Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction?
Abstract
1. Introduction and Background
2. Materials and Methods
3. Results and Discussion
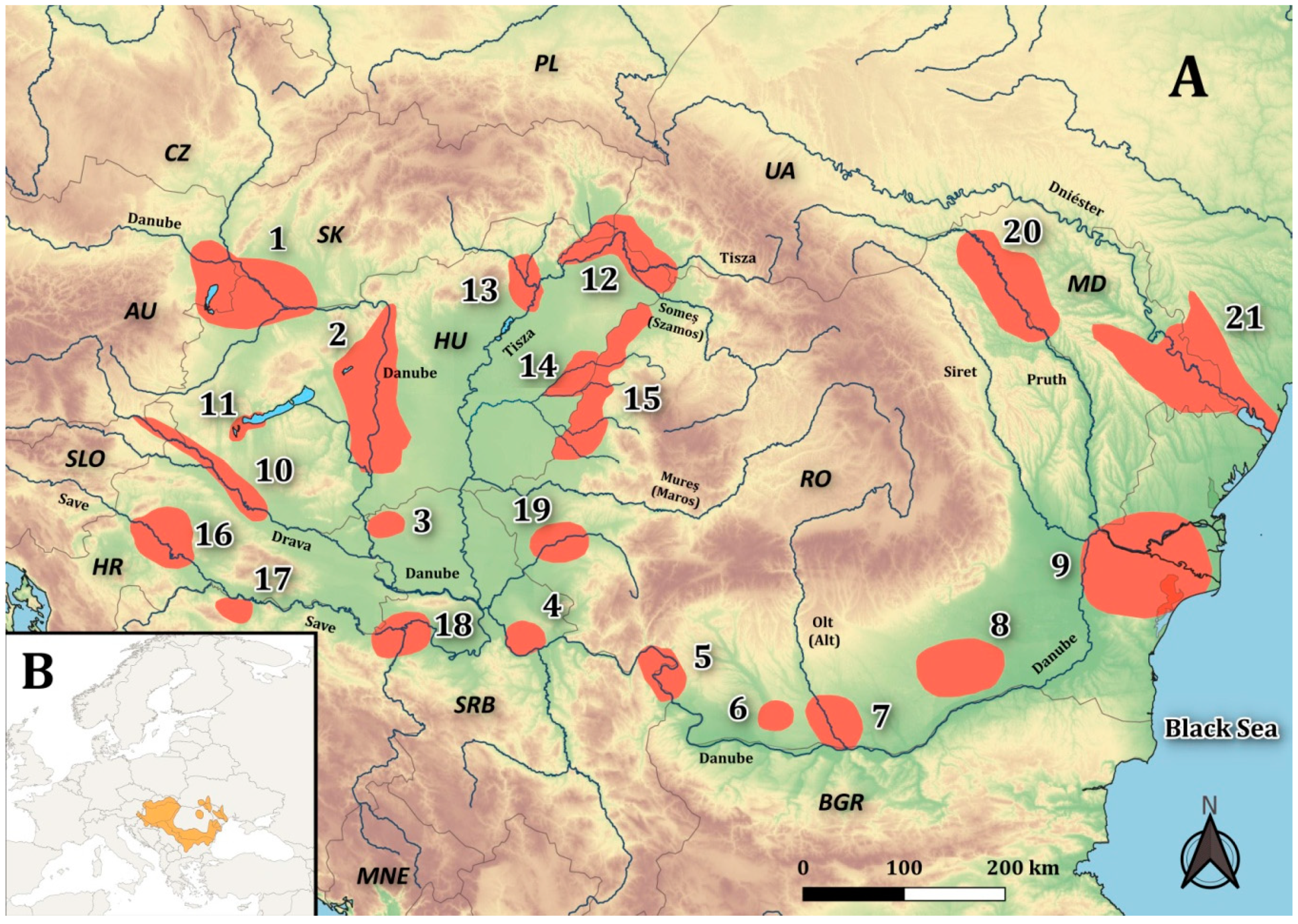
3.1. Wetlands in Austria and Its Borders with Slovakia and Hungary
- Wetlands in Austria
- Wetlands at the Austria-Slovakia-Hungary Border
3.2. Wetlands of the Danube River in Hungary
3.3. Lugomir Channel System in Danube Drainage in Serbia
3.4. Kraljevac Channel System in Danube Drainage in Serbia
3.5. Wetlands at the Serbia-Romania Border
3.6. Jiu River Lower Part in Romania
3.7. Olt River Lower Part in Romania
3.8. Vedea and Argeş Rivers Lower Part in Romania
3.9. Lower Danube River Basin-Danube River Delta in the Romania-Moldova-Ukraine Border Area
3.10. Mura and Drava River System at the Slovenia-Croatia-Hungary Border
3.11. Zala River, Lake Balaton System in Hungary
3.12. Upper Tisza System in Ukraine and Hungary
3.13. Borsodi-Mezőség Plane, Tisza System in Hungary
3.14. Bihar-Plaine Tisza System in Hungary
3.15. Wetlands at the Romania-Hungary Border
3.16. Lonja and Odra Wetlands in Croatia
3.17. Matura River System in Bosnia and Herzegovina
3.18. Wetlands at the Serbia-Bosnia and Herzegovina Border
3.19. Timiș River System in Romania
3.20. Prut River at the Moldova-Romania Border
3.21. Dniester River at Moldova-Ukraine Border
3.22. Danube Basin Umbra krameri Populations State and Potential Trends under Climate Change Impact
3.23. Umbra krameri Refuge and Stepping Stone Habitats Management Elements Proposals for the Danube Basin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maruyama, S.; Ikoma, M.; Genda, H.; Hirose, K.; Yokohama, T.; Santosh, M. The naked planet earth: Most essential pre-requisite for the origin and evolution of life. Geosci. Front. 2013, 4, 141–165. [Google Scholar] [CrossRef]
- Goncharuk, W.; Goncharuk, V.V. Water is everywhere. It holds everything a key to understanding the universe. D. I. Mendeleev’s law is the prototype of the universe constitution. J. Water Chem. Technol. 2019, 41, 341–346. [Google Scholar] [CrossRef]
- May, R.M. Biological diversity: Differences between land and sea. Phil. Trans. R. Soc. Lond. 1994, 343, 105–111. [Google Scholar]
- Benton, M.J. Biodiversity on land and in the sea. Geol. J. 2001, 36, 211–230. [Google Scholar] [CrossRef]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, 1001127. [Google Scholar] [CrossRef]
- Richard, K.; Geerat, G.; Vermeij, J.; Wainwright, P.C. Biodiversity in water and on land. Curr. Biol. 2012, 22, R900–R903. [Google Scholar]
- Barinova, S.S. Empirical model of the functioning of aquatic ecosystems. Int. J. Oceanogr. Aquac. 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Barinova, S. On the classification of water quality from an ecological point of view. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555581. [Google Scholar] [CrossRef]
- Sender, J.; Maślanko, W.; Różańska-Boczula, M.; Cianfaglione, K. A new multi-criteria method for the ecological assessment of lakes: A case study from the transboundary biosphere reserve ‘West Polesie’ (Poland). J. Limnol. 2017, 76. [Google Scholar] [CrossRef][Green Version]
- Cianfaglione, K. Plant landscape and models of French Atlantic estuarine systems. Extended summary of the doctoral thesis. Transylv. Rev. Syst. Ecol. Res. 2021, 23, 15–36. [Google Scholar] [CrossRef]
- Biggs, J.; von Fumetti, J.S.; Kelly-Quinn, M. The importance of small water bodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Pekárik, L.; Čejka, T.; Čiamporovà-Zatovičovà, Z.; Darolovà, A.; Illèsovà, D.; Illyová, M.; Pastuchovà, Z.; Gatial, E.; Čiampor, F. Multidisciplinary evaluation of the function and importance of the small water reservoirs: The biodiversity aspect. Transylv. Rev. Syst. Ecol. Res. 2009, 8, 105–112. [Google Scholar]
- Schneider-Binder, E. The Habitats Along the Upper Danube in Germany and Changes to Them Induced by Human Impacts, 27–48. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Burga, C.A.; Landolt, E. The Upper Engandine: Headwater Region of the River Inn. A Swiss Hot Spot of Plant Diversity and Premium Tourism Region, 49–64. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Hübl, E. Vegetation and Flora Near the Danube in Austria 65–86. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Cianfaglione, K.; Pedrotti, F. Italy in the Danube Geography: Territoty, Landscape, Environment, Vegetation, Fauna, Culture, Human Management and Outlooks for the Future 87–118. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Anfraž, Č.; Mastnak, N.J. Forest Vegetation Along the Mura River in Slovenia 119–134. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Adámek, Z.; Jurajdová, Z.; Janáč, M.; Zahrádková, S.; Němejcová, D.; Jurajda, P. The Response of Fish Assemblages to Human Impacts Along the Lower Stretch of the Rivers Morava and Dyje (Danube River Basin, Czech Republic) 135–150. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Ćaleta, M.; Mustafić, P.; Zanella, D.; Buj, I.; Marčić, Z.; Mrakovčić, M. Human Impacts on the Dobra River (Croatia) 151–168. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Dekić, R.; Ivanc, A.; Ćetković, D.; Lolić, S. Anthropogenic Impact and Environmental Quality of Different Tributaries of the River VRBAS (Bosnia and Herzegovina) 169–214. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Guti, G. Assessment of Long-Term Changes in the Szigetköz Floodplain of the Danube River 215–240. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Đikanović, V.; Nikčević, M.; Mićković, B.; Hegediš, A.; Mrdak, D.; Pešić, V. Anthropogenic Pressure on Watercourses of the Danube River Basin in Montenegro 241–256. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Lenhardt, M.; Smederevac-Lalić, M.; Hegediš, A.; Skorić, S.; Cvijanović, G.; Višnjić-Jeftić, Ž.; Djikanović, V.; Jovičić, K.; Jaćimović, M.; Jarić, I. Human Impacts on Fish Fauna in the Danube River in Serbia: Current Status and Ecological Implications 257–280. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Mišiková Elexová, E.; Makovinská, J. Assessment of the Aquatic Ecosystem in the Slovak Stretch of the Danube River 281–300. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Maślanko, W.; Ferencz, B.; Dawidek, J. State and Changes of Natural Environment in Polish Part of the Danube River Basin 301–326. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Afanasyev, S.; Lyashenko, A.; Iarochevitch, A.; Lietytska, O.; Zorina-Sakharova, K.; Marushevska, O. Pressures and Impacts on Ecological Status of Surface Water Bodies in Ukrainian Part of the Danube River Basin 327–358. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Bakiu, R. Drina River (Sava’s Tributary of Danube River) and Human Impact in Albania 359–380. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Kostov, V.; Slavenska-Stamenkovic, V.; Ristovska, M.; Stojov, V.; Marić, S. Characteristics of the Danube Drainage Area in the Republic of Macedonia 381–392. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Kenderov, L.; Trichkova, T. Long-Term Changes in the Ecological Conditions of the Iskar River (Danube River Basin, Bulgaria) 393–424. In Human Impact on Danube Watershed Biodiversity in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–437. ISBN 978-3-030-37241-5. [Google Scholar]
- Popa, G.O.; Curtean-Bănăduc, A.; Bănăduc, D.; Florescu, I.E.; Burcea, A.; Dudu, A.; Georgescu, S.E.; Costache, M. Molecular Markers Reveal Reduced Genetic Diversity in Romanian Populations of Brown Trout, Salmo trutta L., 1758 (Salmonidae). Acta Zool. Bulg. 2016, 68, 399–406. [Google Scholar]
- Agenţia Europeană de Mediu (AEM). Available online: https://www.eea.europa.eu/ro/themes/climate/about-climate-change (accessed on 23 July 2021).
- Treut, L.; Somerville, R.; Cubasch, U.; Ding, Y.; Mauritzen, C.; Mokssit, A.; Peterson, T.; Prather, M.; Qin, D.; Manning, M.; et al. Historical overview of climate change science. Earth 2007, 43, 93–127. [Google Scholar]
- Heaviside, C. Understanding the impacts of climate change on health to better manage adaptation action. Atmosphere 2019, 10, 119. [Google Scholar] [CrossRef]
- United Nations, Security Council. Available online: https://www.un.org/press/en/2021/sc14445.doc.htm (accessed on 23 July 2021).
- The Intergovernmental Panel on Climate Change (IPCC-53 BIS). Available online: https://www.ipcc.ch/ (accessed on 23 July 2021).
- Mann, M.E.; Bradley, R.S.; Hughes, M.K. Global-scale temperature patterns and climate forcing over the past six centuries. Nature 1998, 392, 779–787. [Google Scholar] [CrossRef]
- Bates, B.C.; Kundzewicz, Z.W.; Wu, S.; Palutikof, J.P. Climate change and water. In Technical Paper of the Intergovernmental Panel on Climate Change; IPCC Secretariat: Geneva, Switzerland, 2008. [Google Scholar]
- Krassilov, V.; Barinova, S. Sea level—Geomagnetic polarity correlation as consequence of rotation geodynamics. Earth Sci. 2013, 2, 1–8. [Google Scholar] [CrossRef][Green Version]
- Fenoglio, S.; Bo, T.; Cucco, M.; Mercalli, L.; Malacarne, G. Effects of global climate change on freshwater biota: A review with special emphasis on the Italian situation. Ital. J. Zool. 2010, 77, 374–383. [Google Scholar] [CrossRef]
- United Nations Secretary-General Meetings Coverage and Press Releases. Available online: https://www.un.org/press/en/2021/sgsm20847.doc.htm (accessed on 28 August 2021).
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef]
- Kühn, I.; Sykes, M.T.; Berry, P.M.; Thuiller, W.; Piper, J.M.; Nigmann, U. MACIS: Minimization of and adaptation to climate change impacts on biodiversity. GAIA—Ecol. Per. Sci. Soc. 2009, 17, 393–395. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The Role of Aquatic Refuge Habitats for Fish, and Threats in the Context of Climate Change and Human Impact, during Seasonal Hydrological Drought in the Saxon Villages Area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 1998, 9, 261–285. [Google Scholar] [CrossRef]
- Gomi, T.; Nagasaka, M.; Fukuda, T.; Hagihara, H. Shifting of the life cycle and life-history traits of the fall webworm in relation to climate change. Entomol. Exp. Appl. 1998, 125, 179–184. [Google Scholar] [CrossRef]
- Balletto, E.; Barbero, F.; Casacci, L.P.; Cerrato, C.; Patricelli, D.; Bonelli, S. L’Impatto dei Cambiamenti Climatici Sulle Farfalle Italiane; XVIII Convegno Gruppo Per l’Ecologia di Base, G.; Gadio: Alessandria, Italy, 2008; pp. 9–11. [Google Scholar]
- Pounds, J.A.; Fogden, P.L.; Campbell, J.H. Biological response to climate change on a tropical mountain. Nature 1999, 398, 611–615. [Google Scholar] [CrossRef]
- Arthington, A.H.; Balcombe, S.R.; Wilson, G.A.; Thoms, M.C.; Marschall, J.C. Spatial and temporal variation in fish assemblage structure in isolated waterholes during the 2001 dry season of an arid-zone river, Cooper Creek, Australia. Mar. Freshw. Res. 2005, 56, 25–35. [Google Scholar] [CrossRef]
- Barbarossa, V.; Bosmans, J.; Wanders, N.; King, H.; Biekens, M.F.P.; Hujbregts, J.; Schipper, A.M. Threates of global warming to the world’s fresh-eater fishes. Nat. Commun. 2021, 12, 1. [Google Scholar] [CrossRef]
- Krassilov, V.A. Terrestrial Palaeoecology and Global Change; Pensoft: Sophia, Bulgaria, 2003; 464p. [Google Scholar]
- Snoj, A.; Marić, S.; Bajec, S.S.; Berrebi, P.; Janjani, S.; Schöffmann, J. Phylogeographic structure and demographic patterns of brown trout in North-West Africa. Mol. Phylogenet. Evol. 2011, 61, 203–211. [Google Scholar] [CrossRef]
- Zubcov, N.; Zubcov, E.; Schlenk, D. The dynamics of metals in fish from Nistru and Prut rivers (Moldova). Transylv. Rev. Syst. Ecol. Res. 2008, 6, 51–58. [Google Scholar]
- Vassilev, M.; Botev, I. Assessment of the ecological status of some Bulgarian rivers from the Aegean Sea Basin based on both environmental and fish parameters. Transylv. Rev. Syst. Ecol. Res. 2008, 6, 71–80. [Google Scholar]
- Momeu, L.; Battes, K.; Battes, K.; Stoica, I.; Avram, A.; Cîmpean, M.; Pricope, F.; Ureche, D. Algae, macroinvertebrate and fish communities from the Arieş River catchment area (Transylvania, Romania). Transylv. Rev. Syst. Ecol. Res. 2009, 7, 149–180. [Google Scholar]
- Trichcokva, T.; Stefanov, T.; Vassilev, M.; Zivcov, M. Fish species diversity in the rivers of the north-west Bulgaria. Transylv. Rev. Syst. Ecol. Res. 2009, 8, 161–168. [Google Scholar]
- Jeeva, V.; Kumar, S.; Verma, D.; Rumana, S. River fragmentation and connectivity problems in Gange River of upper Himalayas: The effect on the fish communities (India). Transylv. Rev. Syst. Ecol. Res. 2011, 12, 75–90. [Google Scholar]
- Florea, L.; Strătilă, S.D.; Costache, M. The assessment of community interest fish species from protected area ROSCI0229. Transylv. Rev. Syst. Ecol. Res. 2014, 16, 73–96. [Google Scholar] [CrossRef]
- Sosai, A.S. Illegal fishing in southern Mannar Island coastal area (Sri Lanka). Transylv. Rev. Syst. Ecol. Res. 2015, 17, 95–108. [Google Scholar]
- Khoshnood, Z.; Khoshnood, R. Effect of industrial wastewater on fish in Karoon River. Transylv. Rev. Syst. Ecol. Res. 2015, 17, 109–120. [Google Scholar] [CrossRef][Green Version]
- Del Monte-Luna, P.; Lluch-Belda, D.; Arreguín-Sánchez, F.; Lluch-Cota, S.; Villalobos-Ortiz, H. Approaching the potential of world marine fish. Transylv. Rev. Syst. Ecol. Res. 2015, 18, 45–56. [Google Scholar]
- Taiwo, I.O.; Olopade, O.A.; Bamidele, N.A. Heavy metal concentration in eight fish species from Epe Lagoon (Nigeria). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 69–82. [Google Scholar] [CrossRef][Green Version]
- Kar, D. Wetlands and their fish diversity in Assam (India). Transylv. Rev. Syst. Ecol. Res. 2019, 21, 47–94. [Google Scholar] [CrossRef]
- Rios, J.M. Predation by nonnative Rainbow Trout, Oncorhynchus mykiss (Walbaum, 1792), on the native biota from freshwater environment of the Central Andes (Argentina). Transylv. Rev. Syst. Ecol. Res. 2019, 23, 67–72. [Google Scholar] [CrossRef]
- Antonescu, B. In Verbis. 11 August 2021. Available online: https://bogdanantonescu.squarespace.com/; https://www.digi24.ro/stiri/actualitate/romania-in-raportul-cod-rosu-pentru-umanitate-expert-meteo-vom-avea-valuri-de-caldura-precipitatii-intense-si-seceta-1629497 (accessed on 28 August 2021).
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014, Synthesis Report. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 8 August 2021).
- Lupo, A.R.; Kononova, N.K.; Semenova, I.G.; Lebedeva, M.G. A comparison of the characteristic drought during the late 20th and early 21st centuries over Eastern Europe, Western Russia and Central North America. Atmosphere 2021, 12, 1033. [Google Scholar] [CrossRef]
- Kjellström, E.; Nikulin, G.; Hanson, U.; Strandberg, G.; Ullerstig, A. 21st century changes in the European climate: Uncertainties derived from an ensemble of regional climate model simulations. Tellus A 2010, 63, 24–40. [Google Scholar] [CrossRef]
- Wong, W.K.; Beldring, S. Climate change effects on spatiotemporal patterns of hydroclimatological summer droughts in Norway. J. Hydrometeorol. 2011, 12, 1205–1220. [Google Scholar] [CrossRef]
- Cianfaglione, K.; Chelli, S.; Campetella, G.; Wellstein, C.; Cerivellini, M.; Ballelli, S.; Lucarini, D.; Canullo, R.; Jentsch, A. European grasslands gradient and the resilience to extreme climate events: The SIGNAL project in Italy. In Climate Gradients and Biodiversity in Mountains of Italy; Pedrotti, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; p. 175. [Google Scholar]
- Brönnimann, S. Early twentieth-century warming. Nature 2009, 2, 735–736. [Google Scholar] [CrossRef]
- Levitus, S.; Antonov, J.I.; Wang, J.; Delworth, T.L.; Dixon, K.W.; Broccoli, A.J. Anthropogenic warming of Earth’s climate system. Science 2001, 292, 267–270. [Google Scholar] [CrossRef]
- Lloyd-Hughes, B. The impracticality of a universal drought definition. Theor. Appl. Climatol. 2014, 117, 607–611. [Google Scholar] [CrossRef]
- IPCC 2012. Summary for policymakers. In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation; Field, C.B., Barros, V.R., Stocker, D., Qin, D.J., Dokken, K.L., Ebi, M.D., Mastrandrea, K.J., Mach, G.-K., Plattner, S.K., Allen, M.T., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 3–21. [Google Scholar]
- Stahle, D.W. Anthropogenic megadrought, human-driven climate warming worsens an otherwise moderate drought. Science 2020, 368, 238–239. [Google Scholar] [CrossRef]
- Wilhite, D.A.; Pulwarty, R.S. Drought as hazard: Understanding the natural and social context. In Drought and Water Crises; Wilhite, D.A., Pulwarty, R.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Gerald, A.M.; Washington, W.M.; Arblaster, J.M.; Hu, A.; Teng, H.; Tebaldi, C.; Sanderson, B.N.; Lamarque, J.-F.; Conley, A.J.; Strand, W.G.; et al. Climate system response to external forcings and climate change projections in CCSM4. J. Clim. 2007, 25, 3661–3683. [Google Scholar]
- Stagl, J.C.; Hattermann, F.F. Impacts of Climate Change on the Hydrological Regime of the Danube River and Its Tributaries Using an Ensemble of Climate Scenarios. Water 2015, 7, 6139–6172. [Google Scholar] [CrossRef]
- Bisselink, B.; de Roo, A.; Bernhardt, J.; Gelati, E. Future projections of water scarcity in the Danube River Basin due to land use, water demand and climate change. J. Environ. Geogr. 2018, 11, 25–36. [Google Scholar] [CrossRef]
- Mauser, W.; Stolz, R.; Weber, M.; Ebner, M.; Danube River Basin, Climate Change Adaptation. Revision and Update of the Danube Study, Integrating and Editing New Scientific Results in Climate Change Research and the Resulting Impacts on Water Availability to Revise the Existing Adaptation Strategies in the Danube River basin. Final Report. pp. 1–137, 2017–2018. Available online: https://www.icpdr.org/main/sites/default/files/nodes/documents/danube_climate_adaptation_study_2018.pdf (accessed on 28 August 2021).
- Kling, K.; Fuchs, M.; Paulin, M. Runoff conditions in the upper Danube basin under an ensemble of climate change scenarios. J. Hydrol. 2012, 424, 269–277. [Google Scholar] [CrossRef]
- Lucarini, V.; Danihlik, R.; Kriegerova, I.; Speranza, A. Hydrological cycle in the Danube basin in present-day and XXII century simulations by IPCCAR4 global climate models. J. Geophys. Res. Earth Surf. 2008, 113, D9. [Google Scholar] [CrossRef]
- Klein, B.; Lingemann, I.; Nilson, E.; Krahe, P.; Maurer, T.; Moser, H. Key concepts of climate change impact analyses for river basin management in the River Danube. River Syst. 2012, 20, 7–21. [Google Scholar] [CrossRef]
- Szolgayova, E.; Parajka, J.; Blöschi, G.; Bucher, C. Long term variability of the Danube River flow and its relation to precipitation and air temperature. J. Hydrol. 2014, 519, 871–880. [Google Scholar] [CrossRef]
- Santos, I.M.; Herrnegger, M.; Holzmann, H. Seasonal discharge forecasting for the Upper Danube. J. Hydrol. Reg. Stud. 2021, 37, 100905. [Google Scholar] [CrossRef]
- Klein, B.; Lingemann, I.; Krahe, P.; Nilson, E. Possible Changes of the Runoff Regime of the Upper Danube in the 20th and 21st Century, 2012. In Proceedings of the Bundesministerium für Verkehr, Bau und Stadtentwicklung (Hg.) KLIWAS: Impacts of Climate Change on Waterways and Navigation in Germany 2011, Second Status Conference, Federal Ministry of Transport, Building and Urban Development, Berlin, Germany, 25–26 October 2011; Available online: https://core.ac.uk/download/pdf/326240253.pdf (accessed on 28 August 2021).
- Stolz, R.; Prasch, M.; Weber, M.; Koch, F.; Weidinger, R.; Ebner, M.; Mauser, W. Climate change impacts on the water resources in the Danube River basin and possibilities to adapt—The way to an adaptation strategy and its update. J. Environ. Geogr. 2018, 11, 13–24. [Google Scholar] [CrossRef]
- Prasch, M.; Koch, F.; Weidinger, R.; Mauser, W. Danube study—Climate change Adaptation. Study to provide a common basin-wide understanding towards the development of a Climate Change adaptation strategy in Danube River Basin. Final. Rep. 2012, 174. Available online: http://www.icpdr.org/flowpaper/viewer/default/files/Danube%20Climate%20Adaptation%20Study_final.pdf (accessed on 28 August 2021).
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes, a broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 14. [Google Scholar] [CrossRef]
- Stoica, C.; Stănescu, E.; Lucaciu, I.; Gherghe, Ş.; Nicolau, M. Influence of global change on biological assemblages in the Danube Delta. J. Environ. Prot. Ecol. 2013, 14, 2. [Google Scholar]
- Friedrichs-Manthey, M.; Langhans, S.D.; Borgwardt, F.; Hein, T.; Kling, H.; Stanzel, P.; Jähning, S.C.; Domisch, S. 300 years of change for native fish species in the upper Danube River Basin—Historical flow alterations versus future climate change. Project: Global change effects in river ecosystems. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River-Danube Delta-North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna. Sci. Total Environ. 2016, 545, 137–151. [Google Scholar] [CrossRef]
- Curtean-Bǎnǎduc, A.; Bǎnǎduc, D.; Bucşa, C. Watersheds Management (Transylvania/Romania): Implications, risks, solutions. In Strategies to Enhance Environmental Security in Transition Countries, NATO Science for Peace and Security Series C-Environmental Security; Springer: Berlin/Heidelberg, Germany, 2007; pp. 225–238. ISBN 978-1-4020-5994-0. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Marić, S.; Gabor, G.; Didenko, A.; Rey Planellas, S.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Costea, G.; Push, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 2021, 54, 111003. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Didenko, A.; Guti, G.; Bănăduc, D. Telestes souffia (Risso, 1827) species conservation at the eastern limit of range—Vişeu River basin, Romania. Appl. Ecol. Environ. Res. 2018, 16, 291–303. [Google Scholar] [CrossRef]
- Bănăduc, D.; Bănăduc, A.; Lenhardt, M.; Guti, G. “Porţile de Fier/Iron Gates” Gorges area (Danube) fish fauna. Transylv. Rev. Syst. Ecol. Res. 2014, 16, 171–196. [Google Scholar] [CrossRef][Green Version]
- Schiemer, F.; Guti, G.; Keckeis, H.; Staraş, M. Ecological status and problems of the Danube River and its fish Fauna: A Review. Environ. Sci. 2013, 273–299. Available online: https://www.researchgate.net/publication/241899284_Ecological_status_and_problems_of_the_Danube_and_its_fish_fauna_A_review (accessed on 28 August 2021).
- Schiemer, F.; Spindler, T. Endangered fish species of the Danube River in Austria. Regul. Rivers Res. Manag. 1989, 4, 397–407. [Google Scholar] [CrossRef]
- Lelek, A. Threatened Fishes of Europe. In European Committee for the Conservation of Nature and Natural Resources; Council of Europe, Ed.; The Freshwater Fishes of Europe; AULA: Wiesbaden, Germany, 1987; Volume 9, pp. 70–73. [Google Scholar]
- Sommerwerk, N.; Bloesch, J.; Baumgartner, C.; Bittl, T.; Čerba, D.; Csányi, B.; Davideanu, G.; Dokulil, M.; Frank, G.; Grecu, L.; et al. The Danube River Basin. In Rivers of Europe; Tockner, K., Uehlinger, U., Robinson, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 59–112. [Google Scholar]
- Heckel, J.; Kner, R. Die Süsswasserfische der Österreichischen Monarchie mit Rücksicht auf die Angränzenden Länder; Wilhelm Engelmann: Leipzig, Germany, 1858. [Google Scholar]
- Antipa, G. Fauna Ihtiologică a României, Publicaţiile Fondului Adamachi; Inst. de arte grafice "Carol Göbl": Bucureşti, România, 1909; p. 294. [Google Scholar] [CrossRef]
- Buşniţă, T. Die Ichtyofauna des Donauflusses. In Limnologie der Donau—Eine Monographische Darstellung; Liepolt, R., Ed.; Schweizerbartsche Verlagsbuchhandlung: Stuttgart, Germany, 1967; Volume 5, pp. 198–224. [Google Scholar]
- Buşniţă, T. Die wirtschaftliche Bedeutung der Donau. Die Fischerei und Fischwirtschaft. In Limnologie der Donau; Liepolt, R., Ed.; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1967; Volume 4, pp. 26–41. [Google Scholar]
- Schletterer, M.; Kuzovlev, V.V.; Zhenikov, Y.N.; Tuhtan, J.A.; Haidvogl, G.; Friedrich, T.; Górski, K.; Füreder, L. Fish fauna and fisheries of large European rivers: Examples from the Volga and the Danube. Hydrobiologia 2017, 814, 45–60. [Google Scholar] [CrossRef]
- Noble, R.A.A.; Cowx, I.G.; Goffaux, D.; Kestemont, P. Assessing the health of European rivers using functional ecological guilds of fish communities: Standardising species classification and approaches to metric selection. Fish. Manag. Ecol. 2007, 14, 381–392. [Google Scholar] [CrossRef]
- Bănărescu, P. Peşti rari şi cu areal restrâns din fauna ţării noastre şi problemele ocrotirii lor. Ocrotirea Nat. 1965, 9, 5–21. [Google Scholar]
- Available online: https://ec.europa.eu/environment/strategy/biodiversity-strategy-2030_en (accessed on 28 August 2021).
- Marić, S.; Stanković, D.; Wazenbök, J.; Šanda, R.; Erös, T.; Takács, P.; Specziár, A.; Sekulić, N.; Bănăduc, D.; Ćaleta, M.; et al. Phylogeography and population genetics of the European mudminnow (Umbra krameri) with a time-calibrated phylogeny for the family Umbridae. Hydrobiologia 2017, 792, 151–168. [Google Scholar] [CrossRef]
- Bănăduc, D. Umbra krameri Walbaum, 1792, a Natura 2000 protected fish species, in Romania. Acta Ichtiol. Romanica 2008, 3, 33–44. [Google Scholar]
- Gaudant, J. An attempt at the palaeontological history of the European mudminnows (Pisces, Teleostei, Umbridae). Neues Jahrb. Geol. Paläontol. Abh. 2012, 263, 93–109. [Google Scholar] [CrossRef]
- Bănărescu, P. Academiei Republicii Populare Române. Fauna Republicii Populare Române, Pisces-Osteichthyes; Editura Academiei Republicii Populare Române: Bucureşti, România, 1964; Volume 8, 962p. [Google Scholar]
- Movchan, Y.V. Observations on the distribution of Umbra krameri Walbaum, 1792, in the Ukraine (Pisces, Umbridae). Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 1995, 97B, 491–495. [Google Scholar]
- Cocan, D.; Mireşan, V. Ihtiologie Sistematica şi Morfologia Peştilor; Colorama Publishing House: Cluj-Napoca, Romania, 2018; Volume I, p. 559. [Google Scholar]
- Müller, T.; Wilhelm, S.; Imecs, I. Conservarea şi reproducerea artificial a unor specii de peşti de mlaştină periclitate: Ţigănuş, caracudă şi ţipar. Ed. Green Steps Braşov. 2015, 1, 50. [Google Scholar]
- Wanzenböck, J. Current knowledge on the European mudminnow, Umbra krameri Walbaum, 1792 (Pisces: Umbridae). Ann. Nat. Mus. Wien. Ser. B Bot. Und Zool. 1995, 97B, 439–449. [Google Scholar]
- Ansari, A.A.; Singh, G.S.; Lanza, G.R.; Rast, W. (Eds.) Eutrophication: Causes, Consequences and Control; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Gigante, D.; Angiolini, C.; Landucci, F.; Maneli, F.; Nisi, B.; Vaselli, O.; Venanzoni, R.; Lastrucci, L. New occurrence of reed bed decline in southern Europe: Do permanent flooding and chemical parameters play a role? Comptes Rendus. Biol. 2014, 337, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Covaliov, S.; Doroftei, M.; Hanganu, J. Danube Delta Biosphere Reserve (D.D.B.R.): Reed dynamics within actual context. Adv. Environ. Sci. 2010, 2, 69–79. [Google Scholar]
- Oteman, B.; Scrieciu, A.; Bouma, T.J.; Stanica, A.; van der Wal, D. Indicators of Expansion and Retreat of Phragmites Based on Optical and Radar Satellite Remote Sensing: A Case Study on the Danube Delta. Wetlands 2021, 41, 1–15. [Google Scholar] [CrossRef]
- Kováč, V. Reproduction behavior and early development of the European mudminnow, Umbra krameri Walbaum 1792. Folia Zool. 1995, 44, 57–80. [Google Scholar]
- Oţel, V. Atlasul Peştilor din Rezervaţia Biosferei Delta Dunării; Institutul Naţional de Cercetare-Dezvoltare Delta Dunării: Tulcea, Romania, 2007. [Google Scholar]
- Povž, M. Threatened fishes of the world: Umbra krameri Walbaum 1792 (Umbridae). Environ. Biol. Fishes 1995, 43, 232. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
- Sekulic, N.; Budakov, L.; Brankovic, D. Distribution of the European mudminnow Umbra krameri (Umbridae) in Serbia. Ital. J. Zool. 1998, 65, 381–382. [Google Scholar] [CrossRef]
- Takács, P.; Erős, T.; Specziár, A.; Sály, P.; Vitál, Z.; Ferincz, Á.; Molnár, T.; Szabolcsi, Z.; Bíró, P.; Csoma, E. Population Genetic Patterns of Threatened European Mudminnow (Umbra krameri Walbaum, 1792) in a Fragmented Landscape: Implications for Conservation Management. PLoS ONE 2015, 10, e0138640. [Google Scholar] [CrossRef] [PubMed]
- Marić, S.; Stanković, D.; Šanda, R.; Ćaleta, M.; Čolić, S.; Šukalo, G.; Snoj, A. Genetic characterisation of European mudminnow (Umbra krameri) populations from the Sava River system. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 46. [Google Scholar] [CrossRef]
- Covaciu-Marcov, S.-D.; Cupşa, D.; Telcean, I.C. Two new populations of the European mudminnow, Umbra krameri (Actinopterigii: Esociformes: Umbridae), in south-western Romania with the first record in the Banat Region. Acta Ichthyol. Piscat. 2018, 48, 251–255. [Google Scholar] [CrossRef]
- Wanzenböck, J. Wiederentdeckung des Europäischen Hundsfisches, Umbra krameri Walbaum 1792. Osterr. Fisch. 1992, 45, 228–229. [Google Scholar]
- Wanzenböck, J.; Spindler, T. Rediscovery of Umbra krameri Walbaum, 1972, in Austria and subsequent investigations. Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 1995, 97B, 450–457. [Google Scholar]
- Marsili, A.F. Danubius Pannonico Mysicus, Observationibus Geographicis, Astronomicis, Hydrographicis, Historicis, Physicis perlustratus et in sex tomos digestus. Tomus Quartus. Hagae Amstelodami 1726, 17, 92. [Google Scholar]
- Kramer, W.H. Elenchus Vegetabilium et Animalium per Austriam Inferiorem Observatorum; Kessinger Publishing, LLC: Vienna, Austria, 1756; p. 396. [Google Scholar]
- Wanzenböck, J.; Spindler, T. Der Hundsfisch, (Umbra krameri Walbaum 1792) als Zielart für besonders gefährdete Feuchtgebietszonen. Artenschutzprogr. Auftr. BMUJF NÖ Landesregier. 1995. [Google Scholar]
- Spindler, T.; Lebensraummanagement des Hundsfisches (Umbra krameri) im Unteren Fadenbach. Nationalpark Donau-Auen. Wissenschaftliche Reihe, Heft 11. 2006. 24p. Available online: https://www.zobodat.at/pdf/NP-Donauauen-WissR_11_0001-0024.pdf (accessed on 28 August 2021).
- Sehr, M.; Keckeis, H. Habitat use of the European mudminnow Umbra krameri and association with other fish species in a disconnected Danube side arm. J. Fish Biol. 2017, 91, 1072–1093. [Google Scholar] [CrossRef]
- Pekárik, L.; Hajdú, J.; Košo, J. Identifying the key habitat characteristics of threatened European mudminnow (Umbra krameri, Walbaum 1792). Fundam. Appl. Limnol. 2014, 184, 151–159. [Google Scholar] [CrossRef]
- Reckendorfer, W.; Keckeis, S. Ökologische Entwicklungsziele Fadenbach. Wiss. Reihe Natl. Donau-Auen. 2016, 66, 1–49. [Google Scholar]
- Ellmauer, T.; Igel, V.; Kudrnovsky, H.; Moser, D.; Paternoster, D. Monitoring von Lebensraumtypen und Arten von Gemeinschaftlicher Bedeutung in Österreich 2016–2018 und Grundlagenerstellung für den Bericht gemäß Artikel17 der FFH-Richtlinie im Jahr 2019: Teil 1: Artikel 11-Monitoring. Umweltbundesamt GmbH, im Auftrag der österreichischen Bundesländer, Wien. 2020. Available online: https://www.verwaltung.steiermark.at/cms/dokumente/12812743_123331268/74b35f03/REP0735_Band%201_Monitoring.pdf (accessed on 28 August 2021).
- Bíró, P.; Paulovits, G. Distribution and status of Umbra krameri Walbaum, 1792, in the drainage of Lake Balaton, Hungary (Pisces: Umbridae). Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 1995, 97B, 470–477. [Google Scholar]
- Bănăduc, D. Important areas for fish in Romania—Preinventory for a draft list of Natura 2000 sites (SCIs) for five fish species. 2006; 1–26. [Google Scholar]
- Wanzenböck, J.; Keresztessy, K. Zonation of a lentic ecotone and its correspondence to life history strategies in fish. Hydrobiologia 1995, 303, 247–255. [Google Scholar] [CrossRef]
- Wolfram, G.; Kasper, V.; Sigmund, E.; Fürnweger, G. Rote Liste gefährdeter Fische und Neunaugen des Burgenlandes. Studie im Auftrag des Amtes der Burgenländischen Landesregierung, Abt. 4. Wien, 140 p. 2022. Available online: https://www.burgenland.at/fileadmin/user_upload/20220119_Rote_Liste_gefaehrdeter_Fische_Bgld_Jan2022.pdf (accessed on 28 August 2021).
- Benesch, A.R. Wiedereinbürgerung Hundsfisch (Umbra krameri W.) im österreichischen Teil des Hanság/Burgenland. Osterr. Fisch. 2004, 57, 161–165. [Google Scholar]
- Benesch, A.R. Schlussbericht Wiedereinbürgerung Hundsfisch (Umbra krameri W.) Hanság—Burgenland. Final Report of an EU Interreg IIIA Project. 2008. Available online: http://www.parcs.at/npns/pdf_public/2019/37988_20190604_135945_schlussberichthundsfischwiedereinbu776rgerung2008.pdf (accessed on 28 August 2021).
- Sallai, Z. Results of the fish faunistical survey of the Lake Fertő (Neusiedlersee) in 2017–2018. Pisces Hungarici 13: 15–32. 2019. Available online: http://real-j.mtak.hu/19629/1/Pisces_Hungarici_2019_13.pdf#page=15 (accessed on 28 August 2021).
- Kux, Z.; Weisz, T. Ichtyofauna jižní části slovenského Záhoří. Acta Musei Morav. 1961, 46, 178–202. [Google Scholar]
- Kováč, V.; Hensel, K.; Černy, J.; Otahelova, H. Ex-situ protection of Umbra krameri. Final report, Biodiversity Protection Project. FNS IE CU, Bratislava. 1996; 34, 44. [Google Scholar]
- Valachovič, D.; Kováč, V. Ochrana blatniaka tmavého ex situ v CHKO Záhorie. Chrán. Uzem. Slov. 1998, 35, 18–19. [Google Scholar]
- Kux, Z. Příspěvek k poznání ichtyofauny dunajského povodí ČSR. Acta Musei Morav. Sci. Nat. 1957, 42, 67–84. [Google Scholar]
- Mišík, V. Výskyt a rozšírenie blatniaka (Umbra krameri Walbaum 1792) na Slovensku. Biológia 1965, 20, 683–688. [Google Scholar]
- Kopáčik, L. Blatňak obyčajný na južnom Slovensku. Živa 1955, 3, 229–230. [Google Scholar]
- Balon, E.K. Ichtyofauna jazera Lion a Čilizského potoka so zreteľom na zriadenie prírodnej rezervácie. Ochr. Fauny 1967, 1, 15–22. [Google Scholar]
- Brtek, Ľ. K výskytu blatniaka tmavého (Umbra krameri Waldbaum, 1792) na Žitnom ostrove. Ochr. Fauny 1969, 3, 124. [Google Scholar]
- Hensel, K. Ryby (Pisces) priľahlých vôd štátnej prírodej rezervácie Číčovské mŕtve rameno a poznánka k výskytu blatniaka (Umbra krameri Walbaum, 1792) vo vodách Žitného ostrova. (Fish (Pisces) adjacent waters of the state nature reserve Číčovské dead arm and knowledge of the occurrence of mudminnow (Umbra krameri Walbaum, 1792) in the waters of Žitný ostrov.). Sprav. Obl. Podunajského Múz. Komár. 1984, 4, 76–81. [Google Scholar]
- Hajdú, J. Príspevok k výskytu blatniaka európskeho (Umbra krameri, Walbaum 1792) v odvodňovacích kanáloch Žitného ostrova. Ochr. Prírody 2002, 21, 175–181. [Google Scholar]
- Hajdú, J.; Kováč, V. Ichtyofauna vybraných vôd Žitného ostrova. Folia Faun. Slovaca 2002, 7, 75–81. [Google Scholar]
- Májsky, J.; Hajdú, J. Záchrana blatniaka tmavého (Umbra krameri) na Slovensku. Šop SR Banská Bystrica Správ. CHKO Dunajské Luhy 2008, 24. Available online: https://www.sopsr.sk/natura/doc/publikacie/blatniak20.pdf (accessed on 28 August 2021).
- Chyzer, K. Die Fische des Zempliner Kommitates, Jahrb. d. ungarn Karp. Ver. 1882, 9, 12–25. [Google Scholar]
- Vladykov, V. Poissons de la Russia Souscarpathique (Tchécoslovaquie). Mémoires de la Societé Zoologique de France: Paris, France, 1931; p. 29. [Google Scholar]
- Záleský, M. Za tmavci (Umbra Crameri Cuv.) ve vých. Slovensku. Akvaristické Listy 1928, 7, 145–147. [Google Scholar]
- Kirka, A.; Vranovský, M.; Mészáros, J.; Nagy, Š.; Šporka, F. Ichtyologický prieskum riek Východoslovenského kraja. Záverečná Správ. Lab. Ryb. Hydrobiol. Bratisl. 1980, VI-3-4/4-009, 70. [Google Scholar]
- Kokordák, J. Ichtyologické pomery v odvodňovacích kanáloch pri Kamennej Moľve. Poľovníctvo Rybárstvo 1974, 26, 33. [Google Scholar]
- Weisz, T.; Kux, Z. Příspěvek k poznání ichtyofauny řek Laborce, Tople a Popradu. Čas. Morav. Mus. 1959, 44, 119–138. [Google Scholar]
- Žitňan, R. Ichtyofauna československého úseku Tisy. Sborník Východoslovenského Múzea Ser. B 1965, 6, 61–67. [Google Scholar]
- Pekárik, L.; Hajdú, J. Rozšírenie Blatniaka Tmavého (Umbra krameri) na Vybraných Lokalitách Podunajskej Nížiny (Prvá časť správy z výskumu). In Distribution of the Umbra krameri in Selected Localities of the Danubian Lowland (First Part of the Research Report); ŠOP SR. Správa CHK: Bratislava, Slovakia, 2007. [Google Scholar]
- Kubalová, S. Floristický a Fytocenologický Prieskum Zameraný na Charakteristiku Habitatov Druhu Blatniak Tmavý (Umbra krameri) a Charakteristika Environmentálnych Podmienok Skúmaných Lokalít—Záverečná správa. (Floristic and Phytocenological Survey Focused on the Characteristics of Habitats of the Species Umbra krameri and the Characteristics of the Environmental Conditions of the Studied Localities—Final Report. Správa CHKO Dunajské luhy; Dunajská Streda, Slovakia. 2007. [Google Scholar]
- Hajdú, J.; Koščo, J.; Pekárik, L.; Lusková, V.; Lusk, S.; Valachovič, D.; Tomeček, J. Blatniak tmavý (Umbra krameri): Súčasný Stav a perspektívy. [European mudminnow (Umbra krameri): Present status and perspective.] XI. In Proceedings of the Česká Ichtyologická Conference, Brno, Czech, 3–4 December 2008; Kopp, R., Ed.; MZLU: Brno, Czech, 2008; pp. 67–71, ISBN 978-80-7375-246-0. [Google Scholar]
- Wilhelm, S. A Lápi Póc; Erdélyi Múzeum-Egyesület: Kolozsvár, Romania, 2008. [Google Scholar]
- Tatár, S.; Bajomi, B.; Specziár, A.; Tóth, B.; Trenovszki, M.M.; Urbányi, B.; Csányi, B.; Szekeres, J.; Müller, T. Habitat establishment, captive breeding and conservation translocation to save threatened populations of the Vulnerable European mudminnow Umbra krameri. Oryx 2017, 51, 718–729. [Google Scholar] [CrossRef]
- Tatár, S.; Sallai, Z.; Demény, F.; Urbányi, B.; Tóth, B.; Müller, T. A lápi póc fajvédelmi mintaprogram. Halászat 2010, 103, 70–75. [Google Scholar]
- Botta, I. Adatok a lápi póc (Umbra krameri WALBAUM) szaporodásbiológiájához. Halászat 1981, 74, 44–45. [Google Scholar]
- Botta, I. Néhány hazai védett halfaj gyűjtése, tartása bemutatása. Halászat 1981, 74, 18–19. [Google Scholar]
- Sallai, Z. A lápi póc (Umbra krameri Walbaum, 1792) Magyarországi Elterjedése Élőhelyi Körülményeinek és Növekedési Ütemének Vizsgálata a Kiskunsági Kolon-tóban. In A Puszta; “NIMFEA” Természetvédelmi Egyesület: Szarvas, Hungary, 2005; Volume 1, pp. 113–172. [Google Scholar]
- Bankovics, A. Éledező mocsarunk, a Kolon-tó. Búvár 1976, 31, 15–118. [Google Scholar]
- Keresztessy, K.; May, K.; Weiperth, A.; Vad, C.F.; Farkas, J. Long-term fish faunistic research and the population biology of the threatened European mudminnow in two Ramsar wetlands of the Danube-Tisza interfluve. Pisces Hung. 2012, 6, 47–54. [Google Scholar]
- Harka, A.; Sallai, Z. Magyarország Halfaunája; ‘NIMFEA’ Természetvédelmi Egyesüle: Szarvas, Hungary, 2004. [Google Scholar]
- Sallai, Z.; Vajda, Z. A Kiskunság halai. In A Puszta; “NIMFEA” Természetvédelmi Egyesület: Túrkeve, Hungary, 2015; Volume 1, pp. 93–163. [Google Scholar]
- Allendorf, F.W.; Luikart, G. Conservation and the Genetics of Populations; John Wiley Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Brauer, C.J.; Unmack, P.J.; Hammer, M.P.; Adams, M.; Beheregaray, L.B. Catchment-Scale Conservation Units Identified for the Threatened Yarra Pygmy Perch (Nannoperca obscura) in Highly Modified River Systems. PLoS ONE 2013, 8, 82953. [Google Scholar] [CrossRef][Green Version]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Eros, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Harka, Á.; Sallai, Z.; Koščo, J. Az amurgéb (Perccottus glenii) terjedése a Tisza vízrendszerében. In A Puszta; “NIMFEA” Természetvédelmi Egyesület: Túrkeve, Hungary, 2003; Volume 1, pp. 49–56. [Google Scholar]
- Grabowska, J.; Błońska, D.; Kati, S.; Nagy, S.A.; Kakareko, T.; Kobak, J.; Antal, L. Competitive interactions for food resources between the invasive Amur sleeper (Perccottus glenii) and threatened European mudminnow (Umbra krameri). Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 2231–2239. [Google Scholar] [CrossRef]
- Erős, T.; Takács, P.; Sály, P.; Specziár, A.; György, Á.I.; Bíró, P. Az amurgéb (Perccottus glenii Dybowski, 1877) megjelenése a Balaton vízgyűjtőjén. Halászat 2008, 101, 75–77. [Google Scholar]
- Takács, P.; Vitál, Z. Amurgéb (Perccottus glenii Dybowski, 1877) a Duna mentén. Halászat 2012, 105, 16. [Google Scholar]
- Weiperth, A.; Staszny, Á.; Ferincz, Á. Occurrence and spread of non native fish species in the Hungarian section of River Danube—A historical review. Pisces Hung. 2013, 7, 103–112. [Google Scholar]
- Sekulić, N.; Marić, S.; Galambos, L.; Radošević, D.; Krpo-Ćetković, J. New distribution data and population structure of the European mudminnow Umbra krameri in Serbia and Bosnia and Herzegovina. J. Fish Biol. 2013, 83, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Miljanović, B.; Sipos, S.; Pankov, N.; Bajić, A.; Muller, T. New Record of Umbra krameri Walbaum, 1792 in Serbia. In Book of Abstracts–5TH Congress of Ecologists of the Republic of Macedonia with International Participation; Melovski, L., Ed.; Macedonian Ecological Society: Skopje, North Macedonia, 2016; p. 89. [Google Scholar]
- Sekulić, N. Ekološke Karakteristike i Morfološko-genetička Diferencijacija Populacija Crnke (Umbra krameri Walbaum, 1792) sa Područja Bačke, Mačve i Semberije. [Ecological Characteristics and Morphological-Genetic Differentiation in Populations of European Mudminnow (Umbra krameri Walbaum, 1792) from Bačka, Mačva and Semberija]. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2013. [Google Scholar]
- Pančić, J.; Srbiji, R.U. Fish of Serbia; State Printing-Office of Belgrade: Belgrade, Serbia, 1860; Volume 41, p. 136. [Google Scholar]
- Janković, D. Ihtiofauna Đerdapske akumulacije pre i posle izgradnje HEPS Đerdap. Ichthyofauna of the Djerdap Reservoir before and after the construction of HPNS Djerdap. In Proceedings of the Conference “The Return of Life to the Rivers”; Brun, G., Ed.; Ministry of Environmental Protection, Belgrade: Belgrade, Serbia, 1995; pp. 135–146. [Google Scholar]
- Mediului, M. Atlasul cadastrului apelor din România. Ed. Romcart SA București 1992, 1, 239–255. [Google Scholar]
- Ujvári, I. Geografia apelor Romaniei Editura Ştiinţifică. Bucureşti 1972, 1, 591. [Google Scholar]
- Bănărescu, P. Pisces (Peşti) in Cartea Roşie a vertebratelor din România. In Botnariuc N şi Tatole V.; Academia Română: Bucureşti, Romania, 2005; p. 260. ISBN 973-0-03943-7. [Google Scholar]
- Telcean, I.C.; Mihut, R.E.; Cupşa, D. The fishes’ last stand: The fish fauna of Jiu River Gorge, between decades of coal mining and present day hydroenergetic works. J. Prot. Mt. Areas Res. Manag. 2017, 9, 15–21. [Google Scholar] [CrossRef][Green Version]
- Barbu, C. The effects of mining activity over the waters from Jiu valley. J. Appl. Econ. Sci. 2008, 4, 374–381. [Google Scholar]
- Frîncu, R.-M. Long-Term Trends in Water Quality Indices in the Lower Danube and Tributaries in Romania (1996–2017). Int. J. Environ. Res. Public Health 2021, 18, 1665. [Google Scholar] [CrossRef]
- Bănărescu, P. Considerations on the threatened fishes of Europe. Ocrot. Nat. I Med. Înconj. 1993, 37, 87–98. [Google Scholar]
- Bănărescu, P. The present-day conservation status of the freshwater fish fauna of Romania. Ocrot. Nat. Med. Înconj. 1994, 38, 1–16. [Google Scholar]
- Bănărescu, P. Situaţia actual a ihtiofaunei de apă dulce a României sub aspect faunistic, taxonomic şi al protecţiei. Stud. Univ. Vasile Goldiş Arad Ser. Ştiinţele Vieţii 2004, 14, 43–49. [Google Scholar]
- Oţel, V.; Nalbant, T.; Bănărescu, P. Rezultatele investigaţiilor ihtiologice din teritoriul Rezervaţiei Biosferei Delta Dunării în anul 1992. An. St. Inst. Delta Dunării 1993, 2, 145–162. [Google Scholar]
- Băcescu, M. Peştii, aşa cum îi vede ţăranul pescar român. Monogr. Monit. Off. Şi Impr. Statului Impr. Naţională. Bucureşti 1947, 3, 219. [Google Scholar]
- Bănărescu, P.M.; Bănăduc, D. Habitats Directive (92/43/EEC) fish species (Osteichthyes) on the Romanian territory. Acta Ichtiol. Romanica 2007, 2, 43–78. [Google Scholar]
- Movchan, Y.V.; Manilo, L.G.; Smirnov, A.G.; Shcherbukha, A.Y. The Catalogue of Collections of Zoological Museum NMNH, NAS of Ukraine. Cyclostomata and Fishes; NMNH: Kiev, Ukraine, 2003; p. 342. [Google Scholar]
- Litvinchuk, S.N.; Borkin, L.J. Distribution, Ecology and Conservation Status of the Danube Crested Newt Triturus dobrogicus (Amphibia, Salamandridae) in Ukraine and Moldova. Vestnik Zoologii. 2002, 36, 35–44. [Google Scholar]
- Smirnov, A.I.; Tkachenko, V.A. Ichthyodiversity Character as a Biotic Marker of Freshening of the Sasyk (Kunduk) Firth. Zbirnyk Pr. Zool. Muzeyu. 2007, 39, 41–56. [Google Scholar]
- Yu, M.; Dzhurtubayev, V.V.; Zamorov, M.P.; Zamorova, T.V.; Urbanska. Macrozobenthos of the Danube lake Kitay and conditions of its occurrence. Odesa Publ. House Odesa Un-Ty 2019, 1, 170. [Google Scholar]
- Csagoly, P.; Magnin, G.; Mohl, A. Danube, Drava, and Mura Rivers: The “Amazon of Europe”. In The Wetland Book; Finlayson, C., Milton, G., Prentice, R., Davidson, N., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 903–909. [Google Scholar]
- Lóczy, D. Introduction. In The Drava River; Lóczy, D., Ed.; Springer Geography: Cham, Switzerland, 2019; pp. 1–3. [Google Scholar]
- Bănăduc, D. Curtean-Bănăduc, A. Bucharest, Monitoring elements for Zingel streber (Siebold, 1863) in the context of Natura 2000 in Croatia. Rom. J. Biol. Zool. 2014, 59, 59–74. [Google Scholar]
- Bănăduc, D.; Curtean-Bănăduc, A. Rhodeus sericeus amarus Bloch, 1782; monitoring elements in the new Natura 2000 context in Croatia. Transylv. Rev. Syst. Ecol. Res. 2014, 16.1, 185–204. [Google Scholar] [CrossRef]
- Mojsisovics, M.A. Bemerkungen zur ichthyologischen Literatur des Donaugebietes. Mitt. Des Osterr. Fisch. Ver. 1893, 47, 11–12. [Google Scholar]
- Taler, Z. Rasprostranjenje i popis slatkovodnih riba Jugoslavije. Glas. Prir. Muz. Srp. Zemlje 1953, 5–6, 425–455. [Google Scholar]
- Pavletić, J. Rijetka riba-Crnka ili rapa. Ribar. Jugosl. 1954, 9, 62–64. [Google Scholar]
- Hirtz, M. Rječnik narodnih zooloških naziva—Ribe (Pisces). Jugosl. Akad. Znan. I Umjet. Zagreb 1956, 1, 478. [Google Scholar]
- Vuković, T.; Ivanović, B. Slatkovodne ribe Jugoslavije; Zemaljski Musej BiH-Prirodnjačko Odjeljenje: Sarajevo, Bosnia Herzegovina, 1971. [Google Scholar]
- Povž, M. Areal velike senčice Umbra krameri Walbaum, 1792 (Osteichthyes). Sloveniji. Ichthyol. 1984, 16, 43–48. [Google Scholar]
- Povž, M. A contribution to the knowledge on freshwater fish and lamprey of Slovenia—The Mura River. Ichthyos 1987, 5, 1–8. [Google Scholar]
- Povž, M. Conservation of the mudminnov, Umbra krameri Walbaum, in Slovenia. J. Fish Biol. 1990, 37, 243. [Google Scholar] [CrossRef]
- Povž, M. Velika senčica (Umbra krameri Walbaum 1972)—Nova vrsta ribe v Sloveniji. Varst. Narave 1990, 16, 45–48. [Google Scholar]
- Povž, M. Discovery, distribution, and conservation of mudminnow Umbra krameri Walbaum, 1972, in Slovenia. Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 1995, 97B, 478–485. [Google Scholar]
- Mrakovčić, M.; Kerovec, M. Umbra krameri. Ekološki Glas. 1990, 5–6, 68–69. [Google Scholar]
- Mrakovčić, M.; Brigić, A.; Buj, I.; Ćaleta, M.; Mustafić, P.; Zanella, D. Red Book of Freshwater Fish of Croatia; Ministry of Culture, State Institute for Nature Protection: Zagreb, Croatia, 2006; 253p. [Google Scholar]
- Veenvliet, P.; Kus Veenvliet, J. Ribe Slovenskih Celiskih Voda; Priročnik za Določanje; Zavod Symbiosis: Grahovo, Bosnia Herzegovina, 2006; 168p. [Google Scholar]
- Povž, M.; Gregori, A.; Gregori, M. Sladkovodne Ribe in Piškurji v Sloveniji; Zavod Umbra: Ljubljana, Slovenia, 2015; 293p. [Google Scholar]
- Podgornik, S.; Pliberšek, K.; Cokan, B.; Ramšak, L. Monitoring Populacij Izbranih Ciljnih Vrst Rib. Velika senčica. Ljubl. -Šmartno Zavod Ribištvo Slov. 2015. Available online: https://natura2000.gov.si/fileadmin/user_upload/knjiznica/raziskave/Natura_2000_Velika_Sencica_2015.pdf (accessed on 15 June 2022).
- Govedič, M.; Šalamun, A. Inventarizacija rib reke Drave od Maribora do Središča ob Dravi. Centar za Kartografijo Favne in Flore: Miklavž na Dravskem Polju, Slovenia. 2006. 61p. Available online: https://www.ckff.si/projekt.php?pid=9 (accessed on 15 June 2022).
- Delić, A.; Grlica, D.; Razlog-Grlica, J. Nova nalazišta crnke (Umbra krameri Walbaum 1792) u Hrvatskoj. Croat. J. Fish. Ribar. 1997, 55, 93–98. [Google Scholar]
- Jelić, D.; Vucić, M.; Jarak, M. Stručna Podloga za Izradu Akcijskog Plana Upravljanja Strogo Zaštićenom Natura 2000 Vrstom Ribe-Crnka (Umbra krameri) na Području Virovitičko-podravske županije; BIOTA: Zagreb, Croatia, 2019; 47p, Available online: https://www.interreg-danube.eu/uploads/media/approved_project_output/0001/34/87ff5e0e015d0a0049ffc535d28be6b3a09ae801.pdf (accessed on 28 August 2021).
- Jelkić, D.; Opačak, A.; Ozimec, S.; Blažetić, S.; Lužaić, R. Vukajlović, N. A new finding of the European mudminnow (Umbra krameri, Walbaum 1792) in Croatia. Poljoprivreda 2019, 25, 64–68. [Google Scholar] [CrossRef]
- Futó, J.; Futó, E.; Adorján, P.; Simon, E. A Kis-Balaton Térsége (A Balaton-Felvidék Természeti értékei 2.); Balaton-felvidéki Nemzeti Park Igazgatóság: Veszprém, Hungary, 2001; p. 112. ISBN 9630075520. [Google Scholar]
- Kis-Balaton, A. Kis-Balaton története PDF Free Download. Available online: http://www.bfnp.hu/magyar/oldalak/kettos_evfordulo_kis_balaton/ (accessed on 15 January 2022).
- Antal, L.; Csipkés, R.; Müller, Z. A fish stock survey on a few water bodies of the Kis-Balaton area. Pisces Hung. 2009, 3, 95–102. [Google Scholar]
- Wiperth, A.; Ferincz, Á.; Paulovics, G. A lápi póc (Umbra krameri) új lelöhelyei a Kis-Balaton területén. Halászat 2010, 103, 2. [Google Scholar]
- Takács, P.; Maázs, G.; Vitál, Z.; Harka, Á. Aquarium fishes in the outflow of the thermal Lake Hévíz. Pisces Hung. 2015, 9, 59–64. [Google Scholar]
- Heckel, J. Magyarország Édesvízi Halainak Rendszeres Átnézete, Jegyzetekkel s az új Fajok Rövid Leírásával. Fordította s a Tudomány újabbkori Haladásával Bővítette CHYZER Kornél; A magyar orvosok és természetvizsgálók VIII; nagygyűlésének évkönyve: Budapest, Hungary, 1847; pp. 193–216. [Google Scholar]
- Bíró, P. A tó és környékének állatvilága. In ILLÉS I. (szerk.): Tavunk a Balaton; NATURA: Debrecen, Hungary, 1981; pp. 120–133. [Google Scholar]
- Bíró, P. (Changes of Fish Fauna of Kis-Balaton). Hidrol. Tájék. 1994, 34, 32–36. [Google Scholar]
- Keresztessy, K. Recent fish faunistical investigations in Hungary with special reference to Umbra krameri Walbaum, 1972. Ann. Nat. Mus. Wien. Ser. B Bot. Und Zool. 1995, 97, 458–465. [Google Scholar]
- Vidéki, R. A Kis-Balaton Növényzete és Tájtörténete. Válogatás az első Tizenhárom MÉTA-Túrafüzetből 2003–2009; MÉTA-túrafüzetek. MTA Ökológiai és Botanikai Kutatóintézete: Pest, Hungary, 2010; pp. 398–401. Available online: http://real-eod.mtak.hu/id/eprint/11614 (accessed on 15 January 2022).
- Kis-Balaton. A Kis-Balaton Története—PDF Free Download. Available online: https://docplayer.hu/2508704-Kis-balaton-a-kis-balaton-tortenete.html (accessed on 15 January 2022).
- Minden, Amit a Balaton Algáiról Tudni Akart—Vörös Lajos Ökológus Áttekintő Írása|MTA. Available online: https://mta.hu/tudomany_hirei/minden-amit-a-balaton-algairol-tudni-akart-voros-lajos-okologus-attekinto-irasa-110849 (accessed on 15 January 2022).
- Mushtaq, N.; Singh, D.V.; Bhat, R.A.; Dervash, M.A.; Hameed, O.B. Freshwater Contamination: Sources and Hazards to Aquatic Biota. Fresh Water Pollut. Dyn. Remediat. 2020, 27–50. [Google Scholar] [CrossRef]
- Yancheva, V.; Georgieva, E.; Stoyanova, S.; Velcheva, I.; Somogyi, D.; Nyeste, K.; Antal, L. A histopathological study on the Caucasian dwarf goby from an anthropogenically loaded site in Hungary using multiple tissues analyses. Acta Zool. 2020, 101, 431–446. [Google Scholar] [CrossRef]
- Takács, P.; Czeglédi, I.; Ferincz, Á.; Sály, P.; Specziár, A.; Vitál, Z.; Weiperth, A.; Erős, T. Non-native fish species in Hungarian waters: Historical overview, potential sources and recent trends in their distribution. Hydrobiologia 2017, 795, 1–22. [Google Scholar] [CrossRef]
- Weiperth, A.; Czeglédi, I.; Ferincz, Á.; Gál, B.; Sály, P.; Specziár, A.; Staszny, Á.; Takács, P.; Vitál, Z.; Erős, T. Idegenhonos halfajok megjelenése és terjedése. In Magyarország Környezeti Állapota; Herman Ottó Intézet; Nonprofit Kft.: Budapest, Hungary, 2016; pp. 91–105. [Google Scholar]
- Golubets, M.A.; Gavrusevich, A.N.; Zagaykevych, I.K. Zdun, V.I.; Komendar, V.I.; Lugovoy, A.E.; Malinovskiy, K.A.; Milkina, L.I.; Nudelman, M.S.; Odinak, Y.P. Ukrainian Carpathians. Nature. Kyiv Nauk. Dumka 1988, 1, 208. [Google Scholar]
- Kurtiak, F.; Talabishko, Y.; Stegun, V.; Velykopolskiy, I. Ichthyofauna of the Latoryza River basin within the borders of Ukraine. Visnyk Lviv. Un-Ty. Biol. Ser. Iss. 2009, 50, 85–94. [Google Scholar]
- Kurtiak, F.F.; Bondar, P.P. Ichthyofauna of Transcarpathia: Rare categories and protection principles. Nauk. Visnyk Uzhgoorodskogo Un-Tu. Seriya Biologiya. Iss. 2014, 36, 56–58. [Google Scholar]
- Afanasyev, S.A.; Guleikova, L.V.; Konovalenko, O.S. Ecological Potential of the Water Bodies of the Ameliorated Flood Lands of the Rivers of the Transcarpathian Lowland; Iva Publish: Uzhgorod, Ukraine, 2010; 80p. [Google Scholar]
- Kiss, R.Y.; Afanasyev, S.O.; Stankevych-Volosianchuk, O.I. (Eds.) Biodiversity of the Tisza and Tur Interfluve: Assessment of Actual State and Conservation Measures; Uzhgorod, RIK-U Publish: Uzhgorod, Ukraine, 2017; 172p. [Google Scholar]
- Afanasyev, S.A. Lietitskaya, Y.N., Manturova, O.V. Altitude distribution and structural organization of hydrobionts’ communities in the rivers of the mountainous part of the Tisa river basin. Hydrobiol. J. 2013, 49, 16–25. [Google Scholar] [CrossRef]
- Afanasyev, S.O.; Dolinsky, V.L.; Lietytska, O.M.; Savchenko, E.V.; Golub, O.O.; Kykyluk, O.P.; Msnturova, O.V. Substantiation of fish stocking and ichthyoamelioration monitoring of the Beregove polder system. In Actual Problems of Theoretical and Practical Ichthyology, Proceedings of the VII Internat. Ichthyological Conference. Melitopol, Berdians, 10–13 September 2014; Grin Publish: Kherson, Ukraine, 2014. [Google Scholar]
- Polyák, L.; Somogyi, D.; Antal, L.; Nyeste, K. A lápi póc (Umbra krameri) utolsó ismert populációja a Felső-Tisza-vidékén. Halászat 2020, 113, 114. [Google Scholar]
- Hoitsy, G. Adatok a Bodrog és a Bodrogzug hal-ökofaunisztikai felméréséből. In Proceedings of the XVIII. Halászati Tudományos Tanácskozás, Szarvas, Hungary, 15–16 June 1994; pp. 164–172. [Google Scholar]
- Sevcsik, A.; Tóth, B. Lápi póc (Umbra krameri), réticsík (Misgurnus fossilis) és amurgéb (Perccottus glenii) az Öreg-Túr alsó szakaszán. Halászat 2011, 104, 39. [Google Scholar]
- Nyeste, K.; Antal, L.; Abonyi, T.; Somogyi, D. A lápi póc (Umbra krameri) újabb adata az Öreg-Túrból. Halászat 2021, 114, 141. [Google Scholar]
- Simon, T. Montan elemek az Északi-Alföld flόrajában és növénytakarόjában. III. Ann. Biol. Univ. Hung. 1951, 2, 249–286. [Google Scholar]
- Harka, Á.; Sallai, Z.; Wilhelm, S. A Túr és mellékvizeinek halai. Halászat 2003, 96, 37–44. [Google Scholar]
- Sándor, W. A Berettyó és mellékvizei halfaunájának változásai. Pisces Hung. 2007, 1–2, 106–112. [Google Scholar]
- Nyeste, K.; Somogyi, D.; Bereczki, C.; Antal, L. Halmentés a beregi Zsid-tónál. Halászat 2022, 115, 14. [Google Scholar]
- Endes, M.; Harka, Á. A Heves—Borsodi-Síkság Gerincesfaunája; Tiszai Téka 2: Eger, Hungary, 1987. [Google Scholar]
- Király, G.; Molnár, Z.; Bölöni, J.; Vojtkó, A. Magyarország Földrajzi Kistájainak Növényzete [Plantgeography of HUNGARY’SMICROREGIONS]; MTA Ökológiai és Botanikai Kutatóintézete: Vácrátót, Hungary, 2008; p. 248. [Google Scholar]
- Harka, Á. Halfaunisztikai megfigyelések a Bükk hegység déli előterének vízfolyásain. Természet 1992, 46, 108–109. [Google Scholar]
- Harka, Á. Magyarország faunájának új halfaja: Az amurgéb (Perccottus glehni Dybowski, 1877). Halászat 1998, 91, 32–33. [Google Scholar]
- Sallai, Z. Lápi póc (Umbra krameri) a Szelep-érből. Halászat 2022, 105, 14. [Google Scholar]
- Harka, Á.; Györe, K.; Sallai, Z.; Wilhelm, S. A Berettyó halfaunája a forrástól a torkolatig. Halászat 1998, 91, 68–74. [Google Scholar]
- Herman, O. A Magyar Halászat Könyve, I.-II.; Magyar TermészettudományivTársulat: Budapest, Hungary, 1887. [Google Scholar]
- Braun, Á.; Tatár, S.; Tóth, B.; Urbányi, B.; Müller, T. Pond monitoring and cage rearing of crucian carp (Carassius carassius) in pilot study of European mudminnow conservation program. Pisces Hungarici 2018, 12, 37–45. [Google Scholar]
- Antal, L.; Czeglédi, I.; Mozsár, A.; Halasi-Kovács, B. Terjed az amurgéb (Perccottus glenii) a Berettyó hazai vízgyűjtőjén. Halászat 2011, 3–4, 84. [Google Scholar]
- Posea, G. Enciclopedia Geografică a României; Editura Stiinţifică şi Enciclopedică: Bucureşti, Romania, 1982. [Google Scholar]
- Bănărescu, P.M.; Oţel, V.; Wilhelm, A. The present status of Umbra krameri Walbaun in Romania. Ann. Nat. Mus. Wien. Ser. B Bot. Zool. 1995, 97, 496–501. [Google Scholar]
- Schwarz, U. 2016: Sava White Book. The River Sava: Threats and Restoration Potential. Radolfzell/Wien: EuroNatur/Riverwatch. Available online: https://www.icpdr.org/main/danube-basin/sava-basin (accessed on 28 August 2021).
- Available online: https://zastita-prirode-smz.hr/zastcena-podrucja/odransko-polje (accessed on 28 August 2021).
- Langhoffer, A. Popis riba koje su prispjele hrv. zemaljskom zoologičkom muzeju od god. 1901. do konca god. 1905. Glas. Hrvat. Naravoslavnog Društva 1908, 20, 114–126. [Google Scholar]
- Zanella, D. Rasprostranjenost i Zaštita Vrste Umbra krameri Walbaum, 1792 u Republici Hrvatskoj. Bachelor’s Thesis, University of Zagreb, Zagreb, Croatia, 1997; pp. 1–56. [Google Scholar]
- Bănăduc, D.; Curtean-Bănăduc, A. Management elements proposal for Sutla Natura 2000 site. Transylv. Rev. Syst. Ecol. Res. 2015, 17, 145–152. [Google Scholar] [CrossRef][Green Version]
- Popijač, A.; Popijač, E.; Pušić, I.; Jelić, D.; Špelić, I. Research of Presence of European Mudminnow (Umbra krameri) within the Lonjsko Polje Nature Park; Oikon: Zagreb, Croatia, 2016; pp. 1–22. Available online: https://oikon.hr/research-presence-european-mudminnow-umbra-krameri-within-lonjsko-polje-nature-park/ (accessed on 28 August 2021).
- Pišl, Z.; Jelić, D.; Lisjak, D.; Pušić, A.; Kalčićek, M. Poredbena Analiza Bogatstva Ihtiofaune i Bioloških Svojstava vode u Gornjem I Srednjem Toku Rijeke Odre; IRES: Zagreb, Croatia, 2016; pp. 1–35. [Google Scholar]
- Grubešić, M.; Trupčević, M.; Margaletić, J.; Pernar, N. The Žutica forest as a model of ingeral natural resource management. Book of Abstracts of the 4. In Proceedings of the International scientific-expert meeting on oil economy, Zagreb, Croatia, 2–5 October 2007; pp. 7–19. [Google Scholar]
- Petronić, S.; Panić, G.; Radošević, D.; Travar, J. Rare and endangered plant and animal species in the Special Natural Reserve "Gromiželj". In Book of Abstracts–5TH Conference on Integrative Protection; Šiljegović, S., Ed.; Republic Institute for Protection of Cultural, Historical and Nature Heritage of Republic of Srpska: Banja Luka, Bosnia Herzegovina, 2010; pp. 199–206. [Google Scholar]
- Maletin, S.; Miljanović, B.; Djukić, N.; Teodorović, I. Naselje riba u specijalnom rezervatu prirode Zasavica [Fish assemblage in the Special Nature Reserve Zasavica]. In Zasavica 2001; Igić, R., Gajin, S., Eds.; Faculty of Natural Sciences and Mathematics, Institute of Biology, University of Novi Sad: Sremska Mitrovica, Serbia, 2001; pp. 70–75. [Google Scholar]
- Simić, V.; Simić, S.; Paunović, M.; Petrović, A.; Stanković, M. Neke Ugrožene Vrste u Specijalnom Rezervatu Prirode “Zasavica” (Umbra Krameri, Pisces i Batrachospermum Gelatinosum, Rhodophyta) [Some Threatened Species in the Special Nature Reserve “Zasavica” (Umbra Krameri, Pisces i Batrachospermum Gelatinosum, Rhodophyta)]; Pokret gorana Sremska Mitrovica: Sremska Mitrovica, Serbia, 2007. [Google Scholar]
- Bănăduc, D.; Stroilă, V.; Curtean-Bănăduc, A. The Fish Fauna of the Timiş River (Banat, Romania) Transylv. Rev. Ecol. Res. 2013, 15, 145–172. [Google Scholar]
- Bănăduc, D.; Stroilă, V.; Curtean-Bănăduc, A. Baki, Carmen Barb, Isabella Serrano, Angela Curtean-Bănăduc. Coştei Hydrographic Diversion Node, a Historical Environment Quality and Biological Resources Accessibility Game Changer; Anthropogenic Induced Problems and Sustainable Solutions—An Ichthyologic Perspective. Transylv. Rev. Ecol. Res. 2021, 23, 87–114. [Google Scholar]
- Burghelea, B.; Bănăduc, D.; Curtean-Bănăduc, A. The Timiş River Basin (Banat, Romania) Natural and Anthropogenic Elements, A Study Case–Management Chalenges. Transylv. Rev. Ecol. Res. 2013, 15, 173–206. [Google Scholar] [CrossRef]
- Available online: https://www.researchgate.net/publication/359237352_Transboundary_Dniester_River_basin_ecological_state_reference_conditions_management_S_Afanasyev_O_Manturova_Eds_Kyiv_2021_-_384_pp (accessed on 28 August 2021).
- Snigirev, S.M.; Medinets, V.I.; Abakumov, A.N.; Karakash, S.F. Investigations of mudminnow Umbra krameri Walbaum, 1792 in the Lower Dniester basin in 2006–2010. In Proceedings of the Ecological and Economic Problems of the Dniester River: Abstracts of International Scientific and Practical Conference, Odesa, Ukraine, 10 October 2010; INVATs Publish: Odesa, Ukraine, 2010. 52p. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bănăduc, D.; Marić, S.; Cianfaglione, K.; Afanasyev, S.; Somogyi, D.; Nyeste, K.; Antal, L.; Koščo, J.; Ćaleta, M.; Wanzenböck, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability 2022, 14, 13493. https://doi.org/10.3390/su142013493
Bănăduc D, Marić S, Cianfaglione K, Afanasyev S, Somogyi D, Nyeste K, Antal L, Koščo J, Ćaleta M, Wanzenböck J, et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability. 2022; 14(20):13493. https://doi.org/10.3390/su142013493
Chicago/Turabian StyleBănăduc, Doru, Saša Marić, Kevin Cianfaglione, Sergey Afanasyev, Dóra Somogyi, Krisztián Nyeste, László Antal, Ján Koščo, Marko Ćaleta, Josef Wanzenböck, and et al. 2022. "Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction?" Sustainability 14, no. 20: 13493. https://doi.org/10.3390/su142013493
APA StyleBănăduc, D., Marić, S., Cianfaglione, K., Afanasyev, S., Somogyi, D., Nyeste, K., Antal, L., Koščo, J., Ćaleta, M., Wanzenböck, J., & Curtean-Bănăduc, A. (2022). Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability, 14(20), 13493. https://doi.org/10.3390/su142013493







