Abstract
Corn (Zea mays L.) is one of the major cereal crops cultivated worldwide. Zinc and selenium are important nutrients for humans and plants, and their deficiency is a cause for concern in most developing countries. Sweet corn fertilized with zinc and selenium can mitigate this problem. Therefore, the objective of this study was to investigate the effects of fertilization with Zn and Se on the yield and quality of sweet corn varieties under different planting densities. The experimental design used was a split-plot based on a randomized complete block design with three replications. Compared to the control, significant differences were recorded in grain yield, leaf area index, and plant height (i.e., Zn/Se + density + variety) treatments. Non-significant differences in the number of kernels per cob, sugar content and crude protein were recorded under different treatments. Significant differences in grain yield, water-soluble sugar, and zinc and selenium content in grain were recorded. Grain yield was higher in Selenium than in Zinc treatments, with a mean difference of 0.05 t ha−1. We conclude that grain yield and selenium content in grain were influenced by selenium foliar application, while water-soluble sugar and zinc content in grain were influenced by foliar zinc application.
1. Introduction
Cereal crops such as corn (Zea mays L.), rice, and wheat account for 45% of the world’s edible dry matter and up to 60% of the daily caloric intake in various developing countries [1]. It has remained a staple food crop worldwide for an extended period [2]. About 94 developing countries in the world get their food calories from corn, wheat, and rice. According to an estimate, about 900 million people out of 4.5 billion people are poor consumers, among whom corn is the most commonly used staple food [3,4]. Poor diet and micronutrient deficiency of zinc and selenium appear to be a big problem in developing countries, leading to food insecurity and low quality food [5,6]. A lack of 51 essential nutrients in the human body can lead to metabolic issues, health complications, malnutrition, and social costs in the community [7,8,9,10]. Micronutrient deficiency has an enormous negative effect on the food and feed production chain [11].
Micronutrient availability for plant uptake relies on different factors, i.e., soil type and crop variety [12]. In China, micronutrient content in soils is very low due to the dominance of calcareous and alkaline soils, and 40% of the land has a micronutrient deficiency. These micronutrient deficiencies, including zinc and selenium, affect crop yield and nutritional value in sweet corn production [13,14,15].
Micronutrients are necessary for plant growth and development because micronutrients help increase grain yields while also improving grain nutrient quality [16,17]. Micronutrients can boost grain yield by 50% while improving macronutrient utilization [18,19]. As a result, micronutrients can play an important role when combined with other nutrients to achieve optimal results. In various cropping zones, specific mineral nutrients, supplemented as chemical fertilizer, have become particularly important for plant growth and production [20]. Plants need zinc and selenium as essential nutrients for their standard and healthy growth and development. The ability to select appropriate genotypes based on genetic differences in Zn uptake between crop varieties and species could be a promising approach to the Zn problem. A foliar spray of Zn and selenium fertilizer improves Zn and selenium concentration in grain [21,22].
Sweet corn throughout the world is widely grown with many adaptable species, which can be grown in all environmental conditions, from arid to very humid areas. Because genes influence the synthesis of endosperm and its use as a vegetable, some species differentiate it from other crops [23].
Sweet corn (Zea mays L.) is a cereal vegetable crop that is highly needed worldwide due to its higher kernel protein, oil, starch, and sugar content compared to other corn types [24]. Sweet corn has mutations that prevent starch synthesis in kernels, which leads to the buildup of a significant quantity of soluble sugars, such as fructose, sucrose, and reducing sugars [25]. Sweet corn is famous for its taste and nutritional value. Its perishable crop products are consumed fresh when in the milking stage or used in the processing industry, with corn kernels canned or used as frozen food immediately after harvest. Sweet corn is rich in selenium, chromium, zinc, copper, nickel, and iron micronutrients [26]. Creamy texture, sweetness, a pleasing aroma, high yield, kernel color, and germination are a few of the best characteristics of sweet corn [27]. Sweet corn’s physiological stages need good water and nutrient supply to achieve high yields [28]. Due to its specialty, sweet corn is well suited for human consumption [29].
In the case of sweet corn, selecting a suitable variety is significant because it impacts sweetness, cob weight, cob length, plant length and sugar content, flavor, texture, etc. [30]. It has also been reported that this variety can cause more noticeable changes in the simulation of ecosystem exchange and latent heat flux [31].
Selenium (Se) is an essential element of the glutathione peroxide component of sweet corn because it can eliminate free radicals and peroxide in cells. Selenium is also a constituent of many other enzymes and proteins [32]. Selenium has physical-chemical and antioxidative properties concerning its reaction to promote plant growth. Selenium’s low concentration encourages plant growth, adding up to the activities of the antioxidant enzymes due to its antioxidant system capacity [33]. Growth stimulation is performed with the selenium element at a low level. At elevated levels, selenium appears to be toxic to many plants. This is due to the non-specific incorporation of selenium into sulfur compounds and oxidative stress. While selenium deficiency is common in areas with low soil selenium, it appears toxic in areas with high soil selenium [34]. Species, phases of development, and physiological conditions are the main cut points of selenium distribution in different plant parts [35].
Several studies have found that applying zinc to plants significantly impacts plant life, consequently increasing the production of carbohydrates and proteins by enhancing the growth of root systems [32]. During periods of drought, this helps the plant to thrive by maximizing the use of soil humidity [36]. Zinc is essential in pollination; the seed sets up water and nutrient transportation from root to shoot, whereas a deficiency results in a reduction in seed formation and yield production [37,38]. Zinc is an enzyme activator, and as a result, many metabolic processes are activated. Zinc helps in nitrogen uptake and influences protein quality, carbon anhydrase activity, and abiotic and biotic stress resistance [5,28,39]. Many researchers worldwide are working to combat zinc deficiency, especially in arid and semi-arid corn, wheat, and rice-growing regions [40,41]. At its early stage, sweet corn is susceptible to biotic and abiotic stress resulting in grain yield reduction [42,43]. Zinc foliar application is a quick and straightforward way of rectifying the plant nutrition status for wheat and maize [44,45]. Using an external supply of zinc can also boost the yield potential of the maize crop. Zinc foliar application promotes maize grain accumulation, and zinc concentration in maize grain correlates with zinc concentration in leaves [46].
Planting density affects grain yield, canopy photosynthesis, and biomass accumulation. It improves solar radiation timing interception. It improves the interval-to-interval interception of solar radiation. When planting density reaches its maximum, grain yield tends to decline, and this is due to the high interplant competition, which limits the supply of carbon and nitrogen and ultimately decreases the kernel number. Hybrid corn has a stable yield for a wide range of planting densities due to tillers that can adjust the yield [47]. Growth and development traits are affected by plant density, with low density plants showing greater photosynthetic CO2 assimilation and higher stomatal conductance. Sustainable crop production can be achieved by maximizing plant density, employing knowledge-based practices, and using efficient fertilizers [48]. A high yield response to higher density is recommended if the maize yield potential is high [49,50,51]. To better balance and synchronize nutrient delivery and crop demands, the 4R principle can be applied to knowledge-based fertilizer use approaches at the correct rate, time, product, and placement. All these combined strategies are effective in yielding [52]. At the same time, high planting density favors taller plants and has fewer tillers per plant, lower fresh shoot biomass, and a greater leaf area index [53]. An increase in planting density is primarily attributed to the rise in yield rather than rising per plant yield, and this is because per plant yield potential remains the same while maize achievement at high planting density improves [54]. Sweet corn yield and quality can be increased by increasing the fertilization amount and density. Previously, research focused on this issue. Few studies have examined the impact of foliar zinc, selenium, and planting density on the quality of a crop’s yield. As a result, this study examined the impact of foliar zinc and selenium applications on sweet corn variety yield and quality at various planting densities.
2. Materials and Methods
2.1. Experimental Location
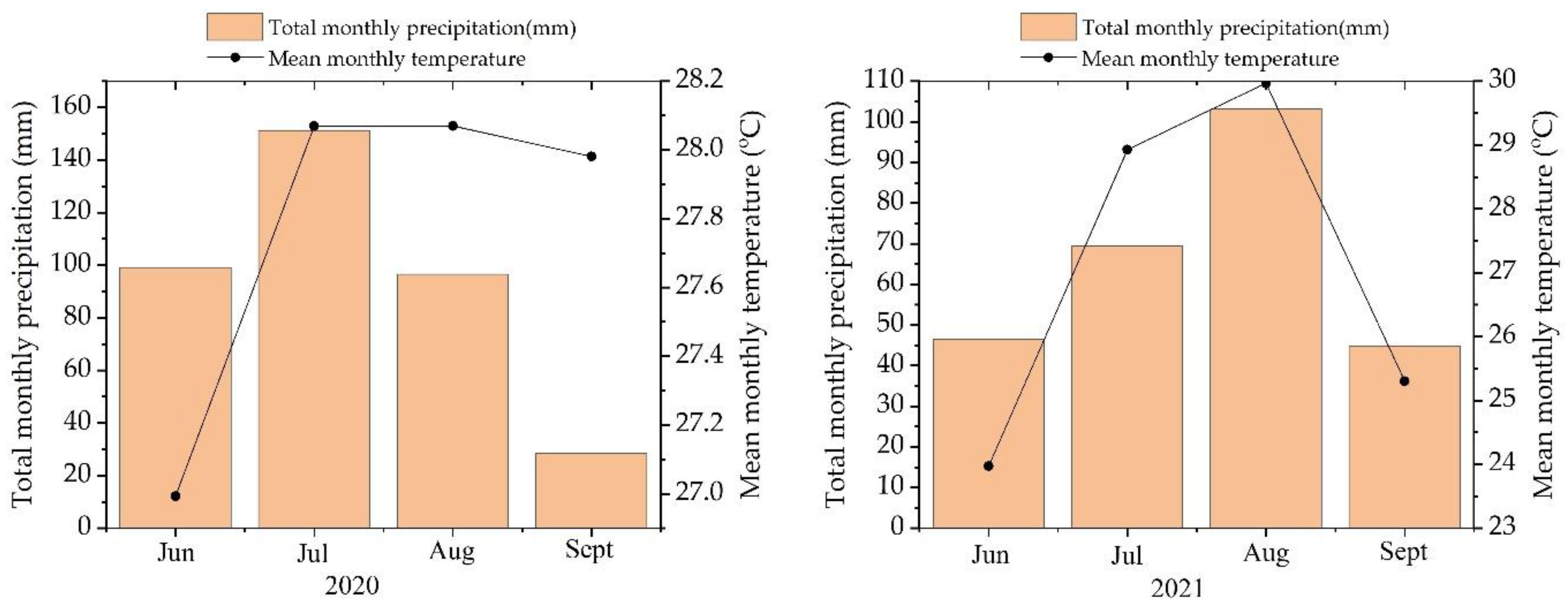
Field experiments were conducted during the summer growing seasons of 2020 and 2021 at the Doukou wheat and corn demonstration research station of Northwest Agriculture and Forestry University, Jingyang County, Xianyang City, Shaanxi Province, China (34°36′ N, 108°52′ E). The area’s altitude is 427.4 m. During the trial period for seasons 2020 and 2021, the mean temperature was 27.7 and 27.04 °C, respectively. The mean precipitation was 93.8 mm for the 2020 season and 65.98 mm for 2021 (Figure 1). A loam soil type was used in the experiment’s location. Seedbed preparation included ploughing and harrowing. Soil samples were taken from five randomly chosen locations before planting. A soil auger was used to drill three times at a depth of 0–20 cm for each point. Samples were air-dried, ground, and sieved before being stored in special bags. The chemical properties of later samples were examined [55], determining the effective components of organic matter, pH, available nitrogen, phosphorus, potassium, zinc, and selenium. The total soil carbon was determined by the Walkley–Black method using potassium dichromate, and the organic matter was calculated by multiplying the total carbon by 1.724. pH was determined by a pH meter. Soil total nitrogen was determined by sodium chloride extraction and the Kjeldahl method. The Olsen method was used to determine the amount of phosphorous available. Available phosphorous was extracted from soil by shaking at 180 rpm for 30 min with a 0.5 M sodium bicarbonate (NaHCO3) solution. After filtering the extractant (150 mm MN 619 G filter paper), the P concentration in the extract was determined using an 882-nm spectrophotometer. Available potassium was extracted from soil by shaking at 200–300 rpm for 30 min with a 1 N ammonium acetate (NH40 Ac) solution. After filtering the extractant, the K concentration of the extract was determined using a flame photometer set to 767 nm. DTPA–TEA–AAS determined the available Zn and Se. As a result, the total nitrogen content was 1.46 g kg−1, available phosphorus content was 17.69 mg kg−1, available potassium content was 189.2 mg kg−1, organic matter content was 18.02 g kg−1, pH 7.9, available zinc content was 0.18 mg kg−1, and total selenium content was 0.10 mg kg−1; see Table 1.
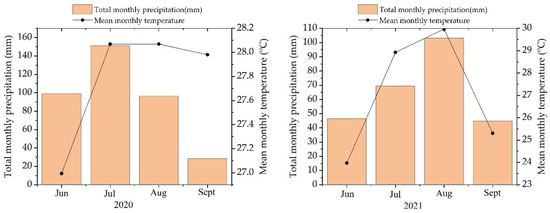
Figure 1.
Meteorological data: Total monthly precipitation and mean monthly temperature; the left side is season 1, and the right side is season 2.

Table 1.
Chemical properties.
Test Materials
Corn varieties Shan tian 2012 (corn varieties bred by Northwest A & F University) and Cai tian nuo 2012 (provided by the Special Corn Research Laboratory of the Agricultural College of Northwest Agriculture and Forestry University). The micro-fertilizers tested were zinc sulfate heptahydrate and selenium-rich ion fertilizer.
2.2. Experimental Design and Treatment
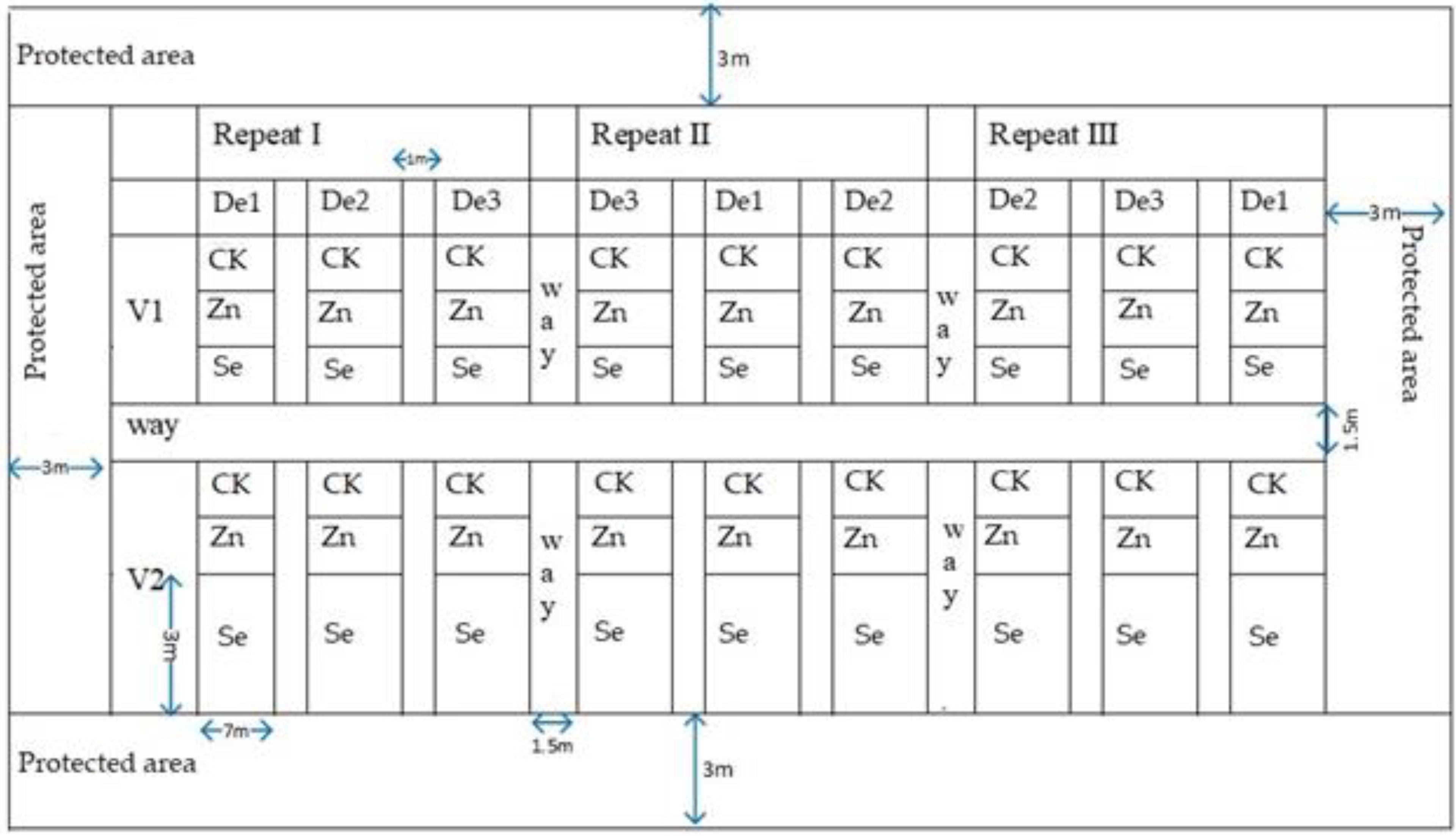
A split-plot based on a randomized complete block design was used for the experimental design. The main areas of each variety were designed with three planting densities (D1), 3.75 × 104 plants ha−1, (D2), 4.4 × 104 plants ha−1, and (D3), 5.25 × 104 plants ha−1. Their copulation was maintained through plant spacing, whereby sweet corn’s sexual expression is strongly influenced by the length of the daily illumination period. It divides sexes into different flowers on the plant, a condition known as monoecy, making outcrossing and copulation easier [56,57]. The trace elements zinc and selenium, as a sub-area, were foliar sprayed on the leaf surface. Water spraying was used as a control. The plot size was 7 m × 3 m per treatment, with 60 cm row spacing. Plant spacings varied between 46 cm, 39 cm, and 33 cm to adjust the density Figure 2. Rotary tillage was conducted. Phosphate fertilizer used diammonium phosphate (N-P2O5-K2O); the ratio was 16:44-0, and the dosage was 375 kg ha−1 before sowing. Nitrogen fertilizer was urea (nitrogen-containing 46.4%, 300 kg ha−1), pressed before the sowing and jointing stages, respectively. The zinc and selenium were sprayed on leaf surfaces, and the concentration of zinc was 2 g L−1. Selenium fertilizer was used according to the instructions for selenium-rich ion fertilizer (fertilizer and water 1:500 preparation). The spraying was performed when plants were at the six-leaf, eight-leaf, and ten-leaf stages. Spraying was performed in calm conditions to reduce evaporation and splashback. The zinc sulfate heptahydrate and selenium-rich ion fertilizer rates were 12 kg ha−1 and 12 L ha−1, respectively. Seed sowing was performed using a hand drill machine, calibrated to plant three seeds per drill. Uniform weeding was performed in plots throughout the crop seasons of 2020 and 2021. Field management was performed according to the actual local production.
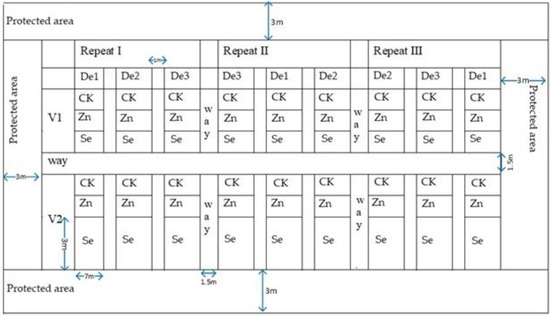
Figure 2.
Field layout. CK: Control, Zn: zinc, Se: selenium, V1: variety 1, V2: variety 2, De1: planting density 1, De2: planting density 2, De3: planting density 3.
2.3. Sampling Procedures and Analysis
In determining leaf area and plant height, six representative sweet corn plants were selected at tassel and maturity stages to determine leaf area and plant height. The length of the midrib and the width of the leaf were measured with a ruler, and the area of a single leaf was obtained by the coefficient method.
In principle growth stages 7 and 8, the fresh-eating of sweet corn, the number of ears in the 3-m sample section in the middle of each plot was counted. The total fresh ears were weighed to calculate the yield of fresh ears; 10 representative ears were selected from each treatment, counting the number of kernels per cob, and the fresh kernels were shelled off. After weighing, the fresh grain yield was calculated.
At the maturity stage, plant height was recorded in the field. Six plants from each plot were selected. A tape measure was used to measure each plant’s height and record the measurement.
The Kjeldahl procedure determines crude protein using a K9840 Kjeldahl Analyser (Hanon Shandong Scientific Instruments Co., Ltd., Jinan, Shandong, China) [58]. There are three important steps in this process: For the first 90 min, the sample was digested in 380 °C boiling concentrated H2SO4 before the catalyst was introduced and allowed to dissolve and oxidize completely. Ammonium sulfate, a byproduct of the nitrogen transformation, was produced. The ammonia was subsequently determined with a volumetric acid solution or by back titration, and the crude protein content was calculated by multiplying the total N by the amount of NaOH solution (lye) [59].
The sugar Brix refractometer, “Brix”, refers to a measurement scale for soluble solids in a liquid. Sweet corn sugar content is one of the factors for quality, and Brix performs the measurement of this quality in a very simple and rapid way to show sucrose content. Fresh kernel in the amount of 12 g from six ears was squeezed to extract sweet corn milk. Then, sugar content was measured after adding the extracted sweet corn milk to the Brix refractometers and recording the result, which was calibrated automatically by the Brix refractometers [39].
Water-soluble sugar content was determined by the Anthrone method: In an anthrone reaction rate assay, mix a sample of 0.5 to 10 g (or dry sample powder 5–1000 mg), put it in a large test tube with 15 mL of distilled water, boil in a boiling water bath for 20 min, remove, cool, and filter the residue several times with distilled water in a 100 mL volumetric flask, constant volume to scale [40].
Zn concentrations were determined using atomic absorption spectrometry (AA320 CRT; Shanghai Analytical Instrument Overall Factory, Shanghai, China) [60]. Material was steeped in nitric acid and then cleaned with deionized water before use. Grains were mixed in a mixer mill (Retsch MM400 GmbH, Haan, Germany). Samples in crucibles were heated until smoke stopped, then placed in a 550 °C furnace for 6 h. Soaked in 5 mL of 1:1 HNO3, the ash was then cleaned and put into a 50 mL volumetric flask with deionized water. Finally, the test solution was measured by atomic absorption spectrometry.
Grains were mixed in a mixer mill (Retsch MM400 GmbH). Thermo Elemental SOLAAR M6 atomic absorption spectrometry was used to measure the selenium level in grain flour following mineralization in HNO3 and HClO4 [61].
2.4. Data Processing
Data were analyzed using SPSS 26.0 and Origin Pro 2021 to make graphs, while the Duncan test method was used for multiple comparisons and significance difference analysis.
3. Results
3.1. Grain Yield, Yield Parameters, and Nutritional Values
The variety affected grain yield, leaf area, plant height, zinc, selenium, and sugar content, while kernels per cob, crude protein, and water-soluble sugar were unaffected (Table 2). Furthermore, grain yield, leaf area, plant height, water-soluble sugar, sugar content, zinc, and selenium were significantly affected by trace elements. Planting density significantly affected leaf area, while grain yield, kernel per cob, plant height, crude protein, water-soluble sugar, zinc, selenium, and sugar content were not significantly affected. In the cases of grain yield, kernel per cob, and leaf area, no significant interactions between variety and planting density were recorded, but it significantly affected zinc. Also, the interaction between trace elements and density affects zinc. Interaction between varieties, trace elements, and planting density significantly affected water-soluble sugar and zinc.

Table 2.
Analysis of variance on yield parameters and nutritional value during two cropping seasons: 2020 and 2021.
For both varieties (Cai tian nuo 2012 and Shan tian 2012) and planting densities, i.e., D1 (3.75 × 104 plants/ha−1), D2 (4.5 × 104 plants/ha−1), and D3 (5.25 × 104 plants/ha−1), there were statistically significant differences between mean grain yields, leaf areas, and plant heights in zinc and selenium treatments as compared to control treatments for both crop seasons, i.e., 2019–2020 and 2010–2021, while there was no statistical difference between the mean kernel per cob in all treatments, varieties, and planting densities for the summer cropping seasons, i.e., 2020 and 2021; see Table 3.

Table 3.
Grain yield, leaf area, plant height, and kernel per cob as affected by foliar application, variety, and planting density.
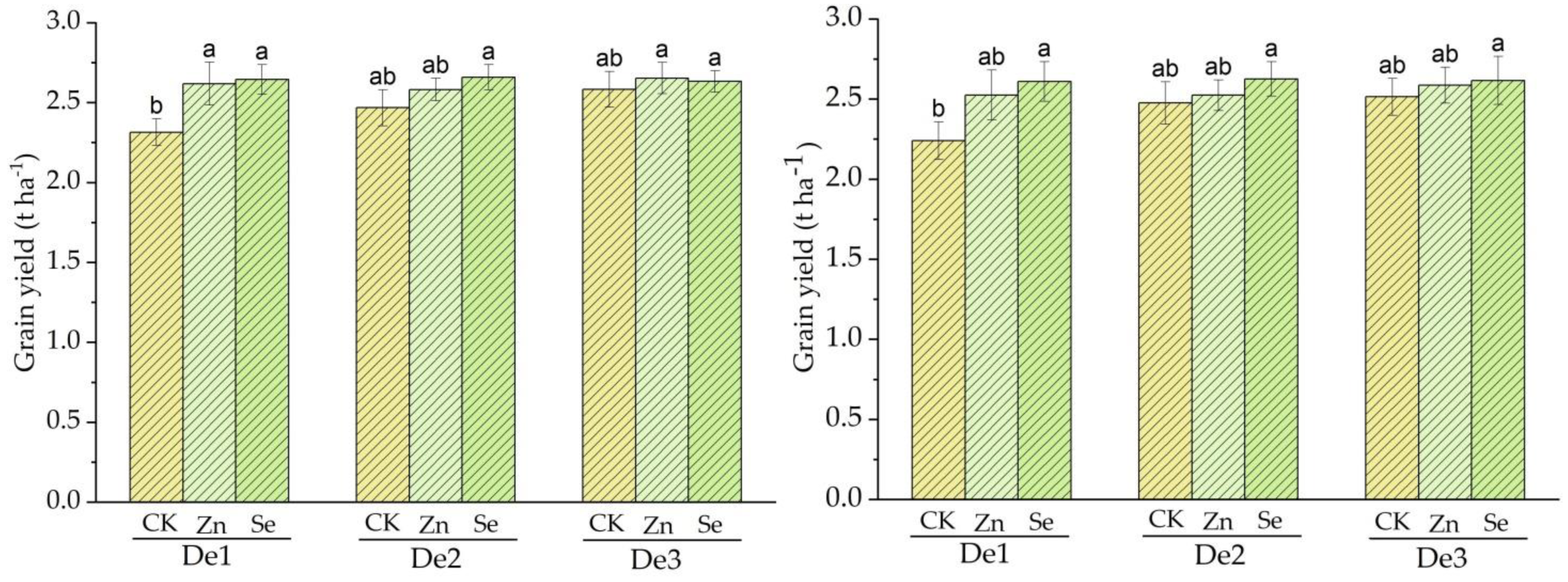
During crop season 2020, the mean grain yields for the control, Zn, and Se treatments were 2.3, 2.61, and 2.64 t ha−1, respectively, in a planting density of 3.75 × 104 plants/ha−1 (DE1). The main grain yield for the control, Zn, and Se treatments was 2.46, 2.59, and 2.66 t ha−1 for a planting density of 4.5 × 104 plants/ha−1 DE2, while the planting density of 5.25 × 104 plants/ha−1 DE3 in the control, Zn, and selenium treatments was 2.58, 2.65 and 2.63 in the first and third treatments, i.e., planting density one (DE1) and two (DE2). The control was different from the zinc and selenium treatments, while the zinc and selenium treatments showed a slight difference from each other.
During crop season 2021, the mean grain yields for the control, Zn and Se treatments were 2.24, 2.52 and 2.61 t ha−1, respectively, in a planting density of 3.75 × 104 plants ha−1 for DE1; the main grain yields for the control, Zn, and Se treatments were 2.52, 2.62 and 2.66 t ha−1 for a planting density of 4.5 × 104 plants ha−1 DE2, while the planting densities of 5.25 × 104 plants ha−1 for DE3 in the control, Zn, and Selenium treatments were 2.51 t ha−1, 2.58 t ha −1 and 2.61 t ha −1, respectively. The second and third planting densities for the control and zinc were different from those for selenium, while for planting density one (D1), the control, zinc, and selenium were different from one another. Compared to the mean grain yield values of seasons one and two, 2021 and 2021, those of season one were higher than those of season two; see Figure 3.
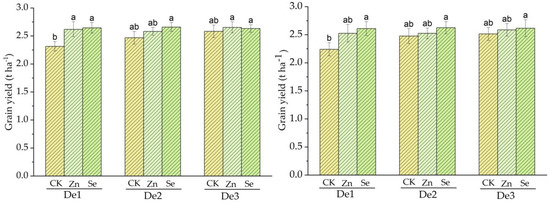
Figure 3.
Treatment effect on grain yield for both cropping seasons; the left side represents season 2019–2020, and the right side represents season 2010–2021. DE1: planting density 1. DE2: planting density 2, DE3: planting density 3, Zn: Zinc, Se: Selenium CK: control. The error bars are the standard error of the mean. Different letters mean there is a statistical difference at p < 0.05.
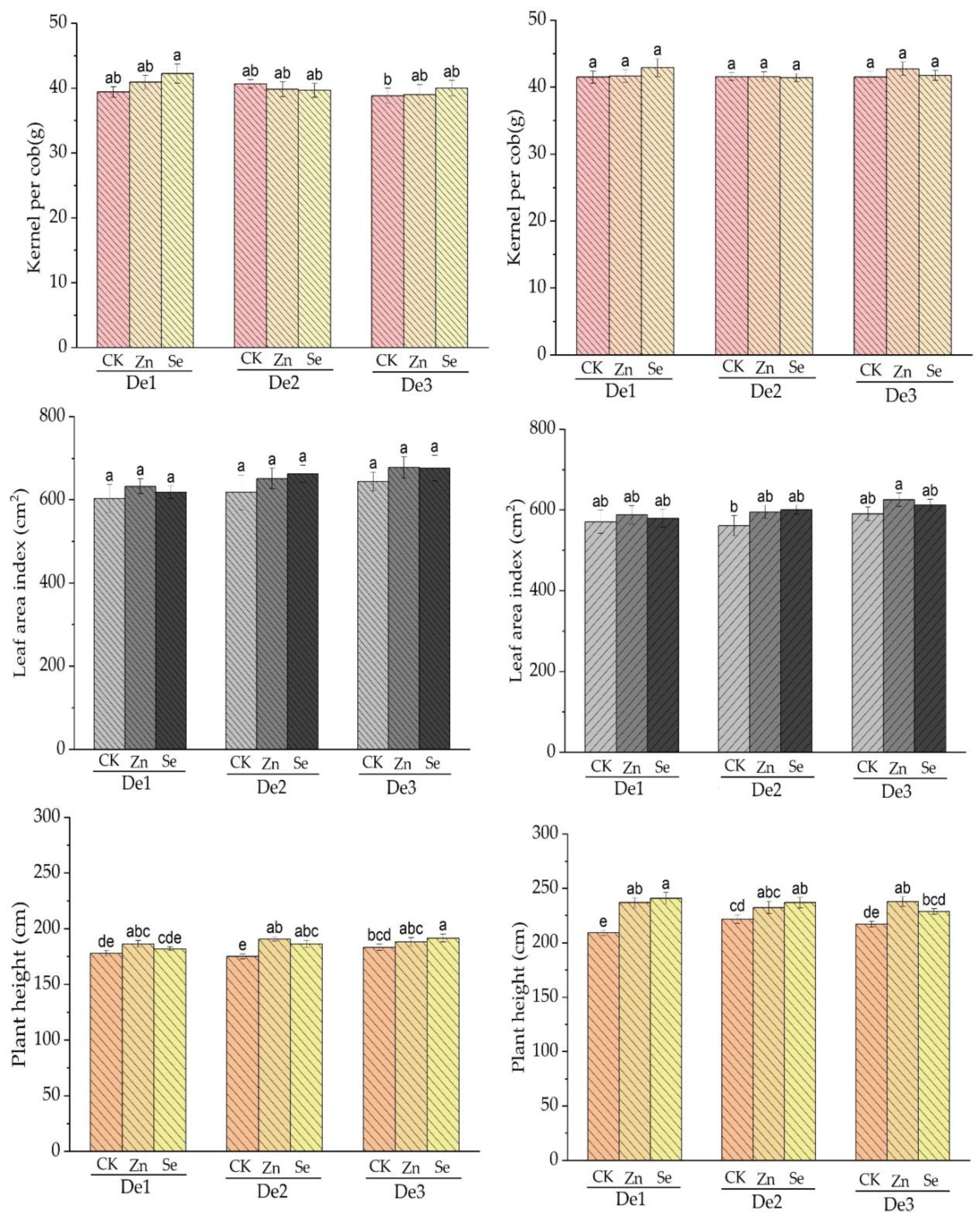
The zinc and selenium treatments had no effect on the number of kernels per cob in crop seasons 2020–2021. For the first crop season (2019–2020) and second crop season (2010–2021), there were no significant variations in leaf areas across treatments in terms of planting density two and three (D2, D3). The zinc and selenium treatments had effects on leaf area. In the case of both crop seasons, i.e., 2019–2020 and 2010–2021, plant heights were positively affected by the zinc and selenium treatments, where the zinc treatment appeared to have more effect than the selenium treatment; see Figure 4.
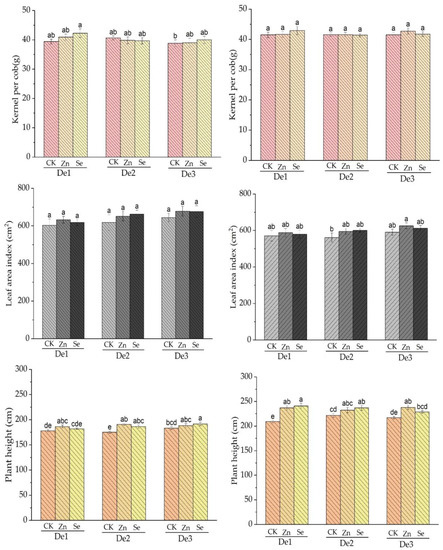
Figure 4.
Effect of the applied treatment on kernel per cob, leaf area index, and plant height for both crop seasons; the left side represents season 2019–2020, and the right side represents season 2010–2021. DE1: planting density 1, DE2: planting density 2, DE3: planting density 3, CK: control, Zn: zinc, Se: selenium. The error bars are the standard error of the mean. Different letters mean there is a statistical difference at p < 0.05.
3.2. Nutritional Values
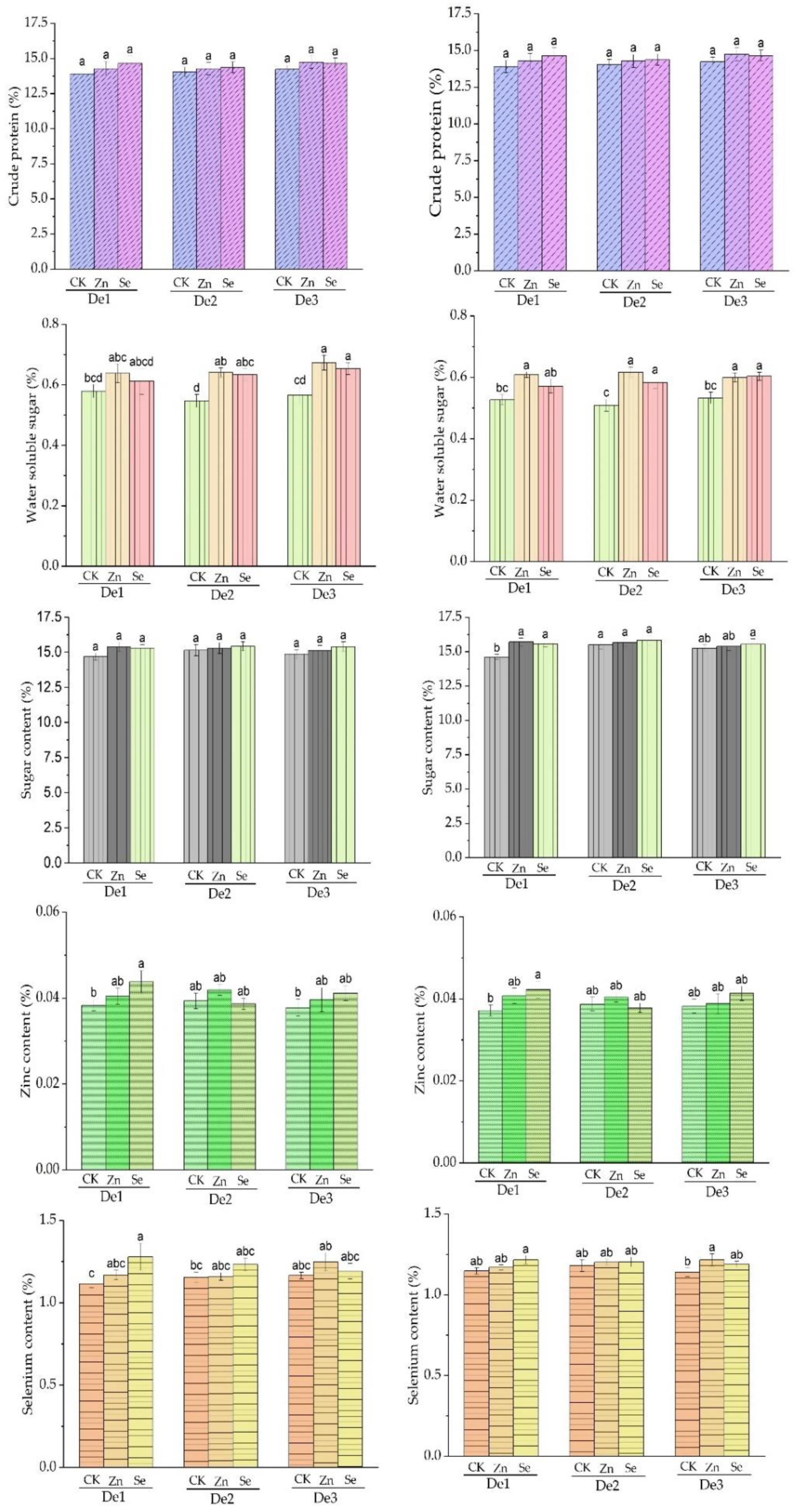
During the 2020 crop season, the foliar application of zinc and selenium was recorded as having statistically significant differences between the means of water-soluble sugar within varieties and planting density, while crude protein and sugar content were not significantly affected when compared to the control treatment. In the crop season of 2021, there were statistically significant effects on crude protein and sugar content of the zinc and selenium treatments, while there were no significant effects on water-soluble sugar: Table 4 and Figure 5.

Table 4.
Crude protein, water-soluble sugar, zinc, selenium, and sugar content are affected by foliar application, variety, and planting densities in seasons 2020 and 2021.
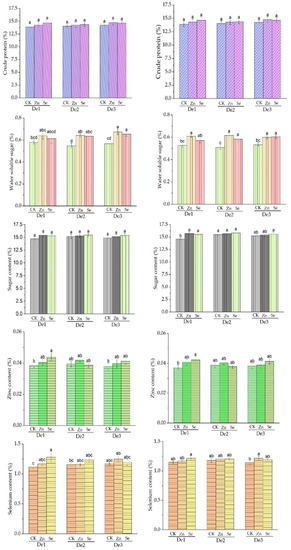
Figure 5.
Crude protein, water-soluble sugar, and sugar contents as affected by the treatment. DE1: planting density 1, DE2: planting density 2, DE3: planting density 3, CK: control, Zn: zinc, Se: selenium. The error bars are the standard error of the mean. Different letters mean there is a statistical difference at p < 0.05.
4. Discussion
This study shows that some statistically significant effects on grain yields were recorded under the different foliar applications of zinc and selenium fertilizer treatments during different cropping seasons. Compared to the second crop season, slightly higher grain yields were recorded in the first cropping season. This variation may be due to seasonal climate change [38].
In both crop seasons, applying zinc and selenium treatments increased grain yields compared to the control treatment. During the first cropping season, grain yields were seen to be more positively affected than during the second cropping season. This reveals that zinc treatment can increase maize grain yield. Potarzycki et al. [62,63] also reported that zinc influences grain yield. Furthermore, as seen in Table 3, selenium also impacted grain yield, as Nawaz et al. [64] reported. Foliar application of Se lowers osmotic potential, which improves turgor pressure and antioxidant system activity and thus increases grain yield [22].
Variety two (Table 3) of both seasons of zinc treatment shows an increase in grain yield as planting density increases. Previous studies also show that an increase in planting density increases grain yield, as reported by Farnham et al. [65], while Gozubenli et al. [66] reported that grain yields increased with increasing planting densities up to 90,000 plants ha−1. A further increase in planting densities decreased the grain yields.
Furthermore, zinc and selenium have a significant role in plant and human life, and zinc affects nitrogen metabolism, photosynthesis, and resistance to abiotic and biotic stresses [63], while plants’ tolerance to UV-induced oxidative stress is enhanced by selenium, which delays senescence and increases growth [67].
The kernel number per cob was not significantly affected by any other treatment during either of the crop seasons. There were slight differences in all treatments and seasons, even in their statistical mean values. This can be corroborated by the study carried out by Germ and Stibilj (2007). Testa et al. [68] also showed that higher planting density negatively affects kernel number and weight per row.
Variety, trace elements, and planting density significantly affected plant height. A slight increase in plant height with increasing plant density was recorded for both crop seasons (Table 3). A previous study by Shelton et al. [69] also reported that plant height increases with higher density. It has also been reported that the application of zinc has significant effects on plant height, such as in Hisham et al. [70]. Similar studies on the effect of selenium have been written by Naseem et al. [71], while the effect of variety on plant height was reported by Fahrurrozi et al. [72].
During both crop seasons, the application of zinc significantly affected the leaf area index (Table 3). During the second crop season, the leaf area recorded was 496.1 cm2, while in the first crop season, the leaf area recorded was 494.7 cm2. The highest value of 707.8 cm2 was recorded in the second crop season, indicating that zinc application increased nitrogen, which increased leaf area. The results are similar to those of Peddapuli et al. [73]. It has also been reported that the application of zinc increases amino acids, tryptophan, and IAA, which ultimately contribute to leaf area expansion [74,75]. In the 2019/2020 season, the leaf area index was not significantly affected, but in season 2020/2021, plant density two and planting density three were significantly affected (Figure 4), indicating that increasing planting density has an impact on the leaf area index. This result is in agreement with the findings of Abuzr et al. [76]. The application of zinc and selenium treatments did not significantly affect the crude protein in either crop seasons compared to the control treatment. During the second crop season, a higher value (15.65%) with zinc application was recorded in planting density three (Table 4). This slight increase in crude protein may be due to seasonal changes compared to the previous season. Zinc is an essential source of protein metabolism [41] and stability due to the vital involvement of zinc in protein synthesis [77]. Planting density had a negative impact on crude protein because it increased nutrient competition, which resulted in lower crude protein levels. Both zinc and selenium treatments did not affect sugar content in the first cropping season. In contrast, in the second cropping season, it was significantly affected. Still, the statistical means show that the highest value was in the first season with variety two, treated with selenium and planting density two. The lowest value, 1.402%, was recorded in season one, treated with water (control) and planting density one. With all that, the influence of variety on sugar content was observed and the results were similar to the previous findings of Li L et al. [30], according to which, sugar content is influenced by the variety and harvesting time. Similar results have also been reported by Haddadi et al. [78]. It has also been reported that differences in varieties and harvesting times affect sugar content, while a delay in harvesting time increases starch content. Zinc and selenium both had positive effects on the sugar content of sweet corn with slight differences in each season, which were, however, not statistically significantly different from each other. The results are similar to the findings of Adamec et al. [79], according to which, the application of zinc at a dose of 30 mL in 10 L of water does not increase the total sugar in corn, and they concluded that genotype has a significant effect on the total sugar in grain at the milking stage.
Selenium foliar spray fertilizer influences the selenium content of the grain quality. At different stages, sprayed selenium fertilizer concentration impacts the selenium content of fresh-eating sweet corn grain, and this reflects our result in Figure 5 for both seasons in density one. Syomina et al. reported that planting density or crowding of crops is directly proportional to reducing their content in the grain [80]. The foliar spraying of selenium fertilizer increases selenium content in sweet corn grain at the large bell and tassel stages [81]. Furthermore, selenium spraying at different concentrations and stages could improve sweet corn grain’s sugar degree, as seen in Table 4. There is no evidence that selenium has any detrimental effects on plant development when used in plant growth supplements. The distribution of selenium in grain for baking purposes is critical [82]. When you compare the inner parts of the grain (the endosperm and germ) to that of the outer parts (the seed coat), you will notice that selenium fertilizer increases selenium in the inner part of the grain, which Lyons et al. support [83]. Moreover, several studies show that selenium accumulation is dependent on both dose and plant growth stage [84].
Biological systems in plants, humans, and animals rely on zinc as a micronutrient. Zinc is essential for protein synthesis, enzyme activation, and membrane integrity in plants [85]. When applied to plants with zinc fertilizer, they increase micronutrients in their edible parts [86]. This is also seen in our result in Figure 5. The zinc content is different compared to that of the control. Furthermore, the foliar application of zinc is the best option for accumulation and improving grain zinc. When foliar zinc fertilizer is applied to plants during the reproductive stage, it is quickly transported to the plant’s reproductive structure. Zinc is absorbed through the leaf epidermis and transported into the grain via phloem via Zn-regulating transporter proteins, resulting in increased Zn bioavailability [87]. Figure 5 shows that density one in both seasons performs well, highlighting planting density and its effect on grain content [80].
5. Conclusions
This two-year study, which had 18 treatments, was conducted to determine the effects of zinc and selenium on different planting densities and varieties of sweet corn. This study showed that there was a significant difference between the control, zinc, and selenium treatments on sweet corn grain yield and nutritional value, i.e., water soluble sugar and the zinc and selenium content on grain. Grain yield and selenium content in grain were positively influenced by selenium foliar application, while water-soluble sugar and the zinc content in grain were positively influenced by foliar zinc application. Furthermore, increasing planting density increased grain yields.
Author Contributions
Conceptualization, B.J.S. and J.H.; methodology, B.J.S. and J.H.; software, B.J.S.; validation, B.J.S., S.G. and R.Z.; formal analysis, B.J.S., S.K.T., S.G. and R.Z.; investigation, B.J.S., S.G. and R.Z.; resources, J.H.; data curation, B.J.S., S.G. and R.Z.; writing—original draft preparation, B.J.S.; writing—review and editing, B.J.S., J.H., R.Z., S.G. and S.K.T.; visualization, B.J.S.; supervision, J.H.; project administration, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shaanxi Province 2021 Annual Innovation Capacity Support Plan (2021 XYSF-12).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Awika, J.M. Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; ACS Publications: Columbus, OH, USA, 2011; pp. 1–13. [Google Scholar]
- Tarighaleslami, M.; Zarghami, R.; Mashadi, A.B.M.; Oveysi, M. Effect of nitrogen fertilizer and water deficit stress on physiological indexes of corn (Zea mays L.). Iran. J. Agron. Plant Breed. 2012, 8, 161–174. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Sec. 2011, 3, 307. [Google Scholar] [CrossRef] [Green Version]
- Yadav, G.S.; Das, A.; Kandpal, B.K.; Babu, S.; Lal, R.; Datta, M.; Das, B.; Singh, R.; Singh, V.; Mohapatra, K.; et al. The food-energy-water-carbon nexus in a maize-maize-mustard cropping sequence of the Indian Himalayas: An impact of tillage-cum-live mulching. Renew. Sustain. Energy Rev. 2021, 151, 111602. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review. J. Soil Sci. Plant Nutr. 2021, 22, 1122–1159. [Google Scholar] [CrossRef]
- Niyigaba, E.; Twizerimana, A.; Mugenzi, I.; Ngnadong, W.A.; Ye, Y.P.; Wu, B.M.; Hai, J.B. Winter wheat grain quality, zinc and iron concentration affected by a combined foliar spray of zinc and iron fertilizers. Agronomy 2019, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Barrett, C.B. Measuring food insecurity. Science 2010, 327, 825–828. [Google Scholar] [CrossRef]
- Branca, F.; Ferrari, M. Impact of micronutrient deficiencies on growth: The stunting syndrome. Ann. Nutr. Metab. 2002, 46, 8–17. [Google Scholar] [CrossRef]
- Golden, M.H. The nature of nutritional deficiency in relation to growth failure and poverty. Acta Paediatr. 1991, 80, 95–110. [Google Scholar] [CrossRef]
- Grujcic, D.; Yazici, A.M.; Tutus, Y.; Cakmak, I.; Singh, B.R. Biofortification of Silage Maize with Zinc, Iron and Selenium as Affected by Nitrogen Fertilization. Plants 2021, 10, 391. [Google Scholar] [CrossRef]
- de Valença, A.W.; Bake, A.; Brouwer, I.D.; Giller, K.E. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Mugenzi, I.; Yongli, D.; Ngnadong, W.A.; Dan, H.; Niyigaba, E.; Twizerimana, A.; Hai, J. Effect of combined zinc and iron application rates on summer maize yield, photosynthetic capacity and grain quality. Int. J. Agron. Agric. Res. 2018, 12, 36–46. [Google Scholar]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, İ.; Kalaycı, M.; Ekiz, H.; Braun, H.J.; Kılınç, Y.; Yılmaz, A. Zinc deficiency as a practical problem in plant and human nutrition in Turkey: A NATO-science for stability project. Field Crop. Res. 1999, 60, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Baloch, Q.B.; Chachar, Q.I.; Tareen, M.N. Effect of foliar application of macro and micro nutrients on production of green chilies (Capsicum annuum L.). J. Agric. Technol. 2008, 4, 177–184. [Google Scholar]
- Panwar, A.S.; Shamim, M.; Babu, S.; Ravishankar, N.; Prusty, A.K.; Alam, N.M.; Singh, D.K.; Bindhu, J.S.; Kaur, J.; Dashora, L.N.; et al. Enhancement in Productivity, Nutrients Use Efficiency, and Economics of Rice-Wheat Cropping Systems in India through Farmer’s Participatory Approach. Sustainability 2019, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of zinc plants. In Zinc in Soils and Plants; Springer: Berlin/Heidelberg, Germany, 1993; pp. 93–106. [Google Scholar]
- Malakouti, M.J. The effect of micronutrients in ensuring efficient use of macronutrients. Turk. J. Agric. For. 2008, 32, 215–220. [Google Scholar]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Tariq, A.; Anjum, S.A.; Randhawa, M.A.; Ullah, E.; Naeem, M.; Qamar, R.; Ashraf, U.; Nadeem, M. Influence of zinc nutrition on growth and yield behaviour of maize (Zea mays L.) hybrids. Am. J. Plant Sci. 2014, 5, 2646–2654. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Zhai, R.; Huang, K.; Tan, H.; Zheng, D.; Huang, A.; Wei, X.; Mo, R.; Xiong, F.; Wei, H.; et al. Effect of Foliar Application of Selenium Fertilizer on Yield, Selenium Content and Heavy Metal Contents of Waxy Maize. Asian Agric. Res. 2021, 12, 40–48. [Google Scholar]
- Revilla, P.; Anibas, C.M.; Tracy, W.F. Sweet Corn Research around the World 2015–2020. Agronomy 2021, 11, 534. [Google Scholar] [CrossRef]
- Burhan, K.; Ertek, A.; Bekir, A. Mineral nutrient content of sweet corn under deficit irrigation. J. Agric. Sci. 2016, 22, 54–61. [Google Scholar]
- Yang, Q.; Yang, X.; Zhang, Q.; Wang, Y.; Song, H.; Huang, F. Quantifying soluble sugar in super sweet corn using near-infrared spectroscopy combined with chemometrics. Optik 2020, 220, 165128. [Google Scholar] [CrossRef]
- Swapna, G.; Jadesha, G.; Mahadevu, P. Sweet corn—A future healthy human nutrition food. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3859–3865. [Google Scholar] [CrossRef]
- Lertrat, K.; Pulam, T. Breeding for Increased Sweetness in Sweet Corn. Int. J. Plant Breed. 2007, 1, 27–30. [Google Scholar]
- Peykarestan, B.; Yarnia, M.; Madani, H.; Rashidi, V.; Abad, H.H.S. Impact of low-alternate furrow irrigiation and zinc sulfate foliar application on grain yield and enrichment of sweet corn hybrids. Pak. J. Bot. 2018, 50, 1005–1011. [Google Scholar]
- Santos, O.F.; Lima, S.F.; Piati, G.L.; Barzotto, G.R.; Gava, R. Irrigation as an alternative to reduce damages caused by defoliation of sweet corn. Hortic. Bras. 2018, 36, 341–345. [Google Scholar] [CrossRef]
- Subaedah, S.; Edy, E.; Mariana, K. Growth, Yield, and Sugar Content of Different Varieties of Sweet Corn and Harvest Time. Int. J. Agron. 2021, 2021, e8882140. [Google Scholar] [CrossRef]
- Li, L.; Vuichard, N.; Viovy, N.; Ciais, P.; Wang, T.; Ceschia, E.; Jans, W.; Wattenbach, M.; Béziat, P.; Gruenwald, T.; et al. Importance of crop varieties and management practices: Evaluation of a process-based model for simulating CO2 and H2O fluxes at five European maize (Zea mays L.) sites. Biogeosciences 2011, 8, 1721–1736. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Z.; Mao, H.; Zhao, H.; Huang, D. Increasing Se concentration in maize grain with soil- or foliar-applied selenite on the Loess Plateau in China. Field Crop. Res. 2013, 150, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, S.; Adeel, M.; Raza, H.; Iqbal, R.; Ahmad, Z.; Naeem, M.; Sheraz, M.; Ahmed, U.; Azmi, U.R. Effects of Foliar Application of Selenium in Maize (Zea mays L.) under Cadmium Toxicity. Biol. Forum 2019, 11, 61–71. [Google Scholar]
- Pilon-Smits, E.A.H. Selenium in Plants. In Progress in Botany; Lüttge, U., Beyschlag, W., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 76, pp. 93–107. [Google Scholar]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in Higher Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabani, J.; Amam, Y. Yield response to water stress at different growth stages of maize hybrids. J. Crop. Prod. Process. 2012, 1, 65–78. [Google Scholar]
- Scot, P.; Aboudrare, A. Adaptation of crop management to water-limited environment. Eur. J. Agron. 2009, 21, 433–446. [Google Scholar]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Sadeghzadeh, B.; Rengel, Z. Zinc in Soils and Crop Nutrition. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Wiley-Blackwell: Oxford, UK, 2011; pp. 335–375. [Google Scholar]
- Alloway, B. Zinc in Soils and Crop Nutrition. Areas of the World with Zinc Deficiency Problems; International Zinc Association Brussels: Brussels, Belgium, 2004; pp. 1–116. [Google Scholar]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef] [Green Version]
- Leach, K.A.; Hameleers, A. The effects of a foliar spray containing phosphorus and zinc on the development, composition and yield of forage maize. Grass Forage Sci. 2001, 56, 311–315. [Google Scholar] [CrossRef]
- Subedi, K.D.; Ma, B.L. Corn crop production: Growth, fertilization and yield. In Agriculture Issues and Policies; Nova Science Publisher: New York, NY, USA, 2009. [Google Scholar]
- Erenoglu, B.; Nikolic, M.; Römheld, V.; Cakmak, I. Uptake and transport of foliar applied zinc (65Zn) in bread and durum wheat cultivars differing in zinc efficiency. Plant Soil 2002, 241, 251–257. [Google Scholar] [CrossRef]
- Grzebisz, W.; Wronska, M.; Diatta, J.B.; Dullin, P. Effect of zinc foliar application at an early stage of maize growth on patterns of nutrients and dry matter accumulation by the canopy. Part I. Zinc uptake patterns and its redistribution among maize organs. J. Elem. 2008, 13, 29–39. [Google Scholar]
- Xia, H.; Kong, W.; Wang, L.; Xue, Y.; Liu, W.; Zhang, C.; Yang, S.; Li, C. Foliar Zn spraying simultaneously improved concentrations and bioavailability of Zn and Fe in maize grains irrespective of foliar sucrose supply. Agronomy 2019, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Tong, L.; Kang, S.; Li, F.; Li, D.; Qin, Y.; Shi, R.; Li, J. Planting density affected biomass and grain yield of maize for seed production in an arid region of Northwest China. J. Arid Land 2018, 10, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Lyu, Y.; Wu, X.; Li, H.; Cheng, L.; Zhang, C.; Yuan, L.; Jiang, R.; Jiang, B.; Rengel, Z.; et al. Grain production versus resource and environmental costs: Towards increasing sustainability of nutrient use in China. J. Exp. Bot. 2016, 67, 4935–4949. [Google Scholar] [CrossRef]
- Du, X.; Wang, Z.; Lei, W.; Kong, L. Increased planting density combined with reduced nitrogen rate to achieve high yield in maize. Sci. Rep. 2021, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Khan, A.; Cai, L.J.; Ahmad, M.I.; Asharf, U.; Jamoro, S.A. Response of maize grown under high plant density; performance, issues and management-a critical review. Adv. Crop. Sci. Technol. 2017, 5, 275. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Li, Y.; Zhang, J.; Liu, P.; Zhao, B.; Dong, S. Increased plant density and reduced N rate lead to more grain yield and higher resource utilization in summer maize. J. Integr. Agric. 2016, 15, 2515–2528. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Feyissa, T.; Ma, H.; Duan, Z.; Zhang, W. Optimizing Plant Density and Balancing NPK Inputs in Combination with Innovative Fertilizer Product for Sustainable Maize Production in North China Plain; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Dhaliwal, D.S.; Ainsworth, E.A.; Williams, M.M. Historical Trends in Sweet Corn Plant Density Tolerance Using Era Hybrids (1930–2010s). Front. Plant Sci. 2021, 12, 1. [Google Scholar] [CrossRef]
- Assefa, Y.; Carter, P.; Hinds, M.; Bhalla, G.; Schon, R.; Jeschke, M.; Paszkiewicz, S.; Smith, S.; Ciampitti, I.A. Analysis of Long Term Study Indicates Both Agronomic Optimal Plant Density and Increase Maize Yield per Plant Contributed to Yield Gain. Sci. Rep. 2018, 8, 4937. [Google Scholar] [CrossRef] [Green Version]
- Piper, C.S. Soil and Plant Analysis; Scientific Jodhpur Publishers: Jodhpur, India, 2019. [Google Scholar]
- Dellaporta, S.L.; Calderon-Urrea, A. The Sex Determination Process in Maize. Science 1994, 266, 1501–1505. [Google Scholar] [CrossRef]
- Ventura, J. Characterization of Maize Sex-Determination Gene Orthologs in Rice (Oryza sativa L. Japonica Cv. Nipponbare); University of Rhode Island: Kingston, RI, USA, 2012. [Google Scholar]
- Beljkaš, B.; Matić, J.; Milovanović, I.; Jovanov, P.; Mišan, A.; Šarić, L. Rapid method for determination of protein content in cereals and oilseeds: Validation, measurement uncertainty and comparison with the Kjeldahl method. Accredit. Qual. Assur. 2010, 15, 555–561. [Google Scholar] [CrossRef]
- Schop, M.; de Vries, S.; Gerrits, W.J.J.; Jansman, A.J.M. In Vitro Enzymatic Protein Hydrolysis Kinetics of Feed Ingredients. Modelling Digestion Kinetics in Pigs. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2020; p. 65. [Google Scholar]
- Isaac, R.A.; Kerber, J.D. Atomic Absorption and Flame Photometry: Techniques and Uses in Soil, Plant, and Water Analysis. In Instrumental Methods for Analysis of Soils and Plant Tissue; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1971; pp. 17–37. [Google Scholar]
- Zhao, A.; Wang, B.; Tian, X.; Yang, X. Combined soil and foliar ZnSO4 application improves wheat grain Zn concentration and Zn fractions in a calcareous soil. Eur. J. Soil Sci. 2020, 71, 681–694. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111 Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Potarzycki, J.; Grzebisz, W. Effect of zinc foliar application on grain yield of maize and its yielding components. Plant Soil Environ. 2009, 55, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Farnham, D.E. Row Spacing, Plant Density, and Hybrid Effects on Corn Grain Yield and Moisture. Agron. J. 2001, 93, 1049–1053. [Google Scholar] [CrossRef]
- Gozubenli, H.; Kilinc, M.; Sener, O.; Konuskan, O. Effects of single and twin row planting on yield and yield components in maize. Asian J. Plant Sci. 2004, 3, 203–206. [Google Scholar] [CrossRef]
- Germ, M.; Stibilj, V. Selenium and plants. Acta Agric. Slov. 2007, 89, 65–71. [Google Scholar] [CrossRef]
- Testa, G.; Reyneri, A.; Blandino, M. Maize grain yield enhancement through high plant density cultivation with different inter-row and intra-row spacings. Eur. J. Agron. 2016, 72, 28–37. [Google Scholar] [CrossRef]
- Shelton, A.C.; Tracy, W.F. Genetic variation and phenotypic response of 15 sweet corn (Zea mays L.) hybrids to population density. Sustainability 2013, 5, 2442–2456. [Google Scholar] [CrossRef] [Green Version]
- Hisham, A.R.A.; Ch’Ng, H.Y.; Rahman, M.M.; Mat, K.; Zulhisyam, A.K. Effects of zinc on the growth and yield of maize (Zea mays L.) cultivated in a tropical acid soil using different application techniques. IOP Conf. Ser. Earth Environ. Sci. 2021, 756, 012056. [Google Scholar] [CrossRef]
- Naseem, M.; Anwar-Ul-Haq, M.; Wang, X.; Farooq, N.; Awais, M.; Sattar, H.; Malik, H.A.; Mustafa, A.; Ahmad, J.; El-Esawi, M.A. Influence of Selenium on Growth, Physiology, and Antioxidant Responses in Maize Varies in a Dose-Dependent Manner. J. Food Qual. 2021, 2021, e6642018. [Google Scholar] [CrossRef]
- Fahrurrozi, F.; Muktamar, Z.; Dwatmadji, D.; Setyowati, N.; Sudjatmiko, S.; Chozin, M. Growth and Yield Responses of Three Sweet Corn (Zea mays L. var. Saccharata) Varieties to Local-based Liquid Organic Fertilizer. Int. J. Adv. Sci. Eng. Inf. Technol. 2017, 6, 319–323. [Google Scholar]
- Peddapuli, M.; Venkateswarlu, B.; Prasad, P.V.N.; Rao, S. Growth and Yield of Sweetcorn as Influenced by Zinc Fertilization. Int. J. Agric. Environ. Biotechnol. 2021, 14, 175–179. [Google Scholar] [CrossRef]
- Safyan, N.; Naderidarbaghshahi, M.R.; Bahari, B. The effect of microelements spraying on growth, qualitative and quantitative grain corn in Iran. Int. Res. J. Appl. Basic Sci. 2012, 3, 2780–2784. [Google Scholar]
- Karrimi, A.S.; Reddy, A.P.K.; Babazoi, F.; Kohistani, T. Growth, yield and post-harvest soil available nutrients in sweet corn (Zea mays L.) as influenced by zinc and iron nutrition. J. Pharmacogn. Phytochem. 2018, 7, 2372–2374. [Google Scholar]
- Abuzar, M.R.; Sadozai, G.U.; Baloch, M.S.; Baloch, A.A.; Shah, I.H.; Javaid, T.; Hussain, N. Effect of plant population densities on yield of maize. J. Anim. Plant Sci. 2011, 21, 692–695. [Google Scholar]
- López-Bellido, L.; López-Bellido, R.J.; Castillo, J.E.; López-Bellido, F.J. Effects of long-term tillage, crop rotation and nitrogen fertilization on bread-making quality of hard red spring wheat. Field Crop. Res. 2001, 72, 197–210. [Google Scholar] [CrossRef]
- Haddadi, M.H. Investigation of characteristics and cultivation of sweet corn: A Review. Int. J. Farming Allied Sci. 2016, 5, 243–247. [Google Scholar]
- Adamec, S.; Andrejiová, A.; Hegedűsová, A.; Šemnicer, M. Evaluation of the foliar nutrition influence on selected quantitative and qualitative paprameters of sugar mayze (Zea mays SK saccharata). Potravin. Slovak J. Food Sci. 2020, 14, 208–215. [Google Scholar] [CrossRef]
- Syomina, S.A.; Paliychuk, A.S.; Gavryushina, I.V.; Lysenko, I.A. Fertilizers, plant density and nutritional properties of corn grain. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; p. 012036. [Google Scholar]
- Huang, A.H.; Huang, K.J.; Peng, J.; Huang, S.H.; Bi, X.C.; Zhai, R.N.; Mo, R.X.; Zheng, D.B.; Zou, C.L.; Wei, X.X.; et al. Effects of foliar spraying of selenium fertilizer on selenium-enriched content, heavy metal content and yield of sweet corn grain. J. South. Agric. 2019, 50, 40–44. [Google Scholar]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [Green Version]
- Lyons, G.H.; Genc, Y.; Stangoulis, J.; Palmer, L.T.; Graham, R.D. Selenium distribution in wheat grain, and the effect of postharvest processing on wheat selenium content. Biol. Trace Elem. Res. 2005, 103, 155–168. [Google Scholar] [CrossRef]
- Radawiec, A.; Rutkowska, B.; Tidaback, J.A.; Gozdowski, D.; Knapowski, T.; Szulc, W. The Impact of Selenium Fertilization on the Quality Characteristics of Spring Wheat Grain. Agronomy 2021, 11, 2100. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Ellis, D.R.; Grusak, M.A. Effect of zinc and manganese supply on the activities of superoxide dismutase and carbonic anhydrase in Medicago truncatula wild type and raz mutant plants. Plant Sci. 2005, 168, 1015–1022. [Google Scholar] [CrossRef]
- Palai, J.B.; Sarkar, N.C.; Jena, J. Effect of zinc on growth, yields, zinc use efficiency and economics in baby corn. J. Pharmacogn. Phytochem. 2018, 7, 1641–1645. [Google Scholar]
- Zulfiqar, U.; Hussain, S.; Ishfaq, M.; Matloob, A.; Ali, N.; Ahmad, M.; Alyemeni, M.; Ahmad, P. Zinc-induced effects on productivity, zinc use efficiency, and grain biofortification of bread wheat under different tillage permutations. Agronomy 2020, 10, 1566. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).