An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth
Abstract
1. Introduction
2. Materials and Methods
2.1. Botanical Material
2.2. Preparation of Aqueous Extract
2.3. Chemical Composition
2.4. HPLC-MS/MS Analysis
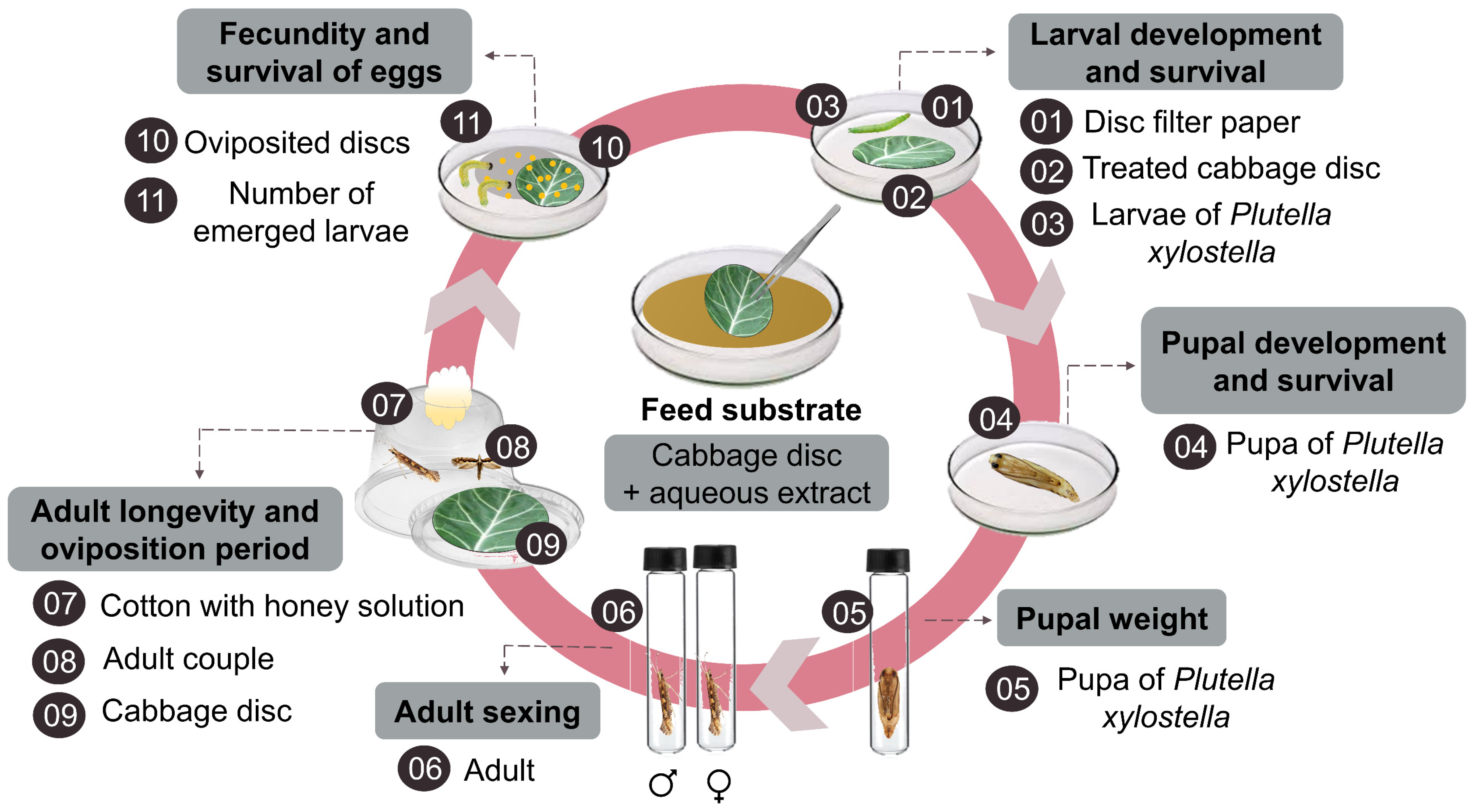
2.5. Rearing of P. xylostella
2.6. Bioactivity of Aqueous Extract on P. xylostella
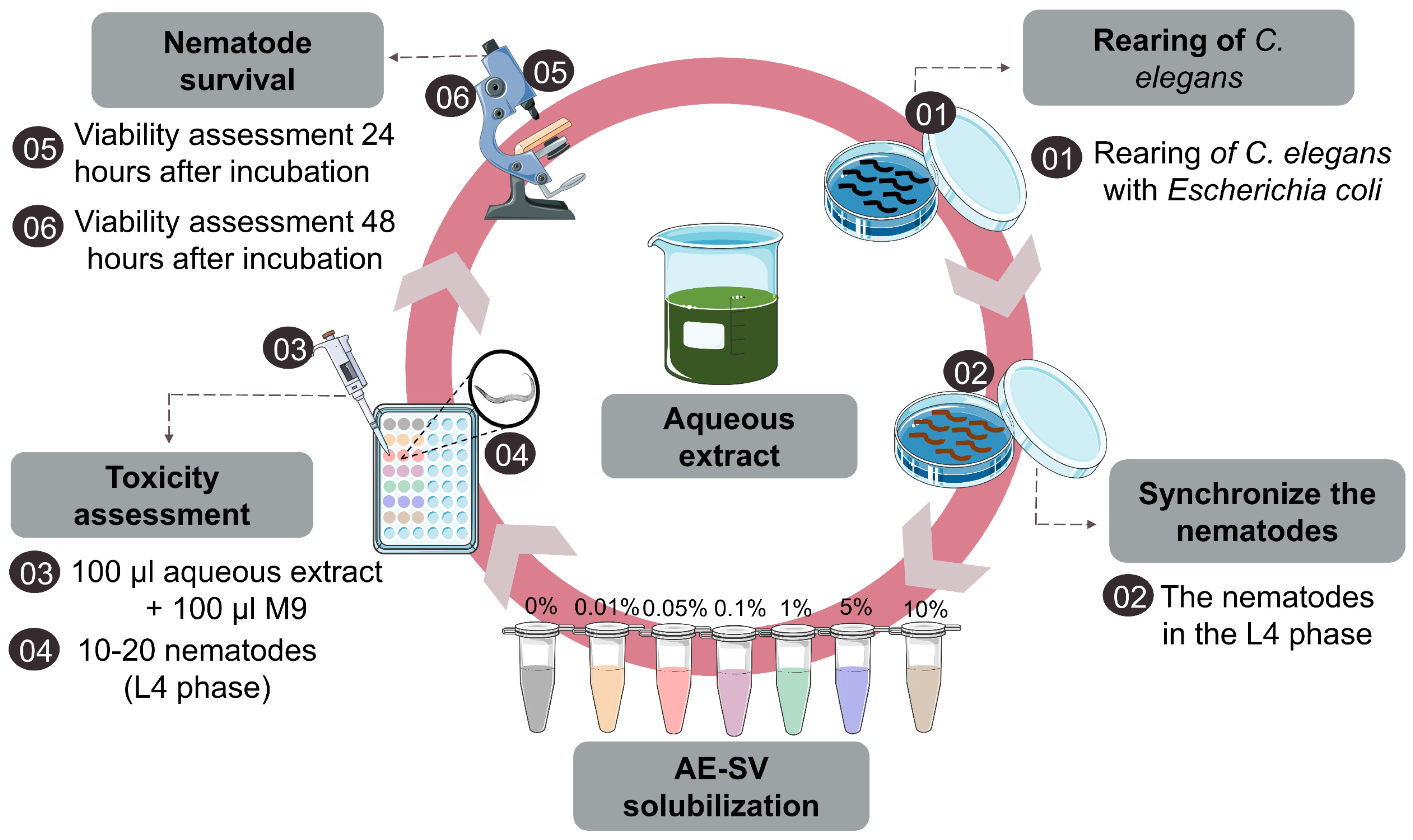
2.7. Rearing of C. elegans
2.8. Toxicity Assessment
3. Results
3.1. Chemical Prolife
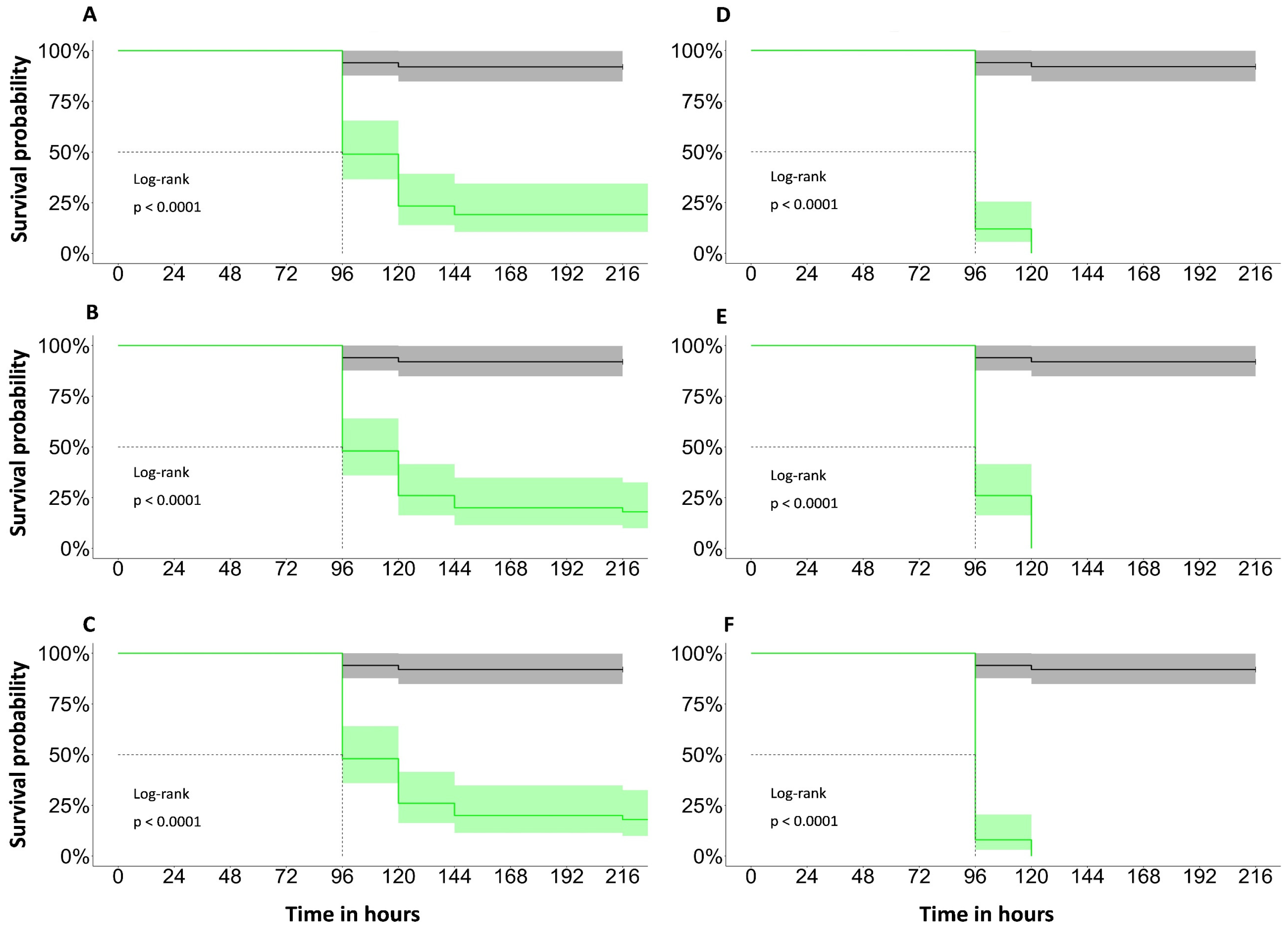
3.2. Bioactivity of AE-S
3.3. Toxicity of AE-S in C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, S.; Handique, G.; Muraleedharan, N.; Dashora, K.; Roy, S.M.; Mukhopadhyay, A.; Babu, A.A. Use of plant extracts for tea pest management in India. Appl. Microbiol. Biotechnol. 2016, 100, 4831–4844. [Google Scholar] [CrossRef] [PubMed]
- Nkechi, E.F.; Ejike, O.G.; Ihuoma, N.J.; Maria-goretti, O.C.; Francis, U.; Godwin, N.; Njokuocha, R. Effects of aqueous and oil leaf extracts of Pterocarpus santalinoides on the maize weevil, Sitophilus zeamais, pest of stored maize grains. Afr. J. Agric. Res. 2018, 13, 617–626. [Google Scholar] [CrossRef]
- Sande, D.; Mullen, J.; Wetzstein, M.; Houston, J. Environmental impacts from pesticide use: A case study of soil fumigation in Florida tomato production. Int. J. Environ. Res. Public Health 2011, 12, 4649–4661. [Google Scholar] [CrossRef]
- Campos, A.P.; Boiça Junior, A.L. Lagartas de Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) submetidas a diferentes concentrações de óleo de nim. Rev. Bras. Milho Sorgo 2012, 11, 137–144. [Google Scholar] [CrossRef]
- Oliveira, F.A.L.D.; Silva, R.O.; Oliveira, N.R.X.D.; Andrade, G.S.; Pereira, F.F.; Zanuncio, J.C.; Coutinho, C.R.; Pastori, P.L. Reproduction of Trichospilus diatraeae (Hymenoptera: Eulophidae) with different densities and parasitism periods in Anticarsia gemmatalis (Lepidoptera: Noctuidae) pupae. Folia Biol. 2018, 43, 103–110. [Google Scholar] [CrossRef]
- Corrêa, J.C.R.; Salgado, H.R.N. Atividade inseticida das plantas e aplicações: Revisão. Rev. Bras. Plantas Med. 2011, 13, 500–506. [Google Scholar] [CrossRef]
- Bandeira, G.N.C.; Camara, A.G.; Moraes, M.M.; Barros, R.; Muhammad, S.; Akhtar, Y. Insecticidal activity of Muntingia calabura extracts against larvae and pupae of diamondback, Plutella xylostella (Lepidoptera, Plutellidae). J. King Saud Univ. Sci. 2013, 25, 83–89. [Google Scholar] [CrossRef]
- Alves, D.S.; Oliveira, D.F.; Santos Júnior, H.M.; Carvalho, D.A.; Santos, M.A.I.; Arvalho, H.W.P. Plant extracts as an alternative to control Leucoptera coffeella (Guérin-èneville) (Lepidoptera: Lyonetiidae). Neotrop. Entomol. 2011, 40, 123–128. [Google Scholar] [CrossRef]
- Shabana, Y.M.; Abdalla, M.E.; Shahin, A.A.; El-Sawy, M.M.; Draz, I.S.; Youssif, A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina Egypt. J. Basic Appl. Sci. 2017, 1, 67–73. [Google Scholar] [CrossRef]
- Capinera, J.L. Encyclopedia of Entomology, 2nd ed.; Springer: Gainesville, FL, USA, 2008; p. 5223. [Google Scholar]
- APRD Arthropod Pesticide Resistance Database. Plutella xylostella. Available online: https://www.pesticideresistance.org/ (accessed on 10 March 2022).
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera:Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Shakeel, M.; Farooq, M.; Nasim, W.; Akram, W.; Khan, F.Z.A.; Jaleel, W.; Zhu, X.; Yin, H.; Li, S.; Fahad, S.; et al. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 14537–14550. [Google Scholar] [CrossRef]
- Aliste, M.; Garrido, I.; Flores, P.; Hellín, P.; Pérez-Lucas, G.; Navarro, S.; Fenoll, J. Photocatalytic degradation four insecticides and their main generated transformation products in soil and pepper crop irrigated with reclaimed agro-wastewater under natural sunlight. J. Hazard. Mater. 2021, 414, 125603. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Roh, J.Y.; Kim, D.H.; Lee, H.S.; Ahn, Y.J. Insecticidal activities of aromatic plant extracts and essential oils against Sitophilus oryzae and Callosobruchus chinensis. J. Stored Prod. Res. 2003, 39, 293–303. [Google Scholar] [CrossRef]
- Menezes, E.L.A. Inseticidas Botânicos: Seus Princípios Ativos, Modo de Ação e Uso Agrícola, 1st ed.; Seropédica: Rio de Janeiro, Brazil, 2005; pp. 4–20. [Google Scholar]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Secur. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review history, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar] [CrossRef]
- Krinski, D.; Massaroli, A.; Machado, M. Potencial inseticida de plantas da família Annonaceae. Rev. Bras. Frutic. 2014, 36, 225–242. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Peres, L.L.S.; Sobreiro, A.I.; Couto, I.F.S.; Silva, R.M.; Pereira, F.F.; Heredia-Vieira, S.C.; Cardoso, C.A.L.; Mauad, M.; Scalon, S.P.Q.; Verza, S.S.; et al. Chemical compounds and bioactivity of aqueous extracts of Alibertia spp. in the control of Plutella xylostella L. (Lepidoptera: Plutellidae). Insects 2017, 8, 125. [Google Scholar] [CrossRef]
- Souza, S.A.; Padial, I.M.P.M.; Couto, I.F.S.; da Silva, M.M.M.; de Carvalho, E.M.; Mussury, R.M. Extrato aquoso de Campomanesia adamantium (Myrtaceae) (Cambess.) O. Berg afeta o desenvolvimento de traça-das-crucíferas. In Meio Ambiente: Impacto do Convívio Entre Vegetação, Animais e Homens 2, 2nd ed.; Teófilo, T., Silva, T., Silva, F., Eds.; Atena Editora: Ponta Grossa, Brazil, 2020; pp. 34–44. [Google Scholar]
- Couto, I.F.S.; Silva, S.V.; Valente, F.I.; Araújo, B.S.; Souza, S.A.; Mauad, M.; Scalon, S.P.Q.; Mussury, R.M. Botanical extracts of the Brazilian savannah affect feeding and oviposition of Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae). J. Agric. Sci. 2019, 11, 322–333. [Google Scholar] [CrossRef]
- Couto, I.F.S.; Souza, S.A.; Valente, F.I.; Silva, R.M.; Scalon, S.P.Q.; Ferreira, F.F.; Silva, S.V.; de Carvalho, E.M.; Mussury, R.M. Changes in the biological characteristics of Plutella xylostella using ethanolic plant extracts. Gesunde Pflanz. 2020, 72, 383–391. [Google Scholar] [CrossRef]
- Silva, R.M.; Santos, L.P.; Silva, G.B.; Miranda, L.O.; Fioratti, C.A.G.; Scalon, S.P.Q.; Mauad, M.; Mussury, R.M. Alibertia spp. (Rubiaceae) extracts interfere with the development and reproduction of Plutella xylostella L. (Lepidoptera: Plutellidae). Gesunde Pflanz. 2020, 72, 351–360. [Google Scholar] [CrossRef]
- Ferreira, E.A.; de Souza, S.A.; Domingues, A.; da Silva, M.M.M.; Padial, I.M.P.M.; de Carvalho, E.M.; Cardoso, C.A.L.; da Silva, S.V.; Mussury, R.M. Phytochemical screening and bioactivity of Ludwigia spp. in the control of Plutella xylostella (Lepidoptera: Plutellidae). Insects 2020, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Padial, I.M.P.M.; Matiasso, A.S.; Souza, S.A.; Mussury, R.M. Efeito de extratos vegetais de Styrax camporum Pohl. sobre a oviposição de Plutella xylostella (L., 1758) (Lepidoptera: Plutellidae). Braz. J. Develop. 2020, 6, 67038–67055. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sujatha, S. Insight of botanical based biopesticides against economically important pest. Int. J. Pharm. Life Sci. 2012, 11, 2138–2148. [Google Scholar]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Penteado, S.R. Defensivos Agrícolas Naturais, 3rd ed.; Grafimagem: Campinas, SP, Brazil, 1999; p. 95. [Google Scholar]
- Castillo-Sánchez, L.E.; Jiménez-Osornio, J.J.; Delgado-Herrera, M.A.; Candelaria-Martínez, B.; Sandoval-Gío, J.J. Effects of the hexanic extract of neem Azadirachta indica against adult whitefly Bemisia tabaci. J. Entomol. Zool. Stud. 2015, 3, 95–99. [Google Scholar]
- Rocha, A.N.; de Souza, S.A.; Fioratti, C.A.G.; Mauad, J.R.C.; Mauad, M.; Mussury, R.M. Tradescantia pallida (Commelinaceae) Promotes Reductions in Plutella xylostella (Lepidoptera: Plutellidae) Populations. Agronomy 2022, 12, 2646. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J.L.D. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Alencar, G.V.; Mendonça, E.S.; Oliveira, T.S.; Jucksch, I.; Cecon, P.R. Percepção ambiental e uso do solo por agricultores de sistemas orgânicos e convencionais na Chapada da Ibiapaba, Ceará. Rev. Econ. Sociol. Rural 2013, 51, 217–236. [Google Scholar] [CrossRef]
- de Mendonça, F.A.C.; da Silva, K.F.S.; dos Santos, K.K.; Ribeiro Júnior, K.A.L.; Sant’Ana, A.E.G. Activities of some Brazilian plants against larvae of the mosquito Aedes aegypti. Fitoterapia 2005, 76, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.A.M.; Paula, J.E.; Espíndola, L.S. Insecticidal activity of cerrado plant extracts on Rhodnius milesi (Carcavallo, Rocha, Galvão & Jurberg) (Hemiptera: Reduviidae), under laboratory conditions. Neotrop. Entomol. 2006, 35, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Simote, S.Y. Estudo Fitoquímico de Helietta puberula (Rutaceae), Simarouba versicolor (Simaroubaceae) e Busca de um Processo de Microencapsulação de Compostos Ativos Visando o Controle de Formigas Cortadeiras. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, SP, Brazil, 2006. [Google Scholar]
- Peñaflor, M.F.G.V.; Almeida, R.N.A.; Simote, S.Y.; Yamane, E.; Bueno, O.; Hebling, M.J.A.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F.; Pagnocca, F. Toxicity of substances isolated from Simarouba versicolor St. Hil. (Simaroubaceae) to the leaf-cutting ant Atta sexdens L. (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus (Singer) Möller. Bioassay 2009, 4, 1–7. [Google Scholar] [CrossRef]
- Barbosa, L.F.; Braz-Filho, R.; Vieira, I.J.C. Chemical constituents of plants from the genus Simaba (Simaroubaceae). Chem. Biodivers. 2011, 8, 2163–2178. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, R.C.G.; Pinto, A.C.; Nunomura, S.M.; Pohlit, A.M. Triterpenos e alcaloide tipo cantinona dos galhos de Simaba polyphylla (Cavalcante) W. W. Thomas (Simaroubaceae). Quim. Nova 2006, 29, 264–268. [Google Scholar] [CrossRef]
- Collins, J.J.; Evason, K.; Kornfeld, K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 1032–1039. [Google Scholar] [CrossRef]
- Cheng, S.C.; Li, W.H.; Shi, Y.C.; Yen, P.L.; Lin, H.Y.; Liao, V.H.C.; Chang, S.T. Antioxidant activity and delayed aging effects of hot water extract from Chamaecyparis obtusa var. formosana leaves. J. Agric. Food Chem. 2014, 62, 4159–4165. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Queirós, L.; Pereira, J.L.; Gonçalves, F.J.M.; Pacheco, M.; Aschner, M.; Pereira, P. Caenorhabditis elegans as a tool for environmental risk assessment: Emerging and promising applications for a “nobelized worm”. Crit. Rev. Toxicol. 2019, 49, 411–429. [Google Scholar] [CrossRef]
- Leelaja, B.C.; Rajini, P.S. Biochemical and physiological responses in Caenorhabditis elegans exposed to sublethal concentrations of the organophosphorus insecticide, monocrotophos. Ecotoxicol. Environ. Saf. 2013, 94, 8–13. [Google Scholar] [CrossRef]
- Zeng, R.; Yu, X.; Tan, X.; Ye, S.; Ding, Z. Deltamethrin affects the expression of voltage-gated calcium channel α1 subunits and the locomotion, egg-laying, foraging behavior of Caenorhabditis elegans. Pestic. Biochem. Physiol. 2017, 138, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.D.; Anderson, G.L.; Williams, P.L. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol. Appl. Pharmacol. 2004, 194, 248–256. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Hope, I.A. Background of Caenorhabditis elegans. In C. elegans: A Practical Approach, 1st ed.; Hope, I.A., Ed.; Oxford Univiversity Pressione: Oxford, UK, 1999; pp. 1–15. [Google Scholar]
- Khweek, A.A.; Amer, A.O. Factors mediating environmental biofilm formation by Legionella pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38. [Google Scholar] [CrossRef]
- Beare, M.H. Fungal and bacterial pathways of organic matter decomposition and nitrogen mineralization in arable soil. In Soil Ecology in Sustainable Agricultural Systems; Brussaard, L., Ferrara-Cerrato, R., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1997; pp. 37–70. [Google Scholar]
- Parodi, D.A.; Jasmine, S.; Yichang, C.; Patrick, A. Reproductive toxicity and meiotic dysfunction following exposure to the pesticides Maneb, Diazinon and Fenarimol. Toxicol. Res. 2015, 4, 645–654. [Google Scholar] [CrossRef]
- Jacques, M.T.; Bornhorst, J.; Soares, M.V.; Schwerdtle, T.; Garcia, S.; Ávila, D.S. Reprotoxicity of glyphosate-based formulation in Caenorhabditis elegans is not due to the active ingredient only. Environ. Pollut. 2019, 252, 1854–1862. [Google Scholar] [CrossRef]
- ASTM International. Standard Guide for Conducting Laboratory Soil Toxicity Tests with the Nematode Caenorhabditis elegans E 2172–01. Available online: https://www.astm.org/Standards/E2172.htm (accessed on 15 February 2021).
- ISO 10872; Water Quality—Determination of the Toxic Effect of Sediment and Soil Samples on Growth, Fertility and Reproduction of Caenorhabditis elegans (Nematoda). ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/46253.html (accessed on 10 February 2021).
- Nass, R.; Hamza, I. The nematode C. elegans as an animal model to explore toxicology in vivo: Solid and axenic growth culture conditions and compound exposure parameters. Curr. Protoc. Toxicol. 2007, 31. Unit1.9. [Google Scholar] [CrossRef] [PubMed]
- Tralau, T.; Riebeling, C.; Pirow, R.; Oelgeschläger, M.; Seiler, A.; Liebsch, M.; Luch, A. Wind of change challenges toxicological regulators. Environ. Health Perspect. 2012, 120, 1489–1494. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Brenton, A.G.; Godfrey, A.R.J. Accurate mass measurement: Terminology and treatment of data. J. Am. Soc. Mass Spectrom. 2010, 21, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.; Thuler, R.T.; Pereira, F.F. Técnica de criação de Plutella xylostella (L., 1758) (Lepidoptera: Yponomeutidae). In Técnicas de Criação de Pragas de Importância Agrícola, em Dietas Naturais, 1st ed.; Pratissoli, D., Ed.; Edufes: Vitória, Brazil, 2012; pp. 65–84. [Google Scholar]
- Logan, J.A.; Wollkind, D.T.; Hoyt, J.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1130–1140. [Google Scholar] [CrossRef]
- Dengg, M.; van Meel, J.C.A. Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J. Pharmacol. Toxicol. Methods 2004, 50, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.C.; Vendramim, J.D. Toxicidad de extractos acuosos de Meliaceae en Spodoptera frugiperda (Lepidoptera: Noctuidae). Manag. Integr. Plagas 1996, 42, 14–22. [Google Scholar]
- Tanzubil, P.B.; McCaffery, A.R. Effects of Azadirachtin on reproduction in the African armyworm (Spodoptera exempta). Entomol. Exp. Appl. 1990, 57, 115–121. [Google Scholar] [CrossRef]
- Costa, E.L.; Silva, N.R.F.P.; Fiúza, L.M. Efeitos, aplicações e limitações de extratos de plantas inseticidas. Acta Biol. Leopoldensia 2004, 26, 173–185. [Google Scholar]
- Reddy, G.V.P.; Tabone, E.; Smith, M.T. Mediation of the host selection and oviposition behavior in the diamondback moth Plutella xylostella and its predator Chrysoperla carnea by chemical cues from cole crops. Biol. Control 2004, 29, 270–277. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Leal-Bertioli, S.C.M.; Bertioli, D.J.; Butt, T.M.; Bordat, D. Caracterização de populações geograficamente distintas da traça-das- crucíferas por susceptibilidade ao Bacillus thuringiensis Berliner e RAPD-PCR. Hortic. Bras. 2004, 22, 607–609. [Google Scholar] [CrossRef]
- Maroneze, D.M.; Gallegos, D.M.N. Efeito de extrato aquoso de Melia azedarach no desenvolvimento das fases imatura e reprodutiva de Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae). Semin. Cienc. Agrar. 2009, 30, 537–550. [Google Scholar] [CrossRef]
- Souza, S.A.; Ferreira, E.A.; Silva, M.M.M.; Padial, I.M.P.M.; Melo, A.M.M.F.; Mussury, R.M. Extrato aquoso de Tabernaemontana solanifolia a. Dc. (Apocynaceae) é uma alternativa efetiva no controle de Traça-das-crucíferas. In Ciências Aplicadas: Princípios Fundamentais, 1st ed.; Ulhôa, J.L.R., Saraiva-Bonatto, E.C., Sousa, A.R., Barbosa, F.C., Gontijo, C.E.O., Pires, P.F., Eds.; Editora Conhecimento Livre: Piracanjuba, Brazil, 2020; pp. 123–140. [Google Scholar] [CrossRef]
- Bisht, K.; Verma, S.; Singh, N.N. Comparison of enhanced artificial diets for mass rearing of Helicoverpa armigera (Hubner) under laboratory conditions. J. Entomol. Zool. Stud. 2018, 6, 2551–2553. [Google Scholar]
- Wang, P.; Furlong, M.J.; Walsh, T.K.; Zalucki, M.P. Moving to keep fit: Feeding behavior and movement of Helicoverpa armigera (Lepidoptera: Noctuidae) on artificial diet with different protein: Carbohydrate ratios. J. Insect Sci. 2019, 19, 20. [Google Scholar] [CrossRef]
- Silva, P.R.C.; Camaroti, J.R.S.L.; Almeida, W.A.; Ferreira, E.C.B.; Paiva, P.M.G.; Barros, R.; Napoleão, T.H.; Pontual, E.V. Schinus terebinthifolia leaf extract is a larvicidal, pupicidal, and oviposition deterring agent against Plutella xylostella. S. Afr. J. Bot. 2019, 127, 124–128. [Google Scholar] [CrossRef]
- Wuyts, N.; Swennen, R.; de Waele, D. Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 2006, 8, 89–101. [Google Scholar] [CrossRef]
- Gonçalves, L.; Spindola, C.B.; Almeida, F. Bacillus thuringiensis var. kurstaki (Bacillaceae): Potencial no controle, no desenvolvimento e reprodução de Oxydia vesulia (Geometridae), em laboratório. Acta Biol. Parana. 2008, 37, 147–163. [Google Scholar] [CrossRef]
- Pingel, R.L.; Lewis, L.C. Field application of Bacillus thuringiensis and Anagrapha falcifera multiple nucleopolyhedrovirus against the corn Earworm, (Lepidoptera: Noctuidae). J. Econ. Entomol. 1997, 90, 1195–1199. [Google Scholar] [CrossRef]
- Latif, Z.; Craven, L.; Hartley, T.G.; Kemp, B.R.; Potter, J.; Rice, M.J.; Waigh, R.D.; Waterman, P.G. An insecticidal quassinoid from the new australian species Quassia sp. aff. bidwillii. Biochem. Syst. Ecol. 2000, 28, 183–184. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Krishna Kumari, G.N.; Gopalakrishnan, G.; Suresh, G.; Wesley, S.D.; Sreelatha, T. Insect antifeedant and growth regulating activities of quassinoids from Samadera indica. Fitoterapia 2001, 72, 568–571. [Google Scholar] [CrossRef]
- Okano, M.; Fukamiya, N.; Lee, K.H. Bioactive quassinoids. Stud. Nat. Prod. Chem. 2000, 23, 285–333. [Google Scholar] [CrossRef]
- Braga, F.C.; Castilho, R.O. Potencialidade do cerrado como fonte de substâncias bioativas e de espécies medicinais para o desenvolvimento de fitoterápicos. In Farmacogn. Coletânea Científica; Souza, G.H.B., Mello, J.C.P., Lopes, N.P., Eds.; UFOP: Ouro Preto, MG, Brazil, 2012; pp. 295–318. [Google Scholar]
- Lidert, Z.; Wing, K.; Polonsky, U.; Imakura, Y.; Okano, M.; Tani, S.; Lin, Y.M.; Kiyokawa, H.; Lee, K.H. Insect antifeedant and growth inhibitory activity of forty-six quassinoids on two species of agricultural pests. J. Nat. Prod. 1987, 50, 442–448. [Google Scholar] [CrossRef]
- Odjo, A.; Piart, J.; Polonsky, J.; Roth, M. Etude de l’effet insecticide de deux quassainoides sur des larves de Locusta migratoria magratoroides R et F (Orthoptera, Acrididae). Comptes Rendus Sci. Paris 1981, 293, 241–244. [Google Scholar]
- Klocke, J.A.; Arisawa, M.; Handa, S.S.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Growth inhibitory, insecticidal and antifeedant effects of some antileukemic and cytotoxic quassinoids on two species of agricultural pests. Experientia 1985, 41, 379–382. [Google Scholar] [CrossRef]
- Napal, G.N.D.; Palacios, S.M. Bioinsecticidal effect of the flavonoids pinocembrin and quercetin against Spodoptera frugiperda. J. Pest Sci. 2015, 88, 629–635. [Google Scholar] [CrossRef]
- Hay, W.T.; Behle, R.W.; Berhow, M.A.; Miller, A.C.; Selling, G.W. Biopesticide synergy when combining plant flavonoids and entomopathogenic baculovirus. Sci. Rep. 2020, 10, 6806. [Google Scholar] [CrossRef] [PubMed]
- Sarria, A.L.F.; Matos, A.P.; Volante, A.C.; Bernardo, A.R.; Cunha, G.O.S.; Fernandes, J.B.; Forim, M.R.; Vieira, P.C.; Silva, M.F.G.F. Insecticidal activity of copper (II) complexes with flavanone derivatives. Nat. Prod. Res. 2021, 36, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Stamp, N.E.; Skrobola, C.M. Failure to avoid rutin diets results in altered food utilization and reduced growth rate of Manduca sexta larvae. Entomol. Exp. Appl. 1993, 68, 127–142. [Google Scholar] [CrossRef]
- Silva, T.R.F.B.; Almeida, A.C.S.; Moura, T.L.; Silva, A.R.; Freitas, S.S.; Jesus, F.G. Effect of the flavonoid rutin on the biology of Spodoptera frugiperda (Lepidoptera: Noctuidae). Acta Sci. Agron. 2016, 38, 165–170. [Google Scholar] [CrossRef]
- Appel, H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef]
- Pandey, A.; Misra, P.; Chandrashekar, K.; Trivedi, P.K. Development of AtMYB12-expressing transgenic tobacco callus culture for production of rutin with biopesticidal potential. Plant Cell Rep. 2012, 31, 1867–1876. [Google Scholar] [CrossRef]
- Salunke, B.K.; Kotkar, H.M.; Mendki, O.S.; Upasani, S.M.; Maheshwari, V.L. Efficacy of flavonoids in controlling Callosobruchus chinensis (L.) (Coleoptera: Bruchidae), a post-harvest pest of grain legumes. Crop Prot. 2005, 24, 888–893. [Google Scholar] [CrossRef]
- Gazzoni, D.L.; Hulsmeyer, A.; Hoffman-Campo, C.B. Efeito de diferentes doses de rutina e de quercetina na biologia de Anticarsia gemmatalis. Pesqui. Agropecu. Bras. 1997, 32, 673–681. [Google Scholar]
- Talukder, F.A.; Islam, M.S.; Hossain, M.S.; Rahman, M.A.; Alam, M.N. Toxicity effects of botanicals and synthetic insecticides on Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). Bangladesh J. Environ. Sci. 2004, 10, 365–371. [Google Scholar]
- Garcez, W.S.; Garcez, F.R.; Silva, L.M.G.E.; Sarmento, U.C. Substâncias de origem vegetal com atividade larvicida contra Aedes aegypti. Rev. Virtual Química 2013, 5, 363–393. [Google Scholar] [CrossRef]
- Neeraj, G.S.; Kumar, A.; Ram, S.; Kumar, V. Evaluation of nematicidal activity of ethanolic extracts of medicinal plants to Meloidogyne incognita (kofoid and white) chitwood under lab conditions. Int. J. Pure Appl. Biosci. 2017, 1, 827–831. [Google Scholar] [CrossRef]
- Padial, I.M.P.M.; de Souza, S.A.; Malaquias, J.B.; Cardoso, C.A.L.; da Silva Pachú, J.K.; Fioratti, C.A.G.; Mussury, R.M. Leaf Extracts of Miconia albicans (Sw.) Triana (Melastomataceae) Prevent the Feeding and Oviposition of Plutella xylostella (Linnaeus, 1758) (Lepidoptera: Plutellidae). Agronomy 2023, 13, 890. [Google Scholar] [CrossRef]
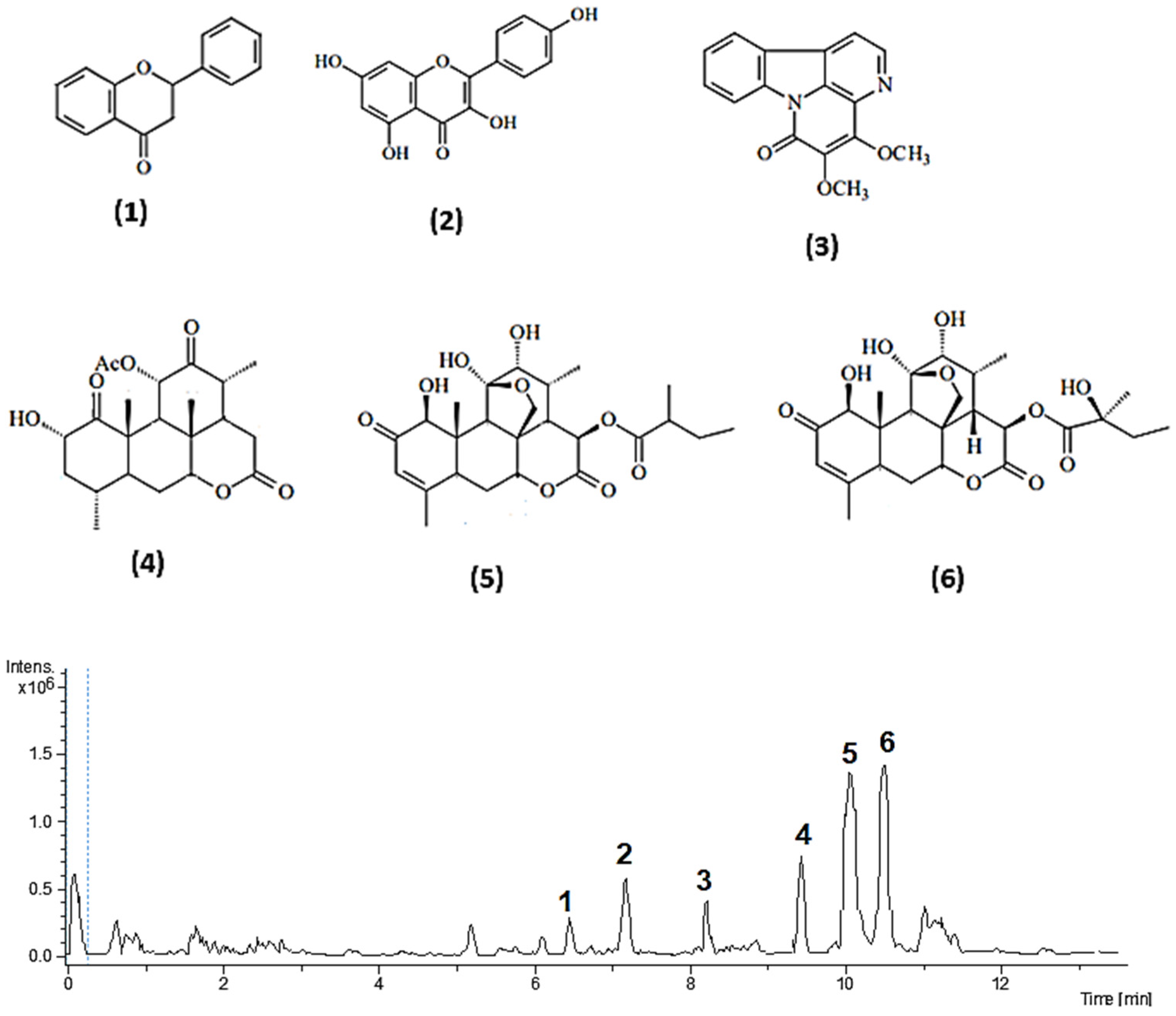


| Peak | Compound | RT (min) | [M+H] m/z | Molecular Formula | MS/MS [M+H] Fragments |
|---|---|---|---|---|---|
| (1) | Flavanone | 6.56 | 224.0837 | C15H12O2 | 223, 195 |
| (2) | Kaempferol | 7.22 | 286.2432 | C15H10O6 | 286, 165, 153, 137, 99 |
| (3) | 4,5-Dimethoxycanthin-6-one | 8.73 | 280.0848 | C16H12N2O3 | 265, 251, 221, 237 |
| (4) | 11-Acetylamarolide | 9.57 | 406.1991 | C22H30O7 | 378, 318, 274, 214 |
| (5) | Ailanthinone | 10.09 | 495.5009 | C25H34O9 | 478, 345, 301, 104 |
| (6) | Glaucarubinone | 10.58 | 494.2151 | C25H34O10 | 493, 375, 345, 301, 117 |
| Concentration (%) | Larval Development (Days) | Larval Mortality (%) |
|---|---|---|
| 0.00 | 6.54 ± 0.24 a | 08.00 ± 03.87 b |
| 0.01 | 5.06 ± 0.27 b | 82.00 ± 05.54 a |
| 0.05 | 4.92 ± 0.66 b | 92.00 ± 03.87 a |
| 0.10 | 4.48 ± 0.41 b | 96.00 ± 02.79 a |
| 1.00 | - | 100.00 ± 0.00 * |
| 5.00 | - | 100.00 ± 0.00 * |
| 10.00 | - | 100.00 ± 0.00 * |
| F/χ2 and P value | F = 19.87; P (>F) < 0.0001 | χ2= 719.95; χ2 (>F) < 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, S.A.; Padial, I.M.P.M.; Domingues, A.; Mauad, J.R.C.; Formagio, A.S.N.; Campos, J.F.; Malaquias, J.B.; Mussury, R.M. An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth. Sustainability 2023, 15, 7759. https://doi.org/10.3390/su15107759
de Souza SA, Padial IMPM, Domingues A, Mauad JRC, Formagio ASN, Campos JF, Malaquias JB, Mussury RM. An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth. Sustainability. 2023; 15(10):7759. https://doi.org/10.3390/su15107759
Chicago/Turabian Stylede Souza, Silvana Aparecida, Isabella Maria Pompeu Monteiro Padial, Alberto Domingues, Juliana Rosa Carrijo Mauad, Anelise Samara Nazari Formagio, Jaqueline Ferreira Campos, José Bruno Malaquias, and Rosilda Mara Mussury. 2023. "An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth" Sustainability 15, no. 10: 7759. https://doi.org/10.3390/su15107759
APA Stylede Souza, S. A., Padial, I. M. P. M., Domingues, A., Mauad, J. R. C., Formagio, A. S. N., Campos, J. F., Malaquias, J. B., & Mussury, R. M. (2023). An Interesting Relationship between the Insecticidal Potential of Simarouba sp. in the Biology of Diamondback Moth. Sustainability, 15(10), 7759. https://doi.org/10.3390/su15107759












