Appraisal of Heavy Metals Accumulation, Physiological Response, and Human Health Risks of Five Crop Species Grown at Various Distances from Traffic Highway
Abstract
1. Introduction
2. Materials and Methods
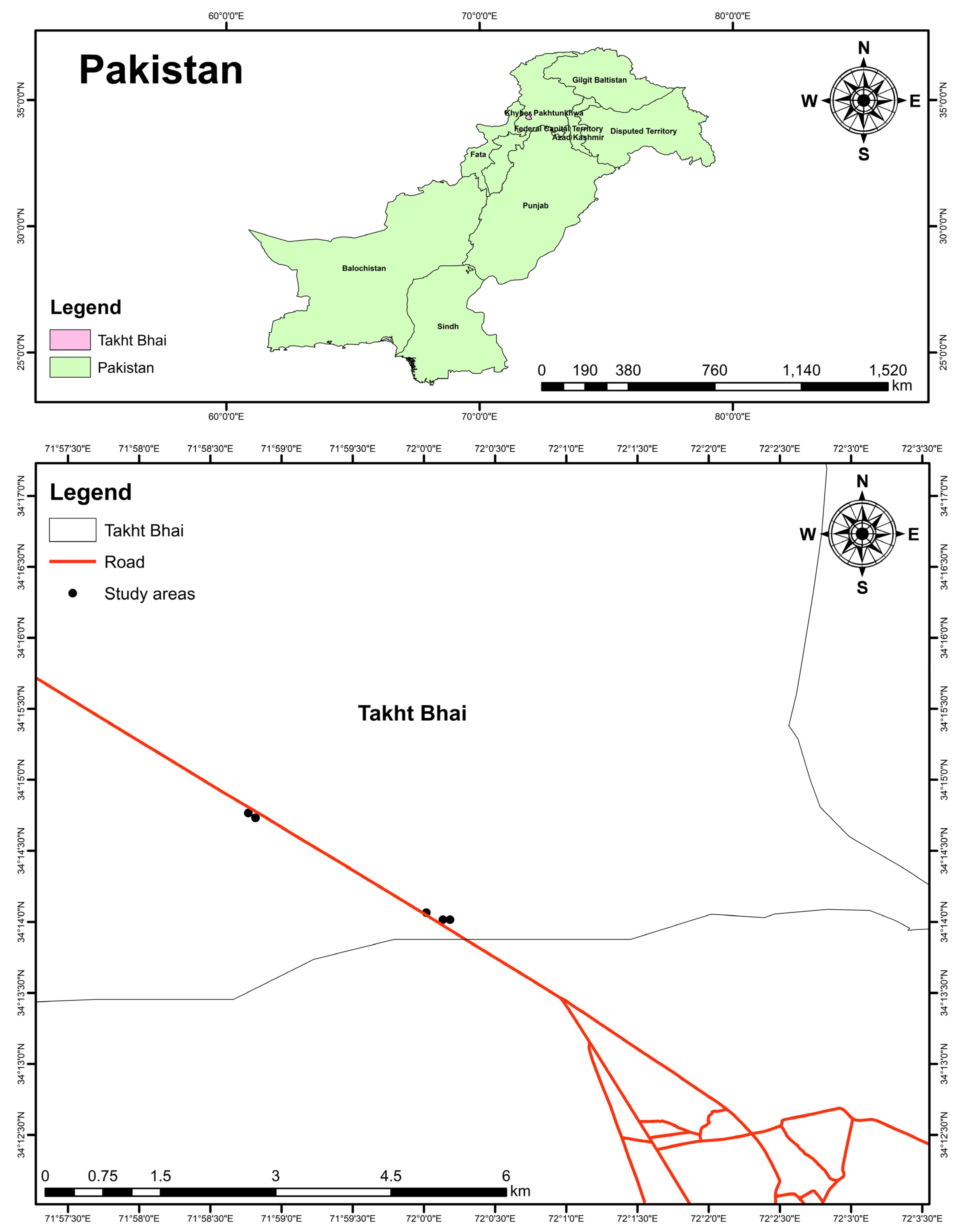
2.1. Site Description and Samples Collection
2.2. Soil and Plant Analysis
2.3. Physiological and Biochemical Analyses of Plants Samples
2.4. Health Risk Assessment
2.4.1. Estimated Daily Intake (EDI)
2.4.2. Target Hazard Quotient (THQ)
2.4.3. Hazard Index (HI)
2.4.4. Target Cancer Risk (TCR)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Soil
3.2. Lead (Pb) Concentration in Plant Tissues
3.3. Pb Translocation and Accumulation in Plants
3.4. Cadmium (Cd) Concentration in Plant Tissues
3.5. Cd Translocation and Bioconcentration in Plant Parts
3.6. Proline Contents in Plants at Various Distances from the Main Highway
3.7. Phenolic Contents in Plants
3.8. Carotenoid and Chlorophyll Contents in Plants
3.9. Correlations among Different Parameters
3.10. Risk Assessment
3.10.1. Estimated Daily Intake of Metals and Health Risk Assessment
3.10.2. Dry Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Ullah, R.; Tsui, M.T.-K.; Chen, H.; Chow, A.; Williams, C.; Ligaba-Osena, A. Microplastics interaction with terrestrial plants and their impacts on agriculture. J. Environ. Qual. 2021, 50, 1024–1041. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M. United Nations Human Settlements Programme: The State of the World’s Cities, 2004/2005: Globalization and Urban Culture. Popul. Dev. Rev. 2005, 31, 592–594. [Google Scholar]
- Bai, J.; Cui, B.; Wang, Q.; Gao, H.; Ding, Q. Assessment of heavy metal contamination of roadside soils in Southwest China. Stoch. Environ. Res. Risk Assess. 2009, 23, 341–347. [Google Scholar] [CrossRef]
- Suzuki, K.; Yabuki, T.; Ono, Y. Roadside Rhododendron pulchrum leaves as bioindicators of heavy metal pollution in traffic areas of Okayama, Japan. Environ. Monit. Assess. 2008, 149, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Müller, H.W.; Abdullah, A.; Abdelgawad, G.; Utermann, J. Urban soil pollution in Damascus, Syria: Concentrations and patterns of heavy metals in the soils of the Damascus Ghouta. Geoderma 2005, 124, 63–71. [Google Scholar] [CrossRef]
- Mabood, F.; Hadi, F.; Jan, A.U.; Ditta, A.; Islam, Z.; Siddiqui, M.H.; Ali, H.M.; Sabagh, A.E.L. Assessment of Pb and Ni and potential health risks associated with the consumption of vegetables grown on the roadside soils in District Swat, Khyber Pakhtunkhwa, Pakistan. Environ. Monit. Assess. 2022, 194, 906. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Road-Deposited Sediment Pollutants: A Critical Review of their Characteristics, Source Apportionment, and Management. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1315–1348. [Google Scholar] [CrossRef]
- Ullah, I.; Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.; Rizwan, M.S.; Jan, A.U.; Ahmad, I. Assessment of health and ecological risks of heavy metal contamination: A case study of agricultural soils in Thall, Dir-Kohistan. Environ. Monit. Assess. 2020, 192, 786. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Chen, J.-Q.; Chai, L.-Y.; Yang, Z.-H.; Huang, S.-H.; Zheng, Y. Environmental impact and site-specific human health risks of chromium in the vicinity of a ferro-alloy manufactory, China. J. Hazard. Mater. 2011, 190, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Alengebawy, A.; Abdelkhalek, S.; Qureshi, S.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Tanvir, B.; Xiukang, W.; Brtnicky, M.; Ditta, A.; Kucerik, J.; Subhani, Z.; Nazir, M.Z.; Radziemska, M.; Saeed, Q.; et al. Co-composted biochar enhances growth, physiological, and phytostabilization efficiency of Brassica napus and reduces associated health risks under Chromium stress. Front. Plant Sci. 2021, 12, 775785. [Google Scholar] [CrossRef]
- Amari, T.; Ghnaya, T.; Abdelly, C. Nickel, cadmium and lead phytotoxicity and potential of halophytic plants in heavy metal extraction. S. Afr. J. Bot. 2017, 111, 99–110. [Google Scholar] [CrossRef]
- Khan, R.U.; Durrani, F.R.; Chand, N.; Anwar, H.; Naz, S.; Farooqi, F.A.; Manzoor, M.N. Effect of Cannabis sativa fortified feed on muscle growth and visceral organs in broiler chicks. Int. J. Biol. Biotechnol. 2009, 6, 179–182. [Google Scholar]
- Raikwar, M.; Kumar, P.; Singh, M.; Singh, A. Toxic effect of heavy metals in livestock health. Vet. World 2008, 1, 28–30. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Alatalo, J.M.; Mahmood, M.; Imtiaz, M.; Ditta, A.; Ali, E.F.; Abdelrahman, H.; Slaný, M.; Antoniadis, V.; et al. Herbal plants- and rice straw-derived biochars reduced metal mobilization in fishpond sediments and improved their potential as fertilizers. Sci. Total. Environ. 2022, 826, 154043. [Google Scholar] [CrossRef]
- Majeed, A.; Muhmood, A.; Niaz, A.; Ditta, A.; Rajpar, M.N. Comparative efficacy of different biochars and traditional manures in the attenuation of cadmium toxicity in rice (Oryza sativa L.). Arab. J. Geosci. 2022, 15, 209. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Wójcik, M. The Influence of Selenium on Root Growth and Oxidative Stress Induced by Lead in Vicia faba L. minor Plants. Biol. Trace Element Res. 2012, 147, 320–328. [Google Scholar] [CrossRef]
- Hadi, F.; Ali, N.; Fuller, M.P. Molybdenum (Mo) increases endogenous phenolics, proline and photosynthetic pigments and the phytoremediation potential of the industrially important plant Ricinus communis L. for removal of cadmium from contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 20408–20430. [Google Scholar] [CrossRef]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Ahmad, I.; Tahir, M.; Daraz, U.; Ditta, A.; Hussain, M.B.; Khan, Z.U.H. Responses and Tolerance of Cereal Crops to Metal and Metalloid Toxicity. In Agronomic Crops; Hassanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 235–264. [Google Scholar] [CrossRef]
- Handique, G.K.; Handique, A.K. Proline accumulation in lemongrass (Cymbopogon flexuosus Stapf.) due to heavy metal stress. J. Environ. Biol. 2009, 30, 299–302. [Google Scholar] [PubMed]
- Ahmad, R.; Hadi, F.; Jan, A.U.; Ditta, A. Straw incorporation in contaminated soil enhances drought tolerance but simultaneously increases the accumulation of heavy metals in rice. Sustainability 2022, 14, 10578. [Google Scholar] [CrossRef]
- Joint FAO/WHO. Expert Standards Program Codex Alimentation Commission; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- ALINORM 01/12A; FAO/WHO Codex Alimentarius Commission. Food Additives and Contaminants. Joint FAO/WHO Food Standards Programme: Geneva, Switzerland, 2001; pp. 1–289.
- Chaoua, S.; Boussaa, S.; El Gharmali, A.; Boumezzough, A. Impact of irrigation with wastewater on accumulation of heavy metals in soil and crops in the region of Marrakech in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 429–436. [Google Scholar] [CrossRef]
- Mahmood, A.; Malik, R.N. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem. 2014, 7, 91–99. [Google Scholar] [CrossRef]
- Jan, F.A.; Ishaq, M.; Ihsanullah, I.; Asim, S. Multivariate statistical analysis of heavy metals pollution in industrial area and its comparison with relatively less polluted area: A case study from the City of Peshawar and district Dir Lower. J. Hazard. Mater. 2010, 176, 609–616. [Google Scholar] [CrossRef]
- Han, L.; Chen, Y.; Chen, M.; Wu, Y.; Su, R.; Du, L.; Liu, Z. Mushroom residue modification enhances phytoremediation potential of Paulownia fortunei to lead-zinc slag. Chemosphere 2020, 253, 126774. [Google Scholar] [CrossRef]
- Szteke, B.; Jedrzejczak, R.; Nieplocha, J.; Tych, W. Influence of the environmental factors on cadmium content in strawberry fruit. Fruit Sci. Rep. 1989, 16, 1–6. [Google Scholar]
- Gebologlu, N.; Cetin, S.C.; Ece, A.; Yilmaz, E.; Flmastas, M. Assessment of lead and cadmium contents of tomatoes and beans grown in the vicinity of highway of Tokat, Turkey. Asian J. Chem. 2005, 17, 730–736. [Google Scholar]
- Ul Abideen, S.N.; Abideen, S.A. Protein level and heavy metals (Pb, Cr, and Cd) concentrations in wheat (Triticum aestivum) and in oat (Avena sativa) plants. Int. J. Innov. Appl. Stud. 2013, 3, 284–289. [Google Scholar]
- Udosen, E.D.; Ukpong, M.E.; Etim, E.E. Concentrations of heavy metals in soil samples within Mkpanak in Ibeno coastal area of Akwa Ibom State, Nigeria. Int. J. Mod. Chem. 2012, 3, 74–81. [Google Scholar]
- Krystofova, O.; Zitka, O.; Krizkova, S.; Hynek, D.; Shestivska, V.; Adam, V.; Hubalek, J.; Mackova, M.; Macek, T.; Zehnalek, J.; et al. Accumulation of cadmium by transgenic tobacco plants (Nicotiana tabacum L.) carrying yeast metallothionein gene revealed by electrochemistry. Int. J. Electrochem. Sci. 2012, 7, 886–907. [Google Scholar]
- Elless, M.P.; Blaylock, M.J. Amendment Optimization to Enhance Lead Extractability from Contaminated Soils for Phytoremediation. Int. J. Phytoremediation 2000, 2, 75–89. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA; New York, NY, USA, 2005. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publication: Oxford, UK, 1974. [Google Scholar]
- Cui, Y.-J.; Zhu, Y.-G.; Zhai, R.-H.; Chen, D.-Y.; Huang, Y.-Z.; Qiu, Y.; Liang, J.-Z. Transfer of metals from soil to vegetables in an area near a smelter in Nanning, China. Environ. Int. 2004, 30, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total. Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzyme in isolated chloroplasts, polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949, 130, 267–272. [Google Scholar]
- Vijayarengan, P. Growth and biochemical variations in radish under zinc applications. Int. Res. J. Plant Sci. 2012, 2, 43–49. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chen, C.; Qian, Y.; Chen, Q.; Li, C. Assessment of Daily Intake of Toxic Elements Due to Consumption of Vegetables, Fruits, Meat, and Seafood by Inhabitants of Xiamen, China. J. Food Sci. 2011, 76, T181–T188. [Google Scholar] [CrossRef]
- Sabir, M.; Baltrėnaitė-Gedienė, E.; Ditta, A.; Ullah, H.; Kanwal, A.; Ullah, S.; Faraj, T.K. Bioaccumulation of heavy metals in a soil–plant system from an open dumpsite and the associated health risks through multiple routes. Sustainability 2022, 14, 13223. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.; Bordean, D.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Antoine, J.M.R.; Fung, L.A.H.; Grant, C.N. Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 2017, 4, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem. 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Fernández-Landero, S.; Giráldez, I.; Fernández-Caliani, J.C. Predicting the relative oral bioavailability of naturally occurring As, Cd and Pb from in vitro bioaccessibility measurement: Implications for human soil ingestion exposure assessment. Environ. Geochem. Health 2021, 43, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Kamunda, C.; Mathuthu, M.; Madhuku, M. Health Risk Assessment of Heavy Metals in Soils from Witwatersrand Gold Mining Basin, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Prasad, F.M. Accumulation of Lead and Cadmium in Soil and Vegetable Crops along Major Highways in Agra (India). E-J. Chem. 2010, 7, 1174–1183. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Khan, K.; Madhavan, T.P.V.; Kshirsagar, R.; Boosi, K.N.; Sadhale, P.; Muniyappa, K. N-terminal disordered domain of Saccharomyces cerevisiae Hop1 protein is dispensable for DNA binding, bridging, and synapsis of double-stranded DNA molecules but is necessary for spore formation. Biochemistry 2013, 52, 5265–5279. [Google Scholar] [CrossRef]
- Perveen, S.; Shah, Z.; Nazif, W.; Shah, S.S.; Ihsanullah, I.; Shah, H.U. Study on accumulation of heavy metals in vegetables receiving sewage water. J. Chem. Soc. Pak. 2011, 33, 220–227. [Google Scholar]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Bakirdere, S.; Yaman, M. Determination of lead, cadmium and copper in roadside soil and plants in Elazig, Turkey. Environ. Monit. Assess. 2007, 136, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-P.; Luo, C.-L.; Gao, Y.; Li, F.-B.; Lin, L.-W.; Wu, C.-A.; Li, X. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China. Environ. Pollut. 2010, 158, 820–826. [Google Scholar] [CrossRef]
- Chen, C.; Dickman, M.B. Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 2005, 102, 3459–3464. [Google Scholar] [CrossRef]
- Amani, A.L. Cadmium induced changes in pigment content, ion uptake, proline content and phosphoenolpyruvate carboxylase activity in Triticum aestivum Seedlings. Aust. J. Basic Appl. Sci. 2008, 2, 57–62. [Google Scholar]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- De, B.; Mukherjee, A.K. Mercury-induced Metabolic changes in Seedlings and Cultured cells of Tomato. Geobios-Jodhpur 1996, 23, 83–87. [Google Scholar]
- Lalk, I.; Dorffling, K. Hardening, abscisic acid, proline and freezing resistance in two winter wheat varieties. Physiol. Plant. 1985, 63, 287–292. [Google Scholar] [CrossRef]
- Ullah, R.; Hadi, F.; Ahmad, S.; Jan, A.U.; Rongliang, Q. Phytoremediation of lead and chromium contaminated soil improves with the endogenous phenolics and proline production in Parthenium, Cannabis, Euphorbia, and Rumex species. Water Air Soil Pollut. 2019, 230, 40. [Google Scholar] [CrossRef]
- Awad, M.; El-Sayed, M.M.; Li, X.; Liu, Z.; Mustafa, S.K.; Ditta, A.; Hessini, K. Diminishing heavy metal hazards of contaminated soil via biochar supplementation. Sustainability 2021, 13, 12742. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Rizwan, M.; Ditta, A.; Irshad, S.; Chen, D.-Y.; Bashir, S.; Mahmood, M.; Li, W.; Imtiaz, M. Biochar, slag and ferrous manganese ore affect lead, cadmium and antioxidant enzymes in water spinach (Ipomoea aquatica) grown in multi-metal contaminated soil. Crop Pasture Sci. 2022. [Google Scholar] [CrossRef]
- Hare, P.; Cress, W. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Maeder, V.; Funk, F.; Frey, B.; Sticher, H.; Frossard, E. Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 2003, 252, 301–312. [Google Scholar] [CrossRef]
- Rastgoo, L.; Alemzadeh, A. Biochemical responses of Gouan (‘Aeluropus littoralis’) to heavy metals stress. Aust. J. Crop Sci. 2011, 5, 375–383. [Google Scholar]
- Ahmad, A.; Hadi, F.; Ali, N. Effective phytoextraction of cadmium (Cd) with increasing concentration of total phenolics and free proline in Cannabis sativa (L) plant under various treatments of fertilizers, plant growth regulators and sodium salt. Int. J. Phytoremediation 2015, 17, 56–65. [Google Scholar] [CrossRef]
- Hadi, F.; Ahmad, A.; Ullah, R. Cadmium Phytoextraction Potential of Ricinus communis significantly Increased with Exogenous Application of Growth Regulators and Macronutrients. Soil Sediment Contam. Int. J. 2021, 30, 663–685. [Google Scholar] [CrossRef]
- Emanuil, N.; Akram, M.S.; Ali, S.; Majrashi, A.; Iqbal, M.; El-Esawi, M.A.; Ditta, A.; Alharby, H.F. Exogenous caffeine (1,3,7-Trimethylxanthine) application diminishes cadmium toxicity by modulating physio-biochemical attributes and improving the growth of spinach (Spinacia oleracea L.). Sustainability 2022, 14, 2806. [Google Scholar] [CrossRef]
- Rahman, S.U.; Xuebin, Q.; Riaz, L.; Yasin, G.; Shah, A.N.; Shahzad, U.; Jahan, M.S.; Ditta, A.; Bashir, M.A.; Rehim, A.; et al. The interactive effect of pH variation and cadmium stress on wheat (Triticum aestivum L.) growth, physiological and biochemical parameters. PLoS ONE 2021, 16, e0253798. [Google Scholar] [CrossRef]
- Öncel, I.; Keleş, Y.; Üstün, A. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Mobin, M.; Khan, N.A. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 2007, 164, 601–610. [Google Scholar] [CrossRef] [PubMed]






| Distance from Highway | pH | EC (μS cm−1) | Soil Texture | Lead (mg kg−1) | Cadmium (mg kg−1) |
|---|---|---|---|---|---|
| 100 m distance | 6.89 ± 0.17 | 591 ± 1.21 | Loamy | 2.67 ± 1.93 | 0.47 ± 0.03 |
| 10–50 m distance | 7.01 ± 0.91 | 612 ± 1.91 | Loamy | 18.84 ± 13.11 | 1.17 ± 0.15 |
| 0–10 m distance | 6.94 ± 0.88 | 618 ± 0.84 | Loamy | 32.02 ± 17.37 | 4.51 ± 0.77 |
| Plant | Distance from the Road (m) | Lead (Pb)Accumulation (µg Dry Biomass−1) | Pb Translocation | Pb Bioconcentration | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaves | EP | R–S | R–L | |||
| Fragaria ananassa | 50–100 m | 1.57 ± 0.22 | 1.29 ± 0.18 | 2.45 ± 0.34 | 5.31 ± 0.74 | 0.43 ± 0.05 | 0.83 ± 0.04 | 0.24 ± 0.003 |
| 10–50 m | 9.53 ± 2.45 | 5.91 ± 1.52 | 16.14 ± 4.15 | 31.58 ± 8.13 | 0.48 ± 0.03 | 0.81 ± 0.07 | 0.30 ± 0.002 | |
| 0–10 m | 50.80 ± 16.9 | 22.22 ± 7.42 | 48.50 ± 16.2 | 121.52 ± 40.5 | 0.38 ± 0.08 | 0.78 ± 0.06 | 0.34 ± 0.010 | |
| Lycopersicon esculentum | 50–100 m | 0.78 ± 0.11 | 0.65 ± 0.09 | 0.78 ± 0.11 | 2.21 ± 0.31 | 0.45 ± 0.06 | 0.59 ± 0.04 | 0.56 ± 0.011 |
| 10–50 m | 5.29 ± 1.36 | 3.28 ± 0.84 | 5.87 ± 1.51 | 14.45 ± 3.72 | 0.53 ± 0.03 | 0.53 ± 0.05 | 0.64 ± 0.010 | |
| 0–10 m | 28.22 ± 9.42 | 12.34 ± 4.12 | 18.31 ± 6.11 | 58.87 ± 19.66 | 0.42 ± 0.07 | 0.45 ± 0.05 | 0.70 ± 0.008 | |
| Triticum aestivum | 50–100 m | 0.10 ± 0.01 | 0.09 ± 0.01 | 0.06 ± 0.01 | 0.25 ± 0.03 | 0.47 ± 0.04 | 0.31 ± 0.05 | 0.11 ± 0.003 |
| 10–50 m | 0.52 ± 0.13 | 0.35 ± 0.09 | 0.37 ± 0.10 | 1.25 ± 0.32 | 0.41 ± 0.08 | 0.36 ± 0.06 | 0.12 ± 0.005 | |
| 0–10 m | 1.60 ± 0.53 | 0.76 ± 0.26 | 0.66 ± 0.22 | 3.03 ± 1.01 | 0.43 ± 0.06 | 0.39 ± 0.03 | 0.08 ± 0.000 | |
| Nicotiana tabacum | 50–100 m | 0.17 ± 0.02 | 0.21 ± 0.03 | 0.21 ± 0.03 | 0.59 ± 0.08 | 0.63 ± 0.02 | 0.66 ± 0.08 | 0.12 ± 0.008 |
| 10–50 m | 2.88 ± 0.74 | 2.62 ± 0.67 | 3.98 ± 1.02 | 9.47 ± 2.44 | 0.71 ± 0.05 | 0.60 ± 0.05 | 0.14 ± 0.003 | |
| 0–10 m | 15.36 ± 5.13 | 9.84 ± 3.29 | 12.41 ± 4.14 | 37.60 ± 12.56 | 0.66 ± 0.02 | 0.71 ± 0.04 | 0.16 ±0.001 | |
| Saccharum officinarum | 50–100 m | 0.84 ± 0.12 | 1.66 ± 0.23 | 1.16 ± 0.16 | 3.67 ± 0.51 | 1.01 ± 0.08 | 0.73 ± 0.07 | 0.44 ± 0.000 |
| 10–50 m | 5.70 ± 1.47 | 8.46 ± 2.18 | 8.70 ± 2.24 | 22.86 ± 5.88 | 1.12 ± 0.10 | 0.79 ± 0.09 | 0.34 ± 0.001 | |
| 0–10 m | 30.38 ± 10.1 | 31.83 ± 10.6 | 27.15 ± 9.07 | 89.35 ± 29.84 | 1.03 ± 0.09 | 0.71 ± 0.03 | 0.43 ± 0.000 | |
| Plant | Distance from Road | Cd Accumulation (µg Dry Biomass−1) | Cd Translocation | Cd Bioconcentration | ||||
|---|---|---|---|---|---|---|---|---|
| Root | Stem | Leaves | EP | R–S | R–L | |||
| Fragaria ananassa | 50–100 m | 0.23 ± 0.03 | 0.19 ± 0.03 | 0.23 ± 0.03 | 0.66 ± 0.09 | 0.43 ± 0.02 | 0.53 ± 0.05 | 0.80 ± 0.04 |
| 10–50 m | 1.03 ± 0.27 | 0.64 ± 0.16 | 1.75 ± 0.45 | 3.42 ± 0.88 | 0.55 ± 0.08 | 0.81 ± 0.03 | 0.83 ± 0.05 | |
| 0–10 m | 8.71 ± 2.91 | 8.32 ± 1.27 | 3.81 ± 2.78 | 20.85 ± 6.96 | 0.40 ± 0.04 | 0.78 ± 0.08 | 0.85 ± 0.07 | |
| Lycopersicon esculentum | 50–100 m | 0.17 ± 0.02 | 0.11 ± 0.02 | 0.17 ± 0.02 | 0.46 ± 0.06 | 0.33 ± 0.03 | 0.51 ± 0.03 | 0.58 ± 0.03 |
| 10–50 m | 1.02 ± 0.26 | 0.63 ± 0.16 | 1.13 ± 0.29 | 2.79 ± 0.72 | 0.43 ± 0.06 | 0.58 ± 0.05 | 0.65 ± 0.08 | |
| 0–10 m | 8.16 ± 2.72 | 5.29 ± 1.19 | 3.14 ± 1.77 | 17.01 ± 5.68 | 0.45 ± 0.08 | 0.53 ± 0.02 | 0.68 ± 0.05 | |
| Triticum aestivum | 50–100 m | 0.24 ± 0.03 | 0.14 ± 0.02 | 0.06 ± 0.01 | 0.45 ± 0.06 | 0.31 ± 0.06 | 0.14 ± 0.01 | 0.17 ± 0.07 |
| 10–50 m | 0.82 ± 0.21 | 0.37 ± 0.09 | 0.24 ± 0.06 | 1.43 ± 0.37 | 0.39 ± 0.03 | 0.19 ± 0.03 | 0.14 ± 0.07 | |
| 0–10 m | 2.51 ± 0.84 | 0.79 ± 0.26 | 0.43 ± 0.14 | 3.73 ± 1.25 | 0.33 ± 0.07 | 0.21 ± 0.02 | 0.35 ± 0.04 | |
| Nicotiana tabacum | 50–100 m | 0.23 ± 0.03 | 0.28 ± 0.04 | 0.29 ± 0.04 | 0.80 ± 0.11 | 0.65 ± 0.08 | 0.66 ± 0.03 | 0.88 ± 0.08 |
| 10–50 m | 0.40 ± 0.22 | 0.37 ± 0.20 | 0.56 ± 0.30 | 1.33 ± 0.72 | 0.71 ± 0.05 | 0.69 ± 0.06 | 0.94 ± 0.07 | |
| 0–10 m | 3.44 ± 1.15 | 2.20 ± 0.74 | 2.78 ± 0.93 | 8.42 ± 2.81 | 0.74 ± 0.03 | 0.63 ± 0.09 | 1.08 ± 0.09 | |
| Saccharum officinarum | 50–100 m | 0.20 ± 0.03 | 0.40 ± 0.06 | 0.27 ± 0.04 | 0.87 ± 0.12 | 1.02 ± 0.09 | 0.71 ± 0.05 | 0.85 ± 0.03 |
| 10–50 m | 0.34 ± 0.12 | 0.51 ± 0.18 | 0.52 ± 0.18 | 1.37 ± 0.48 | 1.08 ± 0.10 | 0.83 ± 0.07 | 0.95 ± 0.05 | |
| 0–10 m | 3.10 ± 0.99 | 2.96 ± 1.04 | 2.64 ± 0.88 | 8.70 ± 2.91 | 1.13 ± 0.08 | 0.74 ± 0.05 | 0.91 ± 0.08 | |
| Plants | Distance from Road | Proline (ppm) | Phenolics (ppm) | Carotenoids (ppm) | Chlorophylls (ppm) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Root | Leaves | Root | Leaves | A | b | a + b | |||
| Fragaria ananassa | 50–100 m | 1.33 ± 0.09 c | 0.93 ± 0.07 c | 0.25 ± 0.02 c | 0.45 ± 0.03 c | 0.61 ± 0.02 a | 11.11 ± 0.78a | 5.22 ± 0.37 a | 16.33 ± 1.14 a |
| 10–50 m | 2.00 ± 0.26 b | 1.40 ± 0.18 b | 0.30 ± 0.04 b | 0.60 ± 0.08 b | 0.45 ± 0.06 b | 10.00 ± 1.30 b | 3.65 ± 0.47 b | 13.65 ± 1.77 b | |
| 0–10 m | 3.33 ± 0.57 a | 2.33 ± 0.40 a | 0.51 ± 0.09 a | 1.34 ± 0.23 a | 0.32 ± 0.10 c | 8.00 ± 1.36 c | 2.61 ± 0.44 c | 10.61 ± 1.80 c | |
| Lycopersicon esculentum | 50–100 m | 0.97 ± 0.07 c | 0.78 ± 0.05 c | 0.07 ± 0.00 c | 0.12 ± 0.01 c | 0.52 ± 0.03 a | 10.23 ± 0.72 a | 4.99 ± 0.35 a | 15.22 ± 1.07 a |
| 10–50 m | 1.56 ± 0.20 b | 1.25 ± 0.16 b | 0.08 ± 0.01 b | 0.16 ± 0.02 b | 0.40 ± 0.05 b | 9.20 ± 1.20 b | 3.75 ± 0.49 b | 12.95 ± 1.68 b | |
| 0–10 m | 1.95 ± 0.33 a | 1.56 ± 0.27 a | 0.14 ± 0.02 a | 0.36 ± 0.06 a | 0.39 ± 0.09 c | 7.36 ± 1.25 c | 2.50 ± 0.42 c | 9.86 ± 1.68 c | |
| Triticum aestivum | 50–100 m | 0.45 ± 0.03 c | 0.36 ± 0.03 c | 0.17 ± 0.01 c | 0.30 ± 0.02 c | 0.48 ± 0.02 a | 8.18 ± 0.57 a | 4.11 ± 0.29 a | 12.29 ± 0.86 a |
| 10–50 m | 0.72 ± 0.09 b | 0.58 ± 0.07 b | 0.20 ± 0.03 b | 0.40 ± 0.05 b | 0.40 ± 0.06 b | 7.36 ± 0.96 b | 3.20 ± 0.42 b | 10.57 ± 1.37 b | |
| 0–10 m | 0.90 ± 0.15 a | 0.72 ± 0.12 a | 0.34 ± 0.06 a | 0.89 ± 0.15 a | 0.33 ± 0.07 c | 5.89 ± 1.00 c | 2.05 ± 0.35 c | 7.94 ± 1.35 c | |
| Nicotiana tabacum | 50–100 m | 1.17 ± 0.08 c | 0.93 ± 0.07 c | 0.09 ± 0.01 c | 0.16 ± 0.01 c | 0.48 ± 0.02 a | 5.79 ± 0.41 a | 3.39 ± 0.24 a | 9.18 ± 0.64 a |
| 10–50 m | 1.87 ± 0.24 b | 1.49 ± 0.19 b | 0.11 ± 0.01 b | 0.22 ± 0.03 b | 0.39 ± 0.06 b | 5.61 ± 0.73 b | 3.32 ± 0.43 b | 8.93 ± 1.16 b | |
| 0–10 m | 2.33 ± 0.40 a | 1.87 ± 0.32 a | 0.19 ± 0.03 a | 0.49 ± 0.08 a | 0.25 ± 0.07 c | 5.22 ± 0.89 c | 2.71 ± 0.46 c | 7.93 ± 1.35 c | |
| Saccharum officinarum | 50–100 m | 1.38 ± 0.10 c | 1.10 ± 0.08 c | 0.16 ± 0.01 c | 0.28 ± 0.02 c | 0.33 ± 0.01 a | 7.34 ± 0.51 a | 3.13 ± 0.22 a | 10.47 ± 0.73 a |
| 10–50 m | 2.21 ± 0.29 b | 1.77 ± 0.23 b | 0.19 ± 0.02 b | 0.37 ± 0.05 b | 0.30 ± 0.04 b | 6.61 ± 0.86 b | 2.50 ± 0.33 b | 9.11 ± 1.18 b | |
| 0–10 m | 2.76 ± 0.47 a | 2.21 ± 0.38 a | 0.31 ± 0.05 a | 0.83 ± 0.14 a | 0.16 ± 0.05 c | 5.28 ± 0.90 c | 1.56 ± 0.27 c | 6.85 ± 1.16 c | |
| Plant Sample | Heavy Metal | Chlorophyll (Leaf) | Carotenoid (Leaf) | Proline (Root) | Proline (Leaf) | Phenolics (Root) | Phenolics (Leaf) |
|---|---|---|---|---|---|---|---|
| Fragaria annanasa | Lead | −0.9627 * | –0.8672 | +0.8679 | +0.9973 | +0.7975 | +0.9802 |
| Cadmium | –0.9973 | –0.8750 | +0.8881 | +0.9894 | +0.9654 | +0.9224 | |
| Lycopersicon esculentum | Lead | –0.9776 | –0.8540 | +0.8429 | +0.887 | +0.9674 | +0.956 |
| Cadmium | –0.9863 | –0.8516 | +0.7293 | +0.9077 | +0.8304 | +0.949 | |
| Triticum aestivum | Lead | –0.981 | –0.888 | +0.881 | +0.9837 | +0.7326 | +0.8698 |
| Cadmium | –0.9811 | –0.8789 | +0.7841 | +0.9756 | +0.8809 | +0.889 | |
| Nicotiana tabacum | Lead | –0.9852 | –0.9688 | +0.7775 | +0.9004 | +0.9281 | +0.9699 |
| Cadmium | –0.9917 | –0.9824 | +0.6725 | +0.7487 | +0.9045 | +0.9912 | |
| Saccharum officinarum | Lead | –0.9913 | –0.9767 | +0.7801 | +0.8736 | +0.9548 | +0.9774 |
| Cadmium | –0.9211 | –0.9192 | +0.6791 | +0.7234 | +0.9456 | +0.9911 |
| Plant Species | Distance from Road | EDI | THQ | HI | CR | TCR | |||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Cd | Pb | Cd | Pb | Cd | ||||
| Strawberry | 50–100 m | 0.0011 | 0.0001 | 0.311 | 0.122 | 0.434 | 3.815 × 10−6 | 1.224 × 10−7 | 3.937 × 10−6 |
| 10–50 m | 0.0064 | 0.0007 | 1.824 | 0.697 | 2.521 | 2.235 × 10−5 | 6.965 × 10−7 | 2.304 × 10−5 | |
| 0–10 m | 0.0206 | 0.0035 | 5.889 | 3.535 | 9.424 | 7.213 × 10−5 | 3.535 × 10−6 | 7.567 × 10−5 | |
| Tomato | 50–100 m | 0.0004 | 0.0001 | 0.119 | 0.082 | 0.201 | 1.459 × 10−6 | 8.160 × 10−8 | 1.540 × 10−6 |
| 10–50 m | 0.0027 | 0.0005 | 0.762 | 0.513 | 1.275 | 9.333 × 10−6 | 5.129 × 10−7 | 9.846 × 10−6 | |
| 0–10 m | 0.0089 | 0.0025 | 2.552 | 2.457 | 5.009 | 3.126 × 10−5 | 2.457 × 10−6 | 3.372 × 10−5 | |
| Wheat | 50–100 m | 0.0000 | 0.0001 | 0.012 | 0.058 | 0.071 | 1.530 × 10−7 | 5.829 × 10−8 | 2.113 × 10−7 |
| 10–50 m | 0.0002 | 0.0002 | 0.060 | 0.178 | 0.238 | 7.344 × 10−7 | 1.778 × 10−7 | 9.122 × 10−7 | |
| 0–10 m | 0.0004 | 0.0004 | 0.118 | 0.356 | 0.474 | 1.448 × 10−6 | 3.555 × 10−7 | 1.804 × 10−6 | |
| Tobacco | 50–100 m | 0.0001 | 0.0002 | 0.035 | 0.166 | 0.201 | 4 × 10−7 | 1.661 × 10−7 | 5.945 × 10−7 |
| 10–50 m | 0.0019 | 0.0003 | 0.550 | 0.271 | 0.821 | 6.732 × 10−6 | 2.710 × 10−7 | 7.003 × 10−6 | |
| 0–10 m | 0.0065 | 0.0015 | 1.853 | 1.451 | 3.304 | 2.270 × 10−5 | 1.451 × 10−6 | 2.415 × 10−5 | |
| Sugarcane | 50–100 m | 0.0008 | 0.0002 | 0.235 | 0.195 | 0.430 | 2.876 × 10−6 | 1.953 × 10−7 | 3.072 × 10−6 |
| 10–50 m | 0.0050 | 0.0003 | 1.429 | 0.300 | 1.729 | 1.750 × 10−5 | 3.002 × 10−7 | 1.780 × 10−5 | |
| 0–10 m | 0.0172 | 0.0016 | 4.911 | 1.632 | 6.543 | 6.016 × 10−5 | 1.632 × 10−6 | 6.179 × 10−5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, S.; Hadi, F.; Jan, A.U.; Ullah, R.; Albalawi, B.F.A.; Ditta, A. Appraisal of Heavy Metals Accumulation, Physiological Response, and Human Health Risks of Five Crop Species Grown at Various Distances from Traffic Highway. Sustainability 2022, 14, 16263. https://doi.org/10.3390/su142316263
Ahmad S, Hadi F, Jan AU, Ullah R, Albalawi BFA, Ditta A. Appraisal of Heavy Metals Accumulation, Physiological Response, and Human Health Risks of Five Crop Species Grown at Various Distances from Traffic Highway. Sustainability. 2022; 14(23):16263. https://doi.org/10.3390/su142316263
Chicago/Turabian StyleAhmad, Shakeel, Fazal Hadi, Amin Ullah Jan, Raza Ullah, Bedur Faleh A. Albalawi, and Allah Ditta. 2022. "Appraisal of Heavy Metals Accumulation, Physiological Response, and Human Health Risks of Five Crop Species Grown at Various Distances from Traffic Highway" Sustainability 14, no. 23: 16263. https://doi.org/10.3390/su142316263
APA StyleAhmad, S., Hadi, F., Jan, A. U., Ullah, R., Albalawi, B. F. A., & Ditta, A. (2022). Appraisal of Heavy Metals Accumulation, Physiological Response, and Human Health Risks of Five Crop Species Grown at Various Distances from Traffic Highway. Sustainability, 14(23), 16263. https://doi.org/10.3390/su142316263







