. Sustainability, 15(6), 5271. https://doi.org/10.3390/su15065271






