Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review
Abstract
1. Introduction
2. Materials and Methods
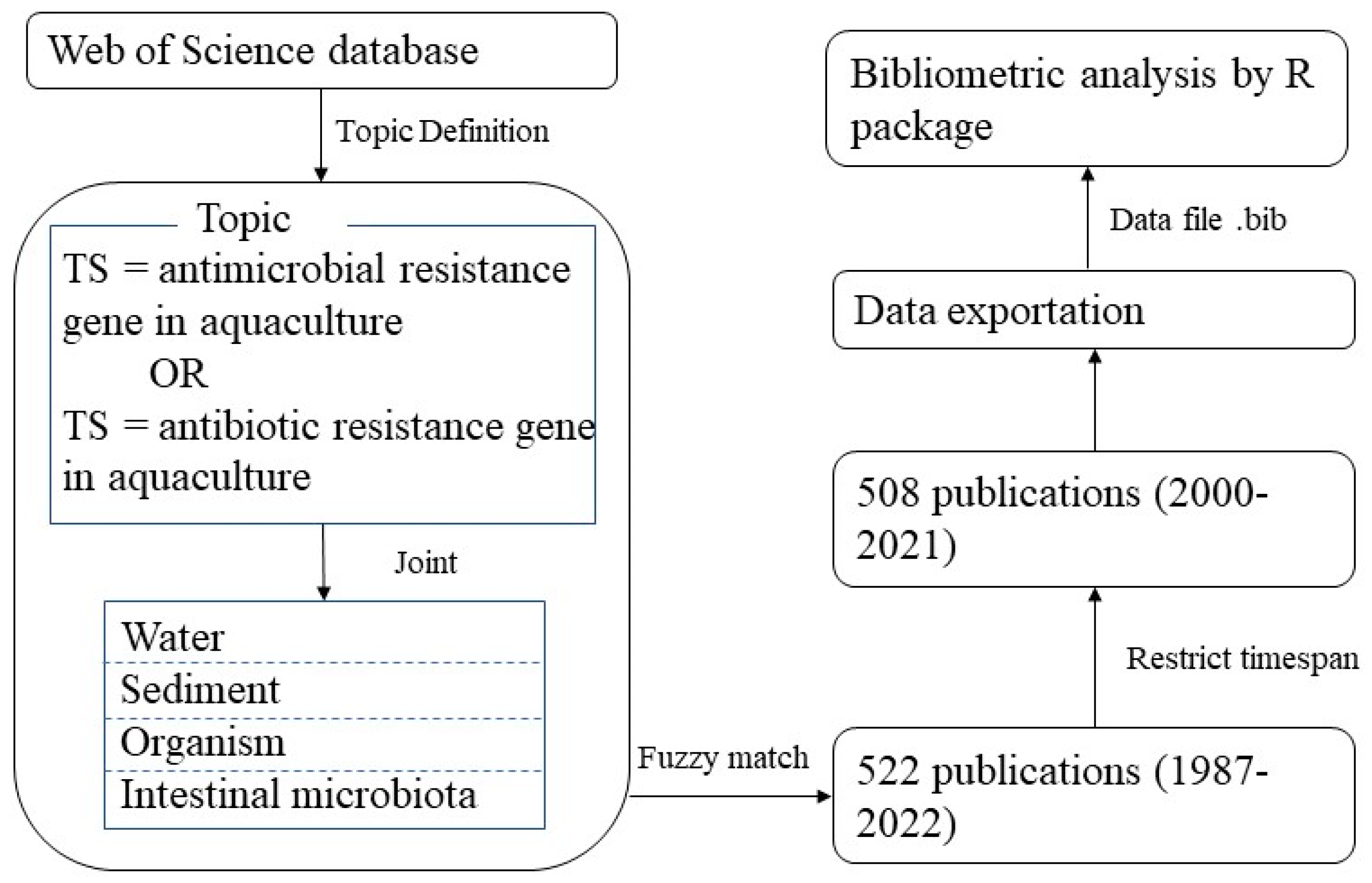
2.1. Data Collection
2.2. Methodology
3. Results and Discussion
3.1. Annual Growth of Publications
3.2. Regional Analysis of Publications
3.3. Analysis of Journals and Highly Cited Publications
3.4. Development of Antimicrobial Resistance in Global Aquaculture
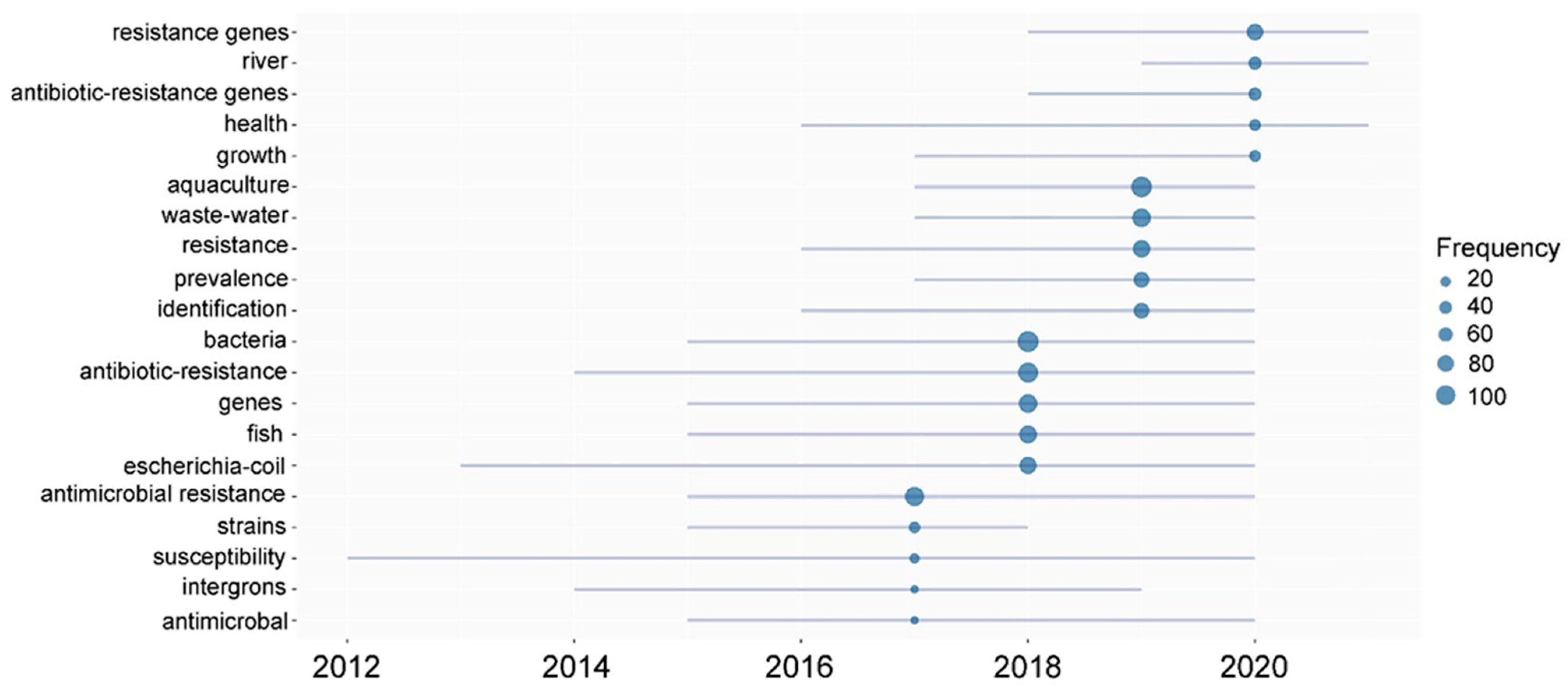
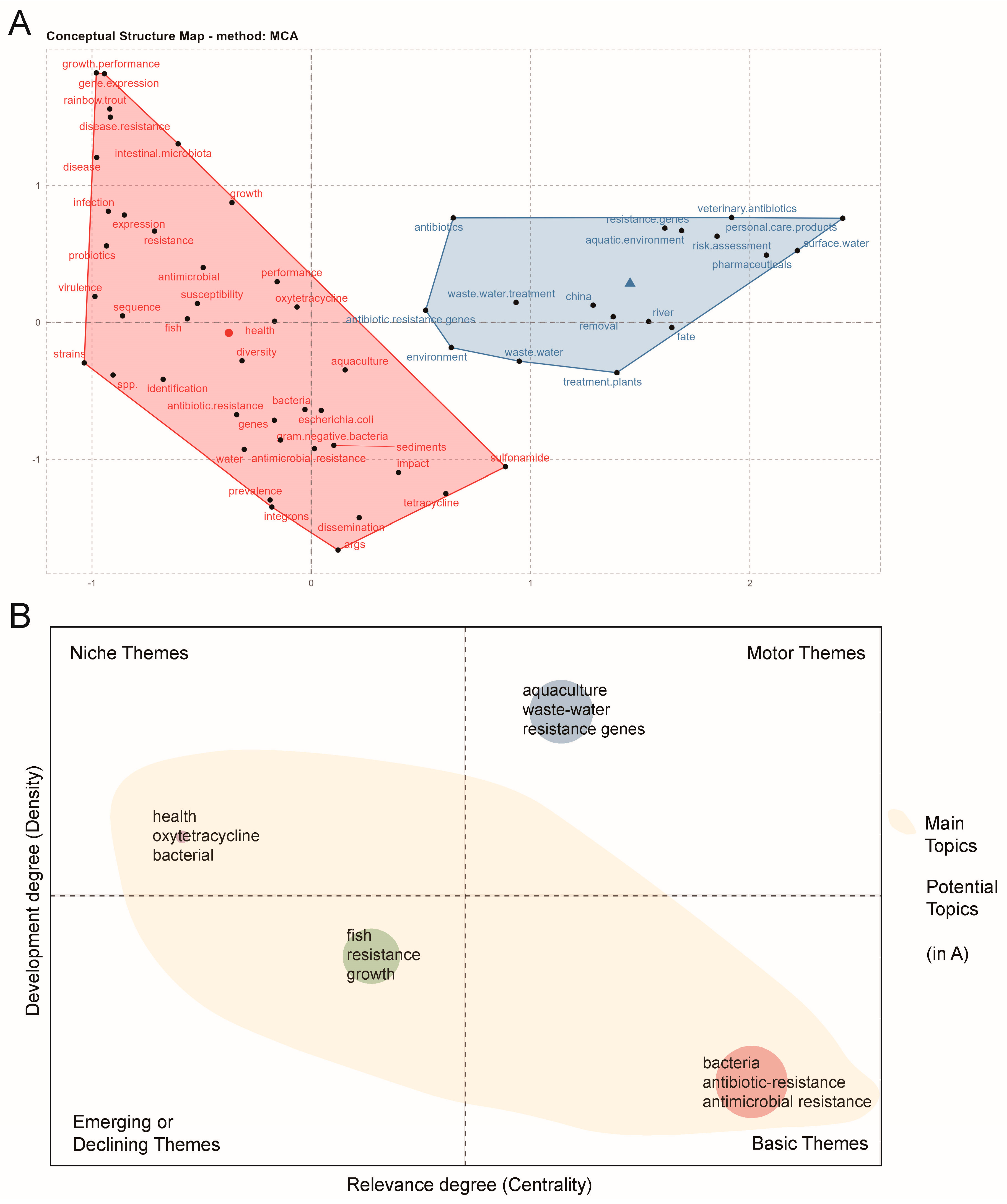
3.5. Current Research Directions of Antimicrobial Resistance in Global Aquaculture
3.6. Futrure Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The state of world fisheries and aquaculture 2020. In Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Shen, X.; Jin, G.; Zhao, Y.; Shao, X. Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci. Total Environ. 2020, 711, 134626. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.M.; Ibrahim, R.; Ahmed, E.-N.G.; El-Bouhy, Z. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Shalaby, A.M.; Khattab, Y.A.; Abdel, R.A.M. Effects of Garlic (Alliumsativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J. Venom Anim. Toxins 2006, 12, 172–201. [Google Scholar] [CrossRef]
- El-Sayed, S.A.A.; Ahmed, S.Y.A.; Abdel-Hamid, N.R. Immunomodulatory and growth performance effects of ginseng extracts as a natural growth promoter in comparison with oxytetracycline in the diets of Nile tilapia (Oreochromis niloticus). Int. J. Livest. Res. 2014, 4, 130–142. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M. Antibiotics use in African aquaculture: Their potential risks on fish and human health. In Current Microbiological Research in Africa; Springer: Berlin/Heidelberg, Germany, 2020; pp. 203–221. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Qiao, F.; Du, Z.Y.; Zhang, M. Influence of long-term feeding antibiotics on the gut health of zebrafish. Zebrafish 2018, 15, 340–348. [Google Scholar] [CrossRef]
- Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquacult. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Huang, L.; Xu, Y.B.; Xu, J.X.; Ling, J.Y.; Chen, J.L.; Zhou, J.L.; Zheng, L.; Du, Q.P. Antibiotic resistance genes (ARGs) in duck and fish production ponds with integrated or non-integrated mode. Chemosphere 2017, 168, 1107–1114. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, Y.B.; Choi, S.; Lee, Y.; Shin, S.G.; Unno, T.; Kim, Y.M. Prevalence of antibiotic resistance genes from effluent of coastal aquaculture, South Korea. Environ. Pollut. 2018, 233, 1049–1057. [Google Scholar] [CrossRef]
- Sun, S.; Korheina, D.K.A.; Fu, H.; Ge, X. Chronic exposure to dietary antibiotics affects intestinal health and antibiotic resistance gene abundance in oriental river prawn (Macrobrachium nipponense), and provokes human health risk. Sci. Total Environ. 2020, 720, 137478. [Google Scholar] [CrossRef]
- Petchiappan, A.; Chatterji, D. Antibiotic resistance: Current perspectives. ACS Omega 2017, 2, 7400–7409. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Nielsen, K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005, 3, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Stokes, H.W.; Gillings, M.R. Gene flow, mobile genetic elements and the recruitment of antibiotic resistance genes into Gram-negative pathogens. FEMS Microbiol. Rev. 2011, 35, 790–819. [Google Scholar] [CrossRef] [PubMed]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-00017. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I.Y.; Wu, N.; Weimer, B.C.; Gao, G.F.; et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol. 2016, 82, 6672–6681. [Google Scholar] [CrossRef]
- Li, B.; Qiu, Y.; Song, Y.; Lin, H.; Yin, H. Dissecting horizontal and vertical gene transfer of antibiotic resistance plasmid in bacterial community using microfluidics. Environ. Int. 2019, 131, 105007. [Google Scholar] [CrossRef]
- McInnes, R.S.; Mccallum, G.E.; Lamberte, L.E.; Van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.X.; Zhao, Z.; Duan, C.; Chen, H.; Wang, M.; Ren, H.; Yin, Y.; Ye, L. Metagenomic analysis revealed the prevalence of antibiotic resistance genes in the gut and living environment of freshwater shrimp. J. Hazard. Mater. 2018, 350, 10–18. [Google Scholar] [CrossRef]
- Li, B.; Yang, Y.; Ma, L.; Ju, F.; Guo, F.; Tiedje, J.M.; Zhang, T. Metagenomic and network analysis reveal wide distribution and co-occurrence of environmental antibiotic resistance genes. ISME. J. 2015, 9, 2490–2502. [Google Scholar] [CrossRef]
- Fu, J.; Yang, D.; Jin, M.; Liu, W.; Zhao, X.; Li, C.; Zhao, T.; Wang, J.; Gao, Z.; Shen, Z. Aquatic animals promote antibiotic resistance gene dissemination in water via conjugation: Role of different regions within the zebra fish intestinal tract, and impact on fish intestinal microbiota. Mol. Ecol. 2017, 26, 5318–5333. [Google Scholar] [CrossRef] [PubMed]
- Américo-Pinheiro, J.H.P.; Bellatto, L.C.; Mansano, C.F.M.; Da Silva, V.D.; Ferreira, L.F.R.; Torres, N.H.; Bilal, M.; Iqbal, H.M. Monitoring microbial contamination of antibiotic resistant Escherichia coli isolated from the surface water of urban park in southeastern Brazil. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100438. [Google Scholar] [CrossRef]
- Storto, D.; Nara, L.B.C.; Kozusny-Andreani, D.I.; Vanzela, L.S.; Mansano, C.F.M.; Bilal, M.; Iqbal, H.; Américo-Pinheiro, J.H.P. Seasonal dynamics of microbial contamination and antibiotic resistance in the water at the Tietê Ecological Park, Brazil. Water Air Soil Pollut. 2021, 232, 257. [Google Scholar] [CrossRef]
- Wallin, J.A. Bibliometric methods: Pitfalls and possibilities. Basic Clin. Pharmacol. Toxicol. 2005, 97, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Van, R.A. Advances in bibliometric analysis: Research performance assessment and science mapping. Bibliometr. Use Abus. Rev. Res. Perform. 2014, 87, 17–28. [Google Scholar]
- Pritchard, A. Statistical bibliography or bibliometrics. J. Doc. 1969, 25, 348–349. [Google Scholar]
- Lawani, S.M. Bibliometrics: Its theoretical foundations, methods and applications. Libri 1981, 31, 294–315. [Google Scholar] [CrossRef]
- Su, Y.; Yu, Y.; Zhang, N. Carbon emissions and environmental management based on Big Data and Streaming Data: A bibliometric analysis. Sci. Total Environ. 2020, 733, 138984. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y. Research trends and areas of focus on the Chinese Loess Plateau: A bibliometric analysis during 1991–2018. Catena 2020, 194, 104798. [Google Scholar] [CrossRef]
- Şahin, S.; Sivri, N.; Akpinar, I.; Çinçin, Z.B.; Sönmez, V.Z. A comprehensive bibliometric overview: Antibiotic resistance and Escherichia coli in natural water. Environ. Sci. Pollut. Res. 2021, 28, 32256–32263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liao, H.; Tu, C. An improved bibliometric analysis on antibiotics in soil research. Bull. Environ. Contam. Toxicol. 2021, 108, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Akpinar, I.; Sivri, N. An alternative material for an effective treatment technique proposal in the light of bibliometric profile of global scientific research on antibiotic resistance and Escherichia coli. Environ. Monit. Assess. 2020, 192, 714. [Google Scholar] [CrossRef] [PubMed]
- Castronovo, C.; Agozzino, V.; Schirò, G.; Mira, F.; Di Bella, S.; Lastra, A.; Antoci, F.; Pennisi, M.; Giudice, E.; Guercio, A. Evaluation of the antimicrobial resistance of different serotypes of Salmonella enterica from livestock farms in Southern Italy. Appl. Sci. 2022, 13, 442. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Mansour, A.M. Bibliometric analysis of global research output on antimicrobial resistance in the environment (2000–2019). Glob. Health Res. Policy 2020, 5, 37. [Google Scholar] [CrossRef]
- Aleixandre-Tudó, J.L.; Castelló-Cogollos, L.; Aleixandre, J.L.; Aleixandre-Benavent, R. Emerging topics in scientific research on global water-use efficiency. J. Agric. Sci. 2019, 157, 480–492. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Humphreys, G.; Fleck, F. United Nations meeting on antimicrobial resistance. WHO Bull. World Health Organ. 2016, 94, 638. [Google Scholar]
- Martinez, J.L. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environ. Pollut. 2009, 157, 2893–2902. [Google Scholar] [CrossRef]
- Larsson, D.J.; Andremont, A.; Bengtsson-Palme, J.; Brandt, K.K.; Dem Roda Husman, A.M.; Fagerstedt, P.; Fick, J.; Flach, C.-F.; Gaze, W.H.; Kuroda, M. Critical knowledge gaps and research needs related to the environmental dimensions of antibiotic resistance. Environ. Int. 2018, 117, 132–138. [Google Scholar] [CrossRef]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; Mckenzie, S.; Walker, P.; Lawrence, R. Aquaculture practices and potential human health risks: Current knowledge and future priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Mao, D.; Luo, Y.; Wang, L.; Xu, B.; Xu, L. Occurrence of sulfonamide and tetracycline-resistant bacteria and resistance genes in aquaculture environment. Water Res. 2012, 46, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-C.; Pan, C.-Y.; Chou, H.-N.; Chen, J.-Y. Using an improved Tol2 transposon system to produce transgenic zebrafish with epinecidin-1 which enhanced resistance to bacterial infection. Fish Shellfish Immunol. 2010, 28, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-Y.; Huang, T.-C.; Wang, Y.-D.; Yeh, Y.-C.; Hui, C.-F.; Chen, J.-Y. Oral administration of recombinant epinecidin-1 protected grouper (Epinephelus coioides) and zebrafish (Danio rerio) from Vibrio vulnificus infection and enhanced immune-related gene expressions. Fish Shellfish Immunol. 2012, 32, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Irshad, S.; Hoseinifar, S.H.; Xiong, H. The functionality of prebiotics as immunostimulant: Evidences from trials on terrestrial and aquatic animals. Fish Shellfish Immunol. 2018, 76, 272–278. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.; Wang, C.; Li, M.; Chen, J.; Xiong, J. Responses of sediment resistome, virulence factors and potential pathogens to decades of antibiotics pollution in a shrimp aquafarm. Sci. Total Environ. 2021, 794, 148760. [Google Scholar] [CrossRef]
- Preena, P.G.; Dharmaratnam, A.; Kumar, V.J.R.; Swaminathan, T.R. Plasmid-mediated antimicrobial resistance in motile aeromonads from diseased Nile tilapia (Oreochromis niloticus). Aquacult. Res. 2021, 52, 237–248. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Duman, M.; Altun, S. Genome analysis and antimicrobial resistance characteristics of Chryseobacterium aquaticum isolated from farmed salmonids. Aquaculture 2021, 535, 736364. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.; Wang, J.; Zhang, Y.; Zhang, P.; Li, X.; Zou, J.; Zhou, A. Interactions of microplastics and antibiotic resistance genes and their effects on the aquaculture environments. J. Hazard. Mater. 2021, 403, 123961. [Google Scholar] [CrossRef]
- Wang, L.; Su, H.; Hu, X.; Xu, Y.; Xu, W.; Huang, X.; Li, Z.; Cao, Y.; Wen, G. Abundance and removal of antibiotic resistance genes (ARGs) in the rearing environments of intensive shrimp aquaculture in South China. J. Environ. Sci. Health Part B 2019, 54, 211–218. [Google Scholar] [CrossRef]
- He, X.; Xu, Y.; Chen, J.; Ling, J.; Li, Y.; Huang, L.; Zhou, X.; Zheng, L.; Xie, G. Evolution of corresponding resistance genes in the water of fish tanks with multiple stresses of antibiotics and heavy metals. Water Res. 2017, 124, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.H.; Rossi, P.; Dinh, H.D.K.; Pham, N.T.A.; Tran, P.A.; Ho, T.T.K.M.; Dinh, Q.T.; De Alencastro, L.F. Analysis of antibiotic multi-resistant bacteria and resistance genes in the effluent of an intensive shrimp farm (Long An, Vietnam). J. Environ. Manag. 2018, 214, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Vilca, F.Z.; Galarza, N.C.; Tejedo, J.R.; Cuba, W.A.Z.; Quiróz, C.N.C.; Tornisielo, V.L. Occurrence of residues of veterinary antibiotics in water, sediment and trout tissue (Oncorhynchus mykiss) in the southern area of Lake Titicaca, Peru. J. Great Lakes Res. 2021, 47, 1219–1227. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, S.; Hong, B.; Li, J.; Han, H.; Qie, G. Antibiotics control in aquaculture requires more than antibiotic-free feeds: A tilapia farming case. Environ. Pollut. 2021, 268, 115854. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Spatiotemporal variations and source tracking of antibiotics in an ecological aquaculture farm in Southern China. Sci. Total Environ. 2021, 763, 143022. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.-R.; Diao, Z.-H.; Sun, K.-F.; Hao, Q.-W.; Liu, S.-S.; Ying, G.-G. Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J. Hazard. Mater. 2018, 343, 140–148. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, L.; Chen, J.; Fan, X.; Xie, S.; Huang, J.; Yu, G. Antibiotic resistance genes and mobile genetic elements in a rural river in Southeast China: Occurrence, seasonal variation and association with the antibiotics. Sci. Total Environ. 2021, 778, 146131. [Google Scholar] [CrossRef]
- Thongsamer, T.; Neamchan, R.; Blackburn, A.; Acharya, K.; Sutheeworapong, S.; Tirachulee, B.; Pattanachan, P.; Vinitnantharat, S.; Zhou, X.-Y.; Su, J.-Q. Environmental antimicrobial resistance is associated with faecal pollution in Central Thailand’s coastal aquaculture region. J. Hazard. Mater. 2021, 416, 125718. [Google Scholar] [CrossRef]
- Zhao, X.; Su, H.; Xu, W.; Hu, X.; Xu, Y.; Wen, G.; Cao, Y. Removal of antibiotic resistance genes and inactivation of antibiotic-resistant bacteria by oxidative treatments. Sci. Total Environ. 2021, 778, 146348. [Google Scholar] [CrossRef]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Zhou, Y.; Zhao, J.; Fu, J.; Jiang, T.; Liu, B.; Chen, F.; Cao, G. High throughput sequencing reveals the abundance and diversity of antibiotic-resistant bacteria in aquaculture wastewaters, Shandong, China. 3 Biotech 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Nadella, R.K.; Panda, S.K.; Rao, B.M.; Prasad, K.P.; Raman, R.; Mothadaka, M.P. Antibiotic resistance of culturable heterotrophic bacteria isolated from shrimp (Penaeus vannamei) aquaculture ponds. Mar. Pollut. Bull. 2021, 172, 112887. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Dong, Q.; Wu, L.; Yang, Y.; Hale, L.; Qin, Z.; Xie, C.; Zhang, Q.; Van Nostrand, J.D.; Zhou, J. Environmental antibiotics drives the genetic functions of resistome dynamics. Environ. Int. 2020, 135, 105398. [Google Scholar] [CrossRef]
- Tartor, Y.H.; El-Naenaeey, E.-S.Y.; Abdallah, H.M.; Samir, M.; Yassen, M.M.; Abdelwahab, A.M. Virulotyping and genetic diversity of Aeromonas hydrophila isolated from Nile tilapia (Oreochromis niloticus) in aquaculture farms in Egypt. Aquaculture 2021, 541, 736781. [Google Scholar] [CrossRef]
- Saticioglu, I.B.; Yardimci, B.; Altun, S.; Duman, M. A comprehensive perspective on a Vagococcus salmoninarum outbreak in rainbow trout broodstock. Aquaculture 2021, 545, 737224. [Google Scholar] [CrossRef]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011, 7, e1002158. [Google Scholar] [CrossRef]
- Suyamud, B.; Lohwacharin, J.; Yang, Y.; Sharma, V.K. Antibiotic resistant bacteria and genes in shrimp aquaculture water: Identification and removal by ferrate (VI). J. Hazard. Mater. 2021, 420, 126572. [Google Scholar] [CrossRef]
- Mateo-Tomas, P.; Olea, P.P.; Sanchez-Barbudo, I.S.; Mateo, R. Alleviating human-wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 2012, 49, 376–385. [Google Scholar] [CrossRef]
- Dien, L.T.; Linh, N.V.; Sangpo, P.; Senapin, S.; St-Hilaire, S.; Rodkhum, C.; Dong, H.T. Ozone nanobubble treatments improve survivability of Nile tilapia (Oreochromis niloticus) challenged with a pathogenic multi-drug-resistant Aeromonas hydrophila. J. Fish Dis. 2021, 44, 1435–1447. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Cochevelou, V.; Dinh, H.D.K.; Breider, F.; Rossi, P. Implementation of a constructed wetland for the sustainable treatment of inland shrimp farming water. J. Environ. Manag. 2021, 279, 111782. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, L.; Ju, Z.; Fu, Y.; Qin, S.; Cui, J. The key environmental influencing factors for the change of sediment bacterial community and antibiotics resistance genes in a long-term polluted lake, China. Ecotoxicology 2021, 30, 1538–1549. [Google Scholar] [CrossRef]
- Sonnenschein, E.C.; Jimenez, G.; Castex, M.; Gram, L. The roseobacter-group bacterium Phaeobacter as a safe probiotic solution for aquaculture. Appl. Environ. Microbiol. 2020, 87, e02581-02520. [Google Scholar] [CrossRef]
- Yang, N.; Song, F.; Polyak, S.W.; Liu, J. Actinonin resistance of pathogenic Vibrio anguillarum in aquaculture. Aquaculture 2021, 541, 736850. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, J.; Chen, X.; Zhang, S.; Xie, M.; Chen, C.; Wu, Z. Screening of intestinal probiotics and the effects of feeding probiotics on the digestive enzyme activity, immune, intestinal flora and WSSV resistance of Procambarus clarkii. Aquaculture 2021, 540, 736748. [Google Scholar] [CrossRef]
- Wu, P.-S.; Liu, C.-H.; Hu, S.-Y. Probiotic Bacillus safensis NPUST1 Administration improves growth performance, gut microbiota, and innate immunity against Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Microorganisms 2021, 9, 2494. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, J.; Lin, G.; Li, M.; Zhu, R.; Zhang, Y.; Mai, K. Effects of Dietary mannan oligosaccharides on non-specific immunity, intestinal health, and antibiotic resistance genes in Pacific white shrimp Litopenaeus vannamei. Front. Immunol. 2021, 12, 772570. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef]
- Pruden, A.; Larsson, D.J.; Amézquita, A.; Collignon, P.; Brandt, K.K.; Graham, D.W.; Lazorchak, J.M.; Suzuki, S.; Silley, P.; Snape, J.R. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef]
- Zou, S.; Xu, W.; Zhang, R.; Tang, J.; Chen, Y.; Zhang, G. Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: Impacts of river discharge and aquaculture activities. Environ. Pollut. 2011, 159, 2913–2920. [Google Scholar] [CrossRef] [PubMed]
- Welch, T.J.; Fricke, W.F.; Mcdermott, P.F.; White, D.G.; Rosso, M.-L.; Rasko, D.A.; Mammel, M.K.; Eppinger, M.; Rosovitz, M.; Wagner, D. Multiple antimicrobial resistance in plague: An emerging public health risk. PLoS ONE 2007, 2, e309. [Google Scholar] [CrossRef] [PubMed]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial manipulations to improve fish health and production–a Mediterranean perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]


| Country | Papers | Average Article Citations |
|---|---|---|
| China | 206 | 23 |
| India | 35 | 12 |
| USA | 28 | 95 |
| UK | 21 | 82 |
| Republic of Korea | 17 | 19 |
| Japan | 16 | 34 |
| Brazil | 11 | 11 |
| Egypt | 11 | 7 |
| Spain | 11 | 114 |
| Portugal | 10 | 32 |
| Journal | h-Index | TC | PY-Start | IF2020 |
|---|---|---|---|---|
| Fish & Shellfish Immunology | 16 | 1271 | 2003 | 4.581 |
| Science of the Total Environment | 16 | 1018 | 2011 | 7.963 |
| PLoS ONE | 12 | 760 | 2007 | 3.24 |
| Environmental Pollution | 11 | 1682 | 2009 | 8.071 |
| Aquaculture | 9 | 382 | 2007 | 4.242 |
| Environmental Science and Pollution Research | 9 | 296 | 2015 | 4.223 |
| Frontiers in Microbiology | 9 | 691 | 2012 | 5.64 |
| Chemosphere | 8 | 351 | 2014 | 7.086 |
| Water Research | 8 | 675 | 2012 | 11.236 |
| Ecotoxicology and Environmental Safety | 7 | 218 | 2014 | 6.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Wang, H.; Wang, C.; Gao, H.; Wang, Y.; Xu, J. Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review. Sustainability 2023, 15, 9012. https://doi.org/10.3390/su15119012
Xiao Y, Wang H, Wang C, Gao H, Wang Y, Xu J. Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review. Sustainability. 2023; 15(11):9012. https://doi.org/10.3390/su15119012
Chicago/Turabian StyleXiao, Yayu, Hongxia Wang, Chen Wang, He Gao, Yuyu Wang, and Jun Xu. 2023. "Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review" Sustainability 15, no. 11: 9012. https://doi.org/10.3390/su15119012
APA StyleXiao, Y., Wang, H., Wang, C., Gao, H., Wang, Y., & Xu, J. (2023). Trends in and Future Research Direction of Antimicrobial Resistance in Global Aquaculture Systems: A Review. Sustainability, 15(11), 9012. https://doi.org/10.3390/su15119012






