Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming through Life Cycle Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Production System
2.1.1. Farming Practice and Culture System
2.1.2. Water Quality
2.1.3. Feed Management
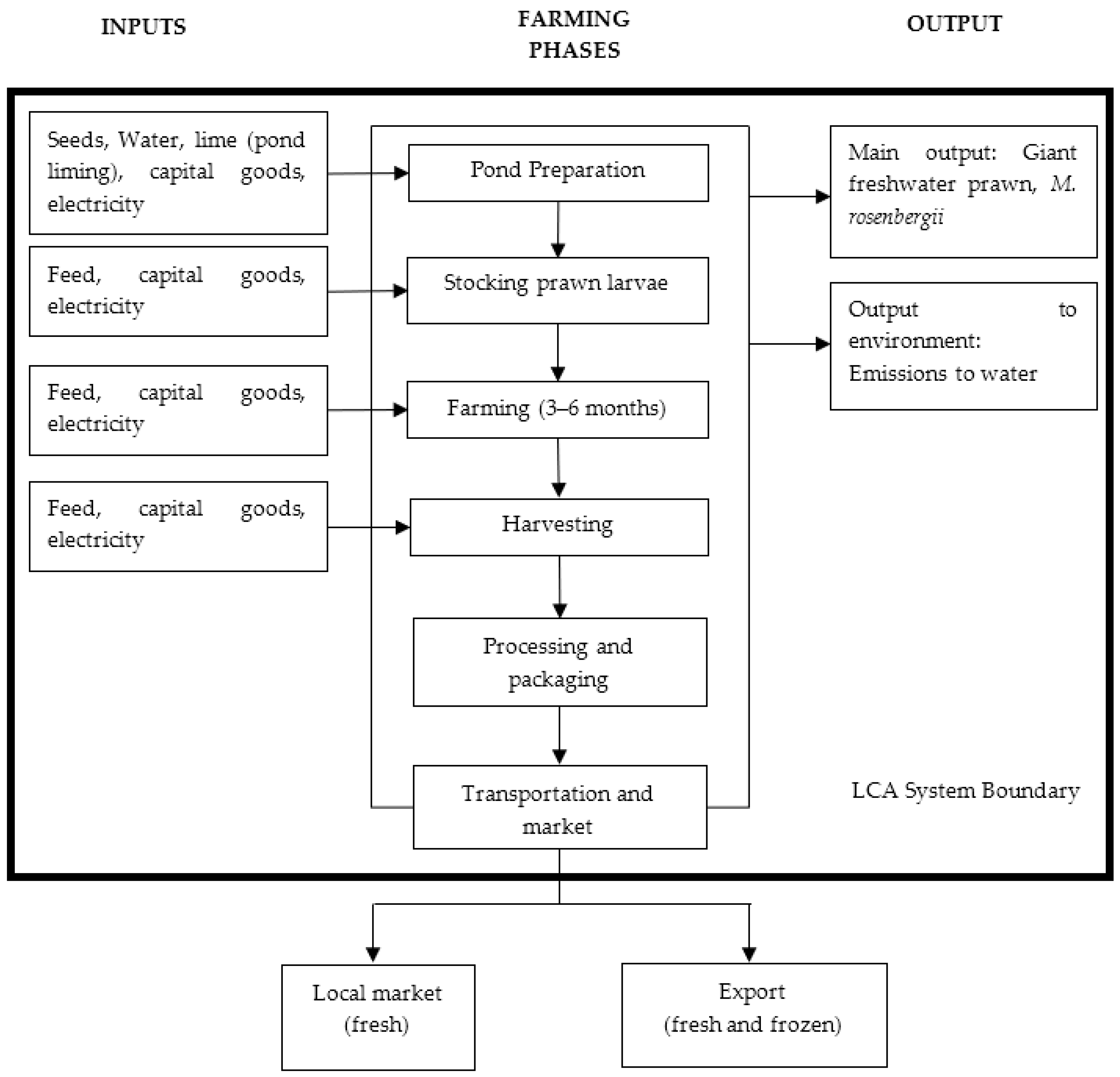
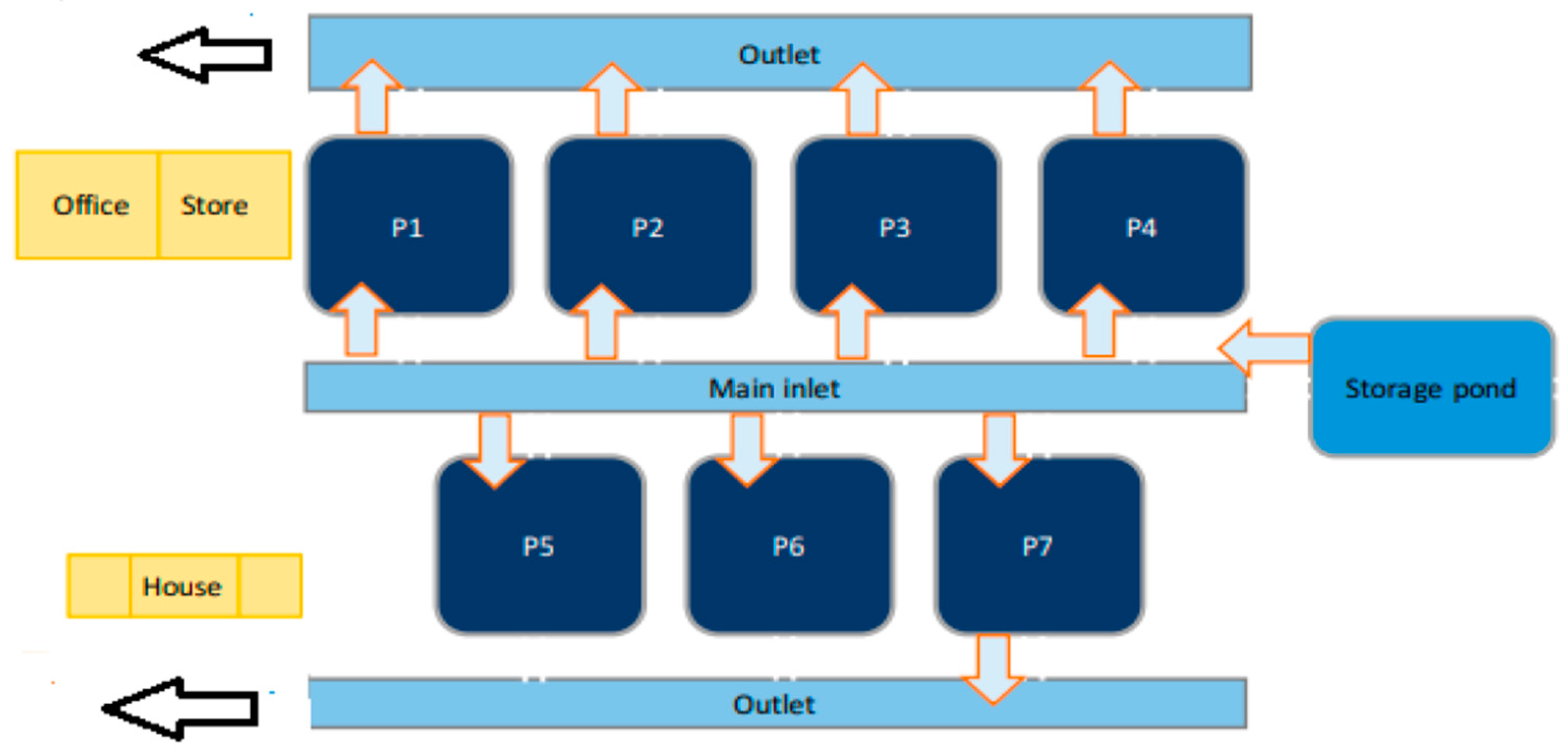
2.2. System Description
2.3. Goal and Scope Definition
2.4. Boundary System
2.5. Data Inventory
2.6. Impact Analysis
- i.
- global warming (kg CO2 equivalent), which assemblage emissions of greenhouse gases
- ii.
- terrestrial acidification (kg SO2 equivalent to air), which assemblage proton increase in natural soils
- iii.
- freshwater eutrophication (kg P equivalent), which assemblage phosphorus increase in freshwater
- iv.
- terrestrial ecotoxicity (kg 1,4-DCB-equivalent to industrial soil), which assemblage hazard weighted increase in natural soil
- v.
- freshwater ecotoxicity (kg 1,4-DCB-equivalent to freshwater), which assemblage hazard weighted increase in freshwater
- vi.
- human carcinogenic activity (kg 1,4-DCB-equivalent to urban air) which assemblage risk increase of cancer disease incidence
- vii.
- human non-carcinogenic activity (kg 1,4-DCB-equivalent to urban air) which assemblage risk increase of non-cancer disease incidence
- viii.
- water consumption (m3 water consumed) which assemblage increase in water consumption
3. Results
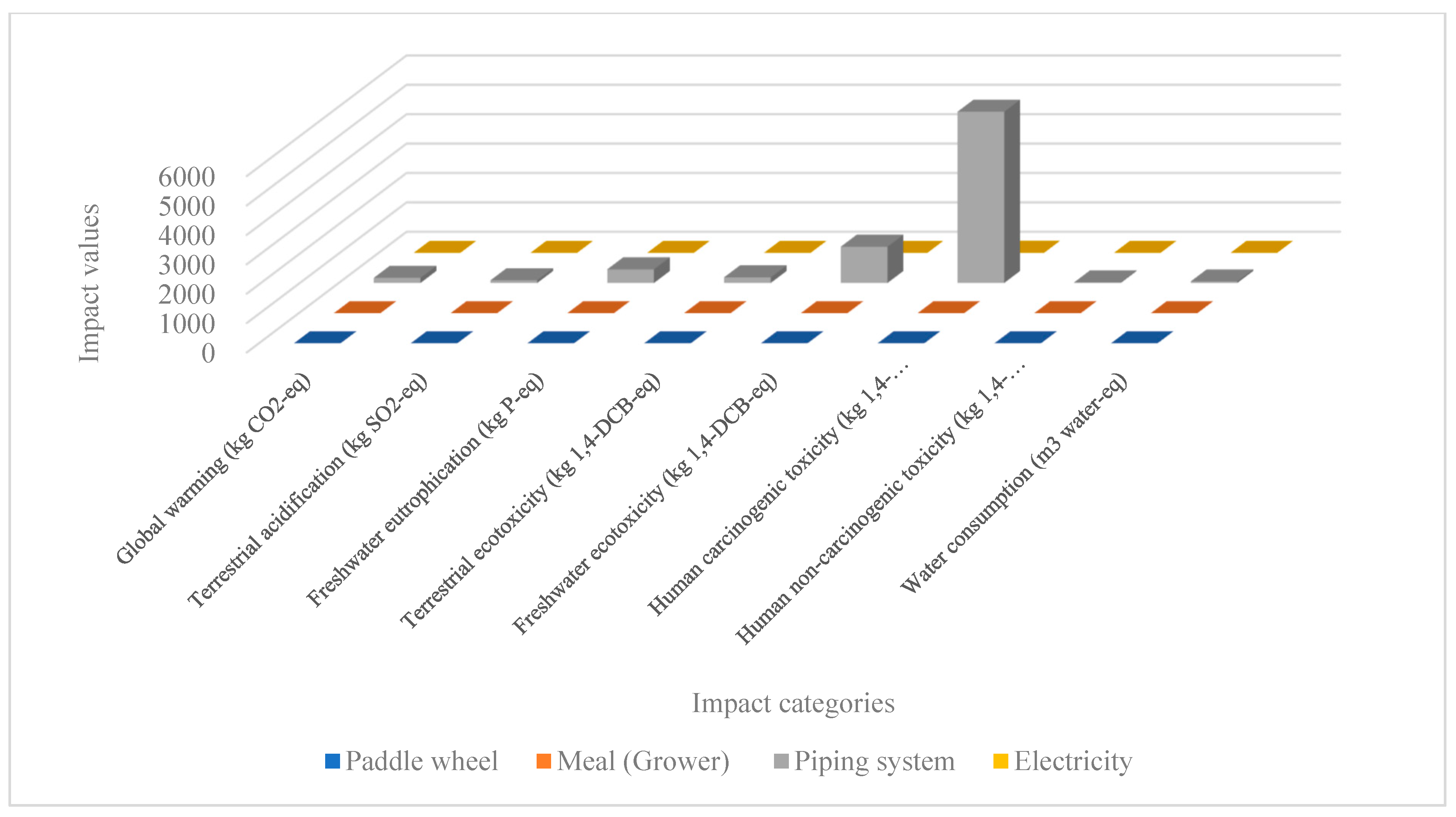
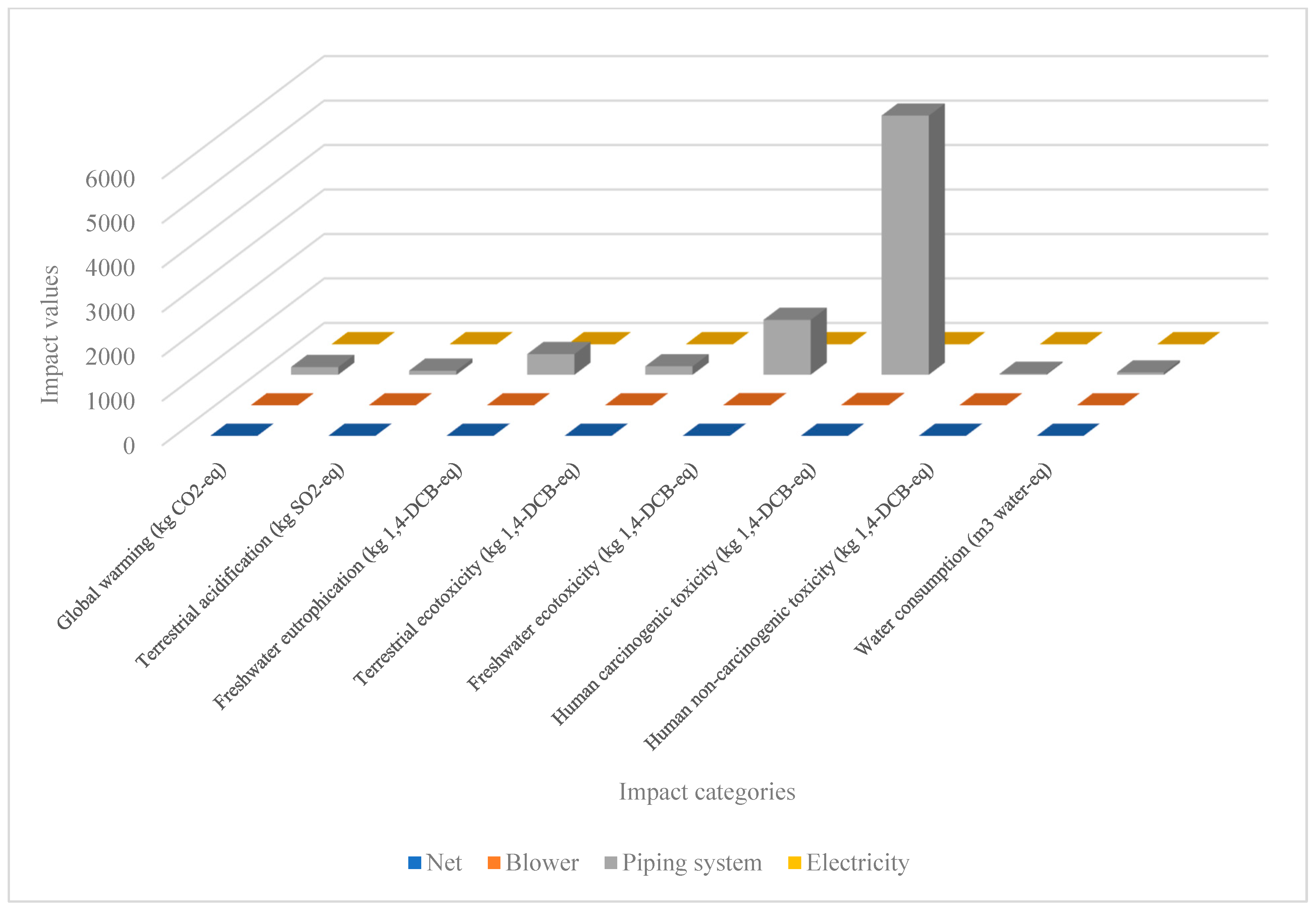
Environmental Impacts
4. Discussion
4.1. Environmental Impact
4.2. Feed Management
4.3. Effluent Management
4.4. Allocations
4.5. Uncertainty Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization: Rome, Italy, 2020; pp. 1–73. [Google Scholar] [CrossRef]
- DOFM. Annual Fisheries Statistics 2020; Department of Fisheries Malaysia: Putrajaya, Malaysia, 2020. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 12 March 2022).
- Jee, K.A. Farming of giant freshwater prawn. In Aquaculture Practices in Malaysia: Malaysian Fish; Society Occasional Publication; Malaysian Fisheries Society: Selangor, Malaysia, 1998. [Google Scholar]
- New, M.B.; Kutty, M.N. Commercial freshwater prawn farming and enhancement around the world. In Freshwater Prawns: Biology and Farming; New, M.B., Valenti, W.C., Tidwell, J.H., D’Abramo, L.R., Kutty, M.N., Eds.; Wiley Online Library: New York, NY, USA, 2010; pp. 346–399. [Google Scholar]
- Ling, S.W. General biology and development of Macrobrachium rosenbergii. FAO Fish Rep. 1969, 57, 589–606. [Google Scholar]
- Ezekiel, B.B.; Firuza, B.M.; Mohammad, L.A.; Subha, B. Analysis of factors for determining suitable site for Giant Freshwater Prawn (Macrobrachium rosenbergii) farming through the local knowledge in Negeri Sembilan of Peninsular Malaysia. Pertanika J. Soc. Sci. Humanit. 2018, 26, 2867–2882. [Google Scholar]
- DOFM. Annual Fisheries Statistics of Malaysia; Department of Fisheries Malaysia: Putrajaya, Malaysia, 2019. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 5 May 2021).
- Hamzah, A.; Kaharudin, M.S.; Balton, M.; Hafizi, W.M.; Nguyen, N.H. Genetic selection for harvest body weight in the Malaysian giant freshwater prawn (Macrobrachium rosenbergii). Int. J. Agric. Technol. 2022, 18, 975–990. [Google Scholar]
- DOFM. Annual Fisheries Statistics, Department of Fisheries Malaysia. 2013. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 5 May 2021).
- DOFM. Annual Fisheries Statistics, Department of Fisheries Malaysia. 2018. Available online: https://www.dof.gov.my/en/resources/fisheries-statistics-i/ (accessed on 5 May 2021).
- Liong, P.C.; Hanafi, H.B.; Merican, Z.O.; Nagaraj, G. Aquaculture development in Malaysia. In Seminar on Aquaculture Development in Southeast Asia; SEAFDEC Aquaculture Department: Iloilo City, Philippines, 1998; pp. 73–90. [Google Scholar]
- Iliyasu, A.; Mohamed, Z.A.; Terano, R. Comparative analysis of technical efficiency for different production culture systems and species of freshwater aquaculture in Peninsular Malaysia. Aquac. Rep. 2016, 3, 51–57. [Google Scholar] [CrossRef]
- Banu, R.; Christianus, A. Giant Freshwater Prawn Macrobrachium rosenbergii farming: A review on its current status and prospective in Malaysia. J. Aquat. Res. Dev. 2016, 7, 423. [Google Scholar] [CrossRef]
- Henares, M.N.P.; Medeiros, M.V.; Camargo, A.F.M. Overview of strategies that contribute to the environmental sustainability of pond aquaculture: Rearing systems, residue treatment, and environmental assessment tools. Rev. Aquacult. 2020, 12, 453–470. [Google Scholar] [CrossRef]
- Wongprawmas, R.; Sogari, G.; Gai, F.; Parisi, G.; Menozzi, D.; Mora, C. How information influences consumers’ perception and purchasing intention for farmed and wild fish. Aquaculture 2022, 547, 737504. [Google Scholar] [CrossRef]
- Santos, A.A.O.; Aubin, J.; Corson, M.S.; Valenti, W.C.; Camargo, A.F.M. Comparing environmental impacts of native and introduced freshwater prawn farming in Brazil and the influence of better effluent management using LCA. Aquaculture 2015, 444, 151–159. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Gentry, R.R.; Halpern, B.S. Global change in marine aquaculture production potential under climate change. Nat. Ecol. Evol. 2018, 2, 1745–1750. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding farmed salmon: Is organic better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- Diana, J.S. Aquaculture production and biodiversity conservation. BioScience 2009, 59, 27–38. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Guinée, J.B.; Kleijn, R.; De Snoo, G.R. Life cycle assessment of aquaculture systems—A review of methodologies. Int. J. Life Cycle Assess. 2012, 17, 304–313. [Google Scholar] [CrossRef]
- Jonell, M.; Henriksson, P.J.G. Mangrove-shrimp farms in Vietnam—Comparing organic systems and conventional systems using life cycle assessment. Aquaculture 2015, 447, 66–75. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Rico, A.; Zhang, W.; Ahmad-Al-Nahid, S.K.; Newton, R.; Phan, L.T.; Zhang, Z.; Jaithiang, J.; Dao, H.M.; Phu, T.M.; et al. Comparison of Asian aquaculture products by use of statistically supported life cycle assessment. Environ. Sci. Technol. 2015, 49, 14176–14183. [Google Scholar] [CrossRef]
- Ottinger, M.; Clauss, K.; Kuenzer, C. Aquaculture: Relevance, distribution, impacts and spatial assessments—A review. Ocean Coast. Manag. 2016, 119, 244–266. [Google Scholar] [CrossRef]
- Curran, M.A. Life Cycle Assessment Handbook: A Guide for Environmentally Sustainable Products; John Wiley & Sons: Chicago, IL, USA, 2012. [Google Scholar]
- EU. Building the Single Market for Green Products Facilitating Better Information on the Environmental Performance of Products and Organisations; European Commission: Brussels, Belgium, 2013; pp. 1–13.
- ISO 14040:2006; Environmental Management—Life Cycle Assessment. International Oganization for Standardization: Geneva, Switzerland, 2006. Available online: https://www.iso.org/obp/ui#iso:std:iso:14040:ed-2:v1:en (accessed on 7 May 2021).
- European Commision. International Reference Life Cycle Data System (ILCD) Handbook: General Guide for Life Cycle Assessment—Detailed Guidance, 1st ed.; Publications Office of the European Union: Luxembourg, 2010; pp. 1–30.
- Bohnes, F.A.; Hauschild, M.Z.; Schlundt, J.; Laurent, A. Life cycle assessments of aquaculture systems: A critical review of reported findings with recommendations for policy and system development. Rev. Aquac. 2018, 11, 1061–1079. [Google Scholar] [CrossRef]
- Siti-Dina, R.P.; Ahmad-Fariz, M.; Abdullah, S. Life cycle assessment of Cockles (Anadara granosa) farming: A case study in Malaysia. EnvironmentAsia 2016, 9, 80–90. [Google Scholar] [CrossRef]
- Dullah, H.; Malek, M.A.; Hanafiah, M.M. Life cycle assessment of Nile Tilapia (Oreochromis niloticus) farming in Kenyir Lake, Terengganu. Sustainability 2020, 12, 2268. [Google Scholar] [CrossRef]
- Na-Nakorn, U.; Jintasataporn, O. Current status & prospects of farming the giant river prawn (Macrobrachium rosenbergii de Man 1879) in Thailand. Aquac. Res. 2020, 43, 1015–1022. [Google Scholar] [CrossRef]
- DOFM. Cadangan Perniagaan Projek Ternakan Udang Galah; Department of Fisheries Malaysia: Putrajaya, Malaysia, 2004; pp. 9–16.
- Zainoddin, J. Manual Kerja Penternakan Udang Galah (Macrobrachium rosenbergii) Dalam Kolam; Department of Fisheries Malaysia: Putrajaya, Malaysia, 2013; pp. 14–15.
- Suhairi, A.; Misri, S. The culture of giant Malaysian freshwater prawn Macrobrachium rosenbergii in the pond. Proceeding Natl. IRPA Semin. (Agr. Sect.) 1992, 2, 187–188. [Google Scholar]
- Baumann, H.; Tillman, A.M. The Hitch Hiker’s Guide to L.C.A: An Orientation in Life Cycle Assessment Methodology and Application; Studentlitteratur: Lund, Sweden, 2004. [Google Scholar]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method. Int. J. Life Cycle Assess. 2016, 22, 138–147. [Google Scholar] [CrossRef]
- Medeiros, M.V.; Aubin, J.; Camargo, A.F.M. Life cycle assessment of fish and prawn production: Comparison of monoculture and polyculture freshwater systems in Brazil. J. Clean. Prod. 2017, 156, 528–537. [Google Scholar] [CrossRef]
- Aubin, J.; Baruthio, A.; Mungkung, R.; Lazard, J. Environmental performance of brackish water polyculture system from a life cycle perspective: A Filipino case study. Aquaculture 2015, 435, 217–227. [Google Scholar] [CrossRef]
- Bosma, R.; Anh, P.T.; Potting, J. Life cycle assessment of intensive striped catfish farming in the Mekong Delta for screening hotspots as input to environmental policy and research agenda. Int. J. Life Cycle Assess. 2011, 16, 903–915. [Google Scholar] [CrossRef]
- Huysveld, S.; Schaubroeck, T.; De Meester, S.P.; Van Langenhove, H.; Van Linden, V.; Dewulf, J. Resource use analysis of Pangasius aquaculture in the Mekong delta in Vietnam using exergetic life cycle assessment. J. Clean. Prod. 2013, 51, 225–233. [Google Scholar] [CrossRef]
- Dekamin, M.; Veisi, H.; Safari, E.; Liaghati, H.; Khoshbakht, K.; Dekamin, M.G. (Life cycle assessment for rainbow trout (Oncorhynchus mykiss) production system: A case study for Iran. J. Clean. Prod. 2015, 91, 43–55. [Google Scholar] [CrossRef]
- Ghamkar, R.; Boxman, S.E.; Main, K.L.; Zhang, Q.; Trotz, M.A.; Hicks, A. Life cycle assessment of aquaculture systems: Does burden-shifting occur with an increase in production intensity? Aquac. Eng. 2021, 92, 102130. [Google Scholar] [CrossRef]
- Lyu, W.; Chen, Q.; Zhou, W. Microplastics in terrestrial environments—Emerging contaminants and major challenges. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Sharma, M.D.; Elangickal, A.I.; Mankar, J.S.; Krupadam, R.J. Assessment of cancer risk of microplastics enriched with polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2020, 398, 122994. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flore, I.; The, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vethaak, A.D.; Lavorante, B.R.; Lundebye, A.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Y.; Leung, J.Y.S.; Huang, W.; Zeng, J.; Tang, Y.; Chen, J.; Shi, A.; Yu, X.; Xu, X.; et al. Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Sci. Total Environ. 2019, 708, 135432. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Diana Ita-Nagy, D.; Kahhat, R. Microplastics in fisheries and aquaculture: Implications to food sustainability and safety. Curr. Opin. Green Sustain. Chem. 2021, 29, 100464. [Google Scholar] [CrossRef]
- Wu, C.; Xiong, X.; Amir, H.H.; Zhang, Y.; Xiangrong, X. A review on source, occurrence, and impacts of microplastics in freshwater aquaculture systems in China. Water Biol. Secur. 2022, 1, 100040. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, S.; Feng, K.; Wang, Q. Occurrence of microplastics in a pond-river-lake connection water system: How does the aquaculture process affect microplastics in natural water bodies. J. Clean. Prod. 2022, 352, 131632. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P.; Sonesson, U.; Scholz, A.; Ziegler, F.; Flysjo, A.; Kruse, S.; Cancino, B.; Silverman, H. Not all salmon are created equal: Life cycle assessment (LCA) of global salmon farming systems. Environ. Sci. Technol. 2009, 43, 8730–8736. [Google Scholar] [CrossRef]
- Jerbi, M.A.; Aubin, J.; Garnaoui, K.; Achour, L.; Kacem, A. Life cycle assessment (LCA) of two rearing techniques of seabass (Dicentrarchus labrax). Aquac. Eng. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Efole, E.T.; Aubin, J.; Mikolasek, O.; Corson, M.S.; Eyango, M.T.; Tchomboue, J.; van der Werf, H.M. Environmental impacts of farm integrating aquaculture and agriculture in Cameroon. J. Clean. Prod. 2012, 28, 208–214. [Google Scholar] [CrossRef]
| Parameter | Range |
|---|---|
| Temperature | 28 °C to 32 °C |
| pH | 6.5–8.5 |
| Dissolved oxygen | >4.0 mg/L |
| Alkalinity | 40–100 ppm CaCO3 |
| Hardness | >50–100 ppm |
| Secchi disc | 30–40 cm |
| Ammonia | <1.0 ppm |
| Nitrate | 1–3 ppm |
| Copper | <0.1 ppm |
| Iron | <0.3 ppm |
| Input/Output | Unit | Amount | Inventory (Ecoinvent 3 Database, SimaPro Version 9.3) | Values (Based on 1-Tonne Live Weight of M. rosenbergii |
|---|---|---|---|---|
| Inputs from technosphere: | ||||
| ha | 1.5 | ||
| ha | 0.1–0.3 | ||
| feet | 4 | ||
| pcs | 300,000 | ||
| km | 389 | ||
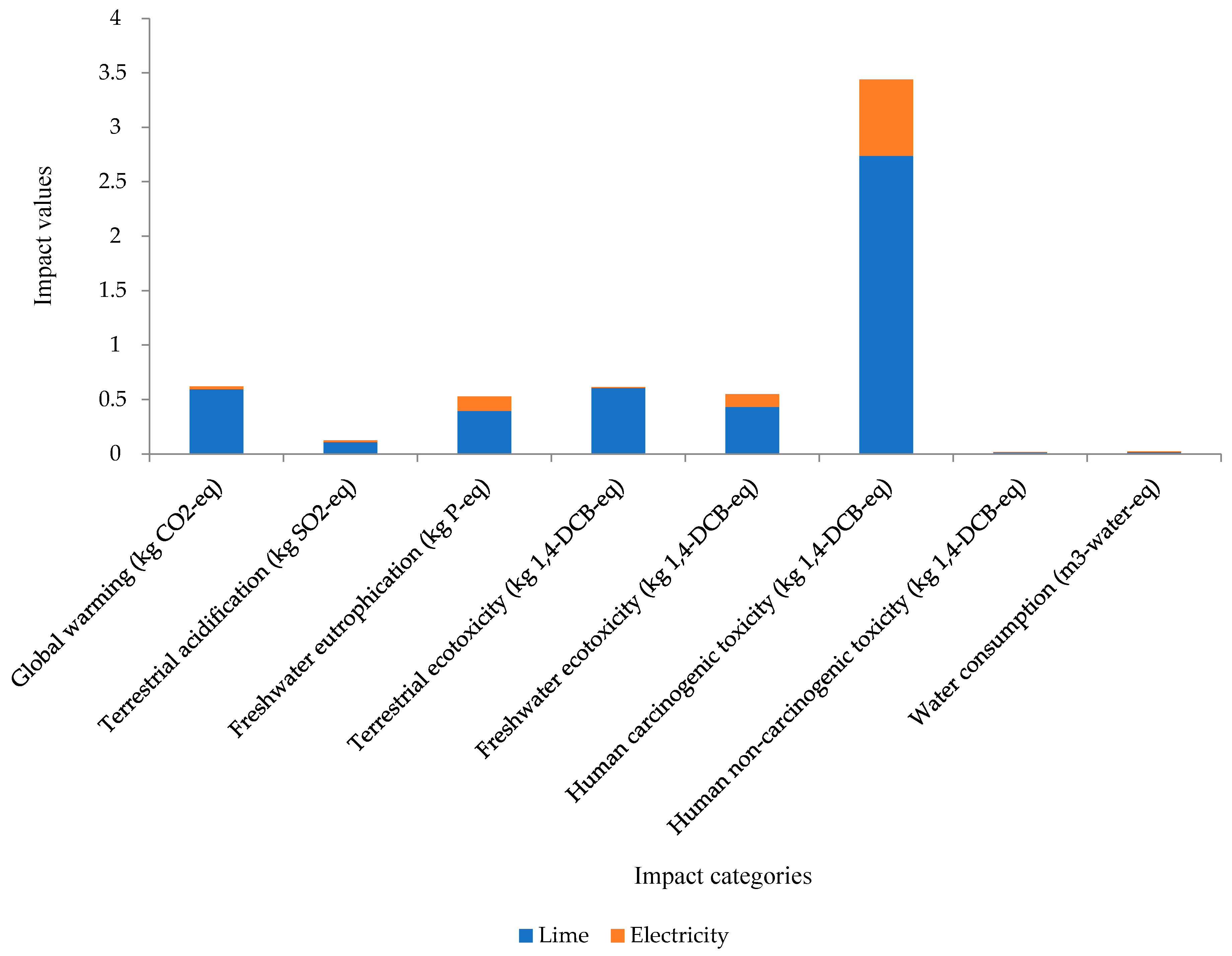
| 25 kg/bag | 15 bags | Lime, hydrated, lose weight {RoW}|market for lime, hydrated, lose weight|APOS, S | 71.9 kg |
| ||||
| 25 kg/bag | 10 bags | Fishmeal, 63–65% protein {GLO}|market for fishmeal, 63–65% protein|APOS, U | 1.102 kg |
| Soybean meal {RoW}|market for soybean meal|APOS, U | 1.653 kg | |||
| Wheat grain, feed {GLO}|market for|APOS, U | 0.480 kg | |||
| 25 kg/bag | 8 bags | Fishmeal, 63–65% protein {GLO}|market for fishmeal, 63–65% protein|APOS, U | 1.026 kg |
| Soybean meal {RoW}|market for soybean meal|APOS, U | 1.438 kg | |||
| Wheat grain, feed {GLO}|market for|APOS, U | 0.240 kg | |||
| 40 kg/bag | 2 bags (final month before harvest) | Sweet corn {RoW}|sweet corn production|APOS, U | 7.670 kg |
| ||||
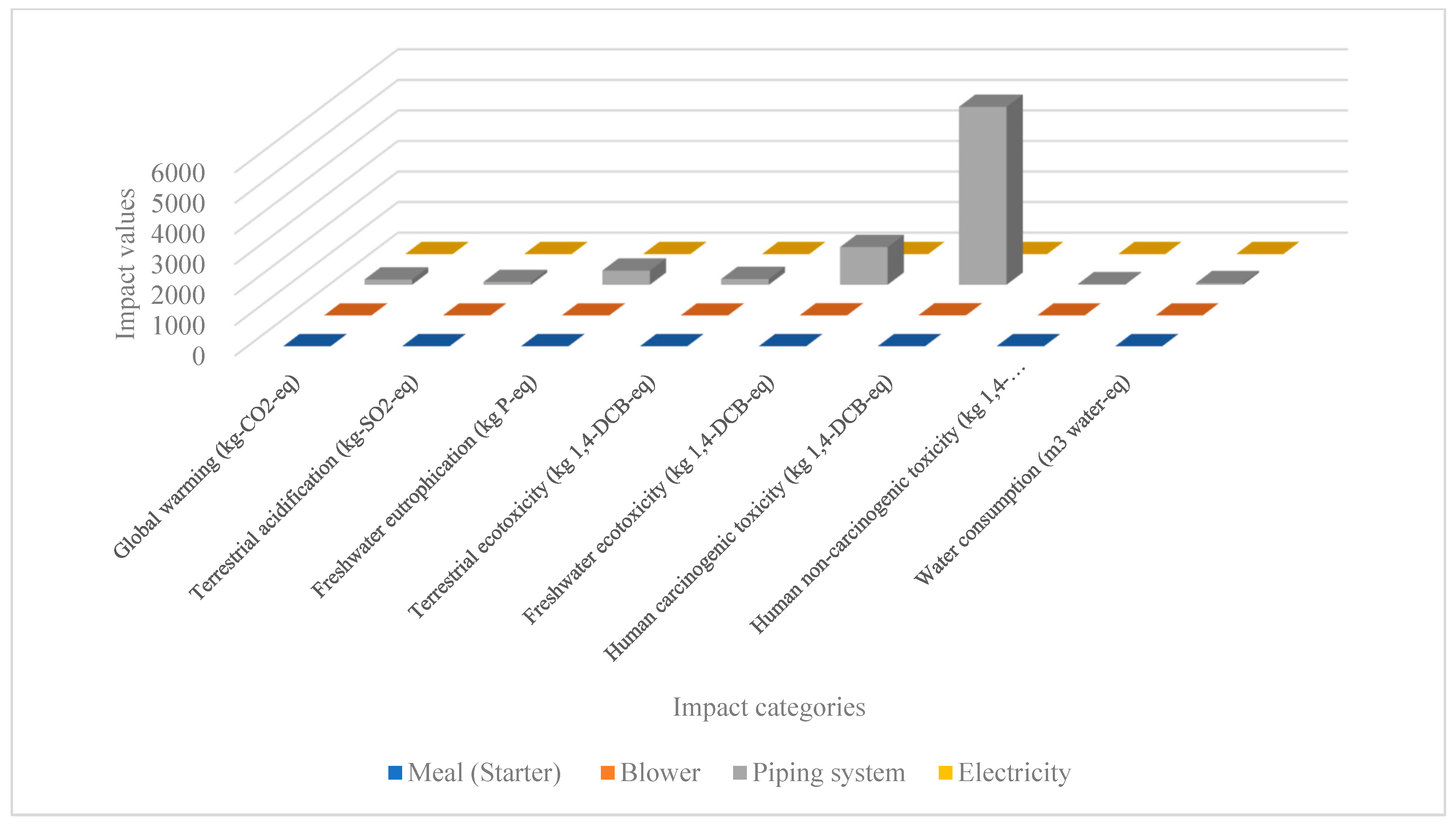
| kg | 17 kg/unit (9 units) | Iron-nickel-chromium alloy {GLO}|market for|APOS, U | 3.451 kg |
| kg | 1.5 kg/unit (9 units) | Polyethylene, high density, granulate {GLO}|market for|APOS, U | 0.862 kg |
| Steel, chromium steel 18/8 {G.L.O.}|market for|APOS, U | 1.055 kg | |||
| kg | 15 kg/unit (4 units) | Nylon 6-6 {RoW}|market for nylon 6-6|APOS, U | 2.876 kg |
| kg | 8001 kg/set (usage of 500 m from the river (water source/supply) to the farm area) | Polyethylene, high density, granulate {GLO}|market for|APOS, U | 767.04 kg |
| kWH | 10,752 kWH/year | Electricity, high voltage {MY}|market for|APOS, S | 227 kWh: pond preparation and harvesting 515 kWh: stocking and farming |
| Output to technosphere: | ||||
| Giant freshwater prawn | kg | 1000 | ||
| Inputs from nature: | ||||
| Hill/stream water (outflow to river without any treatment) | m3 | Estimated: 2.830 × 1010 | ||
| Output to nature: | ||||
| Untreated emissions to water | Nitrate Phosphate pH Temperature DO | 0.015 ppm 0.21 ppm 7.92 26.98 °C 5.2 |
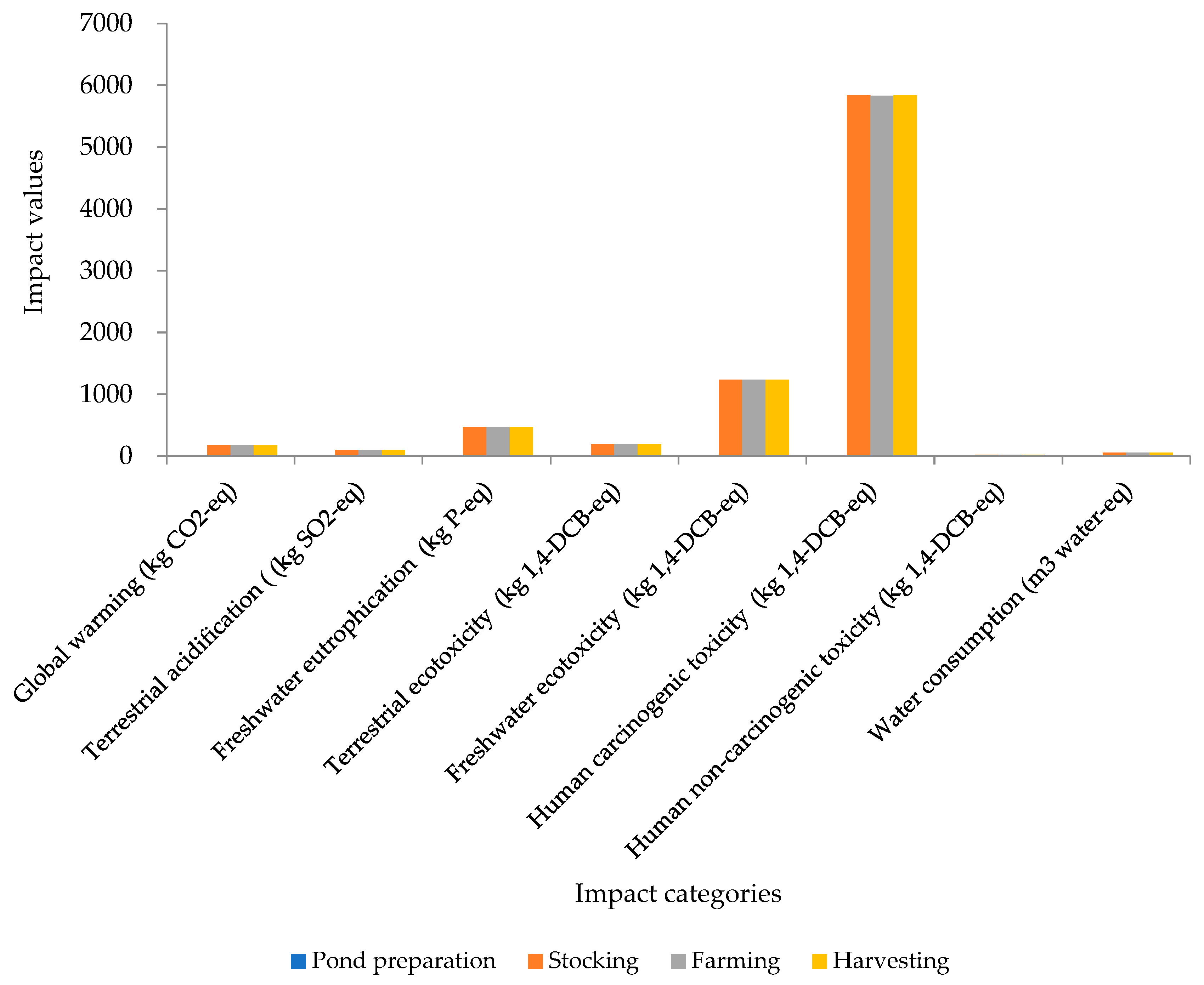
| Environmental Impact (Method: ReCiPe 2016/Midpoint) | Pond Preparation | Stocking | Farming | Harvesting |
|---|---|---|---|---|
| Global warming (kg CO2-eq to air) | 0.619 (0.12%) | 175.427 (33.93%) | 175.446 (33.30%) | 175.432 (33.29%) |
| Terrestrial acidification (kg SO2-eq to air) | 0.125 (0.04%) | 92.986 (33.33%) | 92.860 (33.29%) | 92.988 (33.33%) |
| Freshwater eutrophication (kg P-eq to freshwater) | 0.573 (0.04%) | 465.93 (33.32%) | 465.974 (33.23%) | 465.922 (32.32%) |
| Terrestrial ecotoxicity (kg 1,4-DCB-eq to industrial soil) | 0.614 (0.11%) | 192.226 (33.33%) | 191.626 (33.23%) | 192.222 (33.33%) |
| Freshwater ecotoxicity (kg 1,4-DCB-eq to freshwater) | 0.547 (0.01%) | 1236.683 (33.34%) | 1235.309 (33.30%) | 1236.674 (33.34%) |
| Human carcinogenic toxicity (kg 1,4-DCB-eq to urban air) | 3.439 (0.02%) | 5835.63 (33.33%) | 5831.60 (33.31%) | 5835.632 (33.33%) |
| Human non-carcinogenic toxicity (kg 1,4-DCB-eq to urban air) | 0.0179 (0.03%) | 22.33 (33.33%) | 22.314 (33.31%) | 22.331 (33.33%) |
| Water use (m3 water-eq consumed) | 0.0167 (0.01%) | 53.160 (33.33%) | 53.14 (33.32%) | 53.165 (33.33%) |
| Country | System and Species | Source of Data | Software/ Method | Environmental Impacts | Allocation | References |
|---|---|---|---|---|---|---|
| Brazil | Monoculture & Polyculture: Amazon river prawn (Macrobrachium amazonicum) | Primary data (Experimental) Secondary data | SimaPro v8.0.5/CML-IA version 3.02 method | Climate change Eutrophication Cumulative Energy Demand Land Occupation Acidification Net Primary Production Use (NPPU) Water dependence | System expansion Mass allocation Energy allocation Economic allocation | [38] |
| Philippines | Polyculture (Tilapia, Milkfish, Mud crabs, tiger prawn, wild species | Primary data | SimaPro v.7.0 /Ecoinvent v2.2 database. | Eutrophication Acidification Climate change Land occupation Net primary production use (NPPU) Total cumulative energy demand (TECD) Total human labour | Energy-based allocation Economic allocation | [39] |
| Brazil | Comparison of two monoculture systems (M. rosenbergii and M. amazonicum) | Secondary data from previous studies | SimaPro v.7.3/CML 2001 method | Climate change Eutrophication Acidification Energy use Net primary production use (NPPU) Surface use Water dependence | No allocation | [16] |
| Malaysia | Monoculture (M. rosenbergii) | Primary data (at farm level) Secondary data | SimaPro v.9.3 Method: ReCiPe 2016 | Global warming Terrestrial acidification Freshwater eutrophication Terrestrial ecotoxicity Freshwater ecotoxicity Human carcinogenic Human non-carcinogenic Water consumption | No allocation (monoculture system) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haslawati, B.; Saadiah, I.; Siti-Dina, R.P.; Othman, M.; Latif, M.T. Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming through Life Cycle Assessment. Sustainability 2022, 14, 14776. https://doi.org/10.3390/su142214776
Haslawati B, Saadiah I, Siti-Dina RP, Othman M, Latif MT. Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming through Life Cycle Assessment. Sustainability. 2022; 14(22):14776. https://doi.org/10.3390/su142214776
Chicago/Turabian StyleHaslawati, Baharuddin, Ibrahim Saadiah, Razman Pahri Siti-Dina, Murnira Othman, and Mohd Talib Latif. 2022. "Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming through Life Cycle Assessment" Sustainability 14, no. 22: 14776. https://doi.org/10.3390/su142214776
APA StyleHaslawati, B., Saadiah, I., Siti-Dina, R. P., Othman, M., & Latif, M. T. (2022). Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming through Life Cycle Assessment. Sustainability, 14(22), 14776. https://doi.org/10.3390/su142214776







