Exergy Transfer Analysis of Biomass and Microwave Based on Experimental Heating Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
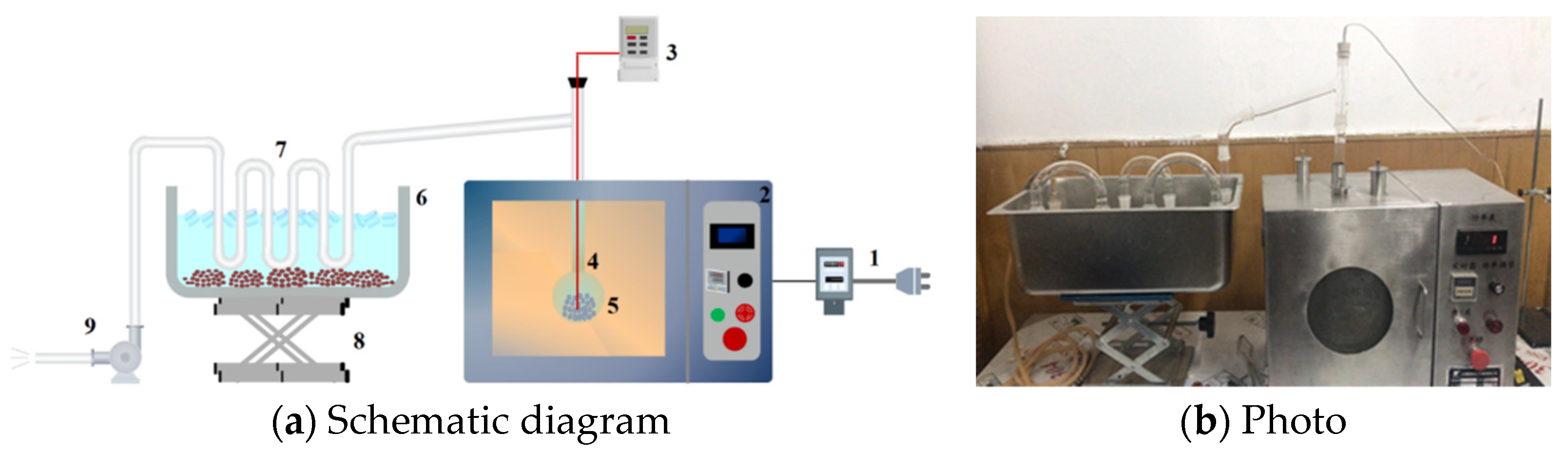
2.2. Experimental Setup
2.3. Experimental Procedures
2.4. Exergy Analysis
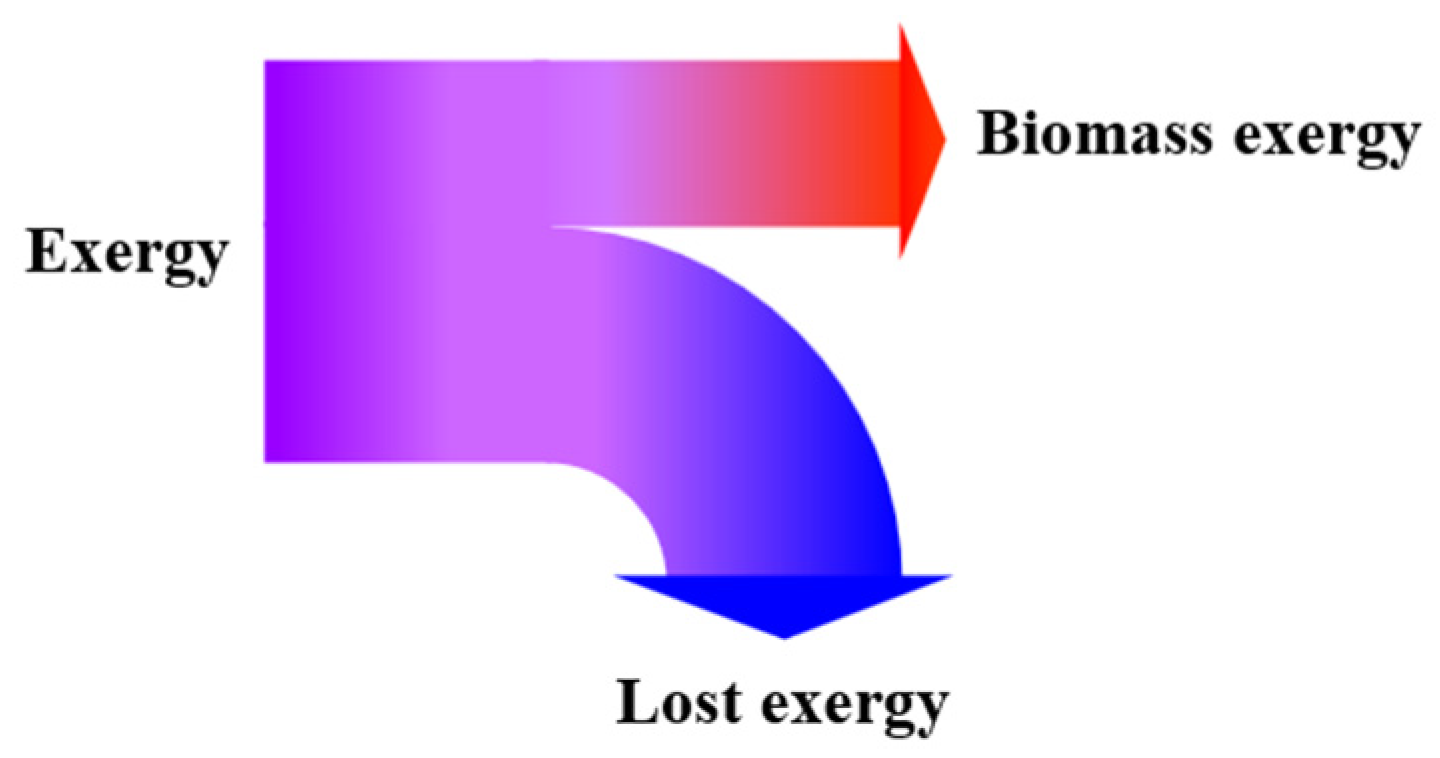
3. Results and Discussion
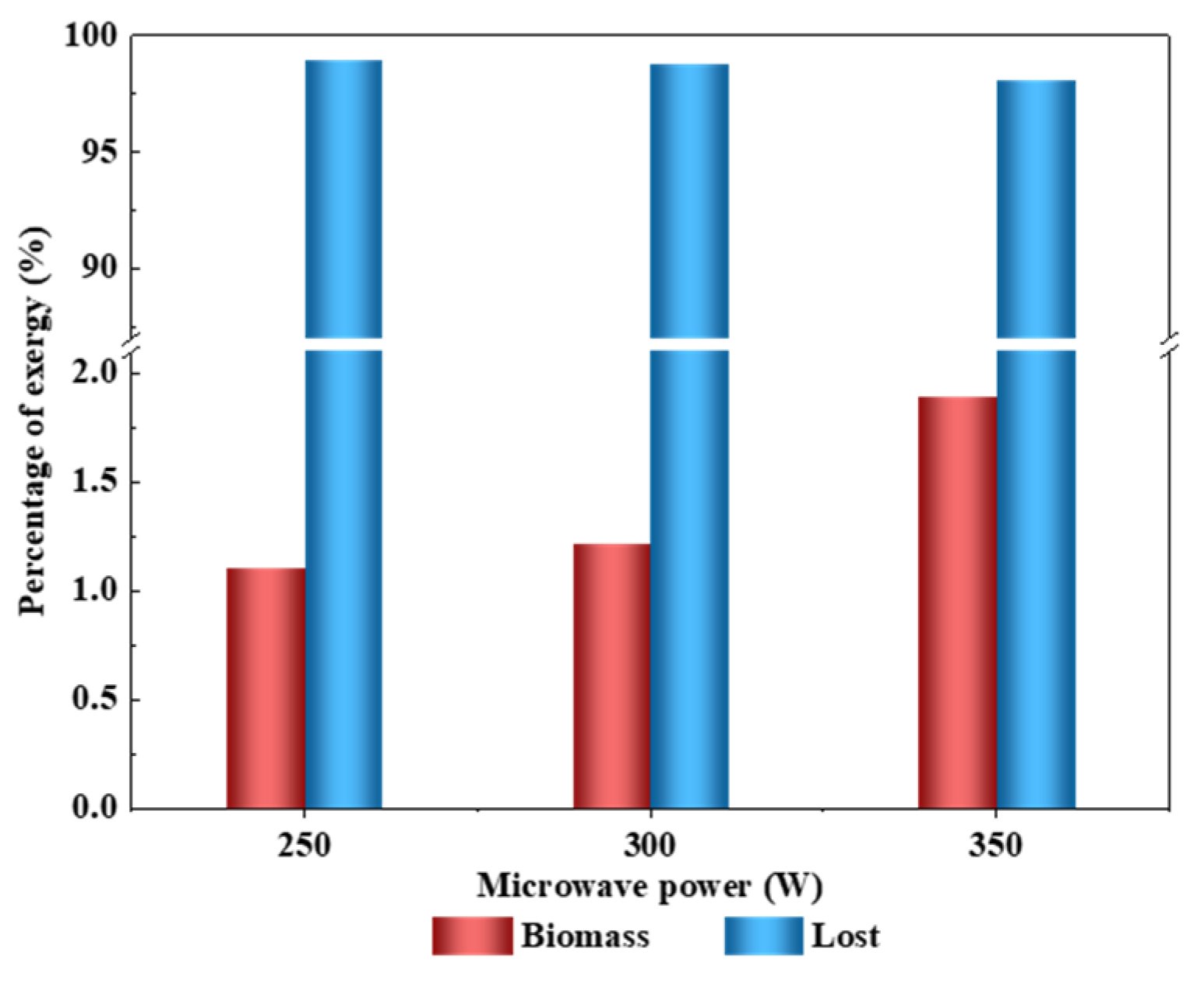
3.1. Effect of Microwave Power
3.1.1. Heating Performance
3.1.2. Exergy Transfer
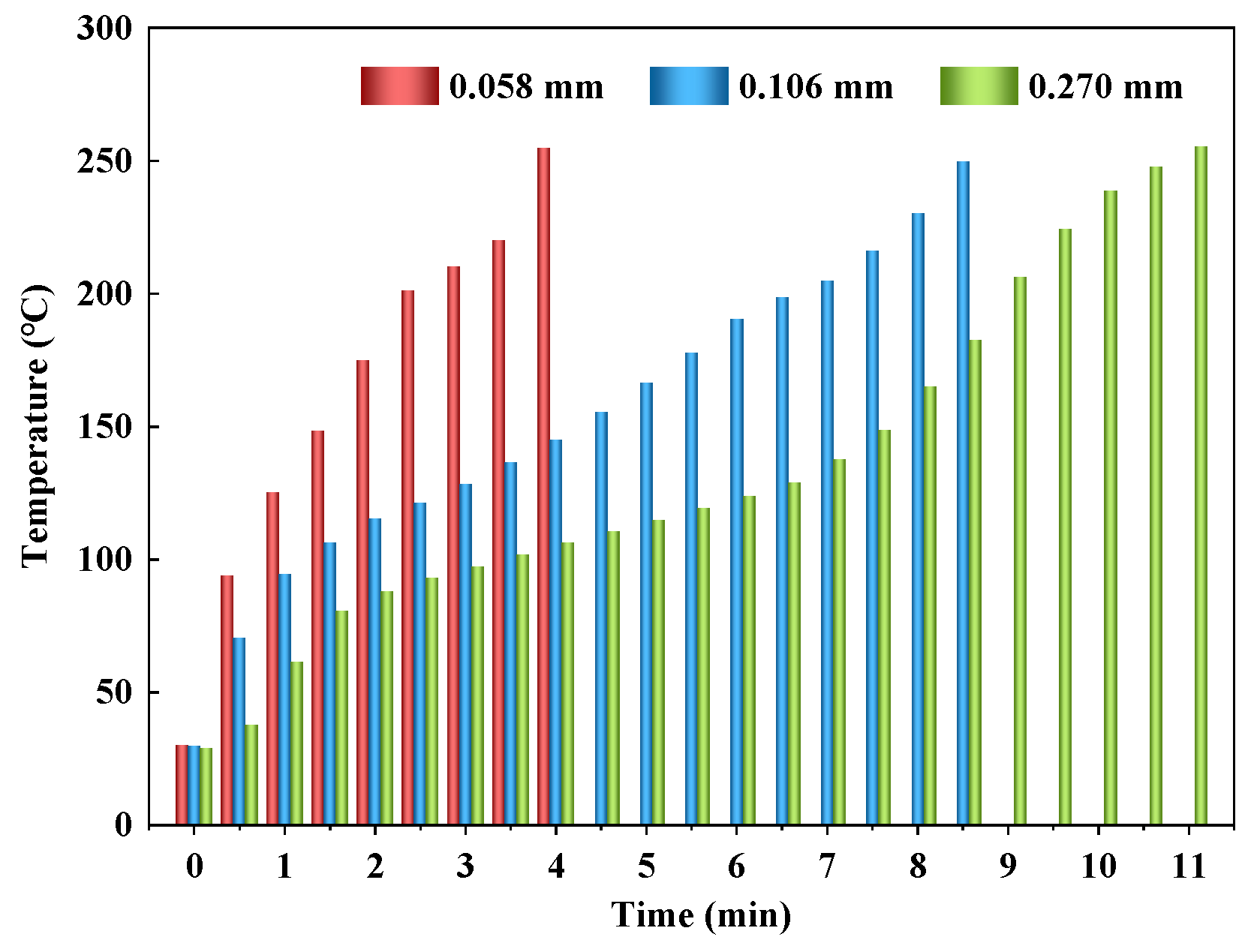
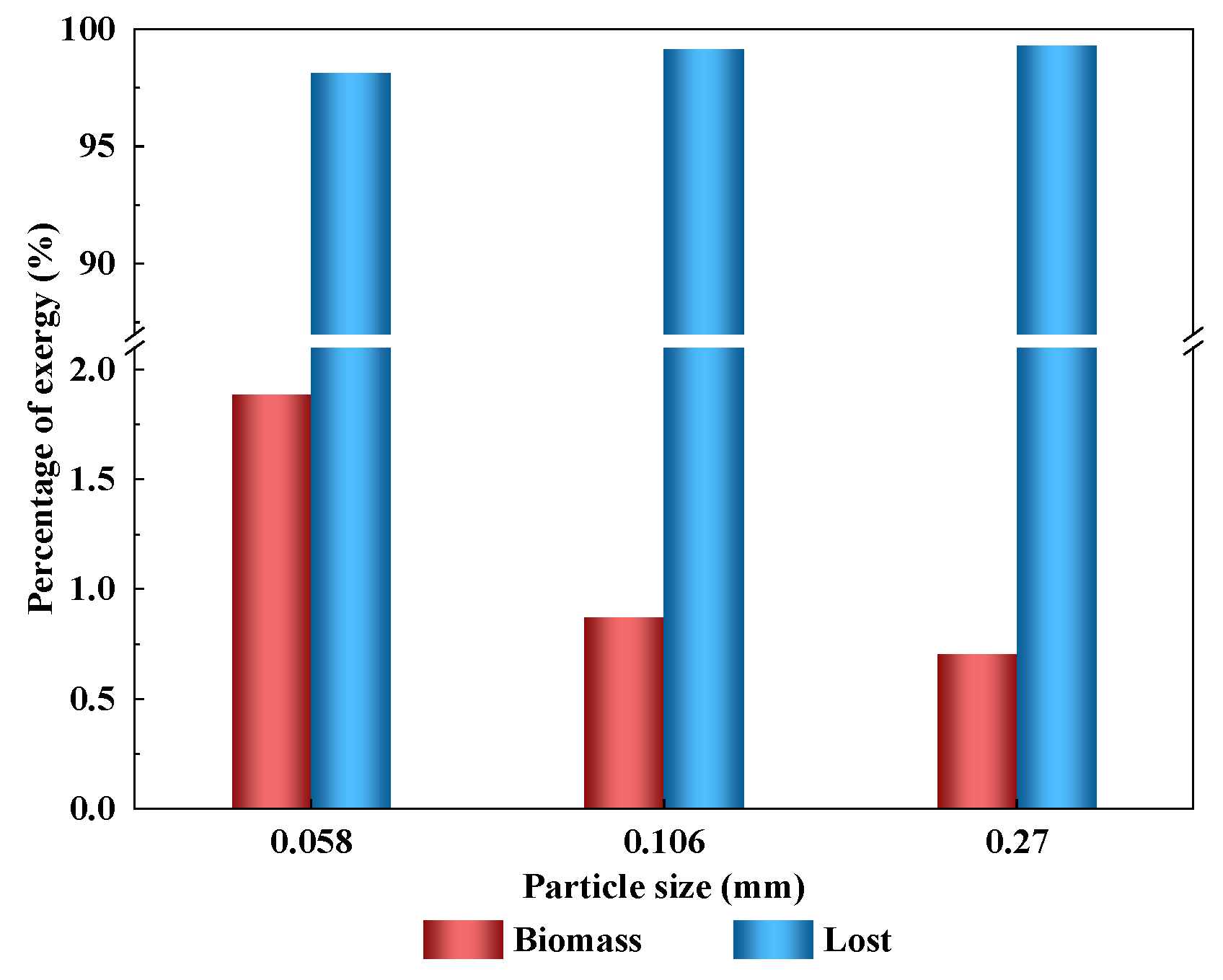
3.2. Effect of Particle Size
3.2.1. Heating Performance
3.2.2. Exergy Transfer
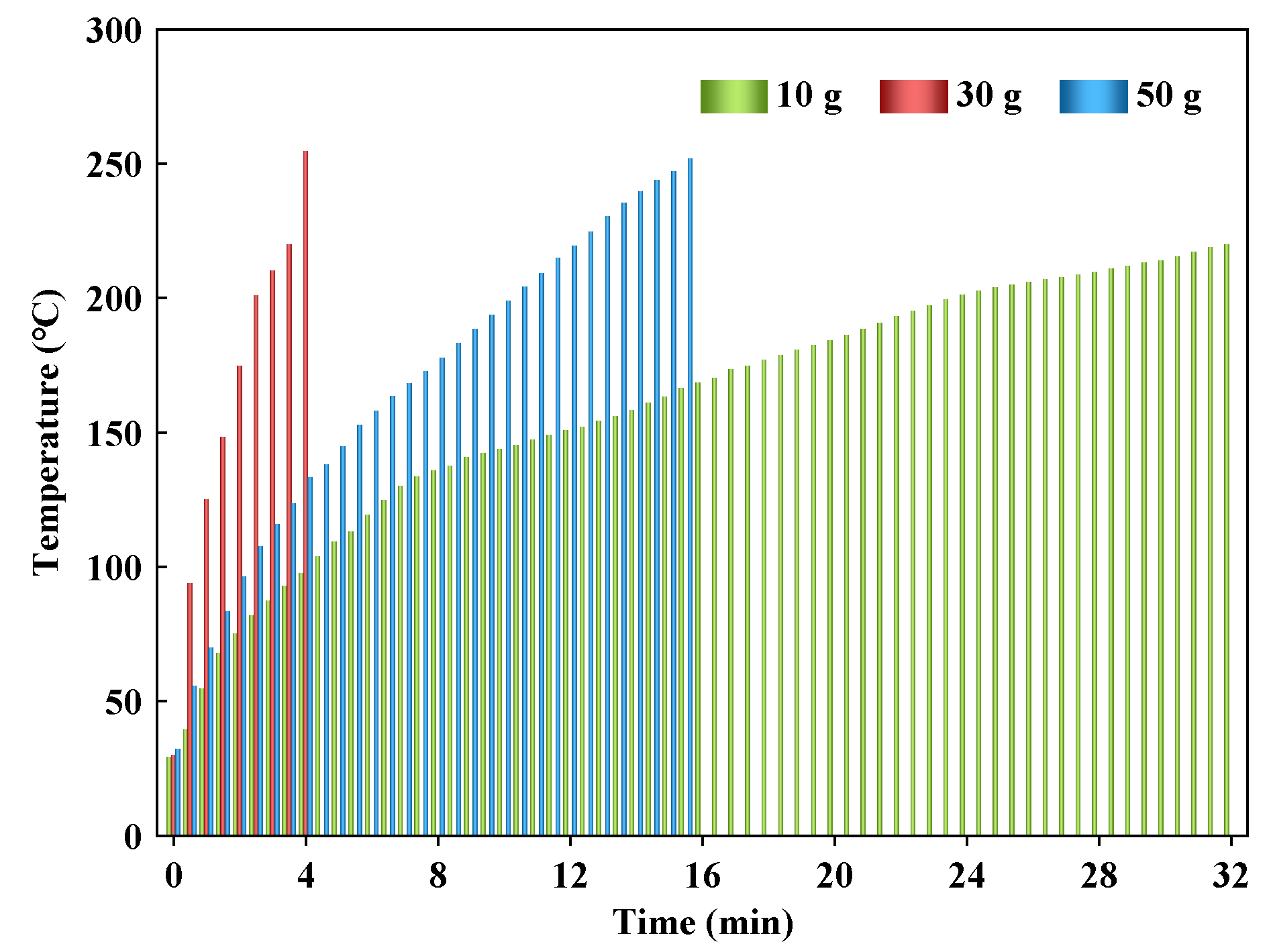
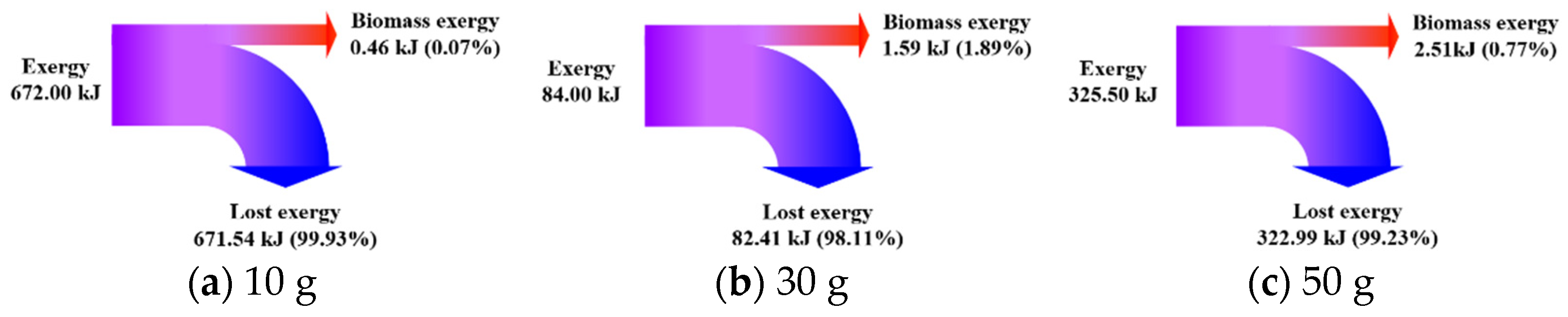
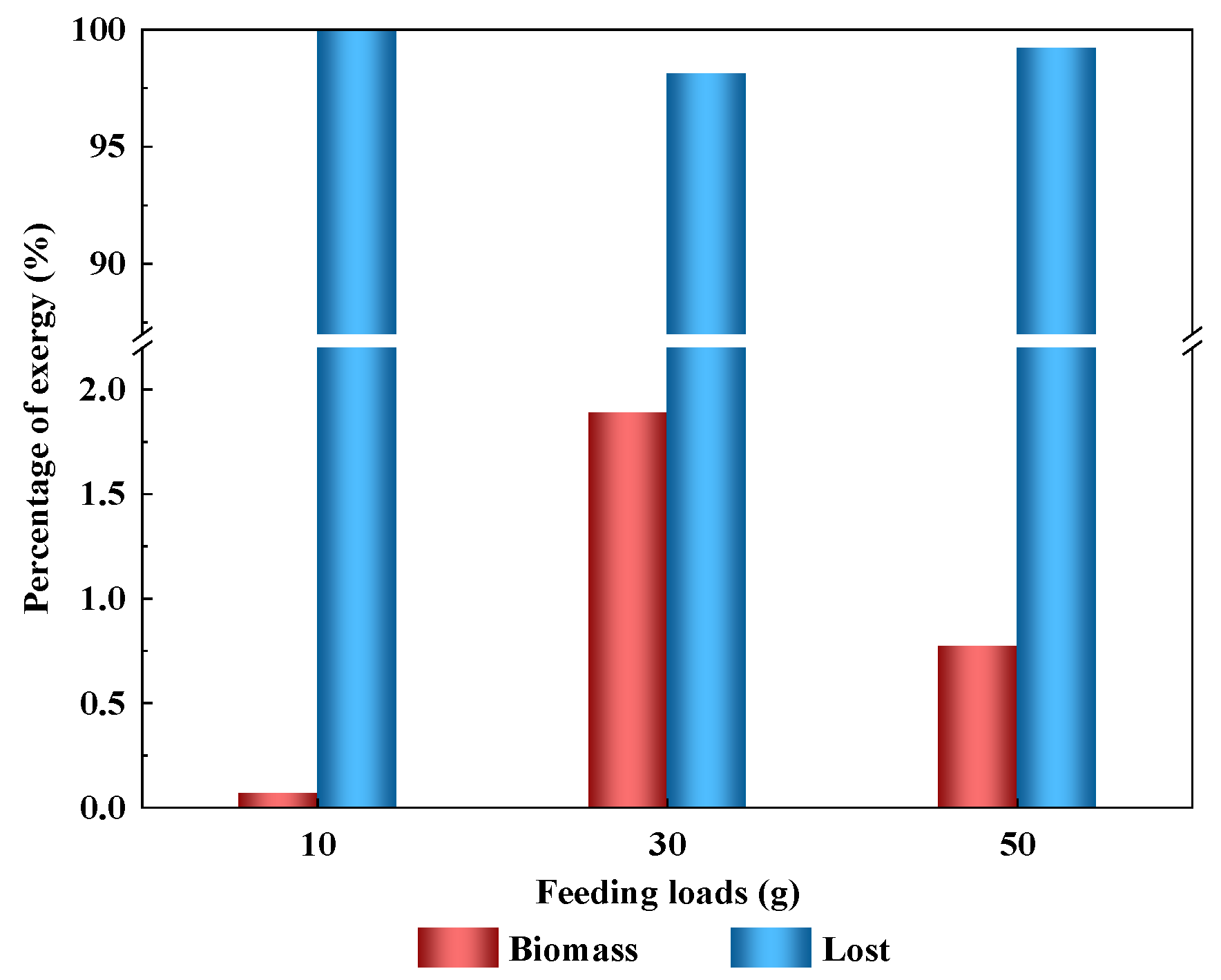
3.3. Effect of Feeding Load
3.3.1. Heating Performance
3.3.2. Exergy Transfer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, W.; Zhang, Y.; Cui, L.; Liu, H.; Maqsood, T. Experimental microwave-assisted air gasification of biomass in fluidized bed reactor. Bioresour. Technol. 2023, 369, 128378. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Sun, Y.; Awasthi, M.K.; Liu, Y.; Xu, X.; Meng, X.; Zhang, H. Effect of different aerobic hydrolysis time on the anaerobic digestion characteristics and energy consumption analysis. Bioresour. Technol. 2021, 320, 124332. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Sun, Y.; Yang, G. One-pot pyrolysis route to Fe-N-Doped carbon nanosheets with outstanding electrochemical performance as cathode materials for microbia. Int. J. Agric. Biol. 2020, 13, 207–214. [Google Scholar]
- Liu, Y.; Fu, W.; Liu, T.; Zhang, Y.; Li, B. Microwave pyrolysis of polyethylene terephthalate (PET) plastic bottle sheets for energy recovery. J. Anal. Appl. Pyrolysis 2022, 161, 105414. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuel production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Yuan, X.; Tian, S.; Wang, X.; Zhang, H.; Han, L. Effect of pyrolysis temperature on composition, carbon fraction and abiotic stability of straw biochars: Correlation and quantitative analysis. Carbon Res. 2022, 1, 17. [Google Scholar] [CrossRef]
- Manzanoa, M.N.; Quiroga, A.G.; Perreault, P.; Madanikashani, S.; Vandewalle, L.A.; Marin, G.B.; Heynderickx, G.J.; Geem, K.M.V. Biomass fast pyrolysis in an innovative gas-solid vortex reactor: Experimental proof of concept. J. Anal. Appl. Pyrolysis 2021, 156, 105165. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Liu, T.; Fu, W.; Li, B. Microwave-assisted pyrolysis of polystyrene for aviation oil production. J. Anal. Appl. Pyrolysis 2022, 162, 105425. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, P.; Liang, S.; Liu, B.; Shuai, Y.; Li, B. Exergy analysis of hydrogen production from steam gasification of biomass: A review. Int. J. Hydrogen Energy 2019, 44, 14290–14302. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Xiong, Q. A review of aviation oil production from organic wastes through thermochemical technologies. Appl. Energy Combust. Sci. 2022, 9, 100058. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Fu, W.; Li, B. A review of biochar prepared by microwave-assisted pyrolysis of organic wastes. Sustain. Energy Technol. Assess. 2022, 50, 101873. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis of Biosolids to Produce Biochars: A Review. Sustainability 2022, 14, 9626. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Liu, S.; Fan, L.; Zhou, N.; Peng, P.; Wang, Y.; Guo, F.; Min, M.; Cheng, Y.; et al. Fast microwave-assisted pyrolysis of wastes for biofuels production—A review. Bioresour. Technol. 2020, 297, 122480. [Google Scholar] [CrossRef] [PubMed]
- Mishra1, R.K.; Mohanty, K. Bio-oil and biochar production using thermal and catalytic pyrolysis of low-value waste neem seeds over low-cost catalysts: Effects of operating conditions on product yields and studies of physicochemical characteristics of bio-oil and biochar. Biochar 2021, 3, 641–656. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg(II) from aqueous solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef]
- Huang, X.; Ren, J.; Ran, J.; Qin, C.; Yang, Z.; Cao, J. Recent advances in pyrolysis of cellulose to value-added chemicals. Fuel Process. Technol. 2022, 229, 107175. [Google Scholar] [CrossRef]
- Koštial, P.; Vlček, J.; Jančíková, Z.K.; Špačková, H.; David, J.; Frischer, R.; Ružiak, I. Effective Ecological and Cheap Heating of Dwelling Spaces. Sustainability 2020, 12, 55. [Google Scholar] [CrossRef]
- Ukanwa, K.S.; Patchigolla, K.; Sakrabani, R.; Anthony, E.; Mandavgane, S. A Review of Chemicals to Produce Activated Carbon from Agricultural Waste Biomass. Sustainability 2019, 11, 6204. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Liu, T.; Omar, M.M.; Li, B. Perspectives into intensification for aviation oil production from microwave pyrolysis of organic wastes. Chem. Eng. Process. Process Intensif. 2022, 176, 108939. [Google Scholar] [CrossRef]
- Putra, P.H.M.; Rozali, S.; Patah, M.F.A.; Idris, A. A review of microwave pyrolysis as a sustainable plastic waste management technique. J. Environ. Manag. 2022, 303, 114240. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Wei, L.; Turner, N.C.; Ma, S.; Yang, M.; Wang, T. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean. Prod. 2020, 250, 119514. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Xiu, S.; Shahbazi, A. Pretreatment and Fractionation of Wheat Straw with Acetic Acid to Enhance Enzymatic Hydrolysis and Ethanol Fermentation. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1230–1238. [Google Scholar] [CrossRef]
- Chougan, M.; Ghaffar, S.H.; Sikora, P.; Mijowska, E.; Kukułka, W.; Stephan, D. Boosting Portland cement-free composite performance via alkali-activation and reinforcement with pre-treated functionalised wheat straw. Ind. Crops Prod. 2022, 178, 114648. [Google Scholar] [CrossRef]
- Grecoa, G.; Videgaina, M.; Stasia, C.D.; Gonzáleza, B.; Manyà, J.J. Evolution of the mass-loss rate during atmospheric and pressurized slow pyrolysis of wheat straw in a bench-scale reactor. J. Anal. Appl. Pyrolysis 2018, 136, 18–26. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Liu, H.; Ma, C.; Song, Z. Microwave pyrolysis of wheat straw: Product distribution and generation mechanism. Bioresour. Technol. 2014, 158, 278–285. [Google Scholar] [CrossRef]
- Yemis, O.; Mazza, G. Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process. Bioresour. Technol. 2012, 109, 215–223. [Google Scholar] [CrossRef]
- Guo, B.; Niu, Z. Specific heat capacity analysis and curve fitting of wheat straw based on DSC. Hubei Agric. Sci. 2014, 53, 5268–5272. [Google Scholar]
- Zhang, Y.; Gao, X.; Li, B.; Zhang, H.; Qi, B.; Wu, Y. An expeditious methodology for estimating the exergy of woody biomass by means of heating values. Fuel 2015, 159, 712–719. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, W.; Liu, H.; Mao, Y.; Ma, C.; Song, Z. Temperature rise and weight loss characteristics of wheat straw under microwave heating. J. Anal. Appl. Pyrolysis 2014, 107, 59–66. [Google Scholar]
- Fu, W.; Dai, J.; Zhang, Y.; Guang, M.; Liu, Y.; Li, B. Heating performances of high density polyethylene (HDPE) plastic particles in a microwave chamber. Sustain. Energy Technol. Assess. 2021, 48, 101581. [Google Scholar] [CrossRef]
- Hong, K.; Fu, W.; Guang, M.; Zhang, Y.; Li, B. Microwave heating performances of low density polyethylene (LDPE) plastic particles. J. Anal. Appl. Pyrolysis 2021, 160, 105356. [Google Scholar] [CrossRef]



| Proximate Analysis (wt.%) | Ultimate Analysis (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | V | A | FC a | C | H | N | S | O a |
| 11.00 | 56.87 | 17.14 | 14.99 | 36.47 | 5.93 | 1.39 | 0.18 | 56.03 |
| Group | Microwave Power (W) | Particle Size (mm) | Feeding Load (g) |
|---|---|---|---|
| 1 | 250 | 0.058 | 30 |
| 2 | 300 | 0.058 | 30 |
| 3 | 350 | 0.058 | 30 |
| 4 | 350 | 0.106 | 30 |
| 5 | 350 | 0.270 | 30 |
| 6 | 350 | 0.058 | 10 |
| 7 | 350 | 0.058 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Liu, C.; Liu, H.; Zhao, W.; Zhang, Y. Exergy Transfer Analysis of Biomass and Microwave Based on Experimental Heating Process. Sustainability 2023, 15, 388. https://doi.org/10.3390/su15010388
Cui L, Liu C, Liu H, Zhao W, Zhang Y. Exergy Transfer Analysis of Biomass and Microwave Based on Experimental Heating Process. Sustainability. 2023; 15(1):388. https://doi.org/10.3390/su15010388
Chicago/Turabian StyleCui, Longfei, Chaoyue Liu, Hui Liu, Wenke Zhao, and Yaning Zhang. 2023. "Exergy Transfer Analysis of Biomass and Microwave Based on Experimental Heating Process" Sustainability 15, no. 1: 388. https://doi.org/10.3390/su15010388
APA StyleCui, L., Liu, C., Liu, H., Zhao, W., & Zhang, Y. (2023). Exergy Transfer Analysis of Biomass and Microwave Based on Experimental Heating Process. Sustainability, 15(1), 388. https://doi.org/10.3390/su15010388






