Effects of Aquatic Plants on Nutrient Concentration in Water and Growth Performance of Fantail Goldfish in an Aquaculture System
Abstract
:1. Introduction
2. Materials and Methods
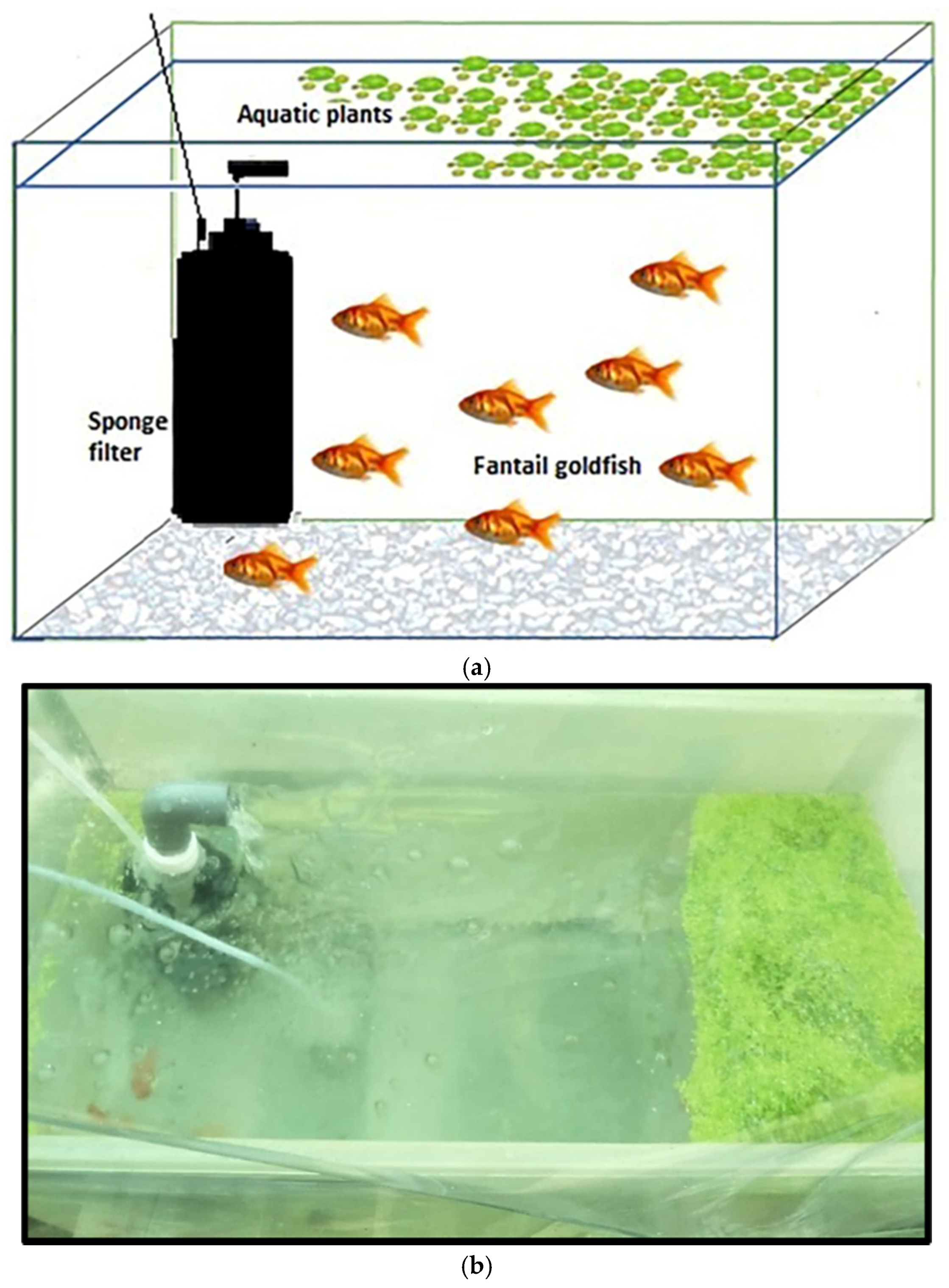
2.1. Experimental Setup
2.2. Measurement of Fantail Goldfish and Aquatic Plants
- Survival Rate (SR%) = (Number of fish at the end of the experiment/Number of fish at the beginning of the experiment) × 100);
- Weight gained (WG%) = [(Final weight, g—Initial weight, g)/Initial weight, g] × 100;
- Specific growth rates (SGR%) = [(Final weight, g—Initial weight, g)/No. of day] × 100;
- Feed Conversion Ratio (FCR%) = [Feed fed (g)/Weight gained (g)] × 100.
2.3. Water Analyses
2.4. Statistical Analysis
3. Results and Discussion
3.1. In Situ Water Quality
3.2. Ex situ Water Quality
3.3. Growth Performance of Fantail Goldfish
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, C. The ornamental fish trade in Malaysia. UTAR Agric. Sci. J. 2016, 2, 4. [Google Scholar]
- Safer, M. Aquatic Invaders of the Pacific Northwest: Carassius auratus (Common Goldfish); Washington Department of Fish and Wildlife Fish: Olympia, WA, USA, 2014; p. 423. [Google Scholar]
- Lim, L.C.; Wong, C.C. Use of the rotifer, Brachionus calyciflorus Pallas, in freshwater ornamental fish larviculture. Hydrobiologia 1997, 358, 269–273. [Google Scholar] [CrossRef]
- DOF (Department of Fisheries Malaysia). List of Registered Ornamental Fish Exporters. 2018. Available online: http://www.dof.gov.my/dof2/resources/user_1/UploadFile/Biosekuriti%20.Revision (accessed on 12 June 2021).
- Lorenzoni, M.; Corboli, M.; Ghetti, L.; Giovanni, P.; Carosi, A. Growth and reproduction of the goldfish Carassius auratus: A case study from Italy. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Invading Nature—Springer Series Invasion Ecology; Gherardi, F., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 2. [Google Scholar] [CrossRef]
- Ota, K.G.; Abe, G. Gold fish morphology as a model for evolutionary developmental biology. WIREs Dev. Biol. 2016, 5, 272–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, C. Guide to Fancy Goldfish, Fishkeeper’s Guides; Interpet Publishing: Dorking, UK, 2002. [Google Scholar]
- Estim, A.; Saufie, S.; Mustafa, S. Water quality remediation using aquaponics sub-systems as biological and mechanical filters in aquaculture. J. Water Process Eng. 2019, 30, 100566. [Google Scholar] [CrossRef]
- Datta, S. Aquarium Water Quality Management; FISHCOOPS Edition: XXVI (III); National Federation of Fishers Cooperative Ltd.: New Delhi, India, 2012; pp. 9–16. [Google Scholar]
- Estim, A. Water Quality Management in a Marine Fish Hatchery System. Ph.D. Dissertation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia, 2010. [Google Scholar]
- Kabir, A.N.; Hossain, M.A.; Rahman, M.S. Use of Duckweed as Feed for Fishes in Polyculture. J. Agric. Rural Dev. 2009, 7, 157–160. [Google Scholar] [CrossRef]
- Ford, T.; Beitinger, T.L. Temperature tolerance in the goldfish, Carassius auratus. J. Ther. Biol. 2005, 30, 147–152. [Google Scholar] [CrossRef]
- Hasan, M.R.; Chakrabarti, R. Use of Algae and Aquatic Macrophytes as Feed in Small-Scale Aquaculture; FAO Fisheries and Aquaculture Technical Paper 531; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Landolt, E. Biosystematic Investigations in the Family of Duckweeds (Lemnaceae), Vol. 2. The Family of Lemnaceae—A Monographic Study, Vol. 1; Veroffentlichungen des Geobotanischen Institutes der E. T. H., Stiftung Rubel: Zurich, Switzerland, 1986; p. 638. [Google Scholar]
- Leng, R.A.; Stambolie, J.H.; Bell, R. Duckweed—A Potential High-Protein Feed Resource for Domestic Animals and Fish; Centre for Duckweed Research and Development University of New England: Armidale, NSW, Australia, 1995; p. 7. [Google Scholar]
- Small, E.; Darbyshire, S.J. Blossoming treasures of Biodiversity: 35. Mosquito ferns (Azolla species)—Tiny ‘super plants’. Biodiversity 2011, 12, 119–128. [Google Scholar] [CrossRef]
- Cagauan, A.G.; Pullin, R.S.V. Azolla in aquaculture: Past, present and future. In Recent Advances in Aquaculture; Muir, J., Roberts, R.J., Eds.; Blackwell Science: Oxford, UK, 1994; pp. 104–130. [Google Scholar]
- Ferdoushi, Z.; Haque, F.; Khan, S.; Haque, M. The effects of two aquatic floating macrophytes (Lemna and Azolla) as biofilters of Nitrogen and Phosphate in fish ponds. Turkish J. Fish. Aquat. Sci. 2008, 8, 253–258. [Google Scholar]
- Das, M.; Rahim, F.I.; Hossain, M.A. Evaluation of fresh Azolla pinata as a low-cost supplemental feed for Thai silver barb Barbonymus gonionotus. Fishes 2018, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Min, C.; Xia-shi, L.; Chungchu, L. Research on some functions of Azolla in CELSS system. Acta Astronaut. 2008, 63, 1061–1066. [Google Scholar] [CrossRef]
- Hafeez-ur-Rehman, M.; Iqbal, K.J.; Abbas, F.; Mushtaq, F.M.; Rasool, F.; Parveen, S. Influence of feeding frequency on growth performance and body indices of goldfish (Carassius auratus). J. Aquat. Res. Dev. 2015, 6, 1000336. [Google Scholar] [CrossRef] [Green Version]
- Appenroth, K.-J.; Sree, K.S.; Bohm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 2017, 217, 266–273. [Google Scholar]
- Wetzel, R.G.; Likens, G.E. Limnological Analyses, 2nd ed.; Springer: New York, NY, USA, 1991. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Kluwer Academic Publishers: Boston, MA, USA, 1998. [Google Scholar]
- Colt, J.E.; Tamasso, J.R. Hatchery water supply and treatment. In Fish Hatchery Management, 2nd ed.; American Fisheries Society: Bethesda, MD, USA, 2012. [Google Scholar]
- Jones, D.R. The effect of hypoxia and anaemia on the swimming performance of rainbow trout (Salmo gairdneri). J. Exp. Biol. 1971, 55, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Nico, L.G.; Schofield, P.J.; Larson, J.; Makled, T.H.; Fusaro, A. Carassius auratus. USGS Nonindigenous Aquatic Species Database, Gainesville: 2014. Available online: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=508 (accessed on 30 June 2021).
- Latha, Y.P.; Lipton, A.P. Water quality management in gold fish (Carassius auratus) rearing tanks using different filter materials. Ind. Hydrobiol. 2007, 10, 301302. [Google Scholar]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; The World Bank: Washington, DC, USA, 1993; p. 76. [Google Scholar]
- Hove, C.V. Azolla and Its Multipurpose Uses with Emphasis on Africa; FAO: Rome, Italy, 1989; p. 53. [Google Scholar]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Summerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Cayuga Aqua Ventures: New York, NY, USA, 2002. [Google Scholar]
- Sengupta, S.; Medda, C.; Dewanji, A. The impact of duckweed growth on water quality in sub-tropical ponds. Environmentalist 2010, 30, 353–360. [Google Scholar] [CrossRef]
- Lake, R.G.; Hinch, S.G. Acute effects of suspended sediment angularity on juvenile coho salmon (Oncorhynchus kisutch). Can. J. Fish. Aquat. Sci. 1999, 56, 862–867. [Google Scholar] [CrossRef]
- Wilber, D.H.; Clarke, D.G. Biological effects of suspended sediments: A review of suspended sediment impacts on fish and shellfish with relation to dredging activities in estuaries. N. Am. J. Fish. Manag. 2001, 21, 855–858. [Google Scholar] [CrossRef]
- Bilotta, G.S.; Brazier, R.E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Res. 2008, 42, 2849–2861. [Google Scholar] [CrossRef]
- Redding, J.M.; Schreck, C.B.; Everest, F.H. Physiological effects on Coho Salmon and steelhead of exposure to suspended solids. Tran. Amer. Fish. Soc. 1987, 116, 737–744. [Google Scholar] [CrossRef]
- Welch, E.B.; Lindell, T. Ecological effects of wastewater. In Applied Limnology and Pollution Effects; E & FN Spon: New York, NY, USA, 1992; p. 425. [Google Scholar]
- Meade, J.W. Allowable ammonia for fish culture. Prog. Fish.-Cult. 1985, 47, 135–145. [Google Scholar] [CrossRef]
- Masser, M.P.; James Rakocy, J.; Thomas, M.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: An Overview of Critical Considerations; SRAC Publication No. 452; SRAC Publication: Stoneville, MS, USA, 1999. [Google Scholar]
- Davidson, J.; Good, C.; Welsh, C.; Summerfelt, T. Comparing the effects of high vs. low nitrate on the health, performance, and welfare of juvenile rainbow trout Oncorhynchus mykiss within water recirculating aquaculture systems. Aquac. Eng. 2014, 59, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Buzby, K.; Lin, L.S. Scaling aquaponic systems: Balancing plant uptake with fish output. Aquac. Eng. 2014, 63, 39–44. [Google Scholar] [CrossRef]
- Yildiz, H.Y.; Robaina, L.; Pirhonen, J.; Mente, E.; Dominguez, D.; Parisi, G. Fish welfare in aquaponic system: Its relation to water quality with an emphasis on feed and feces-a review. Water 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Kroupova, H.; Máchová, J.; Svobodová, Z.; Piačková, V.; Smutná, M. The ability of recovery in common carp after nitrite poisoning. Vet. Med.-Czech. 2006, 51, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Eddy, F.B.; Kunzlik, P.A.; Bath, R.N. Uptake and loss of nitrite from the blood of rainbow trout, Salmo gairdneri Richardson, and Atlantic salmon, Salmo salar L., in fresh water and in dilute seawater. J. Fish. Biol 1983, 23, 105–116. [Google Scholar] [CrossRef]
- Cameron, J.N. Methaemoglobin in erythrocytes of rainbow trout. Comp. Biochem. Physiol. 1971, 40, 743–749. [Google Scholar] [CrossRef]
- Bowser, P.R.; Falls, W.W.; VanZandt, J.; Collier, N.; Phillips, J.D. Methemoglobinemia in channel catfish: Methods of prevention. Prog. Fish.-Cult. 1983, 45, 154–158. [Google Scholar] [CrossRef]
- Landolt, E.; Kandeler, R. The Family of Lemnaceaea Monographic Study; Stiftung Ruebel: Zuerich, Switzerland, 1987; pp. 638–650. [Google Scholar]
- Goopy, J.; Murray, P. A review of the role of duckweed in nutrient reclamation and as a source of animal feed. Asian-Austral. J. Anim. Sci. 2003, 16, 297–305. [Google Scholar] [CrossRef]
- Galkina, N.V.; Abdullaev, D.A.; Zacharova, V.L. Biological and feed features of duckweeds. Uzbekistan Biol. J. 1965, 3, 44–47. [Google Scholar]
- Gaigher, I.G.; Short, R. An evaluation of duckweed (Lemnaceae) as a candidate for aquaculture in South Africa. In Aquaculture 1980 Report Series No. 15; Wamsley, R.D., Wan, J.G., Eds.; CSIRO: Canberra, Australia, 1986; Volume 15, pp. 81–90. [Google Scholar]
- Fasakin, E.A.; Balogum, A.M.; Fasuru, B.E. Use of duckweed, Spirodela polyrrhiza L. Schleiden, as a protein feedstuff in practical diets for tilapia, Oreochromis niloticus L. Aquac. Res. 1999, 30, 313–318. [Google Scholar] [CrossRef]
- Robinette, H.R.; Brunson, M.W.; Day, E.J. Use of duckweed in diets of channel catfish. Proc. Annu. Conf.-Southeast. Assoc. Fish Wildl. Agencies 1980, 34, 108–114. [Google Scholar]
- Al-Khalaifah, H.S.; Amer, S.A.; Al-Sadek, D.M.M.; Khalil, A.A.; Zaki, E.M.; El-Araby, D.A. Optimizing the Growth, Health, Reproductive Performance, and Gonadal Histology of Broodstock Fantail Goldfish (Carassius auratus) by Dietary Cacao Bean Meal. Animals 2020, 10, 1808. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Ahmed, S.A.; Ibrahim, R.E.; Al-Gabri, N.A.; Osman, A.; Sitohy, M. Impact of partial substitution of fish meal by methylated soy protein isolates on the nutritional, immunological, and health aspects of Nile tilapia, Oreochromis niloticus fingerlings. Aquaculture 2020, 518, 734871. [Google Scholar] [CrossRef]
- Kapinga, I.B.; Limbu, S.M.; Madalla, N.A.; Kimaro, W.H.; Mabiki, F.P.; Lamtane, H.A.; Tamatamah, R.A. Dietary Aspilia mossambicensis and Azadirachta indica supplementation alter gonadal characteristics and histology of juvenile Nile tilapia (Oreochromis niloticus). Aquac. Res. 2019, 50, 573–580. [Google Scholar] [CrossRef]
- Kareem, Z.H.; Abdelhadi, Y.M.; Christianus, A.; Karim, M.; Romano, N. Effects of some dietary crude plant extracts on the growth and gonadal maturity of Nile tilapia (Oreochromis niloticus) and their resistance to Streptococcus agalactiae infection. Fish. Physiol. Biochem. 2016, 42, 757–769. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Treatment | |||
|---|---|---|---|---|
| tREA | n | Azolla | Duckweed | Control |
| DO (mg/L) | 24 | 6.95 ± 1.00 | 7.39 ± 2.03 | 7.20 ±1.28 |
| pH | 24 | 7.80 ± 0.41 | 7.81 ± 0.39 | 7.89 ± 0.46 |
| Temperature (°C) | 24 | 32.03 ± 1.68 | 32.21 ± 1.63 | 32.42 ± 1.71 |
| TSS (mg/L) | 24 | 23.022 ± 8.900 a | 26.268 ± 13.414 a | 41.026 ± 37.099 b |
| NH3-N (mg/L) | 24 | 0.055 ± 0.037 a | 0.093 ± 0.067 b | 0.098 ± 0.075 b |
| N02-N (mg/L) | 24 | 0.028 ± 0.060 a | 0.009 ± 0.006 a | 0.012 ± 0.009 a |
| NH3-N (mg/L) | 24 | 0.822 ± 0.823 a | 0.924 ± 0.918 a | 1.653 ± 1.648 b |
| PO4-P (mg/L) | 24 | 0.913 ± 0.815 ab | 0.672 ± 0.817 a | 1.401 ± 1.488 b |
| Parameter | Treatment | ||
|---|---|---|---|
| tREA | Azolla | Duckweed | Control |
| Initial weight (g) | 2.39 ± 0.17 a | 2.27 ± 0.27 a | 2.24 ± 0.21 a |
| Final weight (g) | 4.79 ± 0.10 b | 5.18 ± 0.14 c | 3.69 ± 0.23 a |
| Initial length (cm) | 5.05 ± 0.11 a | 5.20 ± 0.08 a | 5.16 ± 0.09 a |
| Final length (cm) | 6.32 ± 0.11 a | 6.57 ± 0.13 b | 6.15 ± 0.10 a |
| Weight gain (%) | 106.07 ± 27.92 a | 130.22 ± 37.58 a | 71.93 ± 31.64 a |
| Length gain (%) | 24.99 ± 2.26 a | 27.26 ± 9.95 a | 19.708 ± 8.18 a |
| SGR (%/day) | 5.10 ± 0.90 ab | 5.93 ± 1.07 b | 3.20 ± 1.23 a |
| Survival (%) | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Feed intake (g) | 111.80 ± 2.44 a | 132.12 ± 6.55 a | 72.18 ± 6.21 a |
| FCR | 1.09 ± 0.233 a | 1.07 ± 0.28 a | 1.17 ± 0.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslan, M.N.A.M.; Estim, A.; Venmathi Maran, B.A.; Mustafa, S. Effects of Aquatic Plants on Nutrient Concentration in Water and Growth Performance of Fantail Goldfish in an Aquaculture System. Sustainability 2021, 13, 11236. https://doi.org/10.3390/su132011236
Roslan MNAM, Estim A, Venmathi Maran BA, Mustafa S. Effects of Aquatic Plants on Nutrient Concentration in Water and Growth Performance of Fantail Goldfish in an Aquaculture System. Sustainability. 2021; 13(20):11236. https://doi.org/10.3390/su132011236
Chicago/Turabian StyleRoslan, Mohd Naqib Azfar Mohd, Abentin Estim, Balu Alagar Venmathi Maran, and Saleem Mustafa. 2021. "Effects of Aquatic Plants on Nutrient Concentration in Water and Growth Performance of Fantail Goldfish in an Aquaculture System" Sustainability 13, no. 20: 11236. https://doi.org/10.3390/su132011236
APA StyleRoslan, M. N. A. M., Estim, A., Venmathi Maran, B. A., & Mustafa, S. (2021). Effects of Aquatic Plants on Nutrient Concentration in Water and Growth Performance of Fantail Goldfish in an Aquaculture System. Sustainability, 13(20), 11236. https://doi.org/10.3390/su132011236







