Environmental Risk from Organic Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Residues
- -
- Almond tree pruning (AP).
- -
- Commercial brown peat (CP).
- -
- Hay straw (HS).
- -
- Olive tree (Olea europaea L.) pruning (OP).
- -
- Pomegranate (Punica granatum L.) peel (PG).
- -
- Pine (Pinus halepensis) needle fall (PN).
- -
- Date palm (Phoenix dactylifera L.) leaf pruning (PP).
- -
- Sewage sludge compost (SC).
- -
- Vine (Vitis vinifera) pruning (VP).
2.2. Residue Characterization and Methods
2.3. Statistical Analysis
3. Results and Discussion
3.1. Elemental Composition
3.2. Soluble Elements and Solubility Index
3.3. Potential Toxicity
3.4. HM Contribution to Soil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils. Status of the World’s Soil Resources (SWSR)—Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy, 2015. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Voluntary Guidelines for Sustainable Soil Management; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- El-Ramady, H.R.; Alshaal, T.A.; Amer, M.; Domokos-Szabolcsy, E.; Elhawat, N.; Prokisch, J.; Fári, M. Soil Quality and Plant Nutrition. In Sustainable Agriculture Reviews 14: Agroecology and Global Change; Lichtfouse, E., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 14, pp. 345–447. [Google Scholar] [CrossRef]
- El Chami, D.; Daccache, A.; El Moujabber, M. How Can Sustainable Agriculture Increase Climate Resilience? A Systematic Review. Sustainability 2020, 12, 3119. [Google Scholar] [CrossRef]
- Rabary, B.; Sall, S.; Letourmy, P.; Husson, O.; Ralambofetra, E.; Moussa, N.; Chotte, J.L. Effects of living mulches or residue amendments on soil microbial properties in direct seeded cropping systems of Madagascar. Appl. Soil Ecol. 2008, 39, 236–243. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, T.; Navarro-Pedreño, J.; Gómez Lucas, I.; Almendro-Candel, M.B. Land Recycling, Food Security and Technosols. J. Geogr. Res. 2021, 4, 3. [Google Scholar] [CrossRef]
- Rokia, S.; Séré, G.; Schwartz, C.; Deeb, M.; Fournier, F.; Nehls, T.; Damas, O.; Vidal-Beaudet, L. Modelling agronomic properties of Technosols constructed with urban wastes. Waste Manag. 2014, 34, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Coull, M.; Butler, B.; Hough, R.; Beesley, L. A Geochemical and Agronomic Evaluation of Technosols Made from Construction and Demolition Fines Mixed with Green Waste Compost. Agronomy 2021, 11, 649. [Google Scholar] [CrossRef]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F.; Ciaccia, C. Recycling agricultural wastes and by-products in organic farming: Biofertilizers production, yield performance and carbon footprint analysis. Sustainability 2019, 11, 3824. [Google Scholar] [CrossRef]
- Fortunati, S.; Morea, D.; Mosconi, E.M. Circular economy and corporate social responsibility in the agricultural system: Cases study of the Italian agri-food industry. Agric. Econ. 2020, 66, 489–498. [Google Scholar] [CrossRef]
- FAO; FIDA; OMS; PMA y UNICEF. El Estado de la Seguridad Alimentaria y la Nutrición en el Mundo 2021. In Transformación de los Sistemas Alimentarios en Aras de la Seguridad Alimentaria, Una Nutrición Mejorada y Dietas Asequibles y Saludables para Todos; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Loizia, P.; Voukkali, I.; Zorpas, A.A.; Navarro Pedreño, J.; Chatziparaskeva, G.; Inglezakis, V.J.; Vardopoulos, I.; Doula, M. Measuring the level of environmental performance in insular areas, through key performed indicators, in the framework of waste strategy development. Sci. Total Environ. 2021, 753, 141974. [Google Scholar] [CrossRef]
- Montanarella, L.; Panagos, P. The relevance of sustainable soil management within the European Green Deal. Land Use Policy 2021, 100, 104950. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Navarro-Pedreño, J.; Jeguirim, M.; Dimitriou, G.; Almendro Candel, M.B.; Argirusis, C.; Vardopoulos, I.; Loizia, P.; Chatziparaskeva, G.; Papamichael, I. Crisis in leadership vs waste management. Euro-Mediterr. J. Environ. Integr. 2021, 6, 80. [Google Scholar] [CrossRef]
- Parr, J.F.; Colacicco, D. Organic materials as alternative nutrient sources. In Energy in Plant Nutrition and Pest Control; Elsevier Science Publishers: Amsterdam, The Netherlands, 1987; Volume 4, pp. 81–99. [Google Scholar]
- Oueriemmi, H.; Kidd, P.S.; Trasar-Cepeda, C.; Rodríguez-Garrido, B.; Zoghlami, R.I.; Ardhaoui, K.; Prieto-Fernández, Á.; Moussa, M. Evaluation of Composted Organic Wastes and Farmyard Manure for Improving Fertility of Poor Sandy Soils in Arid Regions. Agriculture 2021, 11, 415. [Google Scholar] [CrossRef]
- Cavalli, E.; Lange, A.; Cavalli, C.; Behling, M. Decomposition and release of nutrients from crop residues on soybean-maize cropping systems. Rev. Bras. Cienc. Agrar. Recife 2018, 13, e5527. [Google Scholar] [CrossRef]
- Cole, J.C.; Smith, M.W.; Penn, C.J.; Cheary, B.S.; Conaghan, K.J. Nitrogen, phosphorus, calcium, and magnesium applied individually or as a slow release or controlled release fertilizer increase growth and yield and affect macronutrient and micronutrient concentration and content of field-grown tomato plants. Sci. Hortic. 2016, 211, 420–430. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Global Soil Doctors Programme—A Farmer-to-Farmer Training Programme. In Fertilizer Training Aid Modules; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Hossain, M.Z.; Von Fragstein und Niemsdorff, P.; Heß, J. Effect of Different Organic Wastes on Soil Properties and Plant Growth and Yield: A Review. Sci. Agric. Bohem. 2017, 48, 224–237. [Google Scholar]
- Rico Hernández, J.R.; Navarro-Pedreño, J.; Gómez Lucas, I. Evaluation of plant waste used as mulch on soil moisture retention. SJSS Span. J. Soil Sci. 2016, 6, 2. [Google Scholar] [CrossRef]
- Anwar, Z.; Irshad, M.; Fareed, I.; Saleem, A. Characterization and Recycling of Organic Waste after Co-Composting A Review. J. Agric. Sci. 2015, 7, 4. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Soils for Nutrition: State of the Art; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Oliver, M.A.; Gregory, P.J. Soil, food security and human health: A review. Eur. J. Soil Sci. 2015, 66, 257–276. [Google Scholar] [CrossRef]
- Deeb, M.; Groffman, P.M.; Blouin, M.; Egendorf, S.P.; Vergnes, A.; Vasenev, V.; Cao, D.L.; Walsh, D.; Morin, T.; Séré, G. Using constructed soils for green infrastructure—Challenges and limitations. Soil 2020, 6, 413–434. [Google Scholar] [CrossRef]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Guerra, F.; Trevizam, A.R.; Muraoka, T.; Chaves Marcante, N.; Canniatti-Brazaca, S.G. Heavy metals in vegetables and potential risk for human health. Sci. Agric. 2011, 69, 54–60. [Google Scholar] [CrossRef]
- Jiwan, S.; Ajay, K. Effects of Heavy Metals on Soil, Plants, Human Health and Aquatic Life. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Navarro García, G. Los Elementos Químicos y la Vida Vegetal. Química Agrícola. El Suelo y los Elementos Químicos Esenciales para la Vida Vegetal, 2nd ed.; Ediciones Mundi-Prensa: Madrid, Spain, 2003; pp. 135–150. [Google Scholar]
- Alloway, B. Heavy Metals in Soils Trace Metals and Metalloids in Soils and their Bioavailability, 3rd ed.; Springer: London, UK, 2013. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, T.; Navarro-Pedreño, J.; Gómez, I.; Jordán Vida, M.M.; Bech Borras, J.; Zorpas, A.A. Urban areas, human health and Technosols for the Green Deal. Environ. Geochem. Health. 2021, 43, 5065–5086. [Google Scholar] [CrossRef] [PubMed]
- Tella, M.; Doelsch, E.; Letourmy, P.; Chataing, S.; Cuoq, F.; Bravin, M.N.; Saint Macary, H. Investigation of potentially toxic heavy metals in different organic wastes used to fertilize market garden crops. Waste Manag. 2013, 33, 184–192. [Google Scholar] [CrossRef]
- Moral, R.; Navarro-Pedreño, J.; Gómez, I.; Mataix, J. Quantitative analysis of organic wastes: Effects of sample preparation in the determination of metals. Commun. Soil Sci. Plant Anal. 1996, 27, 753–761. [Google Scholar] [CrossRef]
- Jamroz, E.; Bekier, J.; Medynska-Juraszek, A.; Kaluza-Haladyn, A.; Cwielag-Piasecka, I.; Bednik, M. The contribution of water extractable forms of plant nutrients to evaluate MSW compost maturity: A case study. Nat. Sci. Rep. 2020, 10, 12842. [Google Scholar] [CrossRef] [PubMed]
- Milik, J.; Pasela, R.; Lachowicz, M.; Chalamoński, M. The concentration of trace elements in sewage sludge from wastewater treatment plant in Gniewino. J. Ecol. Eng. 2017, 18, 118–124. [Google Scholar] [CrossRef]
- Alonso, E.; Villar, P.; Santos, A.; Aparicio, I. Fractionation of heavy metals in sludge from anaerobic wastewater stabilization ponds in southern Spain. Waste Manag. 2006, 26, 1270–1276. [Google Scholar] [CrossRef]
- Sypalov, S.A.; Kozhevnikova, A.Y.; Ivanchenkoa, N.L.; Popovaa, Y.A.; Soboleva, N.A. Assessment of Peat Pollution by Heavy Metals Depending on the Depth of Occurrence. Solid Fuel Chem. 2020, 54, 32–36. [Google Scholar] [CrossRef]
- Borgulat, J.; Mętrak, M.; Staszewski, T.; Wiłkomirski, B.; Suska-Malawska, M. Heavy Metals Accumulation in Soil and Plants of Polish Peat Bogs. Pol. J. Environ. Stud. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Musa Ozcan, M.; Dursun, N.; Saglam, C. Heavy metals bounding ability of pomegranate (Punica granatum) peel in model system. Int. J. Food Prop. 2011, 14, 550–556. [Google Scholar] [CrossRef]
- Asam, Z.; Nieminen, M.; Kaila, A.; Laiho, R.; Sarkkola, S.; O’Connor, M.; O’Driscoll, C.; Sana, A.; Rodgers, M.; Zhan, X.; et al. Nutrient and heavy metals in decaying harvest residue needles on drained blanket peat forests. Eur. J. Forest Res. 2014, 133, 969–982. [Google Scholar] [CrossRef]
- Parzych, A.; Mochnacký, S.; Sobisz, Z.; Kurhaluk, N.; Pollákov, N. Accumulation of heavy metals in needles and bark of Pinus species. Folia For. Pol. Ser. A For. 2017, 59, 34–44. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Al-Yahyai, R.; Ahm, M. Heavy metal concentrationes in soils and date palm irrigated by groundwater and treated wastewater. Pak. J. Agric. Sci. 2015, 52, 129–134. [Google Scholar]
- Foereid, B. Nutrients Recovered from Organic Residues as Fertilizers: Challenges to Management and Research Methods. World J. Agric. Soil Sci. 2019, 1, 4. [Google Scholar] [CrossRef]
- European Union. Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, when Sewage Sludge is Used in Agriculture. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31986L0278 (accessed on 10 November 2022).
- Gobierno de España. Real Decreto 1310/1990, de 29 de Octubre, Por el Que se Regula la Utilización de los Lodos de Depuración en el Sector Agrario. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-1990-26490 (accessed on 10 November 2022).
- Gobierno de España. Real Decreto 865/2010, de 2 de Julio, Sobre Sustratos de Cultivo. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2010-11153 (accessed on 10 November 2022).
- European Union. Regulation 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32019R1009 (accessed on 10 November 2022).
- Van der Voet, E.; Salminen, R.; Eckelman, M.; Mudd, G.; Norgate, T.; Hischier, R. Environmental Risks and Challenges of Anthropogenic Metals Flows and Cycles, A Report of the Working Group on the Global Metal Flows to the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2013. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: New York, NY, USA, 2011. [Google Scholar]
- Paradelo, R.; Villada, A.; González, D.; Barral, M.T. Evaluation of the toxicity of heavy metals and organic compounds in compost by means of two germination-elongation tests. Fresenius Environ. Bull. 2010, 19, 5. [Google Scholar]
- Yi, X.Y.; Fang, L.; Yang, X.D.; Ma, L.F.; Liu, M.Y.; Zhang, Q.F.; Ni, K.; Shi, Y.Z. Status of heavy metal in organic fertilizers in main tea growing regions of China. Environ. Sci. 2022. [Google Scholar]
- Naz, S.; Fazio, F.; Habib, S.S.; Nawaz, G.; Attaullah, S.; Ullah, M.; Hayat, A.; Ahmed, I. Incidence of Heavy Metals in the Application of Fertilizers to Crops (Wheat and Rice), a Fish (Common carp) Pond and a Human Health Risk Assessment. Sustainability 2022, 14, 13441. [Google Scholar] [CrossRef]
- Hussain, M.I.; Khan, Z.I.; Akhter, P.; Al-Hemaid, F.M.; Al-Hashimi, A.; Elshikh, M.S.; Ahmad, K.; Yang, H.-H. Potential of Organic Amendments for Heavy Metal Contamination in Soil–Coriander System: Environmental Fate and Associated Ecological Risk. Sustainability 2022, 14, 11374. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Monitoring soil for sustainable development and land degradation neutrality. Environ. Monit. Assess. 2018, 190, 57. [Google Scholar] [CrossRef]
- De Vries, W.; Römkens, P.F.A.M.; Kros, J.; Voogd, J.C.; Schulte-Uebbing, L.F. Impacts of Nutrients and Heavy Metals in European Agriculture. Current and Critical Inputs in Relation to Air, Soil and Water Quality; European Topic Centre-Data Integration: Copenhagen, Denmark, 2022; p. 72. [Google Scholar]
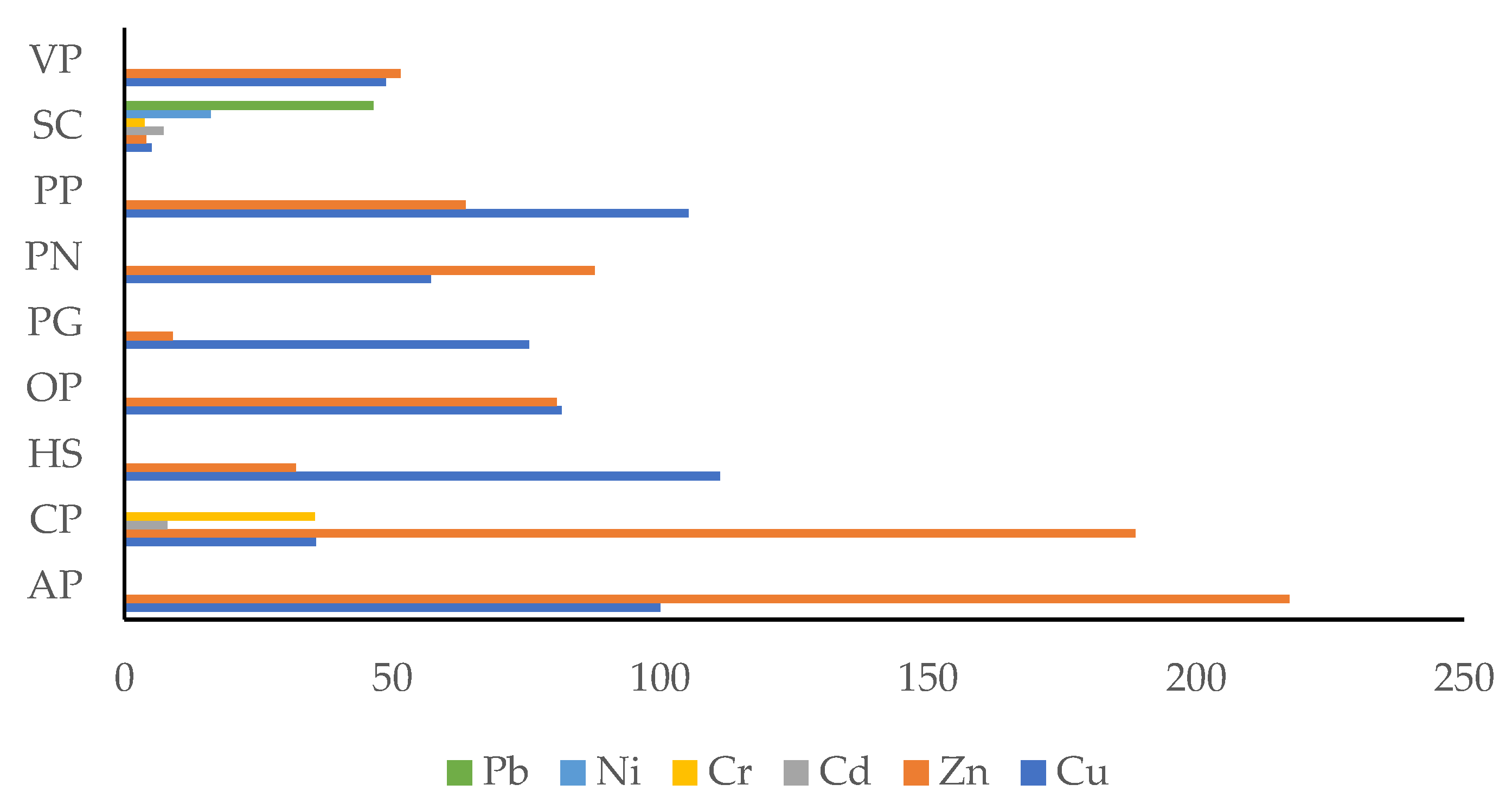

| Residue | Cu (mg kg−1) | Zn (mg kg−1) | Cd (mg kg−1) | Cr (mg kg−1) | Ni (mg kg−1) | Pb (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave. | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | |
| AP | 4.0 a | 0.3 | 4.6 a | 1.7 | nd | - | nd | - | nd | - | nd | - |
| CP | 11.2 | 1.4 | 5.3 ab | 0.7 | 0.629 | 0.005 | 2.8 | 0.4 | nd | - | nd | - |
| HS | 3.6 a | 0.1 | 31.3 | 3.7 | nd | - | nd | - | nd | - | nd | - |
| OP | 4.9 a | 0.3 | 12.4 c | 0.6 | nd | - | nd | - | nd | - | nd | - |
| PG | 5.3 a | 0.2 | 110.8 | 2.0 | nd | - | nd | - | nd | - | nd | - |
| PN | 7.0 a | 0.8 | 11.4 bc | 1.0 | nd | - | nd | - | nd | - | nd | - |
| PP | 3.8 a | 0.4 | 15.7 cd | 0.9 | nd | - | nd | - | nd | - | nd | - |
| SC | 79.7 | 14.6 | 249.5 | 8.4 | 0.686 | 0.108 | 26.4 | 0.7 | 6.2 | 0.5 | 10.8 | 1.9 |
| VP | 8.2 a | 1.3 | 19.4 d | 1.5 | nd | - | nd | - | nd | - | nd | - |
| F 1 | 100.4 *** | 2474 *** | ||||||||||
| Residue | Cu (mg kg−1) | Zn (mg kg−1) | Cd (mg kg−1) | Cr (mg kg−1) | Ni (mg kg−1) | Pb (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ave.σ | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | Ave. | Std. | |
| AP | 0.240 ab | 0.020 | 4.2 ab | 0.3 | nd | - | nd | - | nd | - | nd | - |
| CP | 0.091 a | 0.009 | 4.3 ab | 0.3 | nd | - | nd | - | nd | - | nd | - |
| HS | 1.024 de | 0.267 | 7.8 d | 1.9 | nd | - | nd | - | nd | - | nd | - |
| OP | 1.144 e | 0.187 | 5.6 bc | 0.6 | nd | - | nd | - | nd | - | nd | - |
| PG | 1.797 | 0.329 | 13.9 | 1.0 | nd | - | nd | - | nd | - | nd | - |
| PN | 0.437 b | 0.046 | 4.7 ab | 0.4 | nd | - | nd | - | nd | - | nd | - |
| PP | 0.819 cd | 0.105 | 10.5 | 0.6 | nd | - | nd | - | nd | - | nd | - |
| SC | 0.522 bc | 0.051 | 4.0 a | 0.2 | nd | - | nd | - | nd | - | 0.38 | 0.25 |
| VP | 1.116 de | 0.103 | 6.7 cd | 0.5 | nd | - | nd | - | nd | - | nd | - |
| F 1 | 45.0 *** | 89.6 *** | ||||||||||
| Residue | ICu | IZn | ICd | ICr | INi | IPb |
|---|---|---|---|---|---|---|
| AP | 6 | 90 | - | - | - | - |
| CP | 1 | 82 | - | - | - | - |
| HS | 29 | 25 | - | - | - | - |
| OP | 23 | 45 | - | - | - | - |
| PG | 34 | 13 | - | - | - | - |
| PN | 6 | 42 | - | - | - | - |
| PP | 21 | 67 | - | - | - | - |
| SC | 1 | 2 | - | - | - | 4 |
| VP | 14 | 35 | - | - | - | - |
| Substance | C.D. 86/278/EEC [45] and R.D. 1310/1990 [46] | R.D 865/2010 [47] | EU 2019/1009 [48] | |
|---|---|---|---|---|
| Soil pH < 7 | Soil pH > 7 | |||
| Cu | 1000 | 1750 | 70 | 200 (GM)/300 (OF, L, SI)/600 (OMF) * |
| Zn | 2500 | 4000 | 200 | 500 (GM)/800 (OF, L, SI)/1500 (OMF) * |
| Cd | 20 | 40 | 0.7 | 1.5 (GM, OF)/2 (L, SI)/3-60 1(OMF) * |
| Cr | 1000 | 1500 | 70 | 2 |
| Ni | 300 | 400 | 25 | 50 (GM, OF, SI, OMF)/90 (L) * |
| Pb | 750 | 1200 | 45 | 120 |
| Substance | C.D. 86/278/EEC [45] and R.D. 1310/1990 [46] | UNEP [49] | |||
|---|---|---|---|---|---|
| Soil pH < 7 | Soil pH > 7 | Threshold Value 1 | Lower Guide Value 1 | Higher Guide Value 1 | |
| Cu | 50 | 140/210 * | 100 | 150 | 200 |
| Zn | 150 | 300/450 * | 200 | 250 | 400 |
| Cd | 1 | 3 | 1 | 10 | 20 |
| Cr | 100 | 150 | 100 | 200 | 300 |
| Ni | 30 | 75/112 * | 50 | 100 | 150 |
| Pb | 50 | 300 | 60 | 200 | 750 |
| Residue | Elemental Composition (kg ha−1) | Aqueous Extract (kg ha−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Cd | Cr | Ni | Pb | Cu | Zn | Cd | Cr | Ni | Pb | |
| AP | 0.120 | 0.138 | - | - | - | - | 0.007 | 0.126 | - | - | - | - |
| CP | 0.336 | 0.159 | 0.019 | 0.085 | - | - | 0.003 | 0.129 | - | - | - | - |
| HS | 0.108 | 0.939 | - | - | - | - | 0.031 | 0.234 | - | - | - | - |
| OP | 0.147 | 0.372 | - | - | - | - | 0.034 | 0.168 | - | - | - | - |
| PG | 0.159 | 3.324 | - | - | - | - | 0.054 | 0.417 | - | - | - | - |
| PN | 0.210 | 0.342 | - | - | - | - | 0.013 | 0.141 | - | - | - | - |
| PP | 0.114 | 0.471 | - | - | - | - | 0.025 | 0.315 | - | - | - | - |
| SC | 2.391 | 7.485 | 0.021 | 0.793 | 0.186 | 0.323 | 0.016 | 0.120 | - | - | - | 0.011 |
| VP | 0.246 | 0.582 | - | - | - | - | 0.033 | 0.201 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Espinosa, T.; Navarro-Pedreño, J.; Gómez Lucas, I.; Almendro Candel, M.B.; Pérez Gimeno, A.; Jordán Vidal, M.; Papamichael, I.; Zorpas, A.A. Environmental Risk from Organic Residues. Sustainability 2023, 15, 192. https://doi.org/10.3390/su15010192
Rodríguez-Espinosa T, Navarro-Pedreño J, Gómez Lucas I, Almendro Candel MB, Pérez Gimeno A, Jordán Vidal M, Papamichael I, Zorpas AA. Environmental Risk from Organic Residues. Sustainability. 2023; 15(1):192. https://doi.org/10.3390/su15010192
Chicago/Turabian StyleRodríguez-Espinosa, Teresa, Jose Navarro-Pedreño, Ignacio Gómez Lucas, María Belén Almendro Candel, Ana Pérez Gimeno, Manuel Jordán Vidal, Iliana Papamichael, and Antonis A. Zorpas. 2023. "Environmental Risk from Organic Residues" Sustainability 15, no. 1: 192. https://doi.org/10.3390/su15010192
APA StyleRodríguez-Espinosa, T., Navarro-Pedreño, J., Gómez Lucas, I., Almendro Candel, M. B., Pérez Gimeno, A., Jordán Vidal, M., Papamichael, I., & Zorpas, A. A. (2023). Environmental Risk from Organic Residues. Sustainability, 15(1), 192. https://doi.org/10.3390/su15010192











