Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Proximate Composition
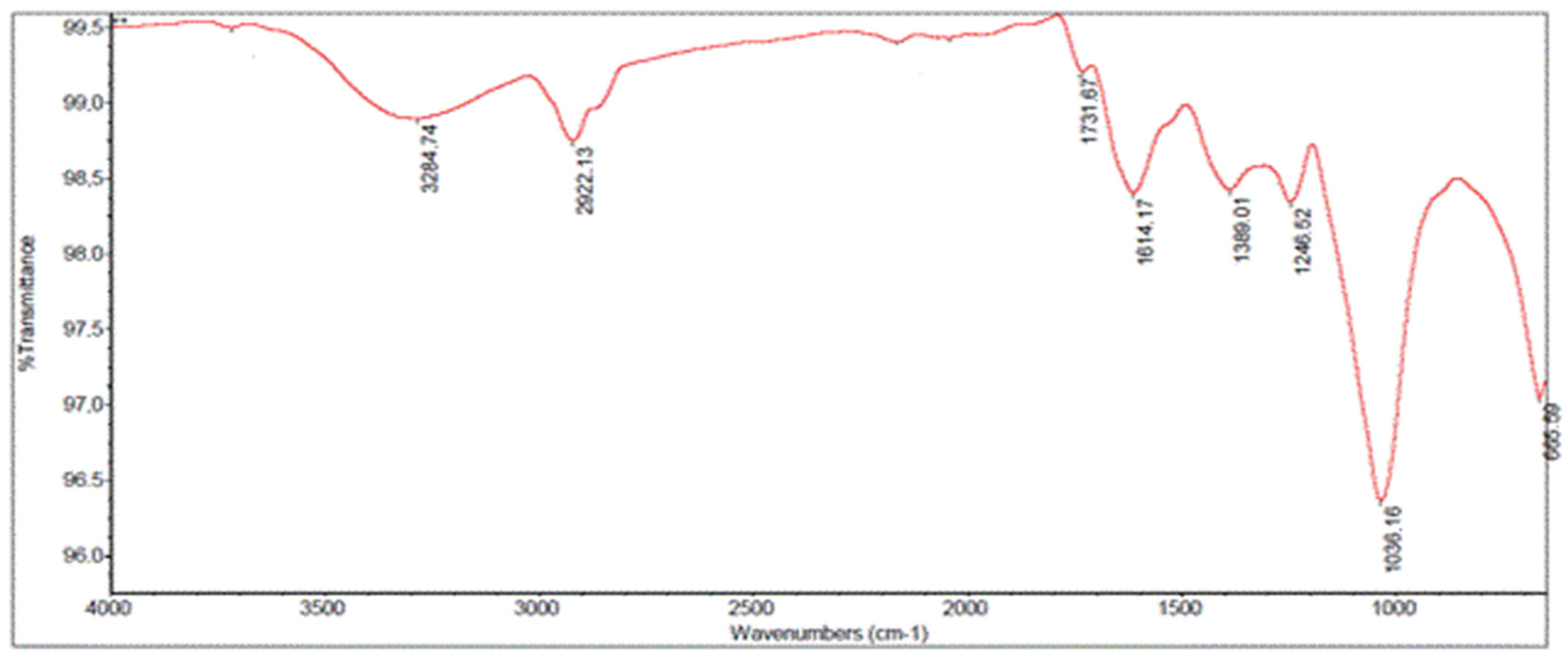
2.3. FTIR Analysis
2.4. Extraction of Phenolic Compounds by Ultrasound-Assisted Extraction and Optimization Procedure
2.5. Determination of Total Phenolic Content
2.6. Profiling of Phenolic Compounds by UHPLC-ESI-QTOF MS
2.7. Design of Fresh Egg Pasta Enriched with Polyphenols from Artichoke By-Products
2.8. Color Measurement and Image Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Artichoke By-Products Characterization
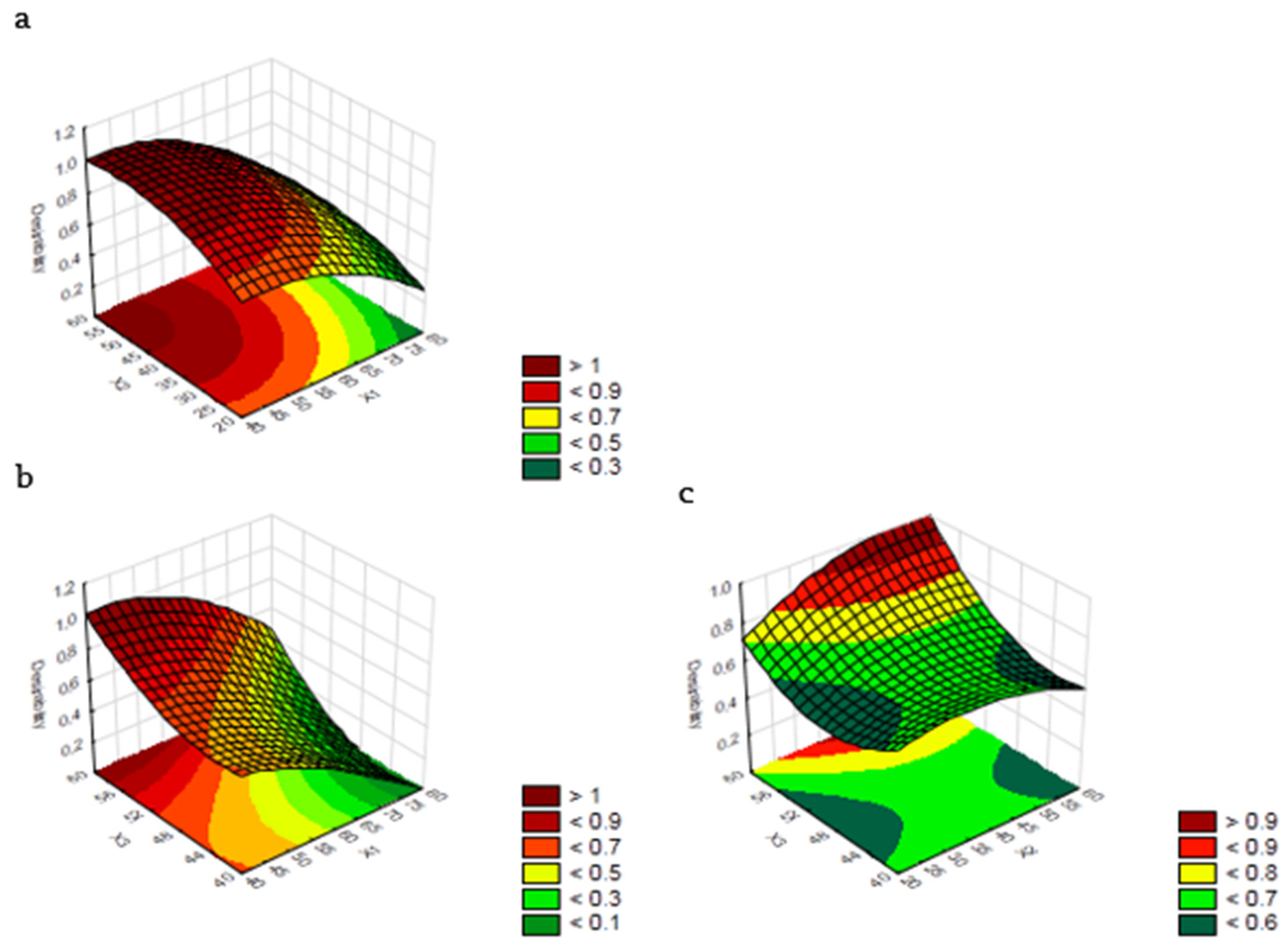
3.2. Optimization of Extracting Parameters and Validation of the RSM Model
3.3. Phenolic Extract Characterization by UHPLC-ESI-QTOF MS
3.4. Effect of Artichoke By-Products Optimal Extract on Fresh Egg Pasta
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development. United Nations. 2015. Available online: https://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed on 23 August 2022).
- FAO. Strategic Framework 2022-31. FAO. 2019. Available online: http://www.fao.org/3/cb7099en/cb7099en.pdf (accessed on 23 August 2022).
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions- The European Green Deal. European Commission. 2019. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 23 August 2022).
- European Commission. Directorate-General for Communication, Circular economy action plan: For a cleaner and more competitive Europe, Publications Office. 2020. Available online: https://data.europa.eu/doi/10.2779/717149 (accessed on 23 August 2022).
- Amoriello, T.; Ciccoritti, R. Sustainability: Recovery and Reuse of Brewing-Derived By-Products. Sustainability 2021, 13, 2355. [Google Scholar]
- Ruggeri, S. The sustainable food design hub: A transdisciplinary approach for ecological transition. In Experiencing and Envisioning Food, Designing for Change; Bonacho, R., Coelho, A., Pires, M., Eidler, M., Massari, S., Alvarez, P., Eds.; Taylor Francis—CRC Press: London, UK, 2023. [Google Scholar]
- Spinelli, S.; Paladino, L.; Costa, C.; Del Nobile, M.A.; Conte, A. Food by-products to fortified pasta: A new approach for optimization. J. Clean. Prod. 2019, 215, 985–991. [Google Scholar]
- Oliviero, T.; Fogliano, V. Food design strategies to increase vegetable intake: The case of vegetable enriched pasta. Trends Food Sci. Technol. 2016, 51, 58–64. [Google Scholar]
- Dias, M.I.; Ferreira, I.C.F.R.; Barreiro, M.F. Microencapsulation of bioactives for food applications. Food Funct. 2015, 6, 1035–1052. [Google Scholar]
- FAOSTAT. 2021. Available online: http://faostat.fao.org (accessed on 23 August 2022).
- De Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar]
- Rottenberg, A.; Zohary, D. The wild ancestry of the cultivated artichoke. Genet. Resour. Crop Evol. 1996, 43, 53–58. [Google Scholar]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke:a functional food and source of nutraceutical ingredients. J. Funct. Food 2009, 1, 131–144. [Google Scholar]
- El Senousy, A.S.; Farag, M.A.; Al-Mahdy, D.A.; Wessjohann, L.A. Developmental changes in leaf phenolics composition from three artichoke cultivars (Cynara scolymus) as determined via UHPLC-MS and chemometrics. Phytochemistry 2014, 108, 67–76. [Google Scholar]
- Fallah Huseini, H.; Kianbakht, S.; Heshmat, R. Cynara scolymus L. in treatment of hypercholesterolemic type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Med. Plants 2012, 11, 58–65. [Google Scholar]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Artichoke (Cynara scolymus L.) byproducts as a potential source of health-promoting antioxidant phenolics. J. Agric. Food Chem. 2002, 50, 3458–3464. [Google Scholar]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Bastida, J.; Viladomat, F.; Codina, C. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2003, 1008, 57–72. [Google Scholar]
- Machado, M.T.; Eça, K.S.; Vieira, G.S.; Menegalli, F.C.; Martínez, J.; Hubinger, M.D. Prebiotic oligosaccharides from artichoke industrial waste: Evaluation of different extraction methods. Ind. Crops Prod. 2015, 76, 141–148. [Google Scholar]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. LWT-Food Sci. Technol. 2020, 132, 109883. [Google Scholar]
- Sabater, C.; Corzo, N.; Olano, A.; Montilla, A. Enzymatic extraction of pectin from artichoke (Cynara scolymus L.) by-products using Celluclast®1.5L. Carbohydr. Polym. 2018, 190, 43–49. [Google Scholar]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar]
- Fernandez, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crops Prod. 2006, 24, 222–229. [Google Scholar]
- Botta, L.; Fiore, V.; Scalici, T.; Valenza, A.; Scaffaro, R. New polylactic acid composites reinforced with artichoke fibers. Materials 2015, 8, 7770–7779. [Google Scholar]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brol, A.; Hallet, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar]
- Carpentieri, S.; Larrea- Wachtendorff, D.; Donsì, F.; Ferrari, G. Functionalization of pasta through the incorporation of bioactive compounds from agri-food by-products: Fundamentals, opportunities, and drawbacks. Trends Food Sci. Technol. 2022, 122, 49–65. [Google Scholar]
- Iadecola, R.; Ciccoritti, R.; Ceccantoni, B.; Bellincontro, A.; Amoriello, T. Optimization of Phenolic Compound Extraction from Brewers’ Spent Grain Using Ultrasound Technologies Coupled with Response Surface Methodology. Sustainability 2022, 14, 3309. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar]
- Pasqualone, A.; Punzi, R.; Trani, A.; Summo, C.; Paradiso, V.M.; Caponio, F.; Gambacorta, G. Enrichment of fresh pasta with antioxidant extracts obtained from artichoke canning by-products by ultrasound-assisted technology and quality characterisation of the end product. Int. J. Food Sci. Technol. 2017, 52, 2078–2087. [Google Scholar]
- Methods No. 104/1, 105/2, 110/1, 136; International Association for Cereal Science and Technology. ICC Standard Methods; ICC: Vienna, Austria, 2003.
- Lee, S.C.; Prosky, L.; DeVries, J.W. Determination of total, soluble, and insoluble, dietary fibre in foods-enzymatic-gravimetric method, MES-TRIS buffer: Collaborative study. J. Assoc. Off. Anal. Chem. 1992, 75, 395–416. [Google Scholar]
- Amoriello, T.; Mellara, F.; Amoriello, M.; Ceccarelli, D.; Ciccoritti, R. Powdered seaweeds as a valuable ingredient for functional breads. Eur. Food Res. Technol. 2021, 247, 2431–2443. [Google Scholar]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of Solvent Type and Extraction Conditions on the Recovery of Phenolic Compounds from Artichoke Waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar]
- Zuorro, A. Response surface methodology analysis of polyphenol recovery from artichoke waste. Am. J. Appl. Sci. 2014, 11, 1463–1471. [Google Scholar]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Reuse potential of artichoke (Cynara scolymus L.) waste for the recovery of phenolic compounds and bioenergy. J. Clean. Prod. 2016, 111, 279–284. [Google Scholar]
- Rabelo, R.S.; Machado, M.T.C.; Martínez, J.; Hubinger, M.D. Ultrasound assisted extraction and nanofiltration of phenolic compounds from artichoke solid wastes. J. Food Eng. 2016, 178, 170–180. [Google Scholar]
- Ciccoritti, R.; Taddei, F.; Gazza, L.; Nocente, F. Influence of kernel thermal pre-treatments on 5-n-alkylresorcinols, polyphenols and antioxidant activity of durum and einkorn wheat. Eur. Food Res. Technol. 2021, 247, 353–362. [Google Scholar]
- Balli, D.; Cecchi, L.; Innocenti, M.; Bellumori, M.; Mulinacci, N. Food by-products valorisation: Grape pomace and olive pomace (pâté) as sources of phenolic compounds and fiber for enrichment of tagliatelle pasta. Food Chem. 2021, 355, 129642. [Google Scholar]
- Zardetto, S.; Fregonese, M.; Pasini, G. Effects of modified atmospheric packaging configuration on spoilage mould growth in damaged packages of fresh pasta. J. Food Eng. 2022, 314, 110760. [Google Scholar]
- Lomonaco, S.; Grassi, M.A.; Vallone, L.; Pistone, V.; Civera, T. Presence of fungal contamination in the production chain of fresh filled pasta, with particular regard to the penicillium genus. Ital. J. Food Saf. 2011, 1, 33. [Google Scholar]
- Yan, X.; Ye, R.; Chen, Y. Blasting Extrusion Processing: The Increase of Soluble Dietary Fiber Content and Extraction of Soluble-fiber Polysaccharides from Wheat Bran. Food Chem. 2015, 180, 106–115. [Google Scholar]
- Quintero Ruiz, N.A.; Paolucci, M.; Siano, F.; Mamone, G.; Picariello, G.; Puppo, M.C.; Volpe, M.G. Characterization of soluble and insoluble fibers in artichoke by-products by ATR-FTIR spectroscopy coupled with chemometrics. Int. J. Food Prop. 2021, 24, 1693–1704. [Google Scholar]
- Lavecchia, R.; Maffei, G.; Paccassoni, F.; Piga, L.; Zuorro, A. Artichoke waste as a source of phenolic antioxidants and bioenergy. Waste Biomass Valorization 2019, 10, 2975–2984. [Google Scholar]
- Peres, R.S.; Armelin, E.; Alemán, C.; Ferreira, C.A. Modified tannin extracted from black wattle tree as an environmentally friendly antifouling pigment. Ind. Crop Prod. 2015, 65, 506–514. [Google Scholar]
- Yılmaz, M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar]
- López-Salas, L.; Borrás-Linares, I.; Quintin, D.; García-Gomez, P.; Giménez-Martínez, R.; Segura-Carretero, A.; Lozano-Sánchez, J. Artichoke by-products as natural source of phenolic food ingredient. Appl. Sci. 2021, 11, 3788. [Google Scholar]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar]
- Huang, D.; Zhou, X.; Si, J.; Gong, X.; Wang, S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016, 10, 56. [Google Scholar]
- Punzi, R.; Paradiso, A.; Fasciano, C.; Trani, A.; Faccia, M.; de Pinto, M.C.; Gambacorta, G. Phenols and antioxidant activity in vitro and in vivo of aqueous extracts obtained by ultrasound-assisted extraction from artichoke by-products. Nat. Prod. Commun. 2014, 9, 1315–1318. [Google Scholar]
- Jiménez-Moreno, N.; Cimminelli, M.J.; Volpe, F.; Ansó, R.; Esparza, I.; Mármol, I.; Ancín-Azpilicueta, C. Phenolic composition of artichoke waste and its antioxidant capacity on differentiated Caco-2 cells. Nutrients 2019, 11, 1723. [Google Scholar]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive characterisation of bioactive phenolic constituents from globe artichoke (Cynara scolymus L.) by HPLC-DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar]
- Farag, M.A.; El-Ahmady, S.H.; Elian, F.S.; Wessjohann, L.A. Metabolomics driven analysis of artichoke leaf and its commercial products via UHPLC-q-TOF-MS and chemometrics. Phytochemistry 2013, 95, 177–187. [Google Scholar]
- Teterycz, D.; Sobota, A.; Kozłowicz, K.; Zarzycki, P. Substitution of semolina durum with common wheat flour in egg and eggless pasta. Acta Sci. Pol. Technol. Aliment. 2019, 18, 439–451. [Google Scholar]
- Biernacka, B.; Dziki, D.; Gawlik- Dziki, U.; Rozylo, R. Common wheat pasta enriched with cereal coffee: Quality and physical and functional properties. LWT-Food Sci. Technol. 2021, 139, 110516. [Google Scholar]
- Parizad, P.A.; Marengo, M.; Bonomi, F.; Scarafoni, A.; Cecchini, C.; Pagani, M.A.; Marti, A.; Iametti, S. Bio-Functional and Structural Properties of Pasta Enriched with a Debranning Fraction from Purple Wheat. Foods 2020, 9, 163. [Google Scholar]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar]
- Angiolillo, L.; Spinelli, S.; Conte, A.; Del Nobile, M.A. Extract from broccoli byproducts to increase fresh filled pasta shelf life. Foods 2019, 8, 621. [Google Scholar]

| Run | X1 | X2 | X3 | X1 (%) | X2 (min) | X3 (°C) | TPCexp (mg GAE/g d.w.) | TPCRSM (mg GAE/g d.w.) |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 40 | 20 | 50 | 18.26 ± 0.04 | 18.26 |
| 2 | 1 | −1 | 0 | 80 | 20 | 50 | 13.79 ± 0.05 | 13.75 |
| 3 | −1 | 1 | 0 | 40 | 60 | 50 | 19.43 ± 0.05 | 19.48 |
| 4 | 1 | 1 | 0 | 80 | 60 | 50 | 13.55 ± 0.07 | 13.55 |
| 5 | −1 | 0 | −1 | 40 | 40 | 40 | 20.07 ± 0.02 | 20.06 |
| 6 | 1 | 0 | −1 | 80 | 40 | 40 | 14.24 ± 0.04 | 14.29 |
| 7 | −1 | 0 | 1 | 40 | 40 | 60 | 22.27 ± 0.04 | 22.22 |
| 8 | 1 | 0 | 1 | 80 | 40 | 60 | 17.54 ± 0.02 | 17.55 |
| 9 | 0 | −1 | −1 | 60 | 20 | 40 | 18.19 ± 0.04 | 18.19 |
| 10 | 0 | 1 | −1 | 60 | 60 | 40 | 16.92 ± 0.08 | 16.88 |
| 11 | 0 | −1 | 1 | 60 | 20 | 60 | 19.04 ± 0.03 | 19.08 |
| 12 | 0 | 1 | 1 | 60 | 60 | 60 | 21.41 ± 0.02 | 21.42 |
| 13 | 0 | 0 | 0 | 60 | 40 | 50 | 18.22 ± 0.02 | 18.22 |
| 14 | 0 | 0 | 0 | 60 | 40 | 50 | 18.22 ± 0.02 | 18.22 |
| 15 | 0 | 0 | 0 | 60 | 40 | 50 | 18.22 ± 0.02 | 18.22 |
| P1 | P2 | P3 | P4 | |
|---|---|---|---|---|
| Semolina (%) | 35 | 35 | 0 | 0 |
| Soft wheat (%) | 35 | 35 | 63 | 63 |
| Eggs (%) | 20 | 20 | 37 | 27 |
| Water (%) | 10 | 0 | 0 | 0 |
| Artichoke extract (%) | 0 | 10 | 0 | 10 |
| Main Peak (cm−1) | Typical Band | Compound Family |
|---|---|---|
| 3284.74 | O–H stretching vibration of the hydroxyl group | Polysaccharide compounds |
| 2922.13 | C–H stretching vibration | Polysaccharides such as inulin |
| 1731.67 | C=O stretching vibration carboxylic acid/ester groups | Lignin or hemicellulose |
| 1614.17 | C–C bonds of aromatic ring | Lignin or polyphenols |
| 1389.01 | CH2 symmetric bending and COH deformation of phenols | Hemicelluloses, celluloses, and phenols |
| 1246.52 | OH group attached to an aromatic ring | S rings and G rings of lignin |
| 1036.16 | C–C stretching/C–O stretching | Polysaccharides such as inulin |
| 665.59 | O–H stretching vibration | Polysaccharides |
| Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|
| X1 | 163.7515 | 1 | 163.7515 | 71,071.23 | <0.0001 |
| X2 | 1.5759 | 1 | 1.5759 | 683.99 | <0.0001 |
| X3 | 44.1731 | 1 | 44.1731 | 19,171.94 | <0.0001 |
| X12 | 14.9372 | 1 | 14.9372 | 6483.05 | <0.0001 |
| X22 | 7.0229 | 1 | 7.0229 | 3048.08 | <0.0001 |
| X32 | 24.0312 | 1 | 24.0312 | 10,429.99 | <0.0001 |
| X1 X2 | 1.5052 | 1 | 1.5052 | 653.29 | <0.0001 |
| X1 X3 | 0.8965 | 1 | 0.8965 | 389.11 | <0.0001 |
| X2 X3 | 9.9736 | 1 | 9.9736 | 4328.74 | <0.0001 |
| Residual | 0.0806 | 35 | 0.0023 | ||
| Lack of fit | 0.0349 | 3 | 0.01164 | 8.1400 | 0.0004 |
| Pure error | 0.0457 | 32 | 0.00143 | ||
| Total SS | 271.5927 | 44 | |||
| R2 | 0.999 | ||||
| Radj2 | 0.998 |
| Peak | Rt (min) | Compounds | Theoretical m/z | Experimental m/z | Molecular Formula | Error (ppm) | mg/g |
|---|---|---|---|---|---|---|---|
| 1 | 4.259 | Caffeoylquinic acid I * | 353.0878 | 353.0882 | C16H18O9 | 1.13 | 0.77 ± 0.04 |
| 2 | 5.859 | 3-O-Caffeoylquinic acid (neochlorogenic acid) * | 353.0878 | 353.0884 | C16H18O9 | 1.70 | 0.56 ± 0.04 |
| 3 | 12.492 | 5-O-Caffeoylquinic acid (chlorogenic acid) * | 353.0878 | 353.0885 | C16H18O9 | 1.98 | 14.1 ± 0.2 |
| 4 | 13.346 | 4-O-Caffeoylquinic acid (cryptochlorogenic acid) * | 353.0878 | 353.0884 | C16H18O9 | 1.70 | 0.67 ± 0.05 |
| 5 | 14.314 | Coumaroylquinic acid I ** | 337.0929 | 337.0937 | C16H18O8 | 2.37 | 0.36 ± 0.02 |
| 6 | 14.747 | Dicaffeoylquinic acid I * | 515.1195 | 515.1204 | C25H24O12 | 1.75 | 0.26 ± 0.02 |
| 7 | 14.864 | Caffeoylquinic acid dimer I * | 705.1672 | 705.1688 | C32H34O18 | 2.27 | 0.18 ± 0.01 |
| 8 | 15.231 | Coumaroylquinic acid II ** | 337.0929 | 337.0935 | C16H18O8 | 1.80 | 0.096 ± 0.005 |
| 9 | 16.831 | Quercetin 3-O-rutinoside (rutin) *** | 609.1461 | 609.1467 | C27H30O16 | 0.98 | 0.038 ± 0.003 |
| 10 | 16.998 | Dicaffeoylquinic acid glucoside I * | 677.1723 | 677.1733 | C31H34O17 | 1.48 | 0.29 ± 0.03 |
| 11 | 17.182 | Dicaffeoylquinic acid glucoside II * | 677.1723 | 677.1737 | C31H34O17 | 2.07 | 0.67 ± 0.05 |
| 12 | 19.449 | Kaempferol-rutinoside *** | 593.1512 | 593.1518 | C27H30O15 | 1.01 | 0.090 ± 0.003 |
| 13 | 19.683 | Dicaffeoylquinic acid II * | 515.1195 | 515.1206 | C25H24O12 | 2.14 | 1.03 ± 0.08 |
| 14 | 20.066 | Dicaffeoylquinic acid III * | 515.1195 | 515.1195 | C25H24O12 | 0.00 | 18.4 ± 0.3 |
| 15 | 20.383 | Dicaffeoylquinic acid IV * | 515.1195 | 515.1201 | C25H24O12 | 1.16 | 14.4 ± 0.3 |
| 16 | 21.417 | Dicaffeoylquinic acid V * | 515.1195 | 515.1208 | C25H24O12 | 2.52 | 3.5 ± 0.1 |
| 17 | 21.767 | Coumaroyl-caffeoylquinic acid * | 499.1246 | 499.1256 | C25H24O11 | 2.00 | 0.47 ± 0.04 |
| 18 | 21.784 | Coumaroylquinic acid III ** | 337.0929 | 337.0941 | C16H18O8 | 3.58 | 0.076 ± 0.005 |
| L* | a* | b* | |
|---|---|---|---|
| ABP | 61.50 ± 0.62 e | −0.17 ± 0.15 a | 17.76 ± 0.05 f |
| UP1 | 87.90 ± 0.66 b | −2.30 ± 0.18 e | 26.84 ± 1.04 bc |
| UP2 | 88.55 ± 0.41 ab | −2.11 ± 0.03 e | 24.50 ± 0.92 cd |
| UP3 | 89.25 ± 0.48 a | −1.20 ± 0.04 b | 23.00 ± 1.22 de |
| UP4 | 88.58 ± 0.19 ab | −1.26 ± 0.05 b | 22.08 ± 0.69 e |
| CP1 | 87.15 ± 0.39 b | −3.28 ± 0.09 g | 31.86 ± 0.87 a |
| CP2 | 80.37 ± 0.89 d | −1.97 ± 0.06 d | 25.77 ± 0.78 c |
| CP3 | 86.91 ± 0.29 b | −2.77 ± 0.08 f | 27.85 ± 0.31 b |
| CP4 | 83.19 ± 0.31 c | −1.79 ± 0.08 c | 23.79 ± 0.41 d |
| TPC-UPi (mg GAE/g d.w.) | TPC-CPi (mg GAE/g d.w.) | ΔTPC (%) | |
|---|---|---|---|
| P1 | 1.86 ± 0.03 c | 0.54 ± 0.02 c | −71 |
| P2 | 2.05 ± 0.05 a | 0.73 ± 0.04 b | −64 |
| P3 | 1.92 ± 0.03 b | 0.57 ± 0.03 c | −70 |
| P4 | 2.04 ± 0.02 a | 0.91 ± 0.02 a | −55 |
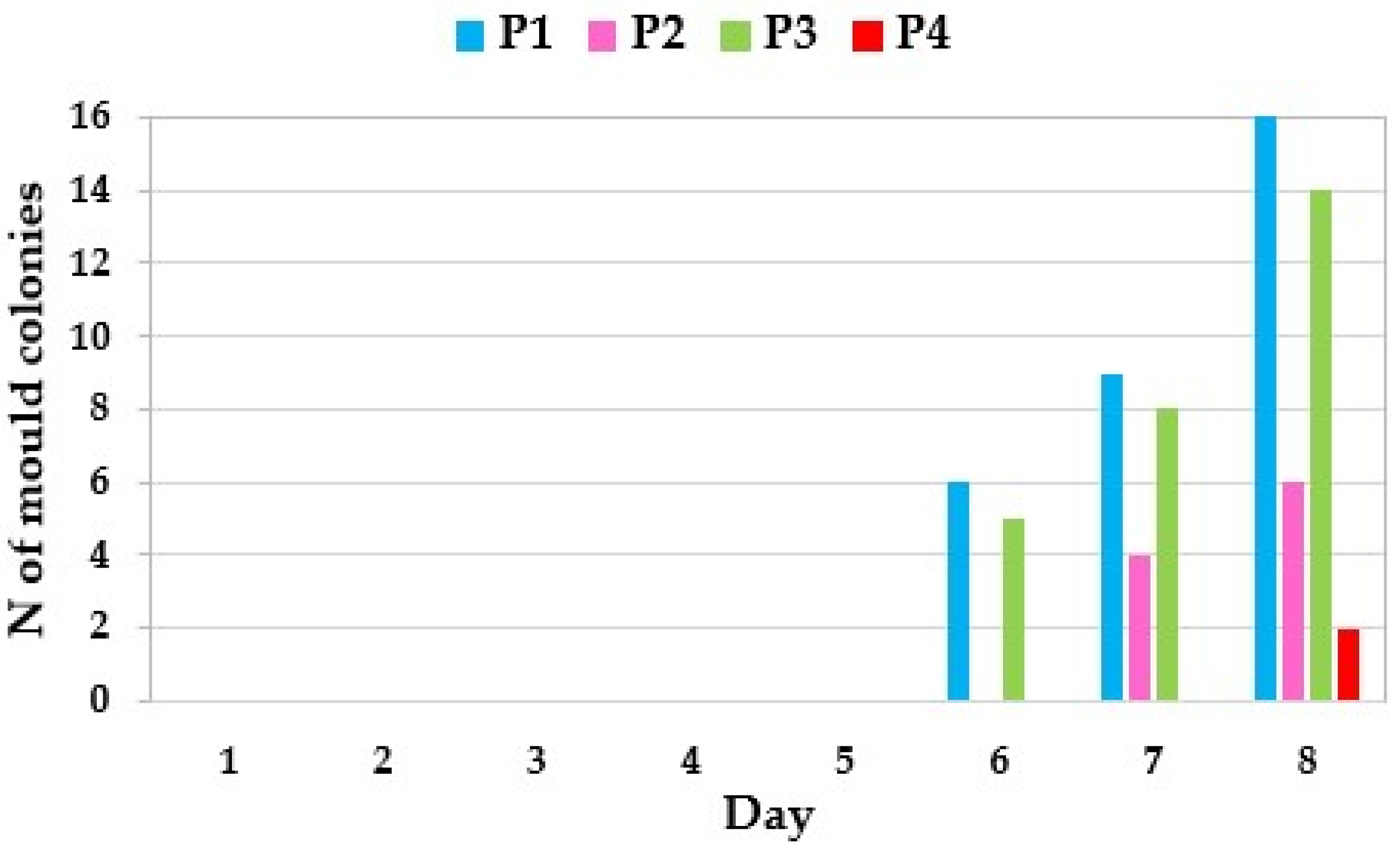
| Factor | Χ2 | Pr > Χ2 |
|---|---|---|
| P | 22.322 | <0.0001 |
| Day | 14.567 | 0.0010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoriello, T.; Mellara, F.; Ruggeri, S.; Ciorba, R.; Ceccarelli, D.; Ciccoritti, R. Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability 2022, 14, 14778. https://doi.org/10.3390/su142214778
Amoriello T, Mellara F, Ruggeri S, Ciorba R, Ceccarelli D, Ciccoritti R. Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability. 2022; 14(22):14778. https://doi.org/10.3390/su142214778
Chicago/Turabian StyleAmoriello, Tiziana, Francesco Mellara, Stefania Ruggeri, Roberto Ciorba, Danilo Ceccarelli, and Roberto Ciccoritti. 2022. "Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project" Sustainability 14, no. 22: 14778. https://doi.org/10.3390/su142214778
APA StyleAmoriello, T., Mellara, F., Ruggeri, S., Ciorba, R., Ceccarelli, D., & Ciccoritti, R. (2022). Artichoke By-Products Valorization for Phenols-Enriched Fresh Egg Pasta: A Sustainable Food Design Project. Sustainability, 14(22), 14778. https://doi.org/10.3390/su142214778









