Effect of Marginal-Quality Irrigation on Accumulation of some Heavy Metals (Mn, Pb, and Zn) in TypicTorripsamment Soils and Food Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Laboratory Analyses
2.4. Quality Control
2.5. Metal Accumulation in Soils and Crop’s Edible Parts
2.6. Data Analyses
3. Results
3.1. Metal Concentrations in Irrigation Water
3.2. Metal Concentrations in the Irrigated Soils
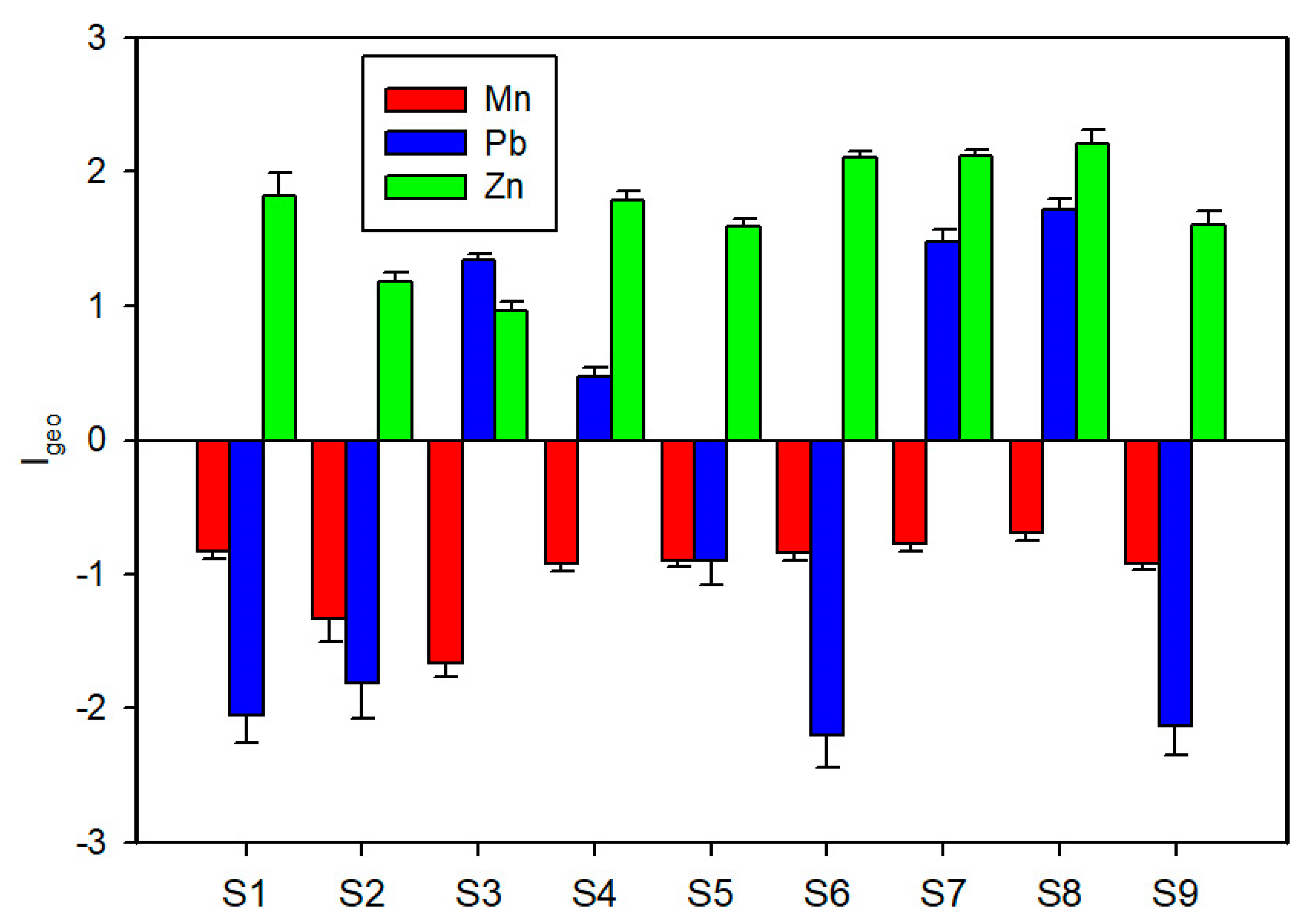
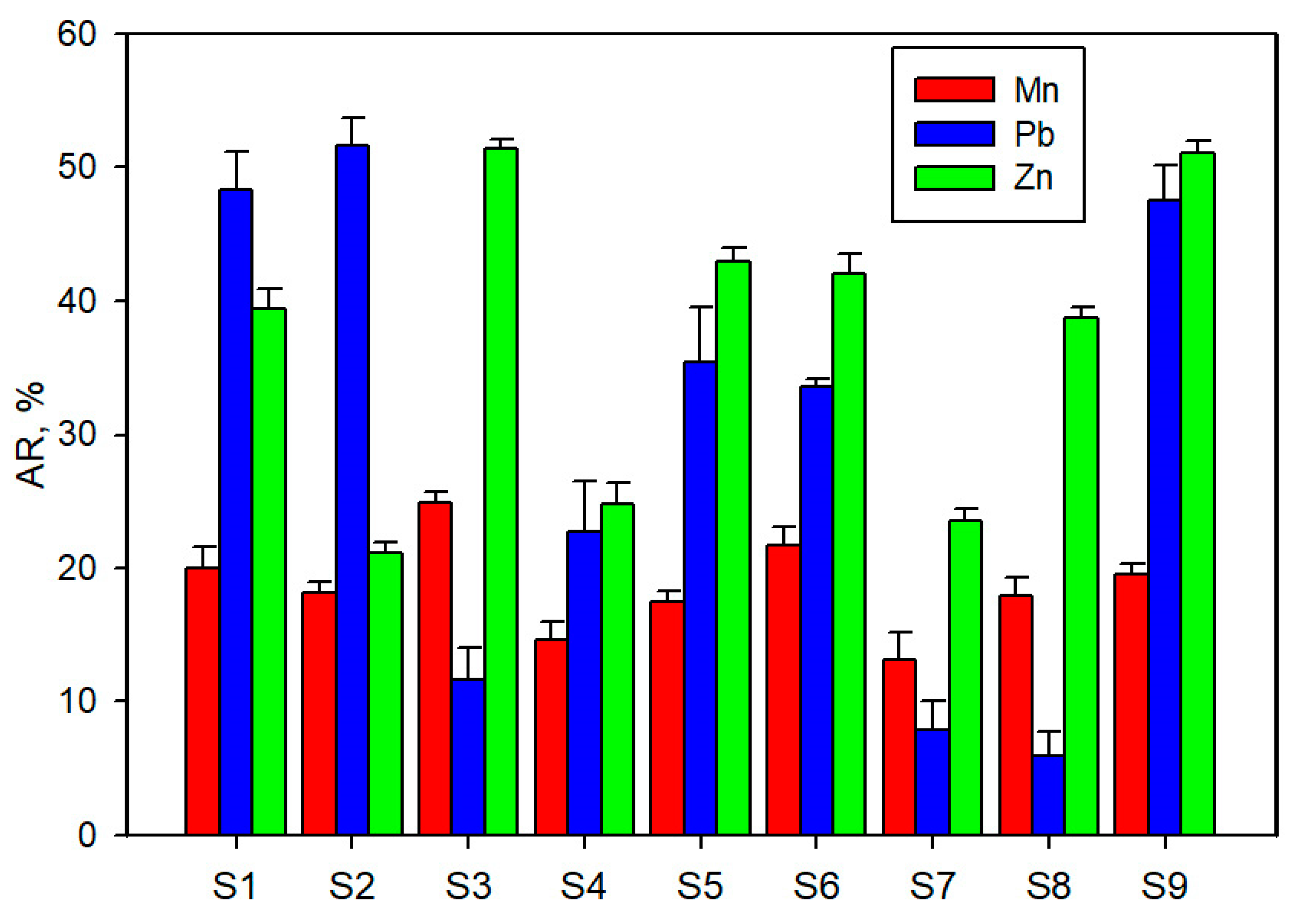
3.3. Metal Geo-Accumulation and Availability Ratio
3.4. Multivariate Statistical Analysis
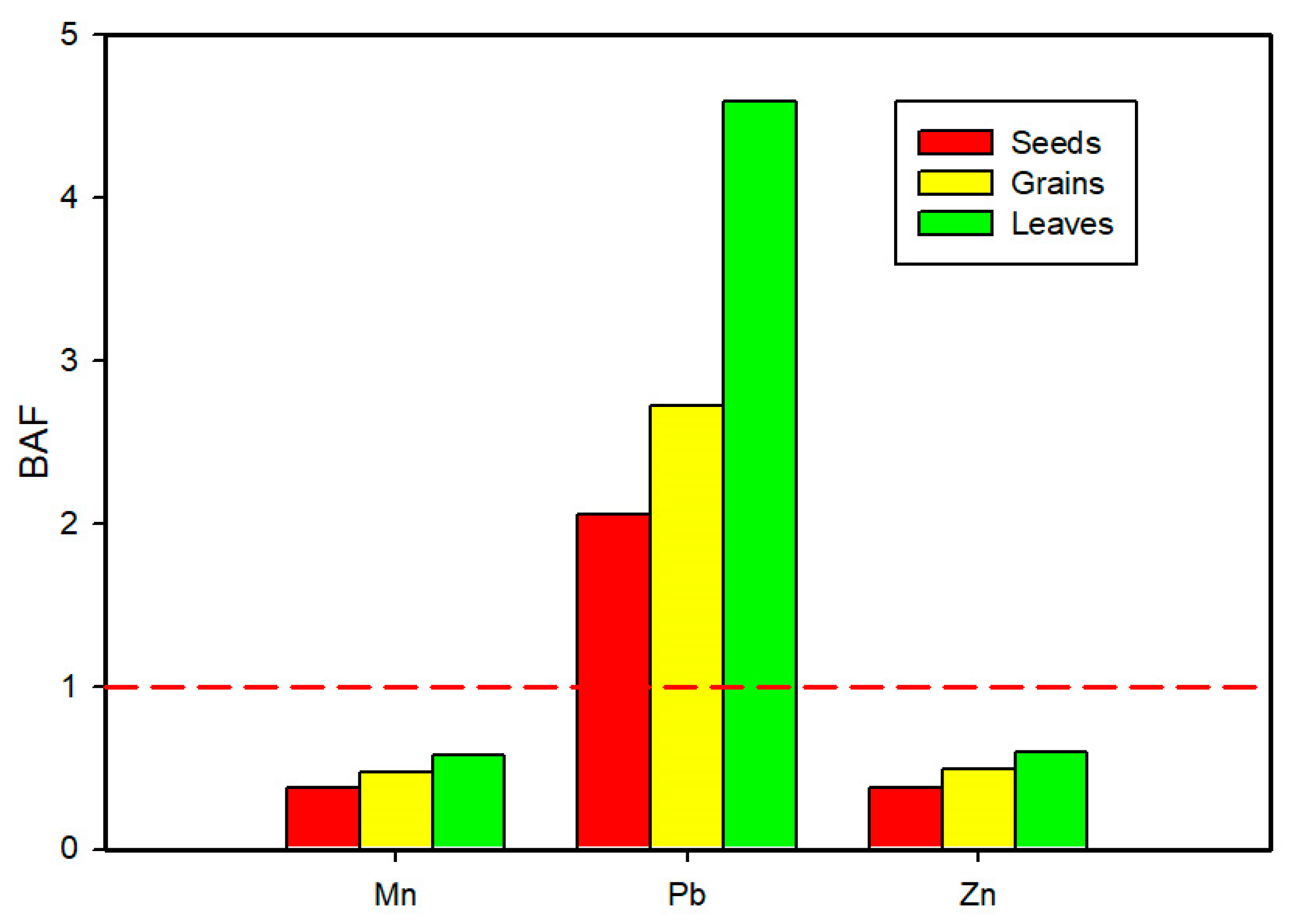
3.5. Metals in the Crop’s Edible Parts
4. Discussion
4.1. Metal Concentrations in Irrigation Water
4.2. Metal Concentrations in the Irrigated Soils
4.3. Metal Geo-Accumulation and Availability Ratio
4.4. Multivariate Statistical Analysis
4.5. Metals in the Crop’s Edible Parts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Gatta, G.; Libutti, A.; Gagliardi, A.; Disciglio, G.; Tarantino, E.; Beneduce, L.; Giuliani, M.M. Wastewater reuse in agriculture: Effects on soil-plant system properties. In Interaction and Fate of Pharmaceuticals in Soil-Crop Systems. The Handbook of Environmental Chemistry; Pérez Solsona, S., Montemurro, N., Chiron, S., Barceló, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 103, pp. 79–102. [Google Scholar] [CrossRef]
- Yadav, R.K.; Dagar, J.C. Innovations in utilization of poor-quality water for sustainable agricultural production. In Innovative Saline Agriculture; Dagar, J.C., Sharma, P.C., Sharma, D.K., Singh, A.K., Eds.; Springer: New Delhi, India, 2016; pp. 219–263. [Google Scholar] [CrossRef]
- Yadav, R.K.; Minhas, P.S.; Khajanchi-La; Dagar, J.C. Potential of wastewater disposal through tree plantations. In Agroforestry for the Management of Waterlogged Saline Soils and Poor-Quality Waters; Dagar, J.C., Minhas, P., Eds.; Springer: New Delhi, India, 2016; pp. 163–179. [Google Scholar] [CrossRef]
- Elgallal, M.; Fletcher, L.; Evans, B. Assessment of potential risks associated with chemicals in wastewater used for irrigation in arid and semiarid zones: A review. Agric. Water Manag. 2016, 177, 419–431. [Google Scholar] [CrossRef]
- Abbas, H.H.; Abuzaid, A.S.; Jahin, H.S.; Kasim, D.S. Assessing the quality of untraditional water sources for irrigation purposes in Al-Qalubiya Governorate, Egypt. Egypt. J. Soil Sci. 2020, 60, 157–166. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press, Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2011; p. 548. ISBN 9781420093681. [Google Scholar]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Phytoremediation of toxic metals present in soil and water environment: A critical review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860. [Google Scholar] [CrossRef] [PubMed]
- Abuzaid, A.S.; Bassouny, M.A.; Jahin, H.S.; Abdelhafez, A.A. Stabilization of lead and copper in a contaminated Typic Torripsament soil using humic substances. CLEAN Soil Air Water 2019, 47, 1800309. [Google Scholar] [CrossRef]
- Riaz, M.U.; Ayub, M.A.; Khalid, H.; AnmarulHaq, M.; Rasul, A.; Zia urRehman, M.; Ali, S. Fate of micronutrients in alkaline soils. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer Nature: Singapore, 2020; pp. 577–613. [Google Scholar] [CrossRef]
- Alamgir, M. The effects of soil properties to the extent of soil contamination with metals. In Environmental Remediation Technologies for Metal-Contaminated Soils; Hasegawa, H., Rahman, I.M.M., Rahman, M.A., Eds.; Springer: Tokyo, Japan, 2016; pp. 1–19. [Google Scholar]
- Huang, J.; Hartemink, A.E. Soil and environmental issues in sandy soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Elbana, T.; Gaber, H.M.; Kishk, F.M. Soil chemical pollution and sustainable agriculture. In The Soils of Egypt; El-Ramady, H., Alshaal, T., Bakr, N., Elbana, T., Mohamed, E., Belal, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 187–200. [Google Scholar] [CrossRef]
- Young, S.D. Chemistry of heavy metals and metalloids in soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 51–95. [Google Scholar] [CrossRef]
- Egwu, G.N.; Agbenin, J.O. Lead enrichment, adsorption and speciation in urban garden soils under long-term wastewater irrigation in northern Nigeria. Environ. Earth Sci. 2013, 69, 1861–1870. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Andersen, M.N.; Wang, G.; Wu, K.; Ye, Z. Domestic wastewater infiltration process in desert sandy soil and its irrigation prospect analysis. Ecotoxicol. Environ. Saf. 2021, 208, 111419. [Google Scholar] [CrossRef] [PubMed]
- Abuzaid, A.S.; Fadl, M.E. Mapping potential risks of long-term wastewater irrigation in alluvial soils, Egypt. Arab. J. Geosci. 2018, 11, 433. [Google Scholar] [CrossRef]
- Elbana, T.A.; Bakr, N.; Elbana, M. Reuse of treated wastewater in Egypt: Challenges and opportunities. In Unconventional Water Resources and Agriculture in Egypt; Negm, A.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 429–453. [Google Scholar] [CrossRef]
- Zidan, M.S.; Daoud, M.A. Agriculture use of marginal water in Egypt: Opportunities and challenges. In Developments in Soil Salinity Assessment and Reclamation: Innovative Thinking and Use of Marginal Soil and Water Resources in Irrigated Agriculture; Shahid, S.S., Abdelfattah, M., Taha, F., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 661–679. [Google Scholar] [CrossRef]
- Elbana, T.A.; Ramadan, M.A.; Gaber, H.M.; Bahnassy, M.H.; Kishk, F.M.; Selim, H.M. Heavy metals accumulation and spatial distribution in long term wastewater irrigated soils. J. Environ. Chem. Eng. 2013, 1, 925–933. [Google Scholar] [CrossRef]
- Abd-Elwahed, M.S. Influence of long-term wastewater irrigation on soil quality and its spatial distribution. Ann. Agric. Sci. 2018, 63, 191–199. [Google Scholar] [CrossRef]
- Yosry, M.; Abo el Abas, Y.M. Distribution pattern of some inorganic pollutants in groundwater and soil of sewage farm at NE Cairo/Egypt. In Water in the Middle East and in North Africa: Resources, Protection and Management; Zereini, F., Jaeschke, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 191–203. [Google Scholar] [CrossRef]
- Abd El-Hameed, A.H.; Naufal, E.H.; El-Husseiny, O.H.; Mohamed, M.K.; Abuzaid, A.S. Land suitability classification of some Qalubiya soils. Ann. Agric. Sci. Moshtohor 2013, 51, 147–158. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; APHA-AWWA-WEF: Washington, DC, USA, 2017. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region, 3rd ed.; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2013. [Google Scholar]
- USEPA. Test Methods for Evaluating Solid Waste. IA: Laboratory Manual Physical/Chemical Methods, SW 846, 3rd ed.; Gov. Print. Office: Washington, DC, USA, 1995.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef]
- Massas, I.; Gasparatos, D.; Ioannou, D.; Kalivas, D. Signs for secondary buildup of heavy metals in soils at the periphery of Athens International Airport, Greece. Environ. Sci. Pollut. Res. 2018, 25, 658–671. [Google Scholar] [CrossRef]
- Sahay, S.; Inam, A.; Iqbal, S. Risk analysis by bioaccumulation of Cr, Cu, Ni, Pb and Cd from wastewater-irrigated soil to Brassica species. Int. J. Environ. Sci. Technol. 2020, 17, 2889–2906. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. FAO Irrigation and Drainage Paper 29 rev 1; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 1994; ISBN 92-5-102263-1. [Google Scholar]
- ECP. Egyptian Code of Practice for the Use of Treated Municipal Wastewater for Agricultural Purposes; The Ministry of Housing Utilities and Urban Communities: Cairo, Egypt, 2015. (In Arabic)
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Alloway, B.J. Bioavailability of elements in soil. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 351–373. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Abdel-Sabour, M.F. Wastewater reuse for irrigation on the desert sandy soil of Egypt: Long-term effect. In Integrated Urban Water Resources Management; Hlavinek, P., Kukharchyk, T., Marsalek, J., Mahrikova, I., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 301–312. [Google Scholar] [CrossRef]
- Abdel El Lateef, E.M.; Hall, J.E.; Lawrence, P.C.; Negm, M.S. Cairo-East Bank effluent re-use study 3—Effect of field crop irrigation with secondary treated wastewater on biological and chemical properties of soil and groundwater. Biologia 2006, 61, S240–S245. [Google Scholar] [CrossRef]
- Mansour, H.; Awad, F.; Saber, M.; Zaghloul, A. Effect of contamination sources on the rate of zinc, copper and nickel release from various soil ecosystems. Bull. Natl. Res. Cent. 2020, 44, 178. [Google Scholar] [CrossRef]
- Yadav, R.K.; Minhas, P.S.; Lal, K.; Chaturvedi, R.K.; Yadav, G.; Verma, T.P. Accumulation of metals in soils, groundwater and edible parts of crops grown under long-term irrigation with sewage mixed industrial effluents. Bull. Environ. Contam. Toxicol. 2015, 95, 200–206. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, M.A.; El-Amier, Y.A.; Abd El-Hamid, H.T. Soil quality and health risk assessment of heavy metals in agricultural areas irrigated with wastewater from Kitchener Drain, Nile Delta, Egypt. J. Sci. Agric. 2017, 1, 158–170. [Google Scholar] [CrossRef][Green Version]
- Abuzaid, A.S.; Jahin, H.S.; Asaad, A.A.; Fadl, M.E.; AbdelRahman, M.A.E.; Scopa, A. Accumulation of potentially toxic metals in Egyptian alluvial soils, berseem clover (Trifolium alexandrinum L.), and groundwater after long-term wastewater irrigation. Agriculture 2021, 11, 713. [Google Scholar] [CrossRef]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 11–50. [Google Scholar] [CrossRef]
- Morgan, J.J. Kinetics of reaction between O2 and Mn(II) species in aqueous solutions. Geochim. Cosmochim. 2005, 69, 35–48. [Google Scholar] [CrossRef]
- Benslimane, S.; Perrot, H.; Bennezar, R.; Bouhidel, K.E. Thermodynamic study of Zn2+ inhibition properties and mechanism on calcium carbonate precipitation by chemical and electrochemical methods. Desalination 2016, 398, 114–120. [Google Scholar] [CrossRef]
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Major Inorganic Pollutants Affecting Soil and Crop Quality, in Soil Pollution—An Emerging Threat to Agriculture; Springer: Singapore, 2017; pp. 75–104. [Google Scholar] [CrossRef]
- Acosta, J.A.; Jansen, B.; Kalbitz, K.; Faz, A.; Martínez-Martínez, S. Salinity increases mobility of heavy metals in soils. Chemosphere 2011, 85, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Aitta, A.; El-Ramady, H.; Alshaal, T.; El-Henawy, A.; Shams, M.; Talha, F.; Brevik, E.C. Seasonal and spatial distribution of soil trace elements around Kitchener drain in the northern Nile Delta, Egypt. Agriculture 2019, 9, 152. [Google Scholar] [CrossRef]
- El-Demerdashe, S.; Dahdoh, M.S.; Hassan, F.A. Sequential extraction of nine trace elements from sludge-amended soils. Fertil. Res. 1995, 41, 77–85. [Google Scholar] [CrossRef]
- Hu, W.; Wang, H.Y.; Zha, T.G.; Pu, J.W.; Wang, Y.H. Metal contamination and fractionation in sewage-irrigated soils along the Liangshui River, Beijing, China. Soil Sediment Contam. 2010, 19, 487–503. [Google Scholar] [CrossRef]
- Beygi, M.; Jalali, M. Assessment of trace elements (Cd, Cu, Ni, Zn) fractionation and bioavailability in vineyard soils from the Hamedan, Iran. Geoderma 2019, 337, 1009–1020. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Bassouny, M.A. Total and DTPA-extractable forms of potentially toxic metals in soils of rice fields, north Nile Delta of Egypt. Environ. Technol. Innov. 2020, 18, 100717. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Wang, X.; Ma, L.; Cheng, Z.; Su, L. Pollution characteristics, spatial distributions, and source apportionment of heavy metals in cultivated soil in Lanzhou, China. Ecol. Indic. 2021, 125, 107507. [Google Scholar] [CrossRef]
- Gasparatos, D.; Mavromati, G.; Kotsovilis, P.; Massas, I. Fractionation of heavy metals and evaluation of the environmental risk for the alkaline soils of the Thriassio plain: A residential, agricultural, and industrial area in Greece. Environ. Earth Sci. 2015, 74, 1099–1108. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Jahin, H.S. Profile distribution and source identification of potentially toxic elements in north Nile Delta, Egypt. Soil Sediment Contam. Int. J. 2019, 28, 582–600. [Google Scholar] [CrossRef]
- Ali, A.M.; Ibrahim, S.M.; Abd El-Hady, Y.A.; Sayed, A.S.A. Assessment of bioavailability of some heavy metals to wheat and faba bean in Sahl El-Tina, Egypt. Agric. Res. 2018, 7, 72–82. [Google Scholar] [CrossRef]
- Farahat, E.A.; Galal, T.M.; Elawa, O.E.; Hassan, L.M. Health risk assessment and growth characteristics of wheat and maize crops irrigated with contaminated wastewater. Environ. Monit. Assess. 2017, 189, 535. [Google Scholar] [CrossRef] [PubMed]
- Galal, T.M.; Khalafallah, A.A.; Elawa, O.E.; Hassan, L.M. Human health risks from consuming cabbage (Brassica oleracea L. var. capitata) grown on wastewater irrigated soil. Int. J. Phytoremediation 2018, 20, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, toxicity, and detoxification in plants. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; Volume 213, pp. 113–136. [Google Scholar] [CrossRef]
- Martinka, M.; Vaculík, M.; Lux, A. Plant cell responses to cadmium and zinc. In Applied Plant Cell Biology: Cellular Tools and Approaches for Plant Biotechnology; Nick, P., Opatrny, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 209–246. [Google Scholar] [CrossRef]
- Sarwar, T.; Shahid, M.; Natasha; Khalid, S.; Shah, A.H.; Ahmad, N.; Naeem, M.A.; Ul Haq, Z.; Murtaza, B.; Bakhat, H.F. Quantification and risk assessment of heavy metal build-up in soil-plant system after irrigation with untreated city wastewater in Vehari, Pakistan. Environ. Geochem. Health 2020, 42, 4281–4297. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shahid, M.J.; Hussain, A.; Rizwan, M.; Ahmad, A.; Adrees, M. Metals phytoextraction by Brassica species. In Approaches to the Remediation of Inorganic Pollutants; Hasanuzzaman, M., Ed.; Springer: Singapore, 2021; pp. 361–384. [Google Scholar] [CrossRef]
- Feleafel, M.N.; Mirdad, Z.M. Hazard and effects of pollution by lead on vegetable crops. J. Agric. Environ. Ethics 2013, 26, 547–567. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. In Heavy Metal Stress in Plants; Gupta, D.K., Corpas, F.J., Palma, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 121–147. [Google Scholar] [CrossRef]
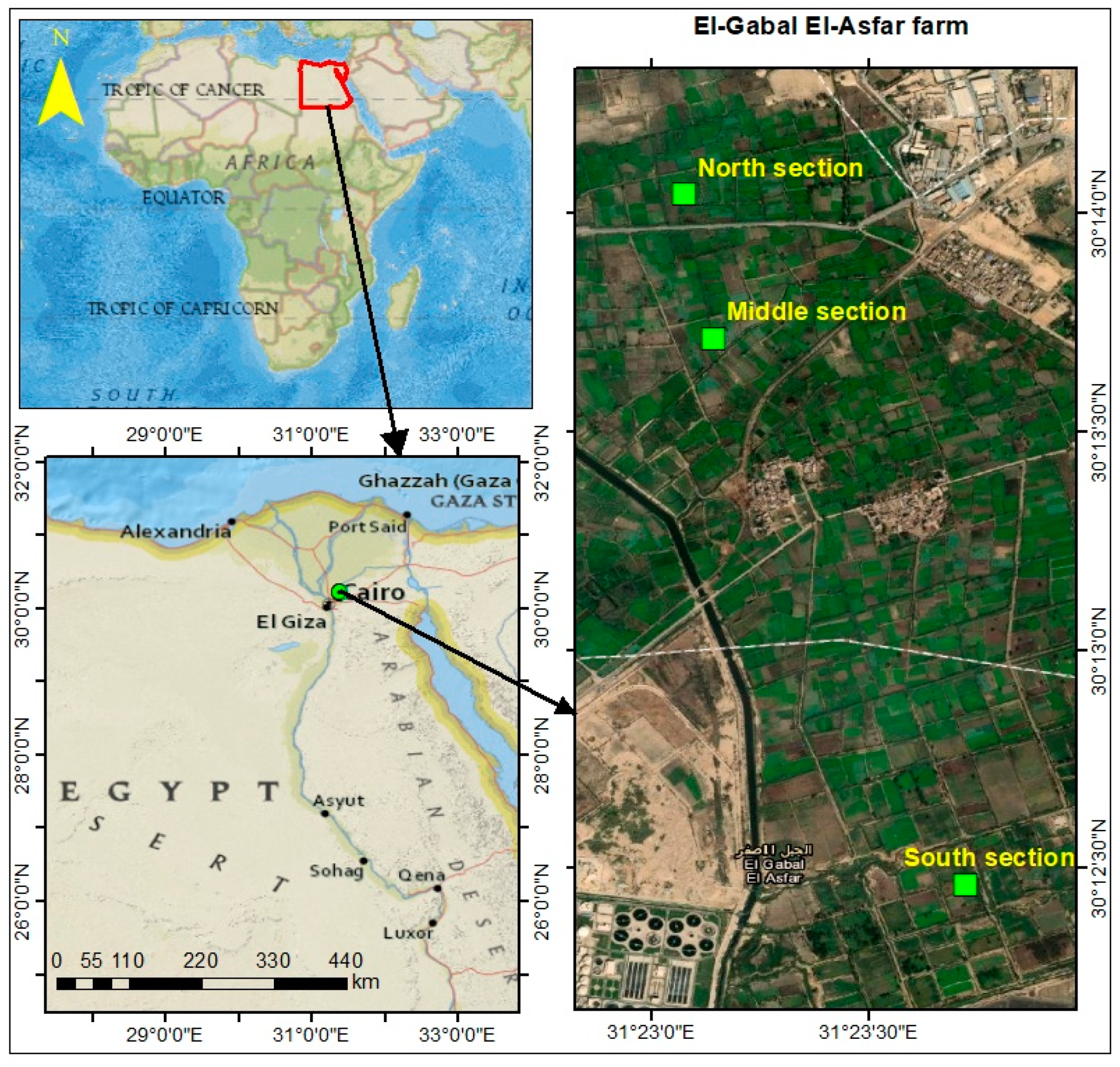


| Section | Site | Code | Irrigation Water | Plant Species |
|---|---|---|---|---|
| South | Plot 5 | 1 | Treated domestic wastewater | Broad bean (Viciafaba) |
| Plot 8 | 2 | Raw domestic wastewater | Wheat (Triticumaestivum L.) | |
| Plot 2 | 3 | Raw domestic wastewater | Cabbage (Brassica oleracea) | |
| Middle | Plot 5 | 4 | Raw domestic wastewater | Wheat (Triticumaestivum L.) |
| Plot 2 | 5 | Treated domestic wastewater | Wheat (Triticumaestivum L.) | |
| Plot 8 | 6 | Well water | Broad bean (Viciafaba) | |
| North | Plot 5 | 7 | Raw domestic wastewater | Cabbage (Brassica oleracea) |
| Plot 2 | 8 | Raw industrial wastewater | Wheat (Triticumaestivum L.) | |
| Plot 8 | 9 | Well water | Broad bean (Viciafaba) |
| Irrigation Water, mg L−1 | Irrigated Soils, mg kg−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Content | DTPA-Extractable Content | ||||||||
| Site | Mn | Pb | Zn | Mn | Pb | Zn | Mn | Pb | Zn |
| 1 | 0.24 f | 0.005 e | 0.18 ab | 410.13 c | 3.11 f | 170.21 c | 77.84 c | 1.43 b | 68.31 c |
| 2 | 0.28 e | 0.020 d | 0.13 b | 277.56 f | 4.15 f | 99.64 g | 49.46 g | 2.17 ab | 21.05 g |
| 3 | 0.25 f | 0.024 c | 0.19 ab | 226.91 g | 38.12 c | 89.73 h | 57.16 f | 4.47 a | 45.55 e |
| 4 | 0.30 de | 0.032 b | 0.16 b | 386.45 e | 20.91 d | 158.92 d | 59.24 f | 4.78 a | 40.52 f |
| 5 | 0.39 ab | 0.004 e | 0.18 ab | 394.53 d | 8.07 e | 138.53 e | 70.32 d | 2.62 ab | 60.09 d |
| 6 | 0.31 cd | 0.004 e | 0.17 b | 408.43 c | 2.72 f | 189.57 b | 91.47 a | 0.91 b | 81.23 a |
| 7 | 0.33 c | 0.034 b | 0.14 b | 427.63 b | 42.87 b | 190.82 b | 64.57 e | 3.41 ab | 45.52 e |
| 8 | 0.41 a | 0.043 a | 0.28 a | 452.72 a | 50.43 a | 198.41 a | 84.29 b | 3.01 ab | 77.51 b |
| 9 | 0.37 b | 0.003 e | 0.18 ab | 388.44 e | 2.91 f | 130.45 f | 77.21 c | 1.25 b | 67.21 c |
| NC | - | - | - | 100–500 | 5–10 | 15–30 | - | - | - |
| MAL | 0.2 1 | 5.0 1 | 5.0 1 | 4000 2 | 200 2 | 300 2 | 5.0 3 | 2.0 3 | 1.25 3 |
| Irrigation Water | Soil Content | r | p-Value |
|---|---|---|---|
| Mn | Total | 0.517 | ** |
| Available | 0.178 | ns | |
| Pb | Total | 0.849 | ** |
| Available | 0.656 | ** | |
| Zn | Total | 0.284 | ns |
| Available | 0.522 | ** |
| Metal | Regression Model | p | R2 |
|---|---|---|---|
| Mn | −70.44 + 11.37pH − 0.51Silt − 0.67Clay + 7.24EC − 0.12 CaCO3 | <0.001 | 0.86 |
| Pb | 425.90 + 5.66OM + 9.61Clay − 7.30Sand | <0.001 | 0.82 |
| Zn | −233.44 + 37.66EC + 30.47pH − 1.02CaCO3 − 1.07Silt | <0.001 | 0.66 |
| pH | EC | OM | CaCO3 | Sand | Silt | Clay | T–Mn | T–Pb | T–Zn | A–Mn | A–Pb | |
| pH | 1.000 | |||||||||||
| EC | 0.061 | 1.000 | ||||||||||
| OM | −0.197 | −0.443 * | 1.000 | |||||||||
| CaCO3 | 0.255 | 0.631 ** | −0.326 | 1.000 | ||||||||
| Sand | −0.449 * | 0.069 | 0.537 ** | −0.124 | 1.000 | |||||||
| Silt | 0.502 ** | 0.109 | −0.557 ** | 0.121 | −0.731 ** | 1.000 | ||||||
| Clay | 0.281 | 0.275 | −0.536 ** | 0.431 * | 0.073 | 0.338 | 1.000 | |||||
| T–Mn | −0.055 | 0.249 | −0.742 ** | 0.482 * | −0.202 | 0.458 * | 0.685 ** | 1.000 | ||||
| T–Pb | −0.049 | 0.502 ** | −0.506 ** | 0.190 | −0.234 | 0.263 | −0.117 | 0.071 | 1.000 | |||
| T–Zn | −0.059 | 0.272 | −0.693 ** | 0.457 * | −0.223 | 0.345 | 0.491 ** | 0.912 ** | 0.254 | 1.000 | ||
| A–Mn | 0.313 | 0.459 * | −0.608 ** | 0.326 | −0.087 | 0.272 | 0.529 ** | 0.704 ** | −0.107 | 0.694 ** | 1.000 | |
| A–Pb | −0.309 | 0.167 | 0.323 | −0.105 | 0.261 | −0.197 | −0.225 | −0.430 * | 0.510 ** | −0.322 | −0.692 ** | 1.000 |
| A–Zn | 0.307 | 0.567 ** | −0.632 ** | 0.268 | −0.088 | 0.246 | 0.472 * | 0.642 ** | −0.034 | 0.616 ** | 0.976 ** | −0.649 ** |
| Parameter | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Eigenvalue | 5.553 | 2.169 | 1.919 | 1.342 |
| Variance, % | 29.907 | 19.083 | 18.919 | 16.573 |
| Cumulative, % | 29.907 | 48.990 | 67.909 | 84.482 |
| Indicator | Eigenvectors | |||
| pH | −0.227 | 0.697 | 0.396 | −0.429 |
| EC | 0.217 | −0.052 | 0.861 | 0.340 |
| OM | −0.742 | −0.473 | −0.277 | −0.162 |
| CaCO3 | 0.196 | 0.074 | 0.803 | 0.015 |
| Sand | −0.139 | −0.927 | 0.097 | −0.123 |
| Silt | 0.315 | 0.830 | 0.029 | 0.020 |
| Clay | 0.554 | 0.051 | 0.375 | −0.235 |
| Total Mn | 0.963 | 0.112 | 0.518 | −0.084 |
| Total Pb | 0.133 | 0.346 | 0.231 | 0.862 |
| Total Zn | 0.920 | 0.097 | 0.580 | 0.055 |
| DTPA-Mn | 0.667 | 0.101 | 0.102 | −0.458 |
| DTPA-Pb | −0.404 | −0.226 | 0.007 | 0.799 |
| DTPA-Zn | 0.613 | 0.102 | 0.139 | −0.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzaid, A.S.; Abdel-Salam, M.A.; Ahmad, A.F.; Fathy, H.A.; Fadl, M.E.; Scopa, A. Effect of Marginal-Quality Irrigation on Accumulation of some Heavy Metals (Mn, Pb, and Zn) in TypicTorripsamment Soils and Food Crops. Sustainability 2022, 14, 1067. https://doi.org/10.3390/su14031067
Abuzaid AS, Abdel-Salam MA, Ahmad AF, Fathy HA, Fadl ME, Scopa A. Effect of Marginal-Quality Irrigation on Accumulation of some Heavy Metals (Mn, Pb, and Zn) in TypicTorripsamment Soils and Food Crops. Sustainability. 2022; 14(3):1067. https://doi.org/10.3390/su14031067
Chicago/Turabian StyleAbuzaid, Ahmed S., Mohamed A. Abdel-Salam, Abeer F. Ahmad, Hala A. Fathy, Mohamed E. Fadl, and Antonio Scopa. 2022. "Effect of Marginal-Quality Irrigation on Accumulation of some Heavy Metals (Mn, Pb, and Zn) in TypicTorripsamment Soils and Food Crops" Sustainability 14, no. 3: 1067. https://doi.org/10.3390/su14031067
APA StyleAbuzaid, A. S., Abdel-Salam, M. A., Ahmad, A. F., Fathy, H. A., Fadl, M. E., & Scopa, A. (2022). Effect of Marginal-Quality Irrigation on Accumulation of some Heavy Metals (Mn, Pb, and Zn) in TypicTorripsamment Soils and Food Crops. Sustainability, 14(3), 1067. https://doi.org/10.3390/su14031067








