Geochemical and Morphological Evaluations of Organic and Mineral Aerosols in Coal Mining Areas: A Case Study of Santa Catarina, Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Strategy
2.2. Analytical Procedures
3. Results
3.1. Particulate Matter Analysis
3.2. Electron Beam Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munawer, M.E. Human health and environmental impacts of coal combustion and post-combustion wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Gent, J.F.; Triche, E.W.; Holford, T.R.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Leaderer, B.P. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003, 290, 1859–1867. [Google Scholar] [PubMed] [Green Version]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, O.; de la Campa, A.M.S.; Amato, F.; Moreno, T.; Silva, L.; de la Rosa, J.D. Physicochemical characterization and sources of the thoracic fraction of road dust in a Latin American megacity. Sci. Total Environ. 2019, 652, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Blackley, D.J.; Halldin, C.N.; Laney, A.S. Continued increase in prevalence of coal workers’ pneumoconiosis in the United States, 1970–2017. Am. J. Public Health 2018, 108, 1220–1222. [Google Scholar] [CrossRef]
- Moreno, T.; Trechera, P.; Querol, X.; Lah, R.; Johnson, D.; Wrana, A.; Williamson, B. Trace element fractionation between PM10 and PM2.5 in coal mine dust: Implications for occupational respiratory health. Int. J. Coal Geol. 2019, 203, 52–59. [Google Scholar] [CrossRef]
- Garcia, K.O.; Teixeira, E.C.; Agudelo-Castañeda, D.M.; Braga, M.; Alabarse, P.G.; Wiegand, F.; Kautzmann, R.M.; Silva, L.F. Assessment of nitro-polycyclic aromatic hydrocarbons in PM1 near an area of heavy-duty traffic. Sci. Total Environ. 2014, 479, 57–65. [Google Scholar] [CrossRef]
- Schneider, I.L.; Teixeira, E.C.; Agudelo-Castañeda, D.M.; e Silva, G.S.; Balzaretti, N.; Braga, M.F.; Oliveira, L.F.S. FTIR analysis and evaluation of carcinogenic and mutagenic risks of nitro-polycyclic aromatic hydrocarbons in PM 1.0. Sci. Total Environ. 2016, 541, 1151–1160. [Google Scholar] [CrossRef]
- Poenar, D.P. Microfluidic and micromachined/MEMS devices for separation, discrimination and detection of airborne par-ticles for pollution monitoring. Micromachines 2019, 10, 483. [Google Scholar]
- Azam, S.; Mishra, D.P. Effects of particle size, dust concentration and dust-dispersionair pressure on rock dust inertant requirement for coal dust explosion suppression in underground coal mines. Process Saf. Environ. Prot. 2019, 126, 35–43. [Google Scholar]
- Haas, E.J. Using self-determination theory to identify organizational interventions to support coal mineworkers’ dust-reducing practices. Int. J. Min. Sci. Technol. 2019, 29, 371–378. [Google Scholar] [CrossRef]
- Su, X.; Ding, R.; Zhuang, X. Characteristics of dust in coal mines in central north China and its research significance. ACS Omega 2020, 5, 9233–9250. [Google Scholar] [PubMed] [Green Version]
- Farmer, J.G.; Broadway, A.; Cave, M.; Wragg, J.; Fordyce, F.; Graham, M.C.; Ngwenya, B.T.; Bewley, R.J. A lead isotopic study of the human bioaccessibility of lead in urban soils from Glasgow, Scotland. Sci. Total Environ. 2011, 409, 4958–4965. [Google Scholar] [CrossRef] [PubMed]
- Amirah, M.; Afiza, A.; Faizal, W.; Nurliyana, M.; Laili, S. Human health risk assessment of metal contamination through consumption of fish. J. Environ. Pollut. Hum. Health 2013, 1, 1–5. [Google Scholar]
- Akinyemi, S.A.; Gitari, W.M.; Petrik, L.F.; Nyakuma, B.B.; Hower, J.C.; Ward, C.R.; Oliveira, M.L.; Silva, L.F. Environmental evaluation and nano-mineralogical study of fresh and unsaturated weathered coal fly ashes. Sci. Total Environ. 2019, 663, 177–188. [Google Scholar]
- Ezemonye, L.I.; Adebayo, P.O.; Enuneku, A.A.; Tongo, I.; Ogbomida, E. Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol. Rep. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Pan, L.; Golden, S.; Assemi, S.; Sime, M.; Wang, X.; Gao, Y.; Miller, J. Characterization of particle size and composition of respirable coal mine dust. Minerals 2021, 11, 276. [Google Scholar] [CrossRef]
- Abbasi, B.; Wang, X.; Chow, J.; Watson, J.; Peik, B.; Nasiri, V.; Riemenschnitter, K.; Elahifard, M. Review of respirable coal mine dust characterization for mass concentration, size distribution and chemical composition. Minerals 2021, 11, 426. [Google Scholar] [CrossRef]
- Mu, M.; Li, B.; Zou, Y.; Wang, W.; Cao, H.; Zhang, Y.; Sun, Q.; Chen, H.; Ge, D.; Tao, H.; et al. Coal dust exposure triggers heterogeneity of transcriptional profiles in mouse pneumoconiosis and vitamin D remedies. Part. Fibre Toxicol. 2022, 19, 1–21. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S. The impacts of coal dust on miners’ health: A review. Environ. Res. 2020, 190, 109849. [Google Scholar] [CrossRef]
- Silva, L.F.; Milanes, C.; Pinto, D.; Ramirez, O.; Lima, B.D. Multiple hazardous elements in nanoparticulate matter from a Caribbean industrialized atmosphere. Chemosphere 2020, 239, 124776. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, W.G.; Green, B.J.; Blachere, F.M.; Martin, S.B.; Law, B.; Jensen, P.; Schafer, M. Sampling and Characterization of Bioaerosols. NIOSH Manual of Analytical Methods, 5th ed.; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2017. [Google Scholar]
- Cerqueira, B.; Vega, F.A.; Silva, L.; Andrade, M.L. Effects of vegetation on chemical and mineralogical characteristics of soils developed on a decantation bank from a copper mine. Sci. Total Environ. 2012, 421, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Nordin, A.P.; da Silva, J.; de Souza, C.T.; Niekraszewicz, L.A.; Dias, J.; da Boit, K.; Oliveira, M.L.; Grivicich, I.; Garcia, A.L.H.; Oliveira, L.F.S.; et al. In vitro genotoxic effect of secondary minerals crystallized in rocks from coal mine drainage. J. Hazard. Mater. 2018, 346, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Flores, D.; Ward, C.; Silva, L.F. Identification of nanominerals and nanoparticles in burning coal waste piles from Portugal. Sci. Total Environ. 2010, 408, 6032–6041. [Google Scholar] [CrossRef]
- Sehn, J.L.; de Leão, F.B.; da Boit, K.; Oliveira, M.; Hidalgo, G.E.; Sampaio, C.H.; Silva, L. Nanomineralogy in the real world: A perspective on nanoparticles in the environmental impacts of coal fire. Chemosphere 2016, 147, 439–443. [Google Scholar] [CrossRef]
- Rojas, J.C.; Sánchez, N.E.; Schneider, I.; Oliveira, M.L.; Teixeira, E.C.; Silva, L.F. Exposure to nanometric pollutants in primary schools: Environmental implications. Urban Clim. 2019, 27, 412–419. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Ramugondo, L.; Matidza, M.; Mhlongo, S.E. Geochemical fractionation of metals and metalloids in tailings and appraisal of environmental pollution in the abandoned Musina Copper Mine, South Africa. Environ. Geochem. Health 2018, 40, 2421–2439. [Google Scholar] [CrossRef]
- Akinyemi, S.A.; Gitari, W.M.; Thobakgale, R.; Petrik, L.F.; Nyakuma, B.B.; Hower, J.C.; Ward, C.R.; Oliveira, M.L.S.; Silva, L.F.O. Geochemical fractionation of hazardous elements in fresh and drilled weathered South African coal fly ashes. Environ. Geochem. Health 2020, 42, 2771–2788. [Google Scholar] [CrossRef]
- Silva, L.; Izquierdo, M.; Querol, X.; Finkelman, R.B.; Oliveira, M.; Wollenschlager, M.; Towler, M.; Pérez-López, R.; Macias, F. Leaching of potential hazardous elements of coal cleaning rejects. Environ. Monit. Assess. 2010, 175, 109–126. [Google Scholar] [CrossRef]
- Silva, L.; Macias, F.; Oliveira, M.; da Boit, M.K.; Waanders, F.B. Coal cleaning residues and Fe-minerals implications. Environ. Monit. Assess. 2010, 172, 367–378. [Google Scholar] [CrossRef]
- Oliveira, M.L.; da Boit, K.; Pacheco, F.; Teixeira, E.C.; Schneider, I.L.; Crissien, T.J.; Pinto, D.C.; Oyaga, R.M.; Silva, L.F. Mul-tifaceted processes controlling the distribution of hazardous compounds in the spontaneous combustion of coal and the effect of these compounds on human health. Environ. Res. 2018, 160, 562–567. [Google Scholar] [PubMed]
- Oliveira, M.L.; da Boit, K.; Schneider, I.L.; Teixeira, E.C.; Borrero, T.J.C.; Silva, L.F. Study of coal cleaning rejects by FIB and sample preparation for HR-TEM: Mineral surface chemistry and nanoparticle-aggregation control for health studies. J. Clean. Prod. 2018, 188, 662–669. [Google Scholar] [CrossRef]
- Meyer, H.; Meischein, M.; Ludwig, A. Rapid assessment of sputtered nanoparticle ionic liquid combinations. ACS Comb. Sci. 2018, 20, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Garzón-Manjón, A.; Meyer, H.; Grochla, D.; Löffler, T.; Schuhmann, W.; Ludwig, A.; Scheu, C. Controlling the amorphous and crystalline state of multinary alloy nanoparticles in an ionic liquid. Nanomaterials 2018, 8, 903. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, J.; Valentim, B.; Ward, C.; Flores, D. Comprehensive characterization of anthracite fly ash from a thermo-electric power plant and its potential environmental impact. Int. J. Coal Geol. 2011, 86, 204–212. [Google Scholar] [CrossRef]
- Quispe, D.; Pérez-López, R.; Silva, L.F.; Nieto, J.M. Changes in mobility of hazardous elements during coal combustion in Santa Catarina power plant (Brazil). Fuel 2012, 94, 495–503. [Google Scholar] [CrossRef]
- Silva, L.F.; Hower, J.C.; Izquierdo, M.; Querol, X. Complex nanominerals and ultrafine particles assemblages in phosphogypsum of the fertilizer industry and implications on human exposure. Sci. Total Environ. 2010, 408, 5117–5122. [Google Scholar]
- Akinyemi, S.; Akinlua, A.; Gitari, W.; Khuse, N.; Eze, P.; Akinyeye, R.; Petrik, L. Natural weathering in dry disposed ash dump: Insight from chemical, mineralogical and geochemical analysis of fresh and unsaturated drilled cores. J. Environ. Manag. 2012, 102, 96–107. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Vega, F.; Silva, L.; Andrade, M. Soil interaction and fractionation of added cadmium in some Galician soils. Microchem. J. 2013, 110, 681–690. [Google Scholar] [CrossRef]
- Giemsa, E.; Soentgen, J.; Kusch, T.; Beck, C.; Münkel, C.; Cyrys, J.; Pitz, M. Influence of local sources and meteorological parameters on the spatial and temporal distribution of ultrafine particles in Augsburg, Germany. Front. Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Hochella, M.F.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.L.; Ward, C.; Izquierdo, M.; Sampaio, C.H.; de Brum, I.A.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F. Chemical composition and minerals in pyrite ash of an abandoned sulphuric acid production plant. Sci. Total Environ. 2012, 430, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahhoseiny, M.; Ardejani, F.D.; Shafaei, S.Z.; Singh, R.; Shokri, B.J. Geochemical characterisation of a pyrite containing coal washing refuse pile produced by the Anjir Tangeh coal washing plant in Zirab, Mazandaran province, Northern Iran. In Proceedings of the 11th International Mine Water Association Congress—Mine Water—Managing the Challenges (IMWA 2011), Aachen, Germany, 4–11 September 2011. [Google Scholar]
- Gasparotto, J.; Chaves, P.R.; Martinello, K.D.B.; da Rosa-Siva, H.T.; Bortolin, R.C.; Silva, L.; Rabelo, T.K.; da Silva, J.; da Silva, F.R.; Nordin, A.P.; et al. Obese rats are more vulnerable to inflammation, genotoxicity and oxidative stress induced by coal dust inhalation than non-obese rats. Ecotoxicol. Environ. Saf. 2018, 165, 44–51. [Google Scholar] [CrossRef]
- Gasparotto, J.; Chaves, P.R.; Martinello, K.D.B.; Oliveira, L.F.S.; Gelain, D.P.; Moreira, J.C.F. Obesity associated with coal ash inhalation triggers systemic inflammation and oxidative damage in the hippocampus of rats. Food Chem. Toxicol. 2019, 133, 110766. [Google Scholar] [CrossRef]
- Ostro, B.; Tobias, A.; Querol, X.; Alastuey, A.; Amato, F.; Pey, J.; Pérez, N.; Sunyer, J. The effects of particulate matter sources on daily mortality: A case-crossover study of Barcelona, Spain. Environ. Health Perspect. 2011, 119, 1781–1787. [Google Scholar] [CrossRef]
- Silva, L.; Moreno, T.; Querol, X. An introductory TEM study of Fe-nanominerals within coal fly ash. Sci. Total Environ. 2009, 407, 4972–4974. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Dotto, G.L.; Hower, J.C. Nanomineralogy of evaporative precipitation of efflorescent compounds from coal mine drainage. Geosci. Front. 2021, 12, 101003. [Google Scholar]
- Gurgueira, S.; Lawrence, J.; Brent, C.; Krishna, M.; Gonzalez-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect. 2002, 110, 749–755. [Google Scholar]
- Ribeiro, J.; Taffarel, S.R.; Sampaio, C.H.; Flores, D.; Silva, L.F. Mineral speciation and fate of some hazardous contaminants in coal waste pile from anthracite mining in Portugal. Int. J. Coal Geol. 2013, 109, 15–23. [Google Scholar]
- Silva, L.F.; DaBoit, K.; Sampaio, C.H.; Jasper, A.; Andrade, M.L.; Kostova, I.J.; Waanders, F.B.; Henke, K.R.; Hower, J.C. The occurrence of hazardous volatile elements and nanoparticles in Bulgarian coal fly ashes and the effect on human health exposure. Sci. Total Environ. 2012, 416, 513–526. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Jasper, A.; Andrade, M.L.; Sampaio, C.H.; Dai, S.; Li, X.; Li, T.; Chen, W.; Wang, X.; Liu, H.; et al. Applied investigation on the interaction of hazardous elements binding on ultrafine and nanoparticles in Chinese anthracite-derived fly ash. Sci. Total Environ. 2012, 419, 250–264. [Google Scholar] [PubMed]
- Silva, L.F.O.; Hower, J.C.; Dotto, G.L.; Marcos, L.S.; Oliveira, M.L.S.; Moreno, A.L. Nanoparticles from evaporite materials in Colombian coal mine drainages. Int. J. Coal Geol. 2020, 230, 103588. [Google Scholar]
- Iavicoli, I.; Leso, V.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vivo studies. J. Nanomater. 2012, 2012, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.F.O.; Hower, J.C.; Dotto, G.L.; Oliveira, M.L.S.; Pinto, D. Titanium nanoparticles in sedimented dust aggregates from urban children’s parks around coal ashes wastes. Fuel 2021, 285, 119162. [Google Scholar]
- Yang, Y.; Chen, B.; Hower, J.; Schindler, M.; Winkler, C.; Brandt, J.; di Giulio, R.; Ge, J.; Liu, M.; Fu, Y.; et al. Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Kornberg, T.; Stueckle, T.; Antonini, J.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Wang, L. Potential toxicity and under-lying mechanisms associated with pulmonary exposure to iron oxide nanoparticles: Conflicting literature and unclear risk. Nanomaterials 2017, 7, 307. [Google Scholar]
- Maher, B.A.; Ahmed, I.A.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar]
- Rajendran, K.; Sen, S.; Suja, G.; Senthil, S.L.; Kumar, T.V. Evaluation of cytotoxicity of hematite nanoparticles in bacteria and human cell lines. Colloids Surf. B Biointerfaces 2017, 157, 101–109. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Querol, X.; da Boit, K.M.; Fdez-Ortiz de Vallejuelo, S.; Madariaga, J.M. Brazilian coal mining residues and sulphide oxidation by Fenton’s reaction: An accelerated weathering procedure to evaluate possible environmental im-pact. J. Hazard. Mater. 2011, 186, 516–525. [Google Scholar]
- Shao, L.; Cao, Y.; Jones, T.; Santosh, M.; Silva, L.F.; Ge, S.; da Boit, K.; Feng, X.; Zhang, M.; Bérubé, K. COVID-19 mortality and exposure to airborne PM2.5: A lag time correlation. Sci. Total Environ. 2021, 806, 151286. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Oliveira, M.L.S. Nanominerals and Ultrafine Particles from Brazilian Coal Fires; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3. [Google Scholar]
- Cutruneo, C.M.N.L.; Oliveira, M.L.S.; Ward, C.R.; Hower, J.C.; de Brum, I.A.S.; Sampaio, C.H.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F.O. A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. Int. J. Coal Geol. 2014, 130, 33. [Google Scholar]
- Dias, C.L.; Oliveira, M.L.S.; Hower, J.C.; Taffarel, S.R.; Kautzmann, R.M.; Silva, L.F.O. Nanominerals and ultrafine particles from coal fires from Santa Catarina, South Brazil. Int. J. Coal Geol. 2014, 122, 50–60. [Google Scholar]
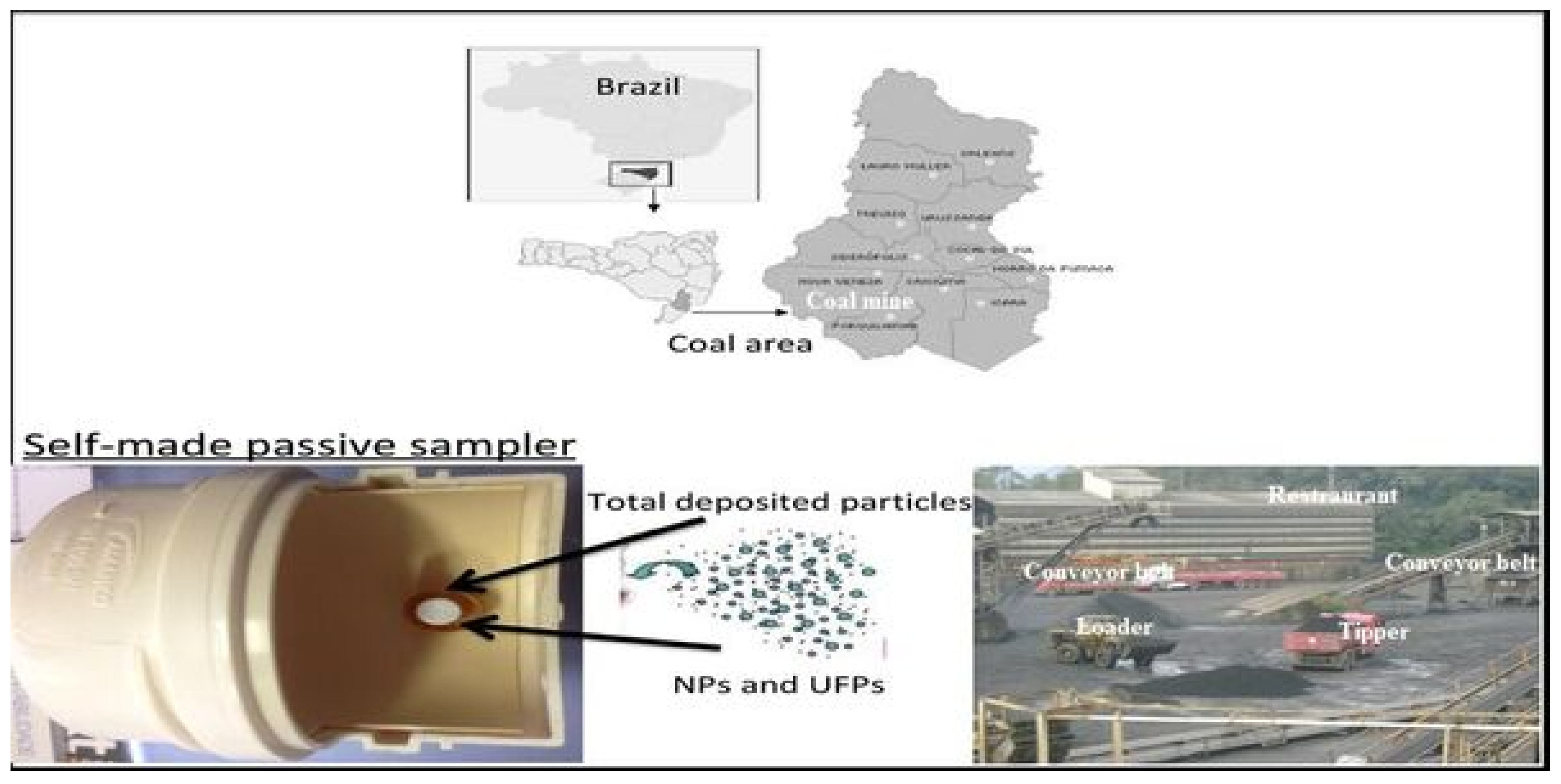
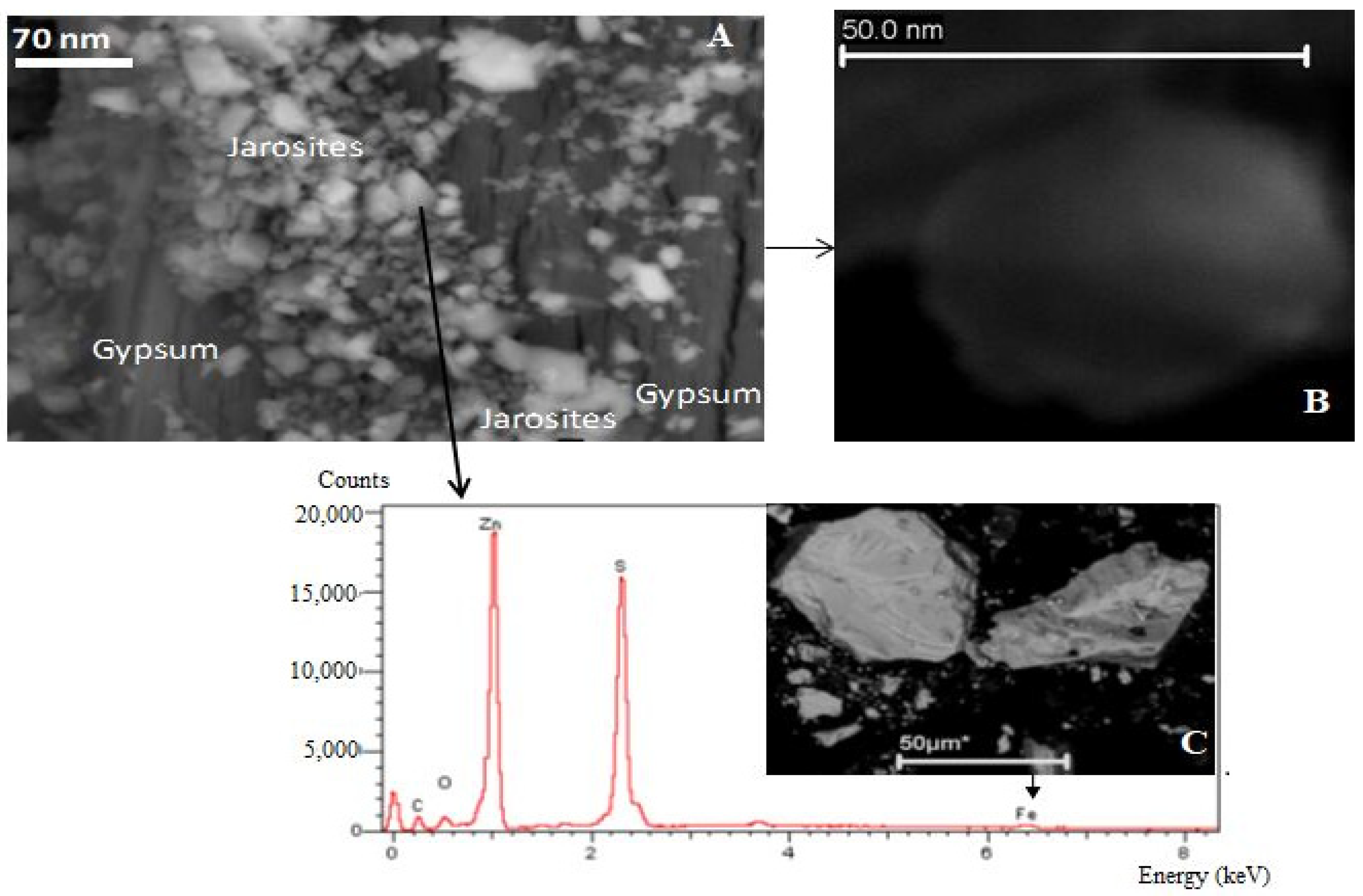
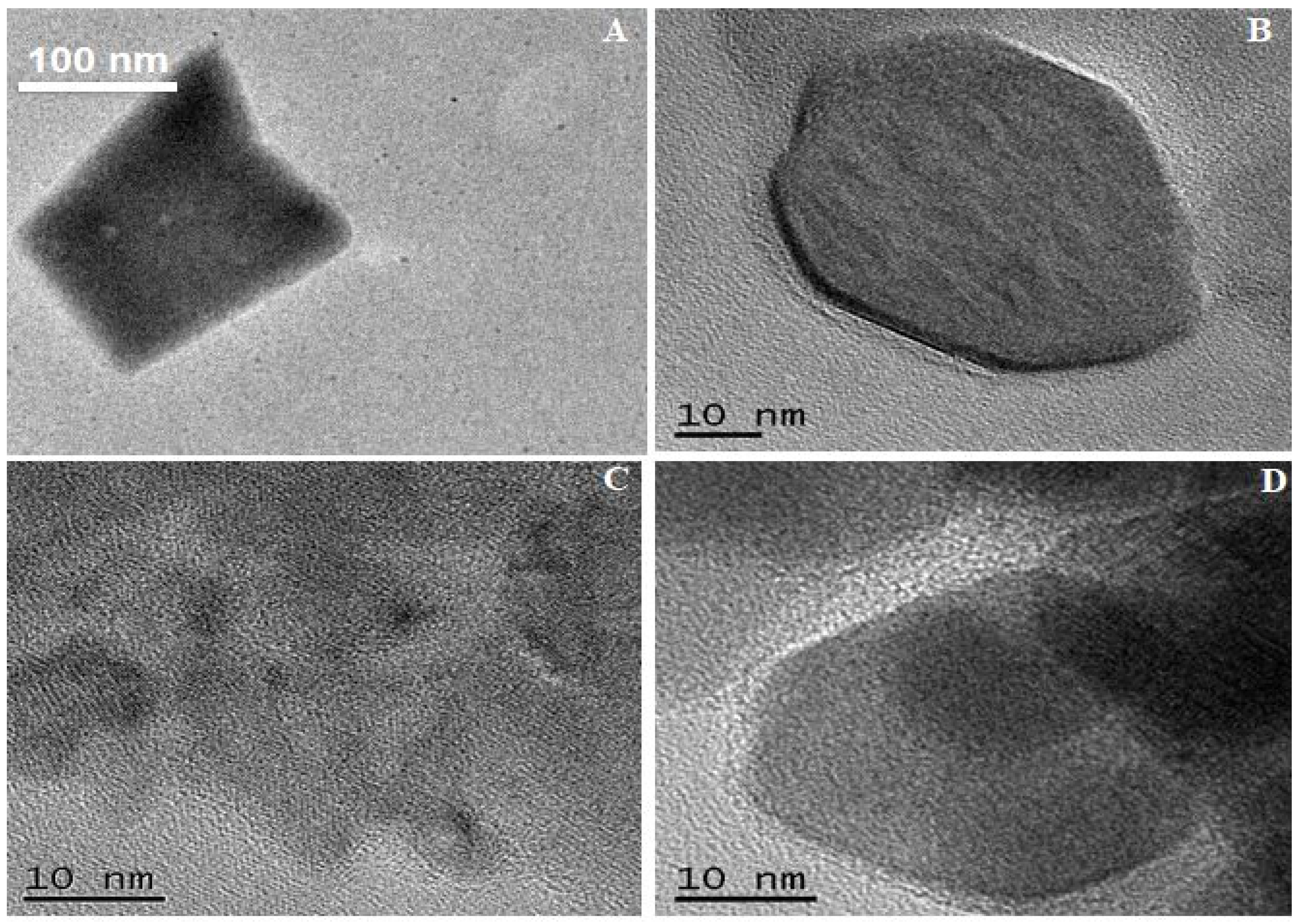

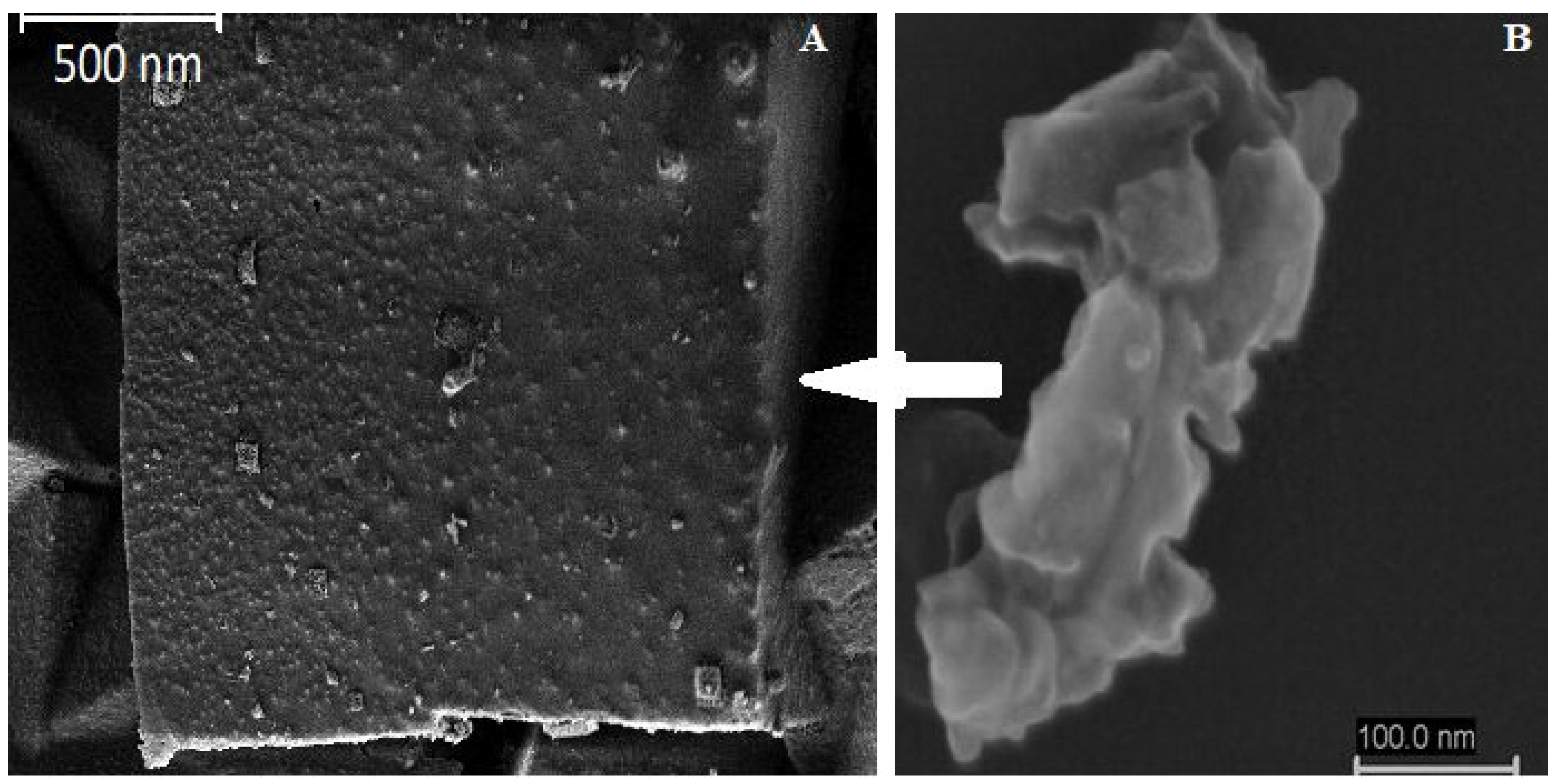
| Sample Names | PM 11 | PM 12 | PM 13 | PM 14 | PM 15 | PM 16 | PM 17 | PM 18 |
|---|---|---|---|---|---|---|---|---|
| Amorphous | B | B | B | B | B | B | B | B |
| Mineral | ||||||||
| Anatase | B | B | B | B | B | B | ||
| Anhydrite | A, B | A, B | B | B | ||||
| Alunogen | B | B | B | |||||
| Barite | B | B | B | B | B | |||
| Calcite | B | A, B | A, B | A, B | A, B | A, B | ||
| Dolomite | B | A, B | A, B | B | A, B | B | B | |
| Epsomite | B | B | B | B | ||||
| Ferrohexahydrite | B | B, M | B, M | M | M | M | M | |
| Gypsum | B, M | B | B | B | B | B | B | |
| Hematite | B | B | B | B | B | B | ||
| Jarosite | B | B | B, M | B | B | B | ||
| Kaolinite | B, M | B | B, M | B, M | B, M | B, M | B, M | |
| Melanterite | B, M | A, B | B | A, B | B | B | A | A |
| Mullite | A, B | B | M | M | M | |||
| Pyrite | B, M | B | B | B | B | |||
| Quartz | B | B, M | M | M | B, M | A, B, M | A, B, M | A, B, M |
| Rutile | A, B, M | A, B | B | A, B | A, B | A, B | A, B | |
| Siderite and Sphalerite | B | B | B | B | B | B | ||
| B | B, M | M | M | B, M | B, M | |||
| Chemical Elements | ||||||||
| Al | E | E | E | E | E | E | E | E |
| As | E | E | E | E | ||||
| Ba | E | E | E | E | E | E | ||
| Br | E | E | E | E | E | |||
| Ca | E | E | E | E | E | E | E | E |
| Cd | E | E | E | E | E | E | E | E |
| Cl | E | E | E | E | E | |||
| Cu | E | E | E | E | E | E | ||
| Co | E | E | E | E | ||||
| Cr | E | E | E | E | E | |||
| Fe | E | E | E | E | E | E | E | E |
| Hg | E | E | E | E | E | |||
| Mn | E | E | E | E | E | E | E | E |
| Ni | E | E | E | E | E | E | E | |
| Pb | E | E | E | E | E | E | E | |
| S | E | E | E | E | ||||
| Sb | E | E | E | E | E | |||
| Se | E | E | E | E | E | E | ||
| Ti | E | E | E | E | E | E | E | E |
| V | E | E | E | E | E | |||
| Zn | E |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinyemi, S.A.; Oliveira, M.L.S.; Nyakuma, B.B.; Dotto, G.L. Geochemical and Morphological Evaluations of Organic and Mineral Aerosols in Coal Mining Areas: A Case Study of Santa Catarina, Brazil. Sustainability 2022, 14, 3847. https://doi.org/10.3390/su14073847
Akinyemi SA, Oliveira MLS, Nyakuma BB, Dotto GL. Geochemical and Morphological Evaluations of Organic and Mineral Aerosols in Coal Mining Areas: A Case Study of Santa Catarina, Brazil. Sustainability. 2022; 14(7):3847. https://doi.org/10.3390/su14073847
Chicago/Turabian StyleAkinyemi, Segun A., Marcos L. S. Oliveira, Bemgba B. Nyakuma, and Guilherme L. Dotto. 2022. "Geochemical and Morphological Evaluations of Organic and Mineral Aerosols in Coal Mining Areas: A Case Study of Santa Catarina, Brazil" Sustainability 14, no. 7: 3847. https://doi.org/10.3390/su14073847
APA StyleAkinyemi, S. A., Oliveira, M. L. S., Nyakuma, B. B., & Dotto, G. L. (2022). Geochemical and Morphological Evaluations of Organic and Mineral Aerosols in Coal Mining Areas: A Case Study of Santa Catarina, Brazil. Sustainability, 14(7), 3847. https://doi.org/10.3390/su14073847







