Abstract
This study describes the use of banana peel (BPEs) and date seed extracts (DSEs) as waste products in the sustainable and eco-friendly biological synthesis of zinc oxide nanoparticles (ZnONPs). ZnONPs_BPE and ZnONPs_DSE were characterized using an ultraviolet-visible spectrophotometer (UV-VIS), Scanning (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), dynamic light scattering (DLS), zeta potential analysis, and Fourier transform infrared (FTIR) spectroscopy. Moreover, the biocompatibility of ZnONPs was analyzed against the normal human skin fibroblast (HSF) cell line. Peaks of UV spectra were 300 nm and 400 nm for ZnONPs-BPE and for ZnONP _DSE, respectively, confirming the ZnONPs’ formation. XRD revealed their hexagonal structure. SEM showed the nanocrystals of ZnONPs_BPE, which are interlinked to one another in a uniform shape, while ZnONPs_DSE appear as large and small chunky crystals. The mean size of ZnONPs_BPE and ZnONPs_DSE was 50 nm and 62 nm using TEM, respectively. On the contrary, their mean size was bigger using DLS with the zeta potential of ZnONPs_BPE = −12.7 mV and ZnONPs_DSE = −5.69 mV. The FTIR analysis demonstrated the presence of carboxyl, hydroxyl, and C–H of cellulose, hemicelluloses, and lignin polymers on ZnONPs surfaces that act as reducing, capping, and stabilizing agents. ZnONPs_BPE (IC50 > 100) have lower cytotoxic effects on HSF cells than ZnONPs_DSE (IC50 = 29.34 μg/mL). The present study indicates the successful synthesis of ZnONPs using agro-wastes that could help in waste management and recycling. Furthermore, ZnONPs_BPE is safe to use for further applications.
1. Introduction
Currently, zinc oxide nanoparticles (ZnONPs) represent important metal oxide NPs which have gained a scientific spotlight due to their unique characteristics. ZnONPs act as a multi-functional agent capable of catalysis, medicinal effects, antimicrobial effects, UV filtration, photochemical capability, and wastewater remediation [1]. Due to their superior characteristics, ZnONPs are widely used as a sunscreen, food additive, food packaging, dietary supplement, and semiconductor material. In addition, ZnONPs are expected to be used for biomedical applications, such as drug delivery, bioimaging, and anticancer agents [2].
Because of the various exposure routes of ZnONPs, they are mainly absorbed as Zn2+ and concentrated in the human liver, kidney, lungs, and spleen. Excess Zn ions generate reactive oxygen species (ROS), in turn inducing oxidative stress that could be a major toxicological mechanism for ZnONPs in different organs [2]. Therefore, ZnONPs must be synthesized in a safe low-toxic way to be used in consumer products and other applications.
There are different methods that are used in the synthesis of ZnONPs, including biological, chemical, hydrothermal, precipitation, microwave, sonication, and solvothermal methods [3]. Although the physical method gives a pure, uniform size and shape of synthesized NPs and does not use toxic chemicals, its disadvantage includes a high cost, high temperature, and it changes NPs’ physicochemical property, and might need exposure to radiation [4]. The hydrothermal synthesis of ZnO nanoparticles involves using high heat (100–1000 °C) and pressure (1–10,000 atm). Although this method produces good-quality crystals, it is expensive and consumes a large amount of energy in production [3]. The solvothermal method is a widely used technique in ZnONPs because it requires a simple setup, relatively low synthesis temperature, and less expensive equipment. On the other hand, this method has disadvantages such as some of the solvents used being toxic and very expensive [3,5]. The chemical synthesis of NPs represents a high-yielding, cost-effective, highly versatile, less dispersed, thermally stable method of NP production that is capable of controlling NP size; however, it gives low purity, uses toxic chemicals, and in turn is hazardous to human beings and the environment [4].
The biosynthetic (green) routes have more advantages over the conventional, physical and chemical, methods [6]. Plant-derived products represent the essential way for ZnONPs green synthesis with or without temperature and co-precipitation agents [7,8]. Phytochemicals, including quinones, organic acids and flavones, and enzymes found in the plant extract facilitate the reduction/oxidation process of the precursor to ZnONPs [7,9]. A wide variety of plant-derived products have been reported for ZnONPs biosynthesis by various studies such as leaves and peels of Punica granatum [10,11], coffee grounds [11], Menta pulegium [12], Costus woodsonii [13], and Phoenix dactylifera waste [14].
The use of agro-waste residues in green nanoparticle synthesis represents a good selection to minimize waste and achieve sustainable development goals. Worldwide, banana represents one of the most important fruits that occupies the fourth world rank of the most significant foodstuffs after rice, corn, and milk [15]. Banana fruit is rich in vitamins, bio-active compounds, starch (13%), crude fat (3.8–11%), crude protein (6–9%), nutrients (K, P, Ca, Mg), cellulose, hemicellulose, and lignin [16]. Banana peel extract contains different concentrations of secondary metabolites such as polyphenols, alkaloids, flavonoids, and saponins. These phytochemical compounds are responsible for the green synthesis of nanoparticles [17]. Banana peel contains a lot of phytonutrients and phytochemicals such as anthocyanins, carotenoids, delphinidin, cyaniding, alpha-carotene, and catecholamines [18,19].
It has been estimated that 23 million date palm trees (19.16%) are present in Saudi Arabia out of a total 120 million on the planet. Date seeds account for 10% of total fruit production, and about 345,000 tons of date palm (23 million trees × 15 kg/tree) are produced per annum in Saudi Arabia [20,21,22]. Generally, palm date seed waste is disposed of in landfills or burned directly on the farm which results in environmental pollution. On the other hand, they comprise important components including cellulose, hemicellulose, and lignin [22]. Date seeds contain a remarkable number of flavonoids, phenolics, anthocyanins, carotenoids, quinones, and sterols, which make them an excellent plant product for ZnONPs synthesis and stabilization [23,24,25].
ZnONPs represent one of the most utilized nanomaterials in consumer products (food packaging and sunscreens) because of their antibacterial potential and superior efficiency in absorbing ultraviolet radiation. Therefore, there is a need to synthesize ZnONPs in an eco-friendly, simple, and sustainable way and to assess their risk to human health. The main objective of the present study is to investigate the use of agro-industrial wastes, banana peel (BPE), and date seed (DSE) extracts, as stabilizing and capping agents for the ZnONPs synthesis, characterization, and then evaluate their cytotoxicity on human skin fibroblast (HSF) cell lines using an (SRB) assay.
2. Materials and Methods
2.1. Banana Peel (BPE) and Date Seed (DSE) Extracts Preparation
Fresh banana and dried date fruits were purchased from a local shop in Taif governate, Saudi Arabia (KSA). Fruits were washed thoroughly using distilled water prior to their use. The peels of banana fruits were isolated and left covered away from the sun until full dryness (for about 48 h). Dried banana peels were ground using a domestic blender into fine powder. On the other hand, date seeds were collected and ground using a Huge Grinder (ALSAIF-ELEC, Model E03407, 220–240 V, 50/60 Hz, 1200 W, Guangdong, China) (Figure 1).
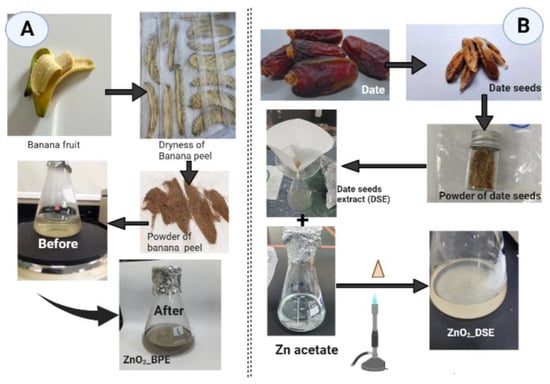
Figure 1.
Schematic illustration of ZnONP preparation using (A) banana peel extract (BPE), and (B) date seed extract (DSE). Created in BioRender.com with agreement number: ZE25G6IWMN, accessed on 4 June 2023.
For each waste product powder, banana peel (BP) and date seed (DS), separately, the following was performed: Each powder was mixed with autoclaved distilled water (with a ratio of 1:10 w/v), boiled for 10 min, filtered, and then cooled down at room temperature for further preparation. Finally, we have obtained banana peel (BPE, pH = 9.91) and date seed (DSE, pH = 6.2) extracts that could be used in ZnO nanoparticle green synthesis.
2.2. Green Synthesis of Zinc Oxide Nanoparticles (ZnONPs)
A total of 95 mL of zinc acetate dihydrate solution 0.01 M Zn(C2H3O2)·2H2O (Sigma-Aldrich, Saint-Louis, MO, USA) was mixed with a volume of 5 mL of each extract, separately. As shown in Figure 1A,B, mixtures were heated with continuous shaking until a color change and powdery precipitates appeared (70 °C, 1 h). For both prepared zinc oxide nanoparticles (ZnONPs), part of them was kept in aqueous form for further ultraviolet-visible (UV-vis) assessment and the rest was transferred to large glass Petri dishes for complete dryness at 60 °C overnight to obtain dry powder for other assessments [11,26].
2.3. Characterization of Prepared ZnONPs
2.3.1. UV–Vis Spectroscopy
The aqueous form of the biologically synthesized ZnONPs_BPE and ZnONPs_DSE nanoparticles was used for UV-vis measurement. The absorption spectra were determined at a wavelength range of 200–800 nm by using a UV–VIS-NIR spectrophotometer (UV-1601, Shimadzu, Kyoto, Japan).
2.3.2. Surface Charge, Morphology, and Size of ZnONPs
Four different techniques were used to determine the size, surface charge, and crystalline shape of the biologically prepared ZnONPs_BPE and ZnONPs_DSE: scanning electron microscopy (SEM), transmission electron microscopy (TEM), dynamic light scattering (DLS), and X-ray diffractometry (XRD). For SEM, the powder of ZnONPs (from both BPE and DSE extracts) was coated with carbon using a Cressington Sputter Coater (108auto, thickness controller MTM-10, UK) for 10 min and then scanned at 20 KV by SEM (JEOL JSM-639OLA, Analytical Scanning Electron Microscope, at Electron microscope unit of Taif University) with magnifications 500× (scale bars = 50 μm), 950× (scale bars = 20 μm), 2000× (scale bars = 10 μm), 2500× (scale bars = 10 μm), 3000× (scale bars = 5 μm), 4000× (scale bars = 5 μm), 5000× (scale bars = 5 μm), and 6000× (scale bars = 2 μm). TEM was used for size measurement, in which images were obtained using a JEOL—JSM-1400 PLUS (JEOL, Tokyo, Japan) at 100 kV. The average size of synthesized ZnONPs was measured from TEM images using ImageJ software. XRD spectra were recorded by CuKα radiation (at 30 kV, and 100 mA) with a wavelength of 1.5406 Å in the 2θ (in the range of 20–80°). The temperature of data collection was 293.00 K. From the X-ray diffraction patterns, the crystalline size was calculated using the Debye–Scherrer equation:
where K is the Scherrer constant, equal to 0.9;
D = Kλ/βcosθ
- λ is the wavelength of the X-rays used, equal to1.5406 Å;
- β is the full width at half maximum (FWHM, in Rad); and θ is the angle at which the refraction takes place [27].
Furthermore, 1 mg of each sample was dispersed in 1 mL of deionized water and then sonicated for 10 min before DLS measurement. The prepared particles were analyzed for their particle size and size distribution in terms of the average volume diameters and polydispersity index by photon correlation spectroscopy using particle size analyzer dynamic light scattering (DLS) (Zetasizer Nano ZN, Malvern Panalytical Ltd., Worcester, UK) at a fixed angle of 173° at 25 °C. The same equipment was used for the determination of zeta potential. Samples were analyzed in triplicate.
2.3.3. Fourier Transforms Infrared Spectroscopy (FTIR)
Fourier transforms infrared spectroscopy (FTIR, Agilent technologies, Santa Clara, CA, USA) at wavelength ranges of 450–4000 cm−1 was used to determine the possible functional groups that are responsible for the synthesis and stabilization of ZnONPs.
2.4. Biocompatibility Assay (SRB)
2.4.1. Cell Culture
Human skin fibroblast (HSF) was obtained from Nawah Scientific Inc., (Mokatam, Cairo, Egypt). Cells were maintained in DMEM medium supplemented with 100 mg/mL of streptomycin, 100 units/mL of penicillin, and 10% of heat-inactivated fetal bovine serum in humidified 5% (v/v) CO2 atmosphere at 37 °C.
2.4.2. Cytotoxicity Assay
Cell viability was assessed by sulforhodamine B (SRB) assay. Aliquots of 100 μL cell suspension (5 × 103 cells) were in 96-well plates and incubated in DMEM medium for 24 h. Cells were treated with another aliquot of 100 μL medium containing nanoparticles (ZnONPs_BPE and ZnONPs_DSE, separately) at various concentrations (0.01–100 μg/mL). After 72 h of nanoparticles exposure, the cells were fixed by replacing the media with 150 μL of 10% trichloroacetic acid (TCA) and were incubated at 4 °C for 1 h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70 μL SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150 μL of TRIS (10 mM) was added to dissolve the protein-bound SRB stain; the absorbance was measured at 540 nm using a BMGLABTECH®—FLUOstar Omega microplate reader (Ortenberg, Germany) [28,29].
3. Results and Discussion
3.1. Characterization of ZnONPs
3.1.1. Color Change and UV–Vis Spectroscopy of Zinc Oxide Nanoparticles
In the present study, we have used the most common agro-wastes worldwide and in Saudi Arabia, banana peel (BPE) and date seed (DSE) extracts, in ZnONP synthesis. Banana occupies the fourth world rank of the most significant foodstuffs [16]. On the other hand, Saudi Arabia has more than 23 million specimens of palm trees [30]. It was reported that KSA palm date fruit production reached about 1,078,300 metric tons in 2010, which represents 14.4% of the world’s production [31]. Therefore, there is a huge amount of banana peel and date seeds that are discarded in the environment. Those agro-wastes could contribute to environmental pollution if not used in recycling or in any eco-friendly sustainable way. BPE and DSE contain different concentrations of phytochemical compounds that can be used in NPs’ green synthesis. Therefore, the use of those wastes in NP synthesis could help in saving raw materials, reducing environmental pollution, waste management, and helping in the synthesis of safe NPs to be used on a large scale.
Visual observation represents the initial confirmation of ZnONP synthesis in the reaction mixture. Prior to incubation, BPE and DSE extracts have a clear color in distilled water, which changed to dark grey and dark yellow, respectively, after Zn2+ addition, which implies its reduction to ZnONPs, as shown in Figure 1. This was evaluated by UV–Vis spectroscopy in the range of 200–800 nm for the suspended nanoparticles. The UV–Vis spectra of ZnONPs_BPE and ZnONPs_DSE showed a sharp peak at 300 nm (Figure 2A) and a broad peak at 400 nm (Figure 2B), respectively, which is the typical characteristic of ZnONPs and confirmed zinc nanoparticle formation. BPE and DSE extracts contain phenolic phytochemicals and flavonoids that are thought to be responsible for Zn2+ reduction to ZnONPs_BPE and ZnONPs_DSE, which was strongly supported by UV-VIS spectroscopy. Likewise, previous studies reported the same range of absorption peaks for ZnONPs biosynthesis with the aid of different plant extracts (such as Cassia fistula, Melia azadarach, Punica granatum, and coffee waste) [11,32,33]. As described by earlier studies, the difference in the UV wavelength of both synthesized ZnONPs could be attributed to their particle size or the capping of phytochemicals from plant extract [34].
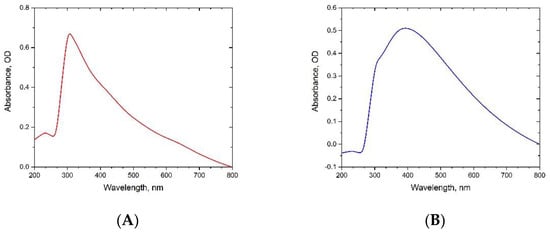
Figure 2.
UV-Visible spectral analysis of green-synthesized ZnONPs_BPE (A) and ZnONPs_DSE (B).
3.1.2. Morphology, Charge, and Size of Zinc Oxide Nanoparticles
The surface morphology of the green-synthesized ZnONPs_BPE and ZnONPs_DSE was studied using SEM, as shown in Figure 3A,B, respectively. The SEM images revealed the presence of nanoparticles’ agglomeration. SEM micrographs showed the nanocrystals of ZnONPs_BPE, which were interlinked to one another in a uniform shape to make large network systems, while SEM images of ZnONPs_DSE showed the formation of large and small chunky crystals of non-homogenized nanoparticles, at different magnifications.
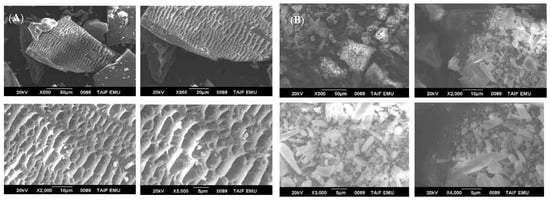
Figure 3.
Scanning electron microscope photos of ZnONPs_BPE (A) and ZnONPs_DSE (B), with different magnifications showing their crystalline shape.
The XRD profiles of ZnONPs_BPE and ZnONPs_DSE showed sharp and distinct peaks of (2θ) 31.7°, 34.4°, 36.2°, 47.5°, 56.6°, 62.8°, 67.9°, and 69.0°, which were indexed as the planes 100, 002, 101,102, 110, 103, 112, and 201 (Figure 4A,B), respectively. These peaks are consistent with wurtzite ZnO according to the Joint Committee on Power Diffraction (JCPD) standards with a card number (36–1451). The XRD pattern revealed the hexagonal lattice structure of the synthesized ZnONPs. On the contrary, other low diffraction peaks could be due to unreacted zinc impurities [35]. The average crystallite size for both ZnONPs_BPE and ZnONPs_DSE was 75.55 nm using the Scherrer equation according to the XRD pattern.
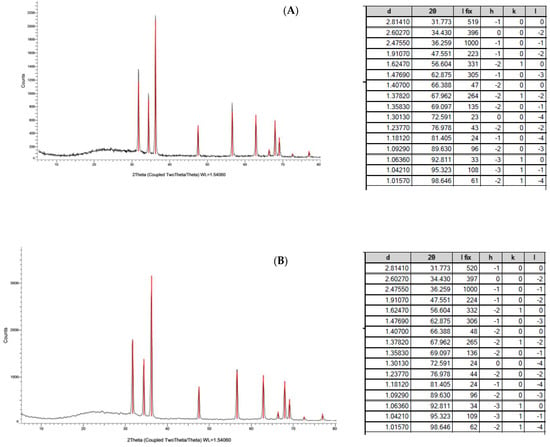
Figure 4.
XRD patterns of green synthesized ZnONPs_BPE (A) and ZnONPs_DSE (B), showing sharp peaks. Table beside each pattern shows their 2 Theta (2θ).
DLS technique was used to determine the hydrodynamic size of synthesized ZnONPs. According to Figure 5, the Z-average size was ZnONPs_BPE = 914.4 nm, ZnONPs_DSE = 2960 nm with a zeta potential of −12.7 mV and −5.69 mV, respectively. On the other hand, TEM images show a smaller size of ZnONPs (Figure 6A,B). The TEM study was conducted to determine the size and crystalline shape of the formed nanoparticles. TEM images of both ZnONPs confirm their spherical shape. The average particle size using TEM images was ZnONPs_BPE = 50 nm and ZnONPs_DSE = 62 nm. From the present data, the TEM analysis revealed a smaller particle size for synthesized ZnONPs in comparison to results obtained from the DLS analysis.
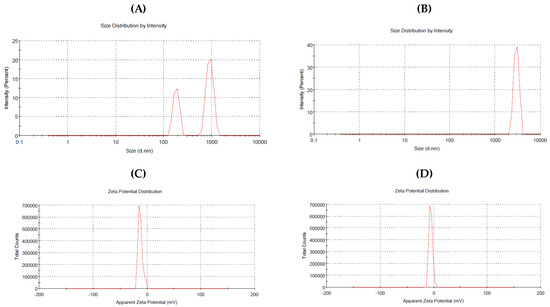
Figure 5.
The average size of biologically synthesized ZnONPs_BPE (A) and ZnONPs_DSE (B) using DLS technique, and the charge on their surfaces (zeta potential) (C,D, respectively).
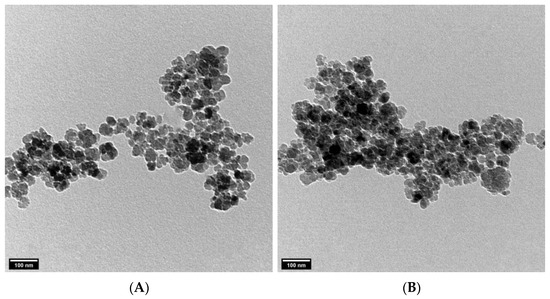
Figure 6.
TEM images of ZnONPs_BPE (A) and ZnONPs_DSE (B) showing their spherical crystalline shape. Scale bar = 100 nm.
In the present study, we have used four different techniques to detect the size, structure, and surface charge of the synthesized zinc oxide nanoparticles. TEM images and XRD patterns showed that the formed ZnONPs were nanocrystalline structures indicating that the used BPE and DSE extracts are responsible for the synthesis, capping, and stabilization of nanoparticles. This was consistent with previous studies that revealed the crystalline shape of the biologically synthesized ZnONPs using various extracts [11,32]. In addition, the hexagonal nanocrystalline with wurtzite structures of the final product was confirmed by the XRD profile that was in good accordance with a card number (36–1451) of JCPD standards, and other previous studies. The size of ZnONPs_BPE and ZnONPs_DSE have slight differences using TEM (50 nm and 62 nm), but there is a large difference between their sizes using DLS (914.4 nm and 2960 nm, respectively). It is reported that nanoparticle size is inversely proportional to their surface negativity (−12.7 mV and −5.69 mV). Low surface negativity leads to a decrease in repulsion force between particles, causing their aggregation into large particles [36]. Moreover, the differences in capping agents that are found in the used extracts (BPE and DSE) could be responsible for the variation in size between ZnONPs_BPE and ZnONPs_DSE [11]. On the other hand, the non-homogeneous distribution of ZnO nanoparticles could be responsible for the detected larger size of ZnONPs using DLS rather than TEM for the same NPs [36]. Moreover, the pH of the used extract affects the size of the formed ZnONPs. Banana peel extract is an alkaline medium with a pH = 9.91, but date seed extract is an acidic medium with a pH = 6.2. Alias et al. have reported that ZnONP powder agglomerates when synthesized in acidic and neutral conditions (pH 6 and 7), but have smaller sizes when synthesized in alkaline media [37].
In previous studies, TEM analysis determined smaller-sized ZnO nanoparticles synthesized from date pulp waste (30 nm) but larger-sized ZnONPs synthesis from banana peels (345.61 ± 39.24 nm), in comparison to the present study [14,38]. The size of the formed NPs was controlled by different factors during synthesis, such as pH, the concentration of zinc acetate, time, and temperature [38,39].
3.1.3. FTIR Analysis
FTIR can identify the functional groups found in the banana peel and date seed extracts that participated in the ZnONP synthesis and their distribution on NPs surfaces. Figure 7A,B illustrate the FTIR absorption spectra of ZnONPs_BPE and ZnONPs_DSE within the range of 4000 to 450 cm−1.
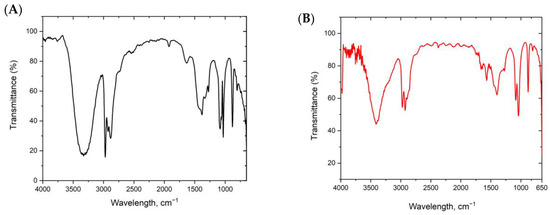
Figure 7.
FTIR spectra of green synthesized ZnONPs_BPE (A) and ZnONPs_DSE (B), showing their functional groups.
Figure 7A shows bands and peaks determined by FTIR analysis of ZnONPs prepared with the help of banana peels. Different peaks indicate the occurrence of multiple functional groups on the surface of NPs. The broad peak at 3640 cm−1 is due to the free hydroxyl group of polymeric compounds. Bands appearing at 3313.4 cm−1 and 2920.3 cm−1 were assigned to the OH stretching and C–H stretching of alkane, respectively. The peak around 1700 cm−1 is attributed to C=O stretching vibrations of carboxylic groups or ester (-COOH, -COOCH3). The peak at around 1580 cm−1 is assigned to the N-H deformation of amines. Sharp peaks at 1460 cm−1 and 1364 cm−1–11,248 cm−1 are assigned to the aromatic ring vibration of lignin, C-H bending of crystalline cellulose and hemicelluloses that are found in the banana peel, while weak bands detected at around 800 cm−1–600 cm−1 were attributed to amine groups [40,41,42,43].
The FTIR spectra for ZnONPs_DSE show many sharp peaks. Peaks at 3367 cm−1, 2924 cm−1, and 2855 cm−1 were assigned to O–H stretching vibrations in hydroxyl groups and asymmetric C–H bands in methyl and methylene groups, respectively. Generally, C–H bands in methyl and methylene groups were attributed to cellulose, hemicellulose, lignin, cutin, and waxes found in the date seed extract. The carbonyl C=O group was represented by a peak at 1744 cm−1 due to either the acetyl and uronic ester groups or the ester linkage of carboxylic groups of the ferulic and p-coumaric acids of hemicelluloses or/and lignin. C=C or C=N vibrations of the aromatic groups are assigned at 1616 cm−1. While C=C stretching of the aromatic skeletal mode was present at 1522 and 1437 cm−1, the peak at 1377 cm−1 is due to cellulose’s C–H stretching. The peak at 1246 cm−1 is assigned to C–O–H deformation and C–O stretching of phenolics groups. The sharp peak at 1061 cm−1 represents hemicellulose and cellulose’s C–O stretching vibration. Finally, the peak at 870 cm−1 represents C–H vibrations of cellulose [44].
3.2. Cytotoxicity Assessment of ZnONPs
Nowadays, it is known that ZnONPs are commonly used in biomedical applications, antibacterial agents, sunscreen, dietary supplements, food additives, and semiconductor materials [2,45]. Therefore, it is important to assess their biocompatibility with biological organisms. In the present study, we have evaluated the cytotoxic potential of ZnONPs_BPE and ZnONPs_DSE on HSF cell lines using an SRB assay. As shown in Figure 8, cell viability significantly decreased after 72 h at concentrations of ZnONPs_DSE in a dose-dependent manner. On the other hand, ZnONPs_BPE treatment does not significantly affect cell viability up to 100 μg/mL. To verify cytotoxicity, the NP concentration that causes 50% growth inhibition of a cell line (IC50) was determined. We have reported that ZnONPs_BPE have lower cytotoxic potentials with IC50 > 100 than ZnONPs_DSE (IC50 = 29.34 μg/mL) (Figure 8).
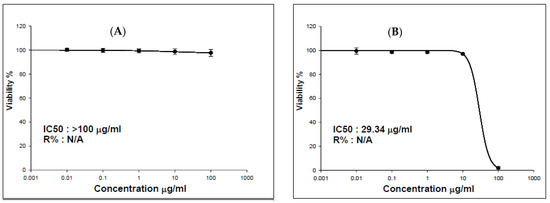
Figure 8.
Percentage of cell viability of HSF according to serial dilutions treatment of ZnONPs_BPE (A) and ZnONPs_DSE (B) after 72 h.
A previous study has revealed that the characteristics of synthesized nanoparticles (size and shape), synthesis methods, and the extract used in capping could affect their cytotoxicity [11]. In agreement with other research articles, biogenic ZnONPs showed high biocompatibility and less toxicity toward normal cells such as normal human HEK-293 cell lines [46] and WI-38 normal lung fibroblast cells [47]. The higher cytotoxic effect of ZnONPs_DSE could be attributed to the type of cell (HSF) that could be sensitive to NPs synthesized using date seed extract. Djaoudene et al. [48] have reported that cell lines such as fibroblast 3T3- L1 are sensitive to DSE at concentrations over 0.25 mg mL−1 using an MTT assay. On the other hand, other studies have reported DSE’s anticancer and antiproliferative potentials in different cancer cell lines [49,50]. These differences may be explained because many studies are performed with purified extracts and not crude extracts as we have done. In addition, the cytotoxic potential of NPs is affected by their size, shape, and surface charge. ZnONPs_DSE exhibited a strong ability to damage the cell membrane of HSF, which in turn caused cell death. ZnONPs_DSE were formed in an acidic medium of the date seed extract (pH = 6.2), which made it more toxic than ZnONPs_BPE (pH = 9.91). Saliani et al. have reported that ZnONPs have a higher toxic effect against bacterial cells (Escherichia coli O157: H7 and Staphylococcus aureus) at acidic pH [51]. Finally, further studies must be conducted to understand the mechanism of action of ZnONPs_BPE and ZnONPs_DSE. In addition, more research studies, such as those concerning antimicrobial and anticancer potential, need to be evaluated for further applications.
4. Conclusions
The present study investigates a cheaper, economical, sustainable, and eco-friendly way to synthesize green ZnONPs using banana peel and date seeds extracts. This way could also help recycle agro-wastes and contribute to waste management. Characterization confirmed their nanocrystalline hexagonal shape. We have reported differences in the shape, size, charge, and, in turn, their cytotoxicity against the normal human skin fibroblast cell line, which could be attributed to the compounds found in the selected extracts. The FTIR technique determined the main functional groups present in BP and DS extracts that are responsible for nanoparticle synthesis, capping, and stabilization. ZnONPs_BPE showed low cytotoxicity against the normal cell line that could be further used in other applications or assessments. This could be attributed to their size, the functional groups that are found on NP surfaces, NP shape, and the surface’s charge. Furthermore, more studies are recommended to determine the cytotoxic mechanism of ZnONPs. Moreover, the antibacterial and anticancer potential of ZnONPs_BPE and ZnONPs_DSE needs to be studied on different cells.
Author Contributions
Conceptualization, N.A.H.; data curation, N.A.H.; funding acquisition, J.S.A.M.; investigation, N.A.H. and J.S.A.M.; methodology, N.A.H., J.S.A.M., F.A.R.A.H., A.W.M. and J.A.A.A.S.; resources, J.S.A.M.; software, N.A.H.; writing—original draft, N.A.H. and J.S.A.M.; writing—review and editing, N.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Z.; Sun, Y.; Xing, J.; Xing, Y.; Meng, A. One step synthesis of Co/Cr-codoped ZnO nanoparticle with superb adsorption properties for various anionic organic pollutants and its regeneration. J. Hazard. Mater. 2018, 352, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Nishimoto, N. Review of Zinc Oxide Nanoparticles: Toxicokinetics, Tissue Distribution for Various Exposure Routes, Toxicological Effects, Toxicity Mechanism in Mammals, and an Approach for Toxicity Reduction. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Droepenu, E.K.; Wee, B.S.; Chin, S.F.; Kok, K.Y.; Maligan, M.F. Zinc Oxide Nanoparticles Synthesis Methods and Its Effect on Morphology: A Review. Biointerface Res. Appl. Chem. 2022, 12, 4261–4292. [Google Scholar]
- Bloch, K.; Pardesi, K.; Satriano, C.; Ghosh, S. Bacteriogenic platinum nanoparticles for application in nanomedicine. Front. Chem. 2021, 9, 624344. [Google Scholar] [CrossRef]
- Zak, A.K.; Razali, R.; Majid, W.H.A.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef]
- Udayabhanu; Nagaraju, G.; Nagabhushana, H.; Suresh, D.; Anupama, C.; Raghu, G.K.; Sharma, S.C. Vitis labruska skin extract assisted green synthesis of ZnO super structures for multifunctional applications. Ceram. Int. 2017, 43, 11656–11667. [Google Scholar] [CrossRef]
- Singh, A.; Gautam, P.K.; Verma, A.; Singh, V.; Shivapriya, P.M.; Shivalkar, S.; Sahoo, A.K.; Samanta, S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020, 25, e00427. [Google Scholar] [CrossRef]
- Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F. Green synthesis of bimetallic copper–silver nanoparticles and their application in catalytic and antibacterial activities. Clean. Technol. Environ. Policy 2020, 22, 269–277. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Process. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Singh, K.; Singh, J.; Rawat, M. Green synthesis of zinc oxide nanoparticles using Punica Granatum leaf extract and its application towards photocatalytic degradation of Coomassie brilliant blue R-250 dye. SN Appl. Sci. 2019, 1, 624. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Hussien, N.A.; Alyamani, A.A.; Morsi, M.M.; AlSufyani, N.M.; Kadi, H.A. Green Synthesis of Zinc Oxide Nanoparticles Using Pomegranate Fruit Peel and Solid Coffee Grounds vs. Chemical Method of Synthesis, with Their Biocompatibility and Antibacterial Properties Investigation. Molecules 2022, 27, 1236. [Google Scholar] [CrossRef]
- Rad, S.S.; Sani, A.M.; Mohseni, S. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb. Pathog. 2019, 131, 239–245. [Google Scholar] [CrossRef]
- Khana, M.M.; Saadaha, N.H.; Khanb, M.E.; Harunsania, M.H.; Tana, A.L.; Cho, M.H. Potentials of Costus woodsonii leaf extract in producing narrow band gap ZnO Nanoparticles. Mater. Sci. Semicond. Process. 2019, 91, 194–200. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Lakshmi, C.M. Production of Ethanol from Banana Waste Plantation by Using Cellulase of Fungal Species Isolated from Banana Plantations in and Around Mysuru District. IJSR 2017, 6, 521–527. [Google Scholar]
- Nik Yusuf, N.A.A.; Rosly, E.S.; Mohamed, M.; Abu Bakar, B.; Yusoff, M.; Sulaiman, M.A.; Ahmad, M.I. Waste Banana Peel and its Potentialization in Agricultural Applications: Morphology Overview. MSF 2016, 840, 394–398. [Google Scholar] [CrossRef]
- Marfu’ah, S.; Rohma, S.M.; Fanani, F.; Hidayati, E.N.; Nitasari, D.W.; Primadi, T.R.; Ciptawati, E.; Sumari, S.; Fajaroh, F. Green Synthesis of ZnO Nanoparticles by Using Banana Peel Extract as Capping agent and Its Bacterial Activity. IOP Conf. Ser. Mater. Sci. Eng. 2020, 833, 012076. [Google Scholar] [CrossRef]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana starch: Production, physicochemical properties, and digestibility—A review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Kanazawa, K.; Sakakibara, H. High content of a dopamine, a strong antioxidant, in Cavendish banana. J. Agric. Food Chem. 2000, 48, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Farooq, A.; Bassyouni, M.I.; Sait, H.H.; El-Wafa, M.A.; Hasan, S.W.; Ani, F.N. Pyrolysis of Saudi Arabian date palm waste: A viable option for converting waste into wealth. Life Sci. J. 2014, 11, 12. [Google Scholar]
- Yahya, S.A.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-Economic Analysis of Fast Pyrolysis of Date Palm Waste for Adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. 2020, 187, 109694. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Bharath, G.; Hai, A.; Rambabu, K.; Banat, F.; Jayaraman, R.; Taher, H.; Bastidas-Oyanedel, J.R.; Ashraf, M.T.; Schmidt, J.E. Systematic production and characterization of pyrolysis-oil from date tree wastes for bio-fuel applications. Biomass Bioenergy 2020, 135, 105523. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of nanosilver Particles synthesized by different approaches and their antimicrobial efficacy. J. Nanomater. 2021, 2021, 12. [Google Scholar] [CrossRef]
- XRD Crystallite (Grain) Size Calculator (Scherrer Equation)—InstaNANO. Available online: https://instanano.com/all/characterization/xrd/crystallite-size/ (accessed on 29 May 2023).
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mc Mahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Allam, R.M.; Al-Abd, A.M.; Khedr, A.; Sharaf, O.A.; Nofal, S.M.; Khalifa, A.E.; Mosli, H.A.; Abdel-Naim, A.B. Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 2018, 291, 77–85. [Google Scholar] [CrossRef]
- Alazmi, A.; Nicolae, S.A.; Modugno, P.; Hasanov, B.E.; Titirici, M.M.; Costa, P.M.F.J. Activated Carbon from Palm Date Seeds for CO2 Capture. Int. J. Environ. Res. Public Health 2021, 18, 12142. [Google Scholar] [CrossRef]
- El-Habba, M.S.; Al-Mulhim, F. The competitiveness of the Saudi Arabian date palm: An analytical study. Afr. J. Agric. Res. 2013, 8, 5260–5267. [Google Scholar]
- Naseer, M.; Aslam, U.; Khalid, B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci. Rep. 2020, 10, 9055. [Google Scholar] [CrossRef]
- Ifeanyichukwu, U.L.; Fayemi, O.E.; Ateba, C.N. Green Synthesis of Zinc Oxide Nanoparticles from Pomegranate (Punica granatum) Extracts and Characterization of Their Antibacterial Activity. Molecules 2020, 25, 4521. [Google Scholar] [CrossRef] [PubMed]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1983. [Google Scholar]
- Heidrun, S.; Hans, A. High-pressure X-ray investigation of zincite ZnO single crystals using diamond anvils with an improved shape. J. Appl. Crystallogr. 2006, 39, 169–175. [Google Scholar]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef]
- Alias, S.S.; Ismail, A.B.; Mohamad, A.A. Effect of pH on ZnO Nanoparticle Properties Synthesized by Sol-Gel Centrifugation. J. Alloys Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Ruangtong, J.; T-Thienprasert, J.; T-Thienprasert, N.P. Green synthesized ZnO nanosheets from banana peel extract possess anti-bacterial activity and anti-cancer activity. Mater. Today Commun. 2020, 24, 101224. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 9, 5. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies; Wiley–Interscience Publication: New York, NY, USA, 1980. [Google Scholar]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; Memon, G.Z.; El-Turki, A.; Allen, G.C. Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloids Surf. B Biointerfaces 2008, 66, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Li, F.T.; Yang, H.; Zhao, Y.; Xu, R. Novel modified pectin for heavy metal adsorption. China Chem. Lett. 2007, 18, 325–328. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Nabili, A.; Fattoum, A.; Passas, R.; Elaloui, E. Extraction and characterization of cellulose from date seeds (Phoenix dactylifera L.). Cell Chem. Tech. 2016, 50, 1015–1023. [Google Scholar]
- Kiani, B.H.; Ajmal, Q.; Akhtar, N.; Haq, I.-U.; Abdel-Maksoud, M.A.; Malik, A.; Aufy, M.; Ullah, N. Biogenic Synthesis of Zinc Oxide Nanoparticles Using Citrullus colocynthis for Potential Biomedical Applications. Plants 2023, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Berehu, H.M.; Anupriy, S.; Khan, M.I.; Chakraborty, R.; Lavudi, K.; Penchalaneni, J.; Mohapatra, B.; Mishra, A.; Patnaik, S. Cytotoxic Potential of Biogenic Zinc Oxide Nanoparticles Synthesized from Swertia chirayita Leaf Extract on Colorectal Cancer Cells. Front. Bioeng. Biotechnol. 2021, 9, 788527. [Google Scholar] [CrossRef]
- Naiel, B.; Fawzy, M.; Halmy, M.W.A.; Mahmoud, A.E. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: Characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci. Rep. 2022, 12, 20370. [Google Scholar] [CrossRef]
- Djaoudene, O.; López, V.; Cásedas, G.; Les, F.; Schisano, C.; Bachir Bey, M.; Tenore, G.C. Phoenix dactylifera L. seeds: A by-product as a source of bioactive compounds with antioxidant and enzyme inhibitory properties. Food Funct. 2019, 10, 4953–4965. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Al-Qahtani, J.H.; Al-Yousef, H.M.; AlSaid, M.S.; Ashour, A.E.; Al-Sohaibani, M.; Rafatullah, S. Proanthocyanidin-Rich Date Seed Extract Protects Against Chemically Induced Hepatorenal Toxicity. J. Med. Food 2015, 18, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidy, N.A.; Al-Zubaidy, A.A.; Sahib, H.B. The anti-proliferative activity of phoenix dactylifera seed extract on MCF-7 breast cancer cell line. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 358–362. [Google Scholar]
- Saliani, M.; Jalal, R.; Kafshdare Goharshadi, E. Effects of pH and Temperature on Antibacterial Activity of Zinc Oxide Nanofluid Against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J. Microbiol. 2015, 8, e17115. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).