An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
Specific Properties of Sampled CAC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MME—Ministry of Mines and Energy, BRAZIL, National Energy Matrix—2030. 2019. Available online: http://antigo.mme.gov.br/pt/web/guest/secretarias/planejamento-e-desenvolvimento-energetico/publicacoes/matriz-energetica-nacional-2030 (accessed on 12 November 2021).
- ANEEL—National Electric Energy Agency. 2021. Available online: https://www.aneel.gov.br (accessed on 13 November 2021).
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Martinello, K.; Oliveira, M.L.S.; Molossi, F.A.; Ramos, C.G.; Teixeira, E.C.; Kautzmann, R.M.; Silva, L.F.O. Direct identification of hazardous elements in ultra-fine and nanominerals from coal fly ash produced during diesel co-firing. Sci. Total Environ. 2014, 470, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Soe, J.T.; Zhang, S.; Ahn, J.W.; Park, M.B.; Ahn, W.S. Synthesis of nanoporous materials via recycling coal fly ash and other solid wastes: A mini review. Chem. Eng. J. 2017, 317, 821–843. [Google Scholar] [CrossRef]
- Kikuchi, R. Application of coal ash to environmental improvement. Resour. Conserv. Recycl. 1999, 27, 333–346. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Mateus, R.; Camões, A.F.; Bragança, L.; Branco, F.G. Comparative environmental life-cycle analysis of concretes using biomass and coal fly ashes as partial cement replacement material. J. Clean. Prod. 2016, 112, 2221–2230. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, C.P.; Zhang, H.; Wang, H.Y. Numerical investigation of slip and fracture instability mechanism of coal- rock parting-coal structure (CRCS). J. Struct. Geol. 2019, 118, 265–278. [Google Scholar] [CrossRef]
- Hossain, M.d.U.; Dong, Y.; Ng, S.T. Influence of supplementary cementitious materials in sustainability performance of concrete industry: A case study in Hong Kong. Case Stud. Constr. Mater. 2021, 15, e00659. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Dyczek, J.; Deja, J. Investigation of the coal fly ashes using IR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 889–894. [Google Scholar] [CrossRef]
- Ahmed, S.; Arocho, I. Analysis of cost comparison and effects of change orders during construction: Study of a mass timber and a concrete building project. J. Build. Eng. 2021, 33, 101856. [Google Scholar] [CrossRef]
- Harihanandh, M.; Viswanathan, K.; Krishnaraja, A. Comparative study on chemical and morphology properties of nano fly ash in concrete. Mater. Today Proc. 2021, 45, 3132–3136. [Google Scholar] [CrossRef]
- Ribeiro, J.; DaBoit, K.; Flores, D.; Kronbauer, M.A.; Silva, L.F. Extensive FE-SEM/EDS, HR-TEM/EDS and ToF-SIMS studies of micron- to nano-particles in anthracite fly ash. Sci. Total Environ. 2013, 452, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.; Nageswara Rao, G. Efficiency of fly ash in concrete. Cem. Concr. Compos. 1993, 15, 223–229. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Palma, L.d.; Cioffi, R. Recycling of non-metallic automotive shredder residues and coal fly-ash in cold-bonded aggregates for sustainable concrete. Compos. Part B Eng. 2017, 116, 46–52. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Boit, K.M.d. Nanominerals and nanoparticles in feed coal and bottom ash: Implications for human health effects. Environ. Monit. Assess. 2010, 174, 187–197. [Google Scholar] [CrossRef]
- Banfield, J.F.; Zhang, H. Nanoparticles in the Environment. Rev. Mineral. Geochem. 2001, 44, 1–58. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Ward, C.R.; Sampaio, C.H.; Querol, X.; Cutruneo, C.M.N.L.; Taffarel, S.R.; Silva, L.F.O. Partitioning of mineralogical and inorganic geochemical components of coals from Santa Catarina, Brazil, by industrial beneficiation processes. Int. J. Coal Geol. 2013, 116, 75–92. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Neckel, A.; Dotto, G.L.; Oliveira, M.L.S. The impact of air pollution on the rate of degradation of the fortress of Florianópolis Island, Brazil. Chemosphere 2020, 251, 126838. [Google Scholar] [CrossRef]
- Oliveira, M.L.S.; Flores, E.M.M.; Dotto, G.L.; Neckel, A.; Silva, L.F.O. Nanomineralogy of mortars and ceramics from the Forum of Caesar and Nerva (Rome, Italy): The protagonist of black crusts produced on historic buildings. J. Clean. Prod. 2021, 278, 123982. [Google Scholar] [CrossRef]
- Trejos, E.M.; Silva, L.F.O.; Hower, J.C.; Flores, E.M.M.; González, C.M.; Pachón, J.E.; Aristizábal, B.H. Volcanic emissions and atmospheric pollution: A study of nanoparticles. Geosci. Front. 2021, 12, 746–755. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Moreno, T.; Querol, X. An introductory TEM study of Fe-nanominerals within coal fly ash. Sci. Total Environ. 2009, 407, 4972–4974. [Google Scholar] [CrossRef]
- Quispe, D.; Pérez-López, R.; Silva, L.F.O.; Nieto, J.M. Changes in mobility of hazardous elements during coal combustion in Santa Catarina power plant (Brazil). Fuel 2012, 94, 495–503. [Google Scholar] [CrossRef]
- Cutruneo, C.M.N.L.; Oliveira, M.L.S.; Ward, C.R.; Hower, J.C.; Brum, I.A.S.d.; Sampaio, C.H.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F.O. A mineralogical and geochemical study of three Brazilian coal cleaning rejects: Demonstration of electron beam applications. Int. J. Coal Geol. 2014, 130, 33–52. [Google Scholar] [CrossRef]
- Cerqueira, B.; Vega, F.A.; Serra, C.; Silva, L.F.O.; Andrade, M.L. Time of flight secondary ion mass spectrometry and high-resolution transmission electron microscopy/energy dispersive spectroscopy: A preliminary study of the distribution of Cu2+ and cu2+/pb2+ on a BT horizon surfaces. J. Hazard. Mater. 2011, 195, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Hower, J.; Izquierdo, M.; Querol, X. Complex nanominerals and ultrafine particles assemblages in phosphogypsum of the fertilizer industry and implications on human exposure. Sci. Total Environ. 2010, 408, 5117–5122. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Jasper, A.; Andrade, M.L.; Sampaio, C.H.; Dai, S.; Li, X.; Li, T.; Chen, W.; Wang, X.; Liu, H.; et al. Applied investigation on the interaction of hazardous elements binding on ultrafine and nanoparticles in Chinese anthracite-derived fly ash. Sci. Total Environ. 2012, 419, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.O.; Oliveira, M.L.S.; Sampaio, C.H.; De Brum, I.A.S.; Hower, J.C. Vanadium and nickel speciation in pulverized coal and petroleum coke co-combustion. Energy Fuels 2013, 27, 1194–1203. [Google Scholar] [CrossRef]
- Ramos, C.G.; Querol, X.; Oliveira, M.L.S.; Pires, K.; Kautzmann, R.M.; Silva, L.F. A preliminary evaluation of volcanic rock powder for application in agriculture as soil a remineralizer. Sci. Total Environ. 2015, 512, 371–380. [Google Scholar] [CrossRef]
- Schneider, I.L.; Teixeira, E.C.; Silva, L.F.O.; Wiegand, F. Atmospheric particle number concentration and size distribution in a traffic–impacted area. Atmos. Pollut. Res. 2015, 6, 877–885. [Google Scholar] [CrossRef]
- Wilcox, J.; Wang, B.; Rupp, E.; Taggart, R.; Hsu-Kim, H.; Oliveira, M.; Cutruneo, C.; Taffarel, S.; Silva, L.F.; Hopps, S.; et al. Observations and assessment of fly ashes from high-sulfur bituminous coals and blends of high-sulfur bituminous and subbituminous coals: Environmental processes recorded at the macro and nanometer scale. Energy Fuels 2015, 29, 7168–7177. [Google Scholar] [CrossRef]
- Civeira, M.S.; Ramos, C.G.; Oliveira, M.L.S.; Kautzmann, R.M.; Taffarel, S.R.; Teixeira, E.C.; Silva, L.F. Nano-mineralogy of suspended sediment during the beginning of coal rejects spill. Chemosphere 2016, 145, 142–147. [Google Scholar] [CrossRef]
- Dalmora, A.C.; Ramos, C.G.; Querol, X.; Kautzmann, R.M.; Oliveira, M.L.S.; Taffarel, S.R.; Moreno, T.; Silva, L.F. Nanoparticulate mineral matter from basalt dust wastes. Chemosphere 2016, 144, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Dalmora, A.C.; Ramos, C.; Oliveira, M.; Teixeira, E.; Kautzmann, R.; Taffarel, S.; De Brum, I.; Silva, L.F. Chemical characterization, nano-particle mineralogy and particle size distribution of basalt dust wastes. Sci. Total Environ. 2016, 539, 560–565. [Google Scholar] [CrossRef] [PubMed]
- León-Mejía, G.; Silva, L.F.O.; Civeira, M.S.; Da Silva, J.; Henriques, J.A.P. Cytotoxicity and genotoxicity induced by coal and coal fly ash particles samples in V79 cells. Environ. Sci. Pollut. Res. 2016, 23, 24019–24031. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Iruretagoiena, A.; De Vallejuelo, S.; De Diego, A.; De Leão, F.; De Medeiros, D.; Oliveira, M.; Taffarel, S.; Arana, G.; Madariaga, J.; Silva, L.F. The mobilization of hazardous elements after a tropical storm event in a polluted estuary. Sci. Total Environ. 2016, 565, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Sehn, J.; De Leão, F.; Da Boit, K.; Oliveira, M.; Hidalgo, G.; Sampaio, C.; Silva, L.F. Nanomineralogy in the real world: A perspective on nanoparticles in the environmental impacts of coal fire. Chemosphere 2016, 147, 439–443. [Google Scholar] [CrossRef]
- Dutta, M.; Saikia, J.; Taffarel, S.R.; Waanders, F.B.; De Medeiros, D.; Cutruneo, C.M.; Saikia, B.K. Environmental assessment and nano-mineralogical characterization of coal, overburden and sediment from Indian coal mining acid drainage. Geosci. Front. 2017, 8, 1285–1297. [Google Scholar] [CrossRef]
- Sánchez-Peña, N.E.; Narváez-Semanate, J.L.; Pabón-Patiño, D.; Fernández-Mera, J.E.; Oliveira, M.L.; Da Boit, K.; Tutikian, B.; Crissien, T.; Pinto, D.; Serrano, I.; et al. Chemical and nano-mineralogical study for determining potential uses of legal Colombian gold mine sludge: Experimental evidence. Chemosphere 2018, 191, 1048–1055. [Google Scholar] [CrossRef]
- León-Mejía, G.; Machado, M.N.; Okuro, R.T.; Silva, L.F.; Telles, C.; Dias, J.; Niekraszewicz, L.; Da Silva, J.; Henriques, J.A.P.; Zin, W.A. Intratracheal instillation of coal and coal fly ash particles in mice induces DNA damage and translocation of metals to extrapulmonary tissues. Sci. Total Environ. 2018, 625, 589–599. [Google Scholar] [CrossRef]
- Nordin, A.P.; Da Silva, J.; De Souza, C.; Niekraszewicz, L.A.B.; Dias, J.F.; Da Boit, K.; Oliveira, M.L.S.; Grivicich, I.; Garcia, A.L.; Silva, L.F.; et al. In vitro genotoxic effect of secondary minerals crystallized in rocks from coal mine drainage. J. Hazard. Mater. 2018, 346, 263–272. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Neckel, A.; Oliveira, M.L.S.; Sampaio, C.H. Atmospheric nanocompounds on Lanzarote Island: Vehicular exhaust and igneous geologic formation interactions. Chemosphere 2020, 254, 126822. [Google Scholar] [CrossRef]
- Silva, L.F.O.; Pinto, D.; Neckel, A.; Oliveira, M.L.S. An analysis of vehicular exhaust derived nanoparticles and historical Belgium fortress building interfaces. Geosci. Front. 2020, 11, 2053–2060. [Google Scholar] [CrossRef]
- Lima, B.D.; Teixeira, E.C.; Hower, J.C.; Civeira, M.S.; Ramírez, O.; Yang, C.; Oliveira, M.L.S.; Silva, L.F.O. Metal-enriched nanoparticles and black carbon: A perspective from the Brazil railway system air pollution. Geosci. Front. 2021, 12, 101129. [Google Scholar] [CrossRef]
- Neckel, A.; Oliveira, M.L.S.; Bolaño, L.J.C.; Maculan, L.S.; Moro, L.; Bodah, E.T.; Moreno-Ríos, A.L.; Bodah, B.W.; Silva, L.F. Biophysical matter in a marine estuary identified by the Sentinel-3B OLCI satellite and the presence of terrestrial iron (Fe) nanoparticles. Mar. Pollut. Bull. 2021, 173, 112925. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Neckel, A.; Silva, L.F.; Dotto, G.L.; Maculan, L.S. Environmental aspects of the depreciation of the culturally significant Wall of Cartagena de Indias—Colombia. Chemosphere 2021, 265, 129119. [Google Scholar] [CrossRef] [PubMed]
- Ravindra Babu, S.; Nguyen, L.S.P.; Sheu, G.R.; Griffith, S.M.; Pani, S.K.; Huang, H.Y.; Lin, N.H. Long-range transport of La Soufrière volcanic plume to the western North Pacific: Influence on atmospheric mercury and aerosol properties. Atmos. Environ. 2022, 268, 118806. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, J.; Li, G.; Wen, S.; Yu, R.; Tang, C.; Feng, X.; Liu, X. Characteristics of Sandstone-type Uranium Mineralization in the Hangjinqi Region of the Northeastern Ordos Basin: Clues from Clay Mineral Studies. Ore Geol. Rev. 2021, 141, 104642. [Google Scholar] [CrossRef]
- Zeng, S.; Shen, Y.; Sun, B.; Zhang, N.; Zhang, S.; Feng, S. Pore structure evolution characteristics of sandstone uranium ore during acid leaching. Nucl. Eng. Technol. 2021, 53, 4033–4041. [Google Scholar] [CrossRef]
- Yue, L.; Jiao, Y.; Fayek, M.; Wu, L.; Rong, H. Micromorphologies and sulfur isotopic compositions of pyrite in sandstone-hosted uranium deposits: A review and implications for ore genesis. Ore Geol. Rev. 2021, 139, 104512. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, J.C.P.; Lo, I.M.C. Life cycle carbon footprint measurement of Portland cement and ready mix concrete for a city with local scarcity of resources like Hong Kong. Int. J. Life Cycle Assess. 2014, 19, 745–757. [Google Scholar] [CrossRef]
- Rafieizonooz, M.; Mirza, J.; Salim, M.R.; Hussin, M.W.; Khankhaje, E. Investigation of coal bottom ash and fly ash in concrete as replacement for sand and cement. Constr. Build. Mater. 2016, 116, 15–24. [Google Scholar] [CrossRef]
- Gao, Z.F.; Long, H.M.; Dai, B.; Gao, X.P. Investigation of reducing particulate matter (PM) and heavy metals pollutions by adding a novel additive from metallurgical dust (MD) during coal combustion. J. Hazard. Mater. 2019, 373, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.S.; Ward, C.R.; Izquierdo, M.; Sampaio, C.H.; Brum, I.A.S.d.; Kautzmann, R.M.; Sabedot, S.; Querol, X.; Silva, L.F.O. Chemical composition and minerals in pyrite ash of an abandoned sulphuric acid production plant. Sci. Total Environ. 2012, 430, 34–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.L.S.; Ward, C.R.; French, D.; Hower, J.C.; Querol, X.; Silva, L.F.O. Mineralogy and leaching characteristics of beneficiated coal products from Santa Catarina, Brazil. Int. J. Coal Geol. 2012, 94, 314–325. [Google Scholar] [CrossRef]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.P.; Gawronski, S.W.; Schröder, P.; Vangronsveld, J. Successes and limitations of phytotechnologies at field scale: Outcomes, assessment and outlook from cost action 859. J. Soils Sediments 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.S.; Choi, Y.J.; Kim, J.G. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere 2009, 77, 1069–1075. [Google Scholar] [CrossRef]
- Ŝaltauskaitė, J.; Kniuipytė, I.; Praspaliauskas, M. Earthworm Eisenia fetida potential for sewage sludge amended soil valorization by heavy metal remediation and soil quality improvement. J. Hazard. Mater. 2022, 424, 127316. [Google Scholar] [CrossRef]
- He, J.; Cai, Y.; Lv, J.; Zhang, L. Primary investigation of quartz as a possible carcinogen in coals of Xuanwei and Fuyuan, high lung cancer incidence area in China. Environ. Earth Sci. 2012, 67, 1679–1684. [Google Scholar] [CrossRef]
- Downward, G.S.; Hu, W.; Rothman, N.; Reiss, B.; Tromp, P.; Wu, G.; Wei, F.; Xu, J.; Seow, W.J.; Chapman, R.S. Quartz in ash, and air in a high lung cancer incidence area in China. Environ. Pollut. 2017, 221, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Downward, G.S.; Hu, W.; Rothman, N.; Reiss, B.; Wu, G.; Wei, F.; Chapman, R.S.; Portengen, L.; Qing, L.; Vermeulen, R. Polycyclic Aromatic Hydrocarbon Exposure in Household Air Pollution from Solid Fuel Combustion among the Female Population of Xuanwei and Fuyuan Counties, China. Environ. Sci. Technol. 2014, 48, 14632–14641. [Google Scholar] [CrossRef]
- Onchoke, K.K. 13C NMR chemical shift assignments of nitrated benzo[a]pyrenes based on two-dimensional techniques and DFT/GIAO calculations. Results Chem. 2021, 3, 100099. [Google Scholar] [CrossRef]
- Navarrete, I.; Vargas, F.; Martinez, P.; Paul, A.; Lopez, M. Flue gas desulfurization (FGD) fly ash as a sustainable, safe alternative for cement-based materials. J. Clean. Prod. 2021, 283, 124646. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Depak, S.R.; Hariharan, K.R.; Abid, S.R.; Murali, G.; Cecchin, D.; Fediuk, R.; Amran, Y.H.M.; Abdelgader, H.S.; Khatib, J.M. Standard and modified falling mass impact tests on preplaced aggregate fibrous concrete and slurry infiltrated fibrous concrete. Constr. Build. Mater. 2021, 298, 123857. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Sun, J.; Bai, X.; Zheng, C. Mineralogy, Chemical Composition, and Microstructure of Ferrospheres in Fly Ashes from Coal Combustion. Energy Fuels 2006, 20, 1490–1497. [Google Scholar] [CrossRef]
- Strzałkowska, E. Morphology, chemical and mineralogical composition of magnetic fraction of coal fly ash. Int. J. Coal Geol. 2021, 240, 103746. [Google Scholar] [CrossRef]
- Matjie, R.H.; French, D.; Ward, C.R.; Pistorius, P.C.; Li, Z. Behaviour of coal mineral matter in sintering and slagging of ash during the gasification process. Fuel Process. Technol. 2011, 92, 1426–1433. [Google Scholar] [CrossRef] [Green Version]
- Valentim, B.; Shreya, N.; Paul, B.; Gomes, C.S.; Sant’ovaia, H.; Guedes, A.; Ribeiro, J.; Flores, D.; Pinho, S.; Suárez-Ruiz, I. Characteristics of ferrospheres in fly ashes derived from Bokaro and Jharia (Jharkand, India) coals. Int. J. Coal Geol. 2016, 153, 52–74. [Google Scholar] [CrossRef]
- Sharonova, O.M.; Anshits, N.N.; Solovyov, L.A.; Salanov, A.N.; Anshits, A.G. Relationship between composition and structure of globules in narrow fractions of ferrospheres. Fuel 2013, 111, 332–343. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Borrego, A.G.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 3. Characterization of magnetic and char concentrates. Fuel 2004, 83, 1563–1583. [Google Scholar] [CrossRef]

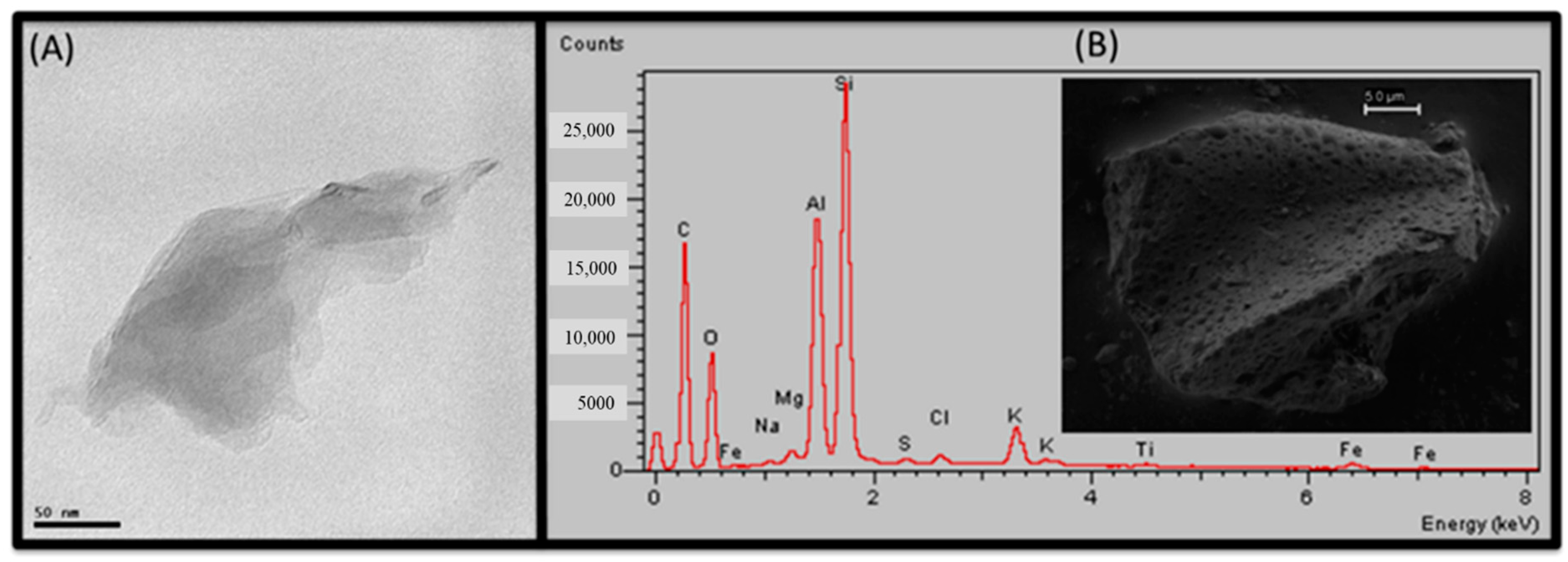
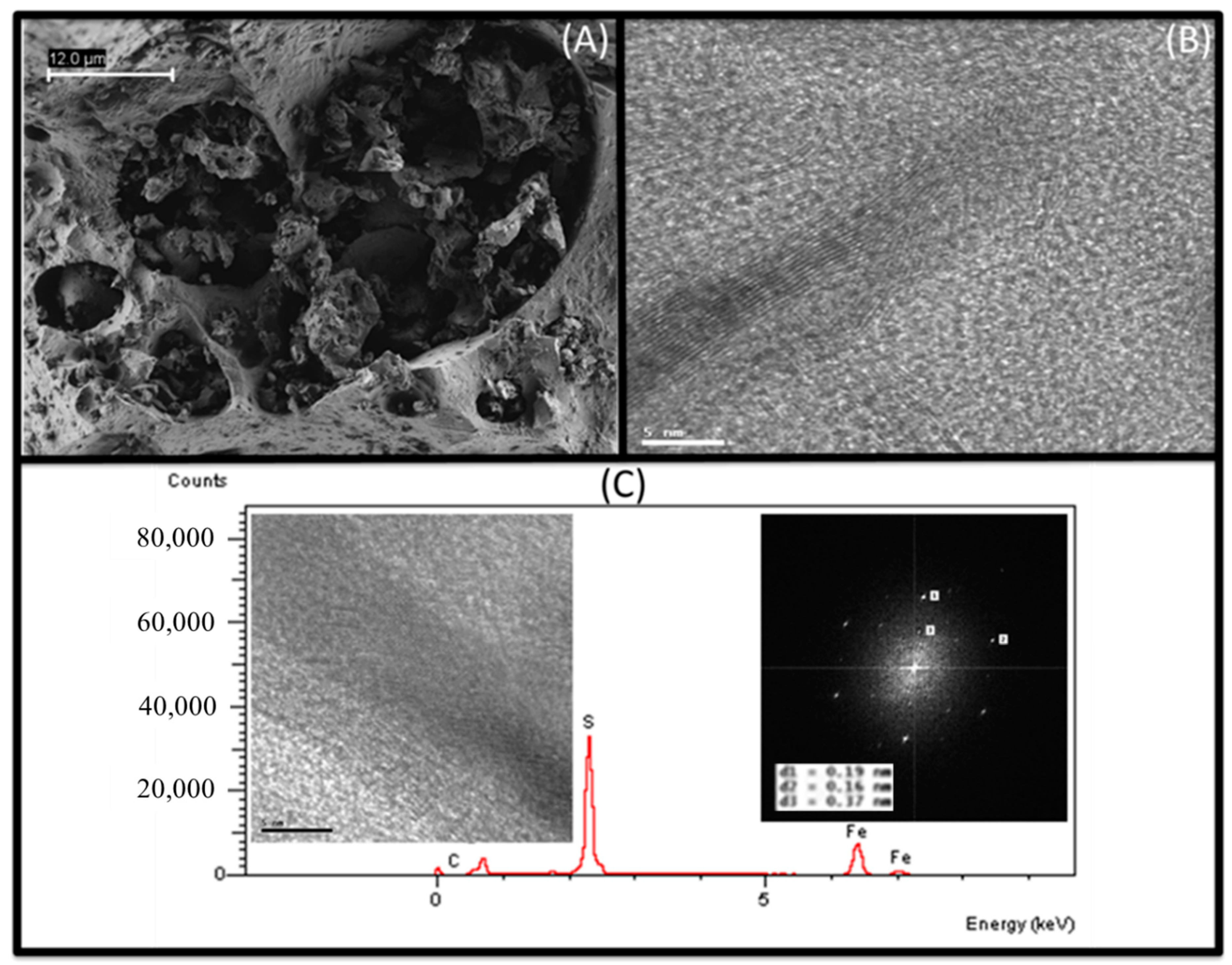
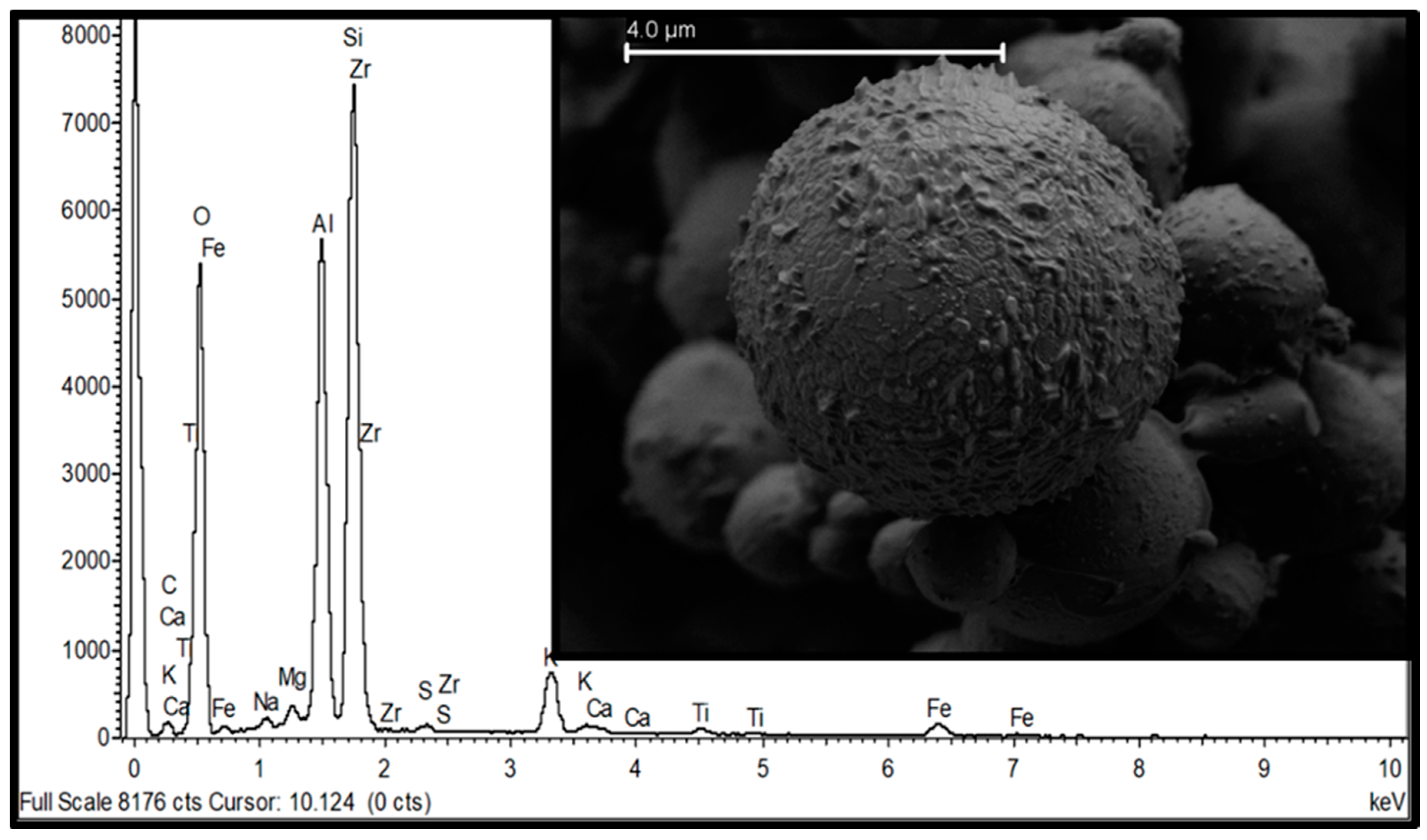
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neckel, A.; Pinto, D.; Adelodun, B.; Dotto, G.L. An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete. Sustainability 2022, 14, 3943. https://doi.org/10.3390/su14073943
Neckel A, Pinto D, Adelodun B, Dotto GL. An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete. Sustainability. 2022; 14(7):3943. https://doi.org/10.3390/su14073943
Chicago/Turabian StyleNeckel, Alcindo, Diana Pinto, Bashir Adelodun, and Guilherme L. Dotto. 2022. "An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete" Sustainability 14, no. 7: 3943. https://doi.org/10.3390/su14073943
APA StyleNeckel, A., Pinto, D., Adelodun, B., & Dotto, G. L. (2022). An Analysis of Nanoparticles Derived from Coal Fly Ash Incorporated into Concrete. Sustainability, 14(7), 3943. https://doi.org/10.3390/su14073943






