Sustainable Management of Salt Slag
Abstract
:1. Introduction
2. Materials and Methods
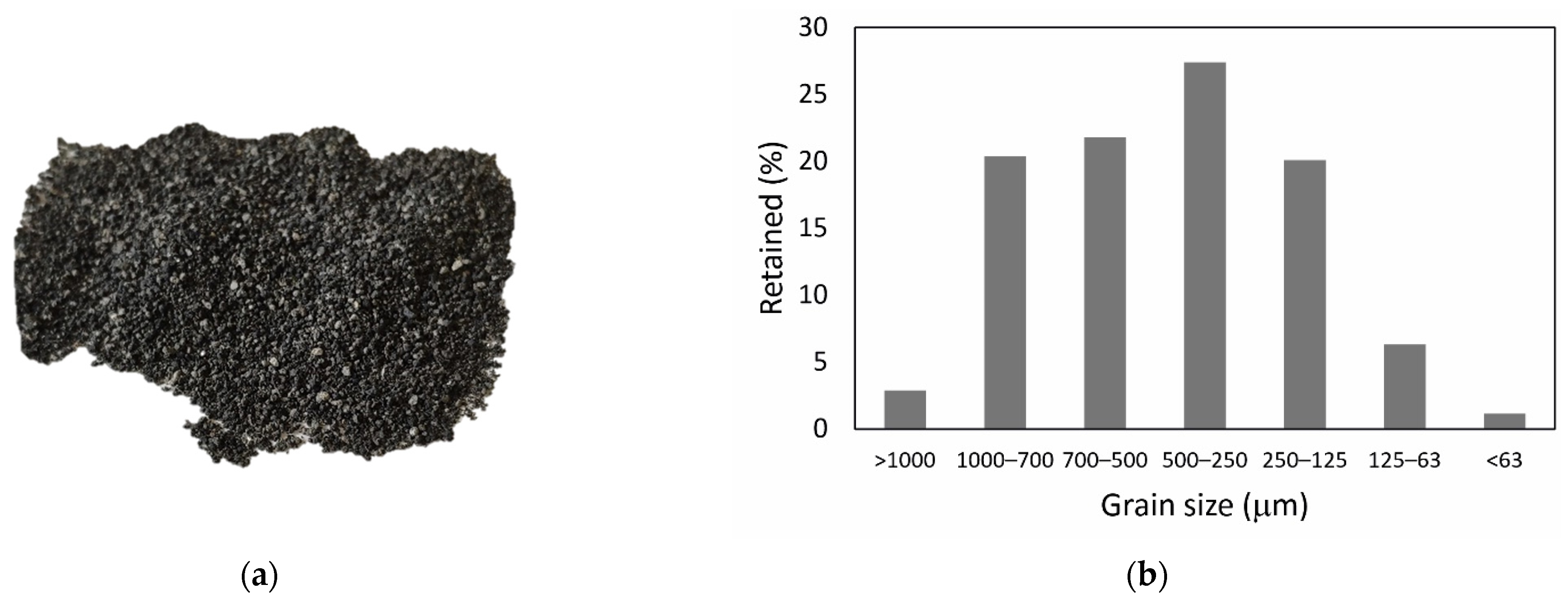
2.1. Raw Materials
2.2. Methods
2.2.1. Hydrolysis of Salt Slag
2.2.2. Synthesis of Zeolite
2.3. Characterization Techniques
3. Results and Discussion
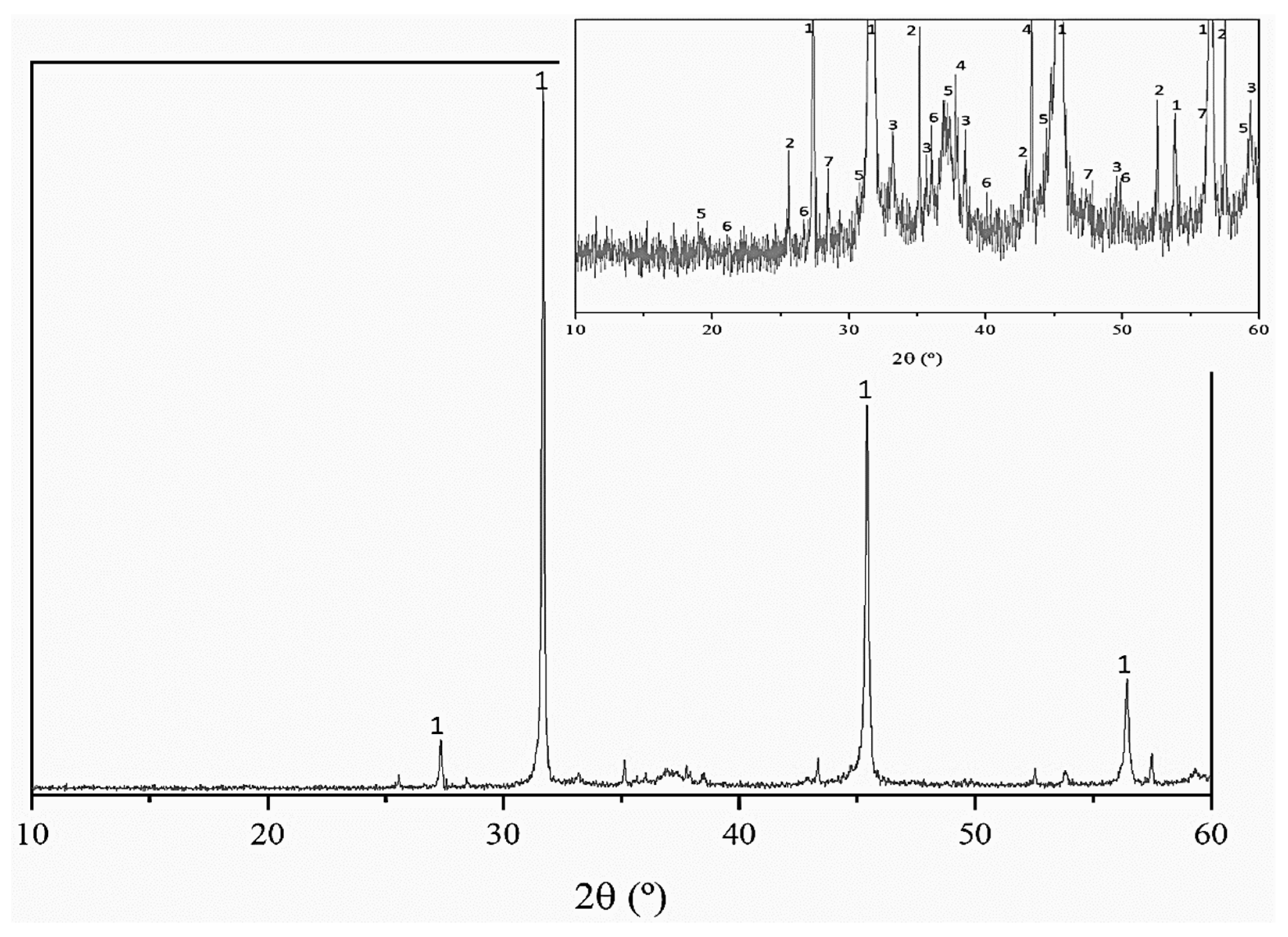
3.1. Hydrolysis of Salt Slag and Characterization of Products
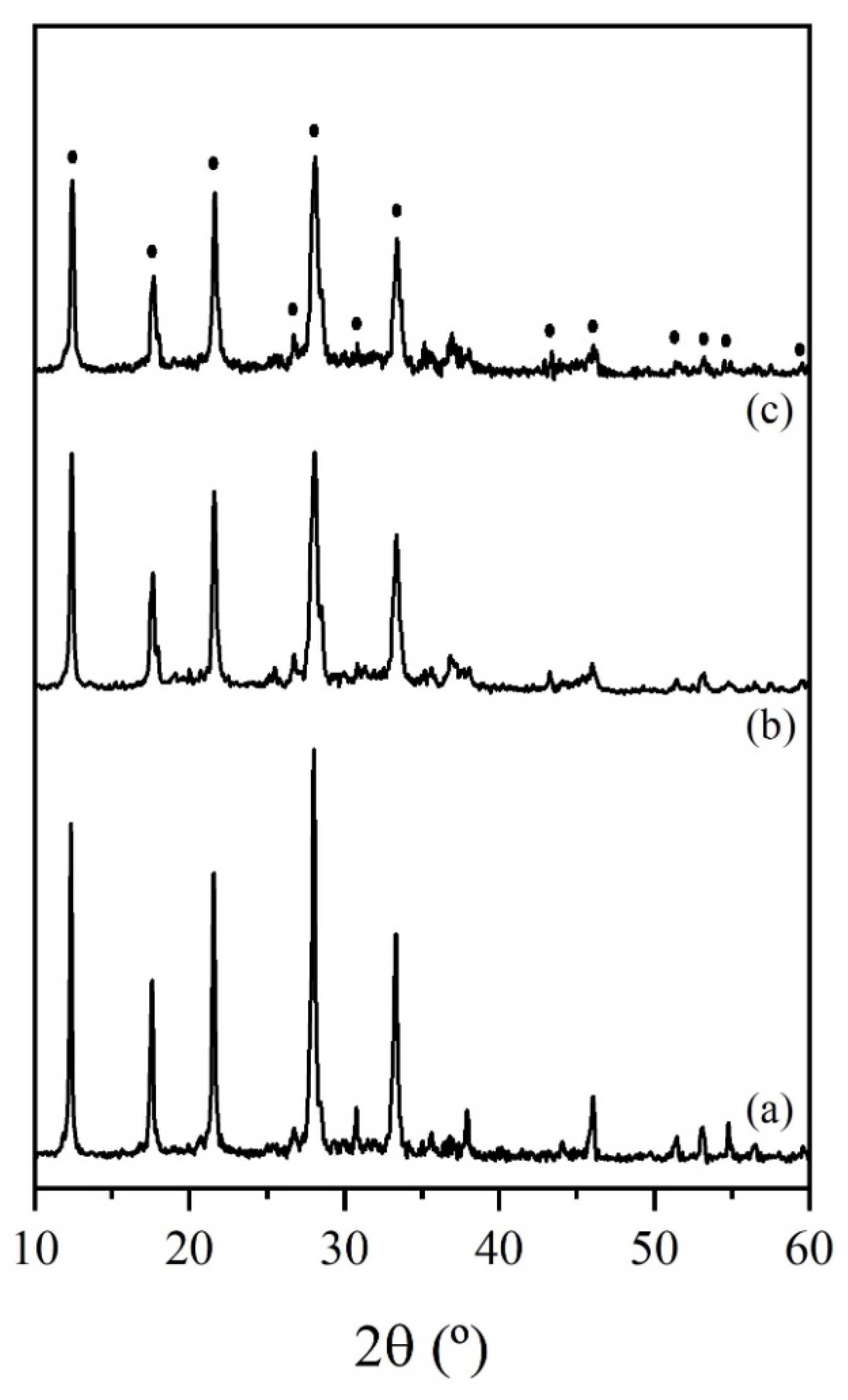
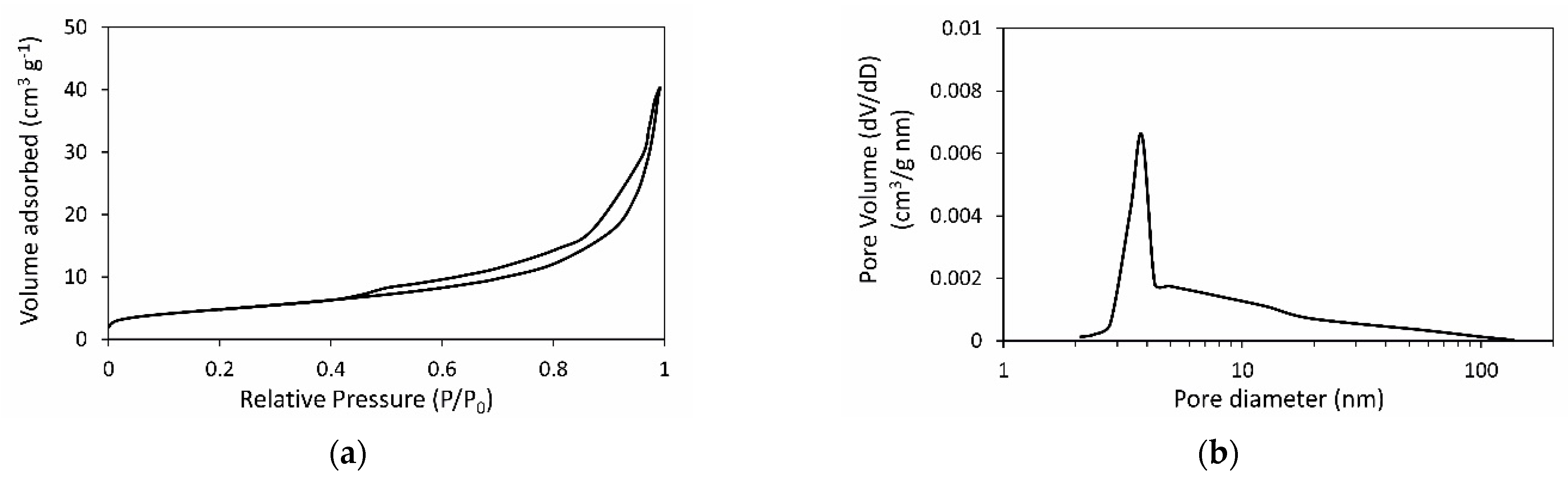
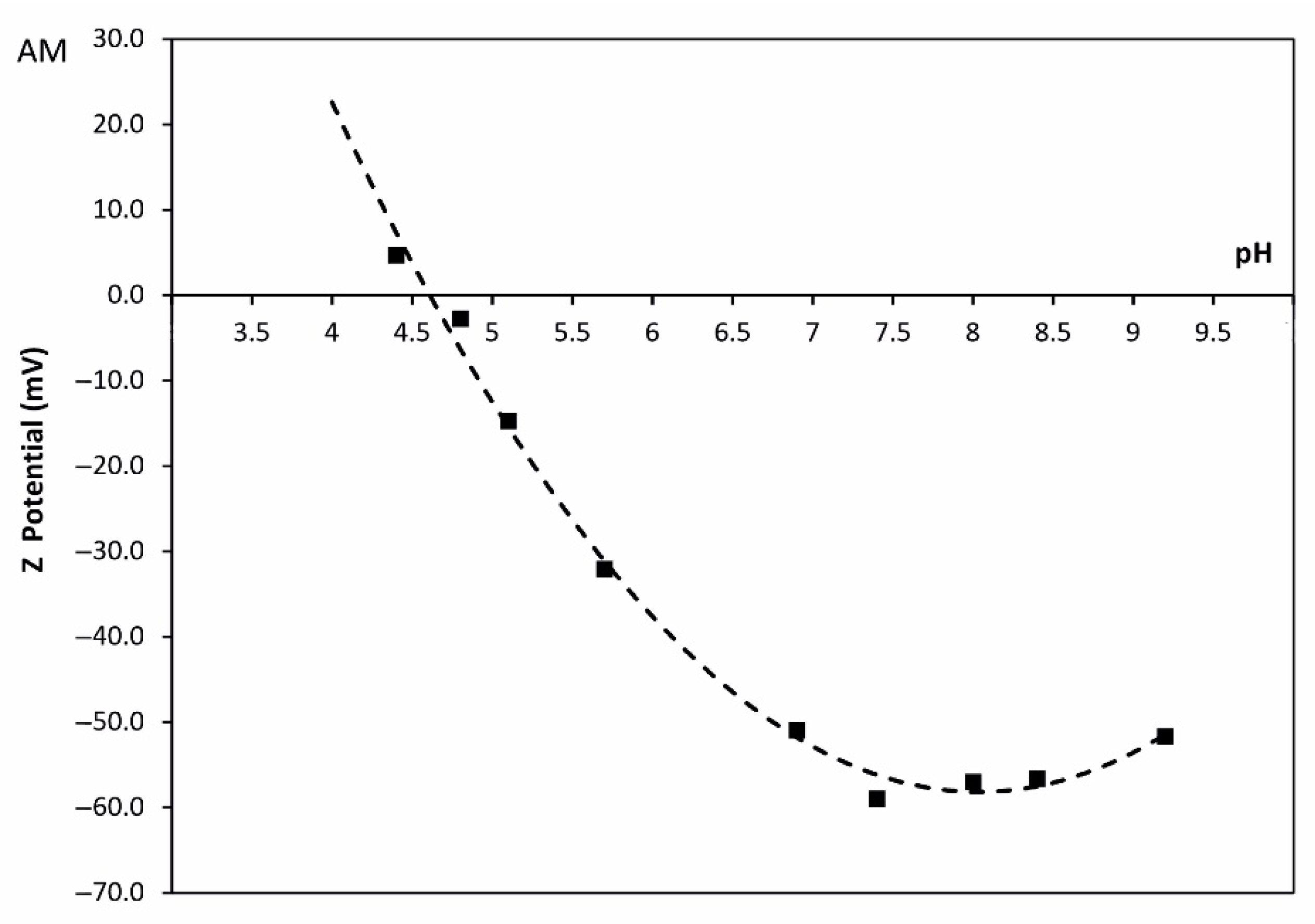
3.2. Synthesis of Zeolite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef] [PubMed]
- López-Delgado, A.; Tayibi, H. Can hazardous waste become a raw material? the case study of an aluminium residue: A review. Waste Manag. Res. 2012, 30, 474–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Circular Aluminium Action Plan. A Strategy for Achieving Aluminium’s Full Potential for Circular Economy by 2030; Brussels, Belgium. 2020. Available online: https://european-aluminium.eu/media/2903/european-aluminium-circular-aluminium-action-plan.pdf (accessed on 2 February 2022).
- European Aluminium Vision 2050. European Aluminium’s Contribution to the EU’s Mid-Century Low-Carbon Roadmap; Brussels, Belgium. 2019. Available online: https://www.european-aluminium.eu/media/2545/sample_vision-2050-low-carbon-strategy_20190401.pdf (accessed on 8 February 2022).
- European Aluminium. European Aluminium & the Sustainable Development Goals. 2019. Available online: https://european-aluminium.eu/media/3253/european-aluminium_connecting-the-sustainability-roadmap-2025-to-the-sdgs-_december_2018_final.pdf (accessed on 10 February 2022).
- European Aluminium Recycling & Circular Economy. Available online: https://european-aluminium.eu/policy-areas/recycling-circular-economy/ (accessed on 10 February 2022).
- European Commission. Efficient Aluminium Salt Cake Recycling Technology. Available online: https://cordis.europa.eu/project/id/314922 (accessed on 15 January 2022).
- Tsakiridis, P.E. Aluminium salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 217–218, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Korili, S.A. Management and valorization of aluminum saline slags: Current status and future trends. Chem. Eng. J. 2016, 289, 74–84. [Google Scholar] [CrossRef]
- Tenorio, J.A.S.; Espinosa, D.C.R. Effect of salt/oxide interaction on the process of aluminum recycling. J. Light Met. 2002, 2, 89–93. [Google Scholar] [CrossRef]
- Ünlü, N.; Drouet, M.G. Comparison of salt-free aluminum dross treatment processes. Resour. Conserv. Recycl. 2002, 36, 61–72. [Google Scholar] [CrossRef]
- Zhang, L. State of the art in aluminum recycling from aluminum dross. TMS Light Met. 2006, 2006, 931–936. [Google Scholar]
- Bruckard, W.J.; Woodcock, J.T. Characterisation and treatment of Australian salt cakes by aqueous leaching. Miner. Eng. 2007, 20, 1376–1390. [Google Scholar] [CrossRef]
- Huang, X.L.; El Badawy, A.; Arambewela, M.; Ford, R.; Barlaz, M.; Tolaymat, T. Characterization of salt cake from secondary aluminum production. J. Hazard. Mater. 2014, 273, 192–199. [Google Scholar] [CrossRef]
- European Waste Catalogue and Hazardous Waste List, Published by the Environmental Protection Agency. Ireland. 2001. Available online: https://v4r7y5k5.stackpathcdn.com/wp-content/uploads/2016/08/EWC_HWL.pdf (accessed on 15 January 2022).
- European Commission. Commission Decision of 18 December 2014 Amending. Decision 2000/532/EC on the List of Waste Pursuant to Directive 2008/98/EC of the European Parliament and of the Council; Brussels, Belgium. 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014D0955 (accessed on 15 January 2022).
- Lysenko, A.P.; Sel’nitsyn, R.S.; Nalivaiko, A.Y. Comprehensive Processing of Saline Aluminum-Containing Slag. Metallurgist 2017, 61, 624–628. [Google Scholar] [CrossRef]
- Galindo, R.; Padilla, I.; Rodríguez, O.; Sánchez-Hernández, R.; López-Andrés, S.; López-Delgado, A. Characterization of Solid Wastes from Aluminum Tertiary Sector: The Current State of Spanish Industry. J. Miner. Mater. Charact. Eng. 2015, 3, 55–64. [Google Scholar] [CrossRef] [Green Version]
- David, E.; Kopac, J. The Assessment of the Recycling Process of Aluminum Hazardous Waste and a New Route of Development. Mater. Today Proc. 2019, 10, 340–347. [Google Scholar] [CrossRef]
- European Commission Efficient Aluminium Salt Cake Recycling Technology. In-House Salt Slag Processing Solution Which Turns Waste into a Product. 2022. Available online: https://cordis.europa.eu/article/id/223757-inhouse-salt-slag-processing-solution-which-turns-waste-into-a-product?msclkid=e4b28d11bf1411eca2aa783d8b270304 (accessed on 15 January 2022).
- Ballon, O. Aluminium Salt Slag Recycling: A Reality Check. In Proceedings of the Mineral Recycing Forum 2017, Rotterdam, The Netherlands, 7–8 March 2017. [Google Scholar]
- Wöhlk, W.; Niederjaufner, G.; Hofmann, G. Recycling of Cover Salt in the Secondary Aluminium Industry. Cryst. Precip. 1987, 99–108. [Google Scholar] [CrossRef]
- Graziano, D.; Hryn, J.N.; Daniels, E.J. The Economics of Salt Cake Recycling. United States. 1996. Available online: https://www.osti.gov/servlets/purl/224304 (accessed on 2 February 2022).
- Hazar, A.B.Y.; Saridede, M.N.; Çiǧdem, M. A study on the structural analysis of aluminium drosses and processing of industrial aluminium salty slags. Scand. J. Metall. 2005, 34, 213–219. [Google Scholar] [CrossRef]
- Davies, M.; Smith, P.; Bruckard, W.J.; Woodcock, J.T. Treatment of salt cakes by aqueous leaching and Bayer-type digestion. Miner. Eng. 2008, 21, 605–612. [Google Scholar] [CrossRef]
- Efficient Aluminium Salt Slag Recycling Technology: Significantly Reducing the Cost and CO2 Impacts of Transport for the Aluminium. 2017. Available online: https://cordis.europa.eu/article/id/223757-inhouse-salt-slag-processing-solution-which-turns-waste-into-a-product (accessed on 25 January 2022).
- Bruckard, W.J.; Woodcock, J.T. Recovery of valuable materials from aluminium salt cakes. Int. J. Miner. Process. 2009, 93, 1–5. [Google Scholar] [CrossRef]
- Xiao, Y.; Reuter, M.A.; Boin, U. Aluminium recycling and environmental issues of salt slag treatment. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2005, 40, 1861–1875. [Google Scholar] [CrossRef]
- Peel, A.; Gibbs, A. Improvements in and Relating to Processing Methods and Processing Apparatus. WO Patent 2018/096358, 31 May 2018. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2018096358 (accessed on 20 January 2022).
- Momza, M.O.; Corsini, T.; Calende, S.; Fracchia, P. Process for the Processing of Slag from Aluminium Scrap and Waste Melting, Recovery of Components Thereof and Treatment of Gasses Generated. U.S. Patent 5013356, 7 May 1991. Available online: https://patents.google.com/patent/US5013356A/en (accessed on 15 January 2022).
- Peng, L.; Mei, Z.; Lidong, T.; Seetharaman, S. Recycling of Aluminum Salt Cake: Utilization of Evolved Ammonia. Metall. Mater. Trans. B 2013, 44, 16–19. [Google Scholar]
- Torres, J. Beneficios del Uso del PAVAL Para la Fabricación de Cementos. 2019. Available online: https://www.ieca.es/producto/beneficios-del-uso-del-paval-la-fabricacion-cementos/ (accessed on 15 January 2022).
- Torrez-Herrera, J.J.; Korili, S.A.; Gil, A. Bimetallic (Pt-Ni) La-hexaaluminate catalysts obtained from aluminum saline slags for the dry reforming of methane. Chem. Eng. J. 2022, 433, 133191. [Google Scholar] [CrossRef]
- Jiménez, A.; Misol, A.; Morato, Á.; Rives, V.; Vicente, M.A.; Gil, A. Optimization of hydrocalumite preparation under microwave irradiation for recovering aluminium from a saline slag. Appl. Clay Sci. 2021, 212, 106217. [Google Scholar] [CrossRef]
- Galindo, R.; López-Delgado, A.; Padilla, I.; Yates, M. Synthesis and characterisation of hydrotalcites produced by an aluminium hazardous waste: A comparison between the use of ammonia and the use of triethanolamine. Appl. Clay Sci. 2015, 115, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Lobo-Recio, M.; Rodrigues, C.; Jeremias, T.; Lapolli, F.R.; Padilla, I.; López-Delgado, A. Highly efficient removal of aluminum, iron, and manganese ions using Linde type-A zeolite obtained from hazardous waste. Chemosphere 2021, 267, 128919. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Chang, Q. Green synthesis of fly ash-based zeolite Y by mixed alkali fusion method. Micro Nano Lett. 2021, 16, 540–545. [Google Scholar] [CrossRef]
- Ren, X.; Qu, R.; Liu, S.; Zhao, H.; Wu, W.; Song, H.; Zheng, C.; Wu, X.; Gao, X. Synthesis of zeolites from coal fly ash for the removal of harmful gaseous pollutants: A review. Aerosol. Air Qual. Res. 2020, 20, 1127–1144. [Google Scholar] [CrossRef] [Green Version]
- López-Delgado, A.; Robla, J.I.; Padilla, I.; López-Andrés, S.; Romero, M. Zero-waste process for the transformation of a hazardous aluminum waste into a raw material to obtain zeolites. J. Clean. Prod. 2020, 255, 120178. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.; Padilla, I.; López-Andrés, S.; López-Delgado, A. Eco-friendly bench-scale zeolitization of an Al-containing waste into gismondine-type zeolite under effluent recycling. J. Clean. Prod. 2017, 161, 792–802. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Hernández, R.; López-Delgado, A.; Padilla, I.; Galindo, R.; López-Andrés, S. One-step synthesis of NaP1, SOD and ANA from a hazardous aluminum solid waste. Microporous Mesoporous Mater. 2016, 226, 267–277. [Google Scholar] [CrossRef]
- ASTM C 136—01 Standard Test Method for Sieve Analysis of Fine and Coarse Aggregates. Available online: https://www.studocu.com/en-us/document/colorado-school-of-mines/water-supply-engineering/astm-c136-2014-standard-test-method-for-sieve-analysis-of-fine-and-coarse-aggregates/8462887 (accessed on 4 December 2021).
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 27, 292–377. [Google Scholar] [CrossRef]
- Taylor, A.M.; Roy, R. Zeolite studies IV: Na-P zeolites and the ion-exchanged derivatives of tetragonal Na-P1. Am. Mineral. 1964, 49, 656–682. [Google Scholar]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of zeolite NaP with controllable morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Azizi, D.; Ibsaine, F.; Dionne, J.; Coudert, L.; Blais, J. Microporous and macroporous materials state-of-the-art of the technologies in zeolitization of aluminosilicate bearing residues from mining and metallurgical industries: A comprehensive review. Microporous Mesoporous Mater. 2021, 318, 111029. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, Y.; Li, D.; Yan, C. Preparation of NaP zeolite block from fly ash-based geoplymer via in situ hydrothermal method. J. Porous Matter 2015, 22, 291–299. [Google Scholar] [CrossRef]
- Ali, I.O.; El-Sheikh, S.M.; Salama, T.M.; Bakr, M.F.; Fodial, M.H. Controllable synthesis of NaP zeolite and its application in calcium adsorption. Sci. China Mater 2015, 58, 621–623. [Google Scholar] [CrossRef] [Green Version]
- Vistuba, J.P.; Nagel-Hassemer, M.E.; Lapolli, F.R.; Lobo-Recio, M.Á. Simultaneous adsorption of iron and manganese from aqueous solutions employing an adsorbent coal. Environ. Technol. 2013, 34, 275–282. [Google Scholar] [CrossRef]
- Espejel-Ayala, F.; Schouwenaars, R.; Durán-Moreno, A.; Ramírez-Zamora, R.M. Use of drinking water sludge in the production process of zeolites. Res. Chem. Intermed. 2014, 40, 2919–2928. [Google Scholar] [CrossRef]
- Gillies, G.; Raj, R.; Kopinke, F.; Georgi, A. Suspension stability and mobility of Trap-Ox Fe-zeolites for in-situ nanoremediation. J. Colloid Interface Sci. 2017, 501, 311–320. [Google Scholar] [CrossRef]
- Sánchez-Hernández, R.; Padilla, I.; López-Andrés, S.; López-Delgado, A. Single and competitive adsorptive removal of lead, cadmium, and mercury using zeolite adsorbent prepared from industrial aluminum waste. Desalin. WATER Treat. 2018, 126, 181–195. [Google Scholar] [CrossRef] [Green Version]


| Al2O3 | SiO2 | Cl | Na2O | MgO | Fe2O3 | CaO | SO3 | K2O | TiO2 | Cr2O3 | MnO | P2O5 | CuO | ZnO | BaO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30.7 | 2.7 | 27.6 | 30.8 | 3.7 | 1.1 | 1.3 | 0.2 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 | 0.2 |
| Hydrolysis Tests | T (°C) | Ratio Slag/Water | Filtrate (Brine) | Recovered NaCl (%) | Recovered NH3 (%) | ||
|---|---|---|---|---|---|---|---|
| pH | I. C. (mS cm−1) | Density (g L−1) | |||||
| 1/10-T25 | 25 | 1/10 | 10.0 ± 0.1 | 64 ± 4 | 1.03 ± 0.01 | 96 | n.r. |
| 1/3-T25 | 25 | 1/3 | 9.9 ± 0.1 | 159 ± 2 | 1.09 ± 0.01 | 89 | n.r. |
| 1/10-T90 | 90 | 1/10 | 10.4 ± 0.1 | 68 ± 3 | 1.03 ± 0.01 | 98 | 77 |
| 1/3-T90 | 90 | 1/3 | 9.6 ± 0.1 | 168 ± 1 | 1.09 ± 0.01 | 80 | 88 |
| Brine | Na | K | Al | Ca | Si | S | P | Sr | Ba | Li | Zn | Mo Cu Cr Pb Mg Mn Ti Ni Co |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/10-T25 | 12,885 | 324 | 42 | 116 | 18 | 14 | 1 | 6 | 19 | 0.4 | <0.2 | <0.2 |
| 1/3-T90 | 39,617 | 640 | 35 | 18 | 15 | 47 | n.d. | n.d. | 4 | <0.2 | 1 | <0.2 |
| Cl | Na2O | Al2O3 | SiO2 | Fe2O3 | CaO | SO3 | K2O | Br | SrO |
|---|---|---|---|---|---|---|---|---|---|
| 55.1 | 43.3 | 0.5 | 0.1 | 0.03 | 0.5 | 0.1 | 0.5 | 0.02 | 0.01 |
| Hydrolyzed Slag | Al2O3 | SiO2 | Cl | Na2O | MgO | Fe2O3 | CaO | SO3 | TiO2 | MnO | CuO | ZnO | BaO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/10-T25 | 67.4 | 4.0 | 2.8 | 2.5 | 7.8 | 2.3 | 1.7 | 0.4 | 0.8 | 0.3 | 0.8 | 0.7 | 0.3 |
| 1/3-T25 | 65.3 | 3.4 | 3.5 | 3.3 | 8.1 | 2.0 | 1.8 | 0.4 | 0.7 | 0.3 | 0.7 | 0.6 | 0.4 |
| 1/10-T90 | 65.3 | 6.1 | 1.9 | 1.8 | 7.0 | 3.1 | 2.4 | 0.5 | 1.1 | 0.3 | 1.0 | 0.8 | 0.5 |
| 1/3-T90 | 62.1 | 5.3 | 7.3 | 7.4 | 6.5 | 2.5 | 1.9 | 0.3 | 0.7 | 0.2 | 0.7 | 0.5 | 0.3 |
| Hydrolyzed SLAG | Zeolite | Experimental Conditions | Yield mZeol (g)/mHS (g) | Mother Liquor | |||
|---|---|---|---|---|---|---|---|
| T (°C) | t (h) | pH | I.C. (mS cm−1) | Density (g L−1) | |||
| 1/10-T25 | Z1/10-120-5h | 120 | 5 | 1.9 | 12.8 ± 0.1 | 145 ± 2 | 1.03 ± 0.01 |
| Z1/10-100-24h | 100 | 24 | 1.8 | 12.9 ± 0.1 | 144 ± 2 | 1.04 ± 0.01 | |
| 1/3-T90 | Z1/3-100-24h | 100 | 24 | 1.8 | 12.8 ± 0.1 | 147 ± 2 | 1.04 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla, I.; Romero, M.; López-Andrés, S.; López-Delgado, A. Sustainable Management of Salt Slag. Sustainability 2022, 14, 4887. https://doi.org/10.3390/su14094887
Padilla I, Romero M, López-Andrés S, López-Delgado A. Sustainable Management of Salt Slag. Sustainability. 2022; 14(9):4887. https://doi.org/10.3390/su14094887
Chicago/Turabian StylePadilla, Isabel, Maximina Romero, Sol López-Andrés, and Aurora López-Delgado. 2022. "Sustainable Management of Salt Slag" Sustainability 14, no. 9: 4887. https://doi.org/10.3390/su14094887
APA StylePadilla, I., Romero, M., López-Andrés, S., & López-Delgado, A. (2022). Sustainable Management of Salt Slag. Sustainability, 14(9), 4887. https://doi.org/10.3390/su14094887









